Abstract
Background ‘Kangaroo mother care’ (KMC) includes thermal care through continuous skin-to-skin contact, support for exclusive breastfeeding or other appropriate feeding, and early recognition/response to illness. Whilst increasingly accepted in both high- and low-income countries, a Cochrane review (2003) did not find evidence of KMC’s mortality benefit, and did not report neonatal-specific data.
Objectives The objectives of this study were to review the evidence, and estimate the effect of KMC on neonatal mortality due to complications of preterm birth.
Methods We conducted systematic reviews. Standardized abstraction tables were used and study quality assessed by adapted GRADE methodology. Meta-analyses were undertaken.
Results We identified 15 studies reporting mortality and/or morbidity outcomes including nine randomized controlled trials (RCTs) and six observational studies all from low- or middle-income settings. Except one, all were hospital-based and included only babies of birth-weight <2000 g (assumed preterm). The one community-based trial had missing birthweight data, as well as other limitations and was excluded. Neonatal-specific data were supplied by two authors. Meta-analysis of three RCTs commencing KMC in the first week of life showed a significant reduction in neonatal mortality [relative risk (RR) 0.49, 95% confidence interval (CI) 0.29–0.82] compared with standard care. A meta-analysis of three observational studies also suggested significant mortality benefit (RR 0.68, 95% CI 0.58–0.79). Five RCTs suggested significant reductions in serious morbidity for babies <2000 g (RR 0.34, 95% CI 0.17–0.65).
Conclusion This is the first published meta-analysis showing that KMC substantially reduces neonatal mortality amongst preterm babies (birth weight <2000 g) in hospital, and is highly effective in reducing severe morbidity, particularly from infection. However, KMC remains unavailable at-scale in most low-income countries.
Keywords: Neonatal mortality, newborn care, preterm births, prematurity, low birthweight, Kangaroo Mother Care, Kangaroo Care, skin-to-skin care
Background
Preterm birth (<37 completed weeks of gestation) is the largest direct cause of neonatal mortality, accounting for an estimated 27% of the 4 million neonatal deaths every year1 and is also the most important risk factor for neonatal deaths for example from infection.2 In high-income countries where tetanus, neonatal infections and intrapartum-related neonatal deaths are rare, preterm birth is the dominant cause of neonatal mortality and morbidity and a major contributor to long term impairment. In low-income countries, whilst deaths directly due to preterm birth are a smaller proportion of deaths, the cause-specific mortality rate is ∼6-fold greater than in high-income countries. For example, the preterm cause-specific neonatal mortality rate in Europe is ∼1.5 per 1000 births, compared with almost 10 per 1000 births in Africa.2 This is a reflection of the lack of even basic care.1 Each year 60 million babies are born outside facilities and even among those born in facilities in low-income countries, few babies who need it receive basic care let alone intensive care with ventilator support.3 Most published trials of neonatal care focus on incremental gains with high-technology care—for example changes in ventilation methods—and have been of limited relevance to the settings with 99% of neonatal deaths.1
The burden on health systems imposed by care of preterm infants in high-income countries is considerable and well recognized. Indeed it is estimated that the cost of care for a single preterm birth in the USA is US$ 51 600.4 This challenge still largely remains invisible in low-income countries but is actually of greater magnitude as preterm birth rates are higher and the resources available fewer, characterized by understaffed hospitals with ill equipped or non-existent neonatal care units which ultimately result in higher neonatal mortality rates.5
In the early 1970s, motivated by problems arising from shortage of incubators and also the impact of mother and newborn separation, Colombian paediatrician Edgar Rey developed a technologically simple method later named ‘Kangaroo mother care’ (KMC).6 KMC has three main components including thermal care through continuous skin-to-skin contact by being tied with a cloth to the front usually of the mother; support for exclusive breastfeeding or other appropriate feeding; and early recognition and response to complications.7 In addition, it is postulated that the baby is colonized by the mother’s commensual organisms reducing the risk of nosocomial infection especially in a hospital environment. KMC can be started after birth as soon as the baby is clinically stable, and can be continued at home until the baby is stronger and begins to wriggle out which is often around the time the baby would have been born if they had been full term.
Acceptance of the KMC method is increasingly widespread and it is considered equivalent to conventional neonatal care for stable preterm infants and more parent and baby friendly.8,9 World Health Organization (WHO) guidelines have been published.10 Uptake of KMC in high-income country neonatal units has been highlighted as a rare example of south-to-north transfer of health interventions.5 However, the most recent Cochrane review of KMC (last updated in 2003) reported no evidence of a mortality effect.11 The lack of convincing mortality benefit has been an impediment to uptake.
Objective
This review aims to assess the effect on neonatal mortality from complications of preterm birth of KMC compared to no care at all or compared to conventional care. A neonatal death in a baby with a birth weight of <2000 g (∼32–34 weeks gestation) is most commonly due to complications of prematurity and hence we assume that deaths in this birth weight category can be considered to be cause-specific for direct complications of preterm deaths.
Methods
Searches
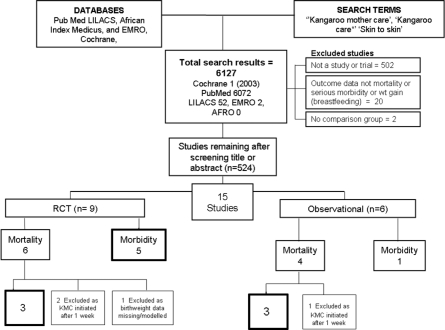
Systematic searches were undertaken of electronic databases including Cochrane Libraries, PubMed, LILACS, African Medicus, EMRO and all World Health Organization Regional Databases and included publications in any language (Figure 1). Online searches of major conference proceedings were also conducted in order to identify unpublished literature. The key search terms included were: ‘Kangaroo Mother Care’, ‘Kangaroo Care’ and ‘Skin to skin care.’ The systematic searches were for studies published between 1968 and 8 September 2009. After initial screening of titles and abstracts we reviewed full-text publications of possible studies.
Figure 1.
Synthesis of study identification in review of the effects of KMC on neonatal morbidity and mortality in preterm labour. Bold boxes signifies new meta-analysis undertaken (searches from 1970 to 9 September 2009)
Inclusion/exclusion criteria, abstraction
We applied the PICO format (Patient, Intervention, Comparison and Outcome) to define the studies to be included as follows. The population of interest were neonates, and the intervention being studied was KMC as defined above (continuous skin-to-skin contact; support for exclusive breastfeeding or other appropriate feeding; and early recognition and response to complications). KMC is commenced once the neonatal is stable irrespective of age, but in some of the early trials KMC was only commenced after the first week of life. Since 75% of neonatal deaths occur in the first week1 these criteria would introduce substantial survival bias and not be comparable with studies examining early initiation of KMC. We therefore excluded trials which only commenced KMC after the first week of life. The comparison population is conventional care which depending on the study setting may be incubator care or more limited care. We sought to identify randomized controlled trials but given the lack of such studies especially in low income settings we also reviewed observational studies fitting the above criteria.
The outcomes of interest were (i) neonatal mortality due to complications of preterm birth as used in International Classification of Disease version 10 and for global estimates for neonatal mortality; and (ii) serious neonatal morbidity related to prematurity (respiratory distress syndrome, pneumonia, septicaemia). Neonatal refers to the first 28 days of life. Where studies only reported mortality for another time period (e.g. infant) we wrote to principal investigators to request the neonatal–specific data. Given that most studies do not report cause specific mortality, or even gestation-specific mortality, a birthweight limit had to be defined as a surrogate. Based on birth weight and gestational age charts and on dataset with cause-specific mortality data by weight and gestational age, a birth weight of ≤2000 g has previously been defined by WHO as an acceptable equivalent for preterm birth likely to be the major underlying cause of death.2 Studies of KMC which reported only weight gain, breastfeeding status or psycho-social outcomes were not analysed here.
All studies meeting the inclusion criteria were double data abstracted into a standardized form. We abstracted key variables with regard to the study identifiers and context, study design and limitations, intervention specifics, and outcome effects. We assessed the quality of each of these studies using a standard approach developed by the Child Health Epidemiology Reference Group (CHERG) based on an adaptation of the GRADE approach.12 For studies which reported mortality outcomes that were not neonatal specific, we contacted the authors to request the neonatal specific data.
Analyses, and summary measures
We planned a priori to conduct three meta-analyses, two for mortality outcomes (one with randomized controlled trials (RCTs) as inputs and one with observational studies), and one for morbidity outcomes (RCTs only). We also planned to undertake additional sensitivity analysis to examine bias that may be introduced by excluding certain studies not meeting our inclusion criteria. We conducted all meta-analysis using STATA version 10.0 statistical software13 and report the Mantel–Haenszel pooled relative risk (RR) and corresponding 95% confidence interval (CI). Heterogeneity between studies was summarized using the I2 statistic. If this statistic exceeded 10% then a random effects analysis was performed as opposed to fixed effects. We summarized the overall quality of evidence for each outcome and each data input type using an adapted version of the GRADE protocol table.12
Results
Our searches identified 6127 titles (Figure 1). After initial screening of titles and abstracts we reviewed 524 papers for the outcomes of interest, including several in French, Spanish and Portuguese. We identified 15 studies of which nine studies8,14–21 were either individually randomized (eight) or cluster randomized trials (one),14 six were observational studies.22–27 All the studies were from low or middle-income countries—Colombia, Ethiopia, Ecuador, Ethiopia, Indonesia, Bangladesh, India, Mexico and South Africa. None of the studies were blinded, as this was not possible for KMC. Some of the studies only tracked pre-discharge mortality, but given the average length of stay of several weeks and the fact that most deaths are in the first few days of life, this is unlikely to result in major bias. The details of each study and quality assessment using GRADE are summarized in Supplementary Table 1.
In all but one of the studies, the intervention was only offered to babies with birth weight <2000 g who were hospital inpatients. In one trial KMC was commenced at home and included babies of all birth weights but there was no mortality benefit detected for babies >2000 g.14 Our inclusion criteria was for only babies <2000 g as an indication of the effect on preterm specific mortality. The only trial including babies with a birth weight >2000 g did find evidence of a mortality effect for such babies. Only two studies included specified a lower weight limit for exclusion of babies for KMC, set at 1000 g.18,27 For all but two studies the KMC was reported to be continuous (i.e. 24-h skin-to-skin contact). In two trials the investigators report that the practice may not have been continuous.14,15
RCTs
We identified nine RCTs, of which six had mortality outcomes (Figure 1, Table 1).8,14–18 In two of the early studies.17,18 KMC was started after babies were one week old or older. Based on our exclusion criteria described above, given the substantial risk of bias, these were excluded from both mortality and morbidity analysis. Concerning the grade quality assessment of RCT trials, Table 1 shows that there were minor limitations in most studies, such as the assessment not being blinded, although by its nature KMC as an intervention cannot be blinded. All but one study specified that data were analysed by intention to treat.14 More details of the potential sources of bias in each trail are given in Supplementary Table 1. The Bangladesh community trial14 had important limitations and was therefore excluded from our primary analyses. The main trial results apply to all babies, not just those <2000 g. However, not all babies in the intervention arm received KMC, and those who did may not have received 24-h KMC.14 Furthermore data on birth weight was missing for the majority of babies (65%) and so the reported estimate of effect in babies below 2000 g is based on ‘modelled data’.14
Table 1.
RCTs identified which compare mortality outcomes in babies receiving KMC to those receiving standard care
| Study | References | Country | Case definition (numbers in trial) | Median day of commencing KMC | Outcome | Design/ limitations |
|---|---|---|---|---|---|---|
| 1 | Charpak et al.,8 1997a | Colombia (facility) | Neonates <2000 g (n = 746) | 4 days | Mortality at 12 months but provided neonatal specific data | RCT—outcome assess not blinded |
| 2 | Suman et al.,15 2008 | India (facility) | Neonates <2000 g (n = 206) | 3.7 days | Mortality at 9 months but provided neonatal specific data | RCT—outcome assess not blinded |
| 3 | Worku et al.,16 2005 | Ethiopia (facility) | Neonates <2000 g (n = 123) | 10 h | Neonatal mortality | RCT—poor description of R and follow up |
| X | Sloan et al.,14 2008 | Bangladesh (community) | All neonates (n = 4165) (<2000 g = 166 and analysis restricted to <2000 g) | 4 h | Neonatal mortality | Cluster RCT, more erratic implementation of KMC. Birthweight data missing for 65%. Possible undercounting of deaths |
| X | Sloan et al.,17 1994a | Ecuador (facility) | Neonates <2000 g (n = 300) | 12.4 days | Mortality at 6 months | RCT—outcome assess not blinded |
| X | Cattaneo et al.,18 1998a | Mexico, Indonesia, Ethiopia (facility) | Neonates 1000–1999 g (n = 285) | 10 days | Pre-discharge mortality | RCT—outcome assess not blinded |
X indicates not included in this analysis because intervention (KMC) only commenced after the first week of life and >75% of deaths in very low birth weight babies occur during this time. See text for details and sensitivity analysis.
aIncluded in Cochrane 2003, Conde-Agudelo A et al.11
Mortality Outcome in RCTs
Among the six RCTs with mortality data, two studies in which the intervention was initiated after 7 days post-delivery were excluded.17,18,21 Two studies8,15 reported mortality at 12 and 9 months, respectively. We wrote to principal investigators of studies that did not report data for the neonatal period to obtain this information.8,15 The Bangladesh community-based KMC trial14 was excluded as discussed above.
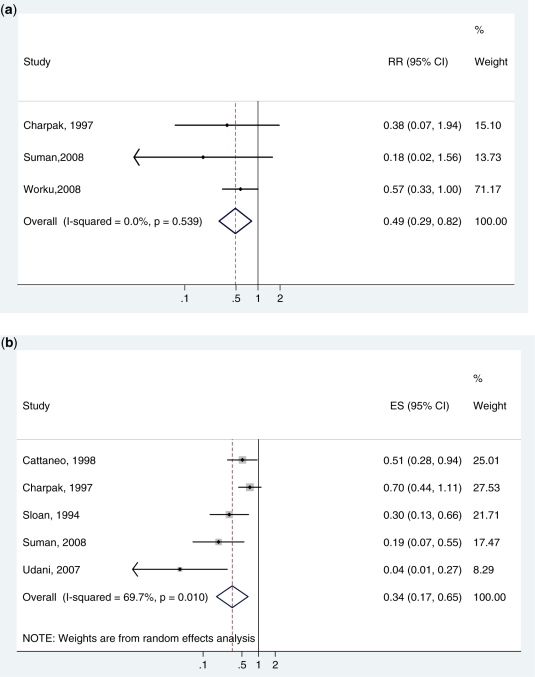
Three studies, all from low/middle-income countries, were thus included in the final meta-analysis8,15,16 (Figure 2a). Only Charpak’s study was in the previous Cochrane mortality meta-analysis but in that analysis the neonatal specific mortality data were not available. The studies were all of moderate or high quality. In our meta-analysis, KMC was associated with a major reduction in neonatal death for babies <2000 g (RR 0.49, 95% CI 0.29–0.82, I2 = 0, 3 studies, 988 infants) (Figure 2a).
Figure 2.
(a) Meta-analysis of three RCTs comparing KMC with standard care showing cause-specific mortality effect for babies of birth weight <2000 g (assumed to be deaths due to direct complications of preterm birth) and excluding studies where KMC was started after the first week of life. (b) A meta-analysis of five RCTs comparing KMC with standard care showing effect on severe morbidity (severe pneumonia, sepsis, jaundice and other severe illness) for babies of birthweight <2000 g and excluding studies where KMC was started after the first week of life. Unpublished neonatal specific data courtesy of authors, Charpak and Suman
We undertook a sensitivity analysis to examine the effect on neonatal mortality of including the two trials with late initiation of KMC.17,18 As expected the estimated risk ratio was closer to 1 (RR 0.64; 95% CI.0.42–0.96), although still a significant mortality effect (plot not shown).
A second sensitivity analysis was undertaken by including the Bangladesh community KMC study14 either with all the babies, or restricted to those <2000 g (modelled data). We estimated a design effect (1.24) from the CIs presented by the authors unadjusted and adjusted for the design effect.14 If restricted to just the babies <2000 g, this had no effect on the point estimate with (RR 0.49; 95% CI 0.32–0.76). If all the babies in the study irrespective of birth weight are included in the meta-analysis there is evidence of heterogeneity (I2 = 55%, P = 0.08). Using a random effects meta-analysis where as the RR is 0.68, the CI is wide (0.38, 1.22), with no significant evidence of a mortality benefit.
Morbidity Outcome in RCTs
Morbidity was defined as severe infection such as sepsis, necrotizing enterocolitis and severe pneumonia although some authors did not give very clear definitions. A total of five studies met the inclusion criteria and were included in the meta-analysis, all studies were of moderate or high quality. All five studies reported reductions in severe morbidity although there is evidence of heterogeneity across trials (I2 = 70%, P = 0.01). KMC was associated with a reduction in serious neonatal morbidity (RR = 0.34, 95% CI 0.17–0.65, five studies, 1520 babies) (Figure 2b).
Observational studies
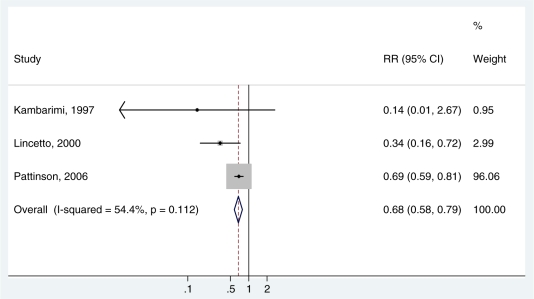
We conducted a further analysis of mortality using three observational studies which had a relevant comparison group (Table 2).23,25,27 One study8 was excluded since KMC was started when babies were 1 week old.22 The meta-analysis showed a reduction in neonatal mortality in babies <2000 g, which was slightly smaller than that observed in the randomized trials (RR 0.68, 95% CI 0.58–0.79, three studies, 8257 infants) (Figure 3). This result was largely driven by a large South African evaluation even though we restricted the inputs sites with comparable before and after audit data (8151 babies).27 The intervention was variably implemented in the different institutions and data collection was through routine mortality audit. Two of the other studies had weight limits <2000 g to commence KMC (160023 and 1800,25 respectively) but these contributed very small numbers of babies compared to the South African study. The Mozambique and Zimbabwean studies reported implementation problems such as non-acceptance by mothers of ‘abnormal’ or ill babies and increased workload on staff.23,25 Effect size was smaller in both these studies, and non significant in the Zimbabwean study.23
Table 2.
Observational studies identified with mortality outcomes comparing babies receiving KMC with those receiving standard care
| Study | References | Country | Case definition (numbers in trial) | Median day of commencing KMC | Outcome | Study limitations |
|---|---|---|---|---|---|---|
| 1 | Pattinson et al.,27 2006 | South Africa | Neonates 1000–1999 g (n = 17855) | Not reported but protocol for early starting | Pre-discharge mortalitya | Before and after routine mortality audit data Intervention variably implemented between sites |
| 2 | Kambarami et al.,23 1998 | Zimbabwe | Neonates <1600 g (n = 74) | 5 | Pre-discharge mortality a | Infants in KMC group were older & heavier—possible source of bias |
| 3 | Lincetto et al.,25 2000 | Mozambique | Neonates <1800 g (n = 32) | 2.9 | Mortality at 3 months (loss to follow up not given)b | implementation challenges reported e.g. resistance of mothers to accept small, ill babies, case managerial problems |
| X | Charpak et al.,22 1994 | Colombia | Neonates <2000 g (n = 332) | 9.1 | Mortality at 12 months (13% loss to follow up)b | KMC infants chosen weighed less, were older at eligibility and had more neonatal complications. Mortality was higher in KMC but reversed after adjustment for weight at birth |
X indicates not included in this analysis because morbidity only assessed after the neonatal period. Pattinson only data from same sites with before/after comparison used.27
aUnderestimate of effect for neonatal period.
bPossible overestimate of effect for neonatal period.
Figure 3.
A meta-analysis of three observational trials comparing KMC with standard incubator care showing cause specific mortality effect for babies of birthweight <2000 g (assumed to be deaths due to direct complications of preterm birth). Pattinson data restricted to sites with comparable before/after data27
Discussion
This is the first meta-analysis presenting evidence of the mortality benefit of KMC. We report a large cause-specific decrease of 51% (95% CI 18–71% reduction) in neonatal deaths with birth weight of <2000 g based on three RCTs (988 babies). A meta-analysis of three observational studies estimated a somewhat smaller effect (32% reduction), although these data are of lower quality and were in usual health system implementation settings. It is evident that KMC has a substantial mortality effect compared with conventional neonatal care, and it is also evident that this mortality benefit is possible even at large scale.27
Our analysis underestimates the overall health benefits as we did not include non-fatal outcomes or effects beyond the neonatal period. The Cochrane review for KMC assessed other outcomes in addition to mortality and morbidity including weight gain, breastfeeding and psycho-social outcomes such as bonding and maternal satisfaction and length of hospital stay. We have not reported on these here given our purpose of estimating mortality effects, but it is clear that there are other positive outcomes for the baby, mother and also the health system in terms of reduced work load for nurses and early discharge from hospital care.
This mortality estimate has a high evidence grade with the three RCTs from low and middle-income countries showing extremely consistent results, with only a slight reduction in quality as assessment was not blinded (Table 3). The meta-analysis of observational trials provides supportive evidence of a substantial mortality reduction, although largely driven by one large study using before and after audit data. We undertook two sensitivity analyses examining the exclusions made but still only applied for babies <2000 g. In both analysis significant evidence of large mortality effects remained (RR 0.60 and 0.62). However if all the normal birthweight babies in the Bangladesh community-based study were included, the uncertainty bounds were so wide that the result was no longer significant.
Table 3.
Quality assessment grade table of studies by outcome, as well as results from corresponding meta-analyses
| Quality assessment |
Summary of findings |
|||||||
|---|---|---|---|---|---|---|---|---|
| No of studies (ref.) | Design | Limitations | Consistency | Directness |
No of events in total |
RR (95% CI) | ||
| Generalizability to population of interest | Generalizability to intervention of interest | Intervention | Control | |||||
| Mortality: RCT data, high quality | ||||||||
| 38,15,16 | RCT | Slight reduction in quality as assessment not blinded | Consistent | All MIC/LICs but comparison group is good incubator care apart from Ethiopian study | Direct – cause specific mortality (BWT <2000 g), although some variability in mortality time period | 17 in 517 | 33 in 471 | RR = 0.49 (0.29–0.82) |
| Mortality: Observational studies, low quality | ||||||||
| 323,25,27 | Observational | Low quality | Consistent direction of effect but some heterogeneity | All MIC/LICs but comparison group is good incubator care | Direct – cause specific mortality (BWT <2000 gms), although some variability in mortality time period | 281 in 4585 | 329 in 3672 | RR = 0.68 (0.58–0.79) |
| Morbidity: RCT data, high quality evidence, but indirect to mortality effect | ||||||||
| 58,15,17,18,19 | RCT | Slight reduction in quality as assessment not blinded | Consistent direction of effect but some heterogeneity | All MIC/LICs but comparison group is good incubator care apart from Ethiopian study | Morbidity | 54 in 782 | 131 in 738 | RR = 0.34 (0.17–0.65) |
LIC, low-income countries; MIC, middle-income countries.
There were several aspects of the studies we examined which mean that the mortality effect we have obtained may be an ‘underestimation’ of the benefit possible in many low-income settings (Box 1). First, the control group in most studies was routine incubator care whereas currently for most of the more than one million neonatal deaths from preterm birth complications, there is often no medical care at all. Secondly, in the earlier trials there was a tendency towards later initiation of KMC with strict restrictions regarding age, weight or clinical status of babies. The practice now is to start KMC earlier as soon as the baby is clinically stable and this should result in a higher impact since the majority of neonatal deaths especially for small babies occur in the first few days of life. Finally, some of the studies only tracked pre-discharge mortality and did not cover the whole neonatal period, giving rise to the possibility of an under-estimation of post-discharge neonatal deaths. However there are also important potential biases that may result in an ‘overestimation’ of effect size, notably the selection basis for starting KMC in that only clinically stable preterm infants qualify to start KMC hence this effect size may not be reflect the reduction possible for all preterm deaths. Only one study specified a lower birth weight limit for starting KMC (1000 g).27 It may be that in settings with no medical care at all for the smallest babies, KMC may be better than nothing—this requires further evaluation.
Box 1 Cause specific mortality effect and quality grade of the estimate for the effect of facility-based KMC.
Cause specific mortality to act on:
Preterm direct complications (within neonatal period)
Cause specific effect and range:
51% reduction (18–71%)
Quality of input evidence:
High (Three RCTS in low/middle-income countries), Mortality and morbidity data consistent
Observational data from large scale implementation trials are consistent
Proximity of the data to cause specific mortality effect:
High (cause specific mortality)
Limitations of the evidence:
Several systematic biases resulting in underestimation of mortality effect
The control group in all these studies is routine incubator care, whereas the group of interest for policy/programmes are babies currently receiving no medical care
Late initiation of KMC/strict restriction to older, stable babies, whereas practice now is to start KMC earlier. Early initiation of KMC for stable babies is likely to be higher impact since up to 50% of neonatal deaths occur on the first day of life
Several studies track pre-discharge mortality only so some underestimation of neonatal mortality reduction
One important bias that may lead to over estimation is survival bias—the sickest babies may die before meeting criteria to commence KMC, or may not meet criteria of being clinically stable.
Where as this review establishes a clear and major impact on neonatal mortality, many questions remain around how to implement. Despite the high impact and apparent feasibility of KMC, few preterm babies in low-income countries currently have access to this intervention. No systematic data on global coverage are available. It appears that, in addition to Colombia, a number of countries in Latin America have made progress in scaling up KMC.17,18 In Asia there are many units now in Indonesia and some in India and Bangladesh but population coverage remains very low in these large countries. Within Africa, South Africa has multiple sites in almost every province27,28 and has employed a low cost model for lower levels in the health system which does not require special units. Malawi has a number of units but all at referral level.29 In most other African countries there are few if any units and these are mainly in capital cities and their presence has depended heavily on local champions to overcome initial resistance. A few countries notably Malawi,29 Tanzania and Ghana now have plans in place to scale up KMC to district hospital or even health centre level. To inform this process it is crucial to understand the constraints to scale up. These constraints may be due to lack of information about effectiveness, or is there reluctance to change current practice even if there are multiple babies per incubator, or perhaps a lack of trust in mothers and letting them onto neonatal units? Is KMC seen as a ‘poor country only’ solution? Formative work around these constraints as well as analyses of cost and potential cost savings on nursing time and length of in-patient stay are needed.
A priority research question concerns community KMC. There is only one study examining KMC initiation at home, in a challenging setting in rural Bangladesh.14 This study demonstrated a substantial mortality benefit for babies <2000 g (or modelled birth weight based on adjusted first weight after birth) but not for normal birthweight babies. At this stage, community initiation of KMC cannot be recommended based on the evidence from this one trial and larger trials in different settings are required. There are ethical concerns regarding increasing care for small babies at home without effective referral care as more babies will be identified who cannot be managed at home. It is important that KMC is not confused with routine skin-to-skin care alone, which is recommended at birth for all babies, whether in facility or at home, although the definitions for this practice and mortality effect data are lacking.
In addition there are no studies in low-income countries of KMC initiation at facility level with effective links to home after discharge. Given the inpatient stay of weeks or even months for very preterm babies, early discharge with effective links to the home would be of benefit to family and facility, but how would this work in practice in weaker health systems and is there a risk of increasing mortality post-discharge?
Conclusion
Evidence has been analysed from a number of RCTs and is consistent with a meta-analysis from large-scale effectiveness evaluations. KMC has a large effect on neonatal mortality and is also effective in reducing morbidity. This evidence is sufficient to recommend the routine use of KMC in facilities for all stable babies <2000 g at birth. The potential effect of KMC is expected to be greatest in low-income countries, where other options for care of preterm babies remain limited with few neonatal care units, often in distant referral hospitals and understaffed and ill-equipped. If KMC were to reach high coverage through implementation at lower levels of the health system, the world’s annual one million neonatal deaths due to preterm birth could be substantially reduced.
Supplementary data
Supplementary data are available at IJE online.
Funding
Bill & Melinda Gates Foundation (grant 43386) to the US Fund for UNICEF to ’Promote evidence-based decision making in designing maternal, neonatal and child health interventions in low- and middle-income countries’; Save The Children USA from the Bill & Melinda Gates Foundation (Grant 50124) for ‘Saving Newborn Lives’. We also acknowledge the Global Alliance for Prevention of Prematurity and Stillbirths (http://www.gappsseattle.org).
Acknowledgements
We are extremely grateful to Prof. Natalie Charpak and Prof. Rao Suman for sharing unpublished data on neonatal-specific outcomes. We thank all members of the Child Health Epidemiology Reference Group for helpful comments and feedback on this work. We also acknowledge Rajiv Bahl of WHO and Abdullah Baqui of Johns Hopkins University, Baltimore, for insightful review of an earlier draft of this paper.
Conflict of interest: None declared.
KEY MESSAGES.
KMC is a simple intervention to care for preterm newborns by tying the baby to the mothers front, providing thermal care through continuous skin to skin contact, increased breastfeeding, reduced infections and early recognition of illness.
Previous reviews have not shown a significant mortality benefit, and included studies where the intervention started after 1 week of age (survival bias) and have combined varying mortality outcomes (predischarge, neonatal, 6 months and infant mortality). In addition several new studies have been published.
Our new meta-analysis of 3 RCTs shows major mortality reduction [51% (18–71%)] for neonatal mortality in babies with birthweight <2000 g, with even greater reductions in serious morbidity.
This evidence is sufficient to recommend the routine use of KMC for all babies <2000 g as soon as they are stable. Up to half a million neonatal deaths due to preterm birth complications could be prevented each year if this intervention were implemented at scale.
Priority research gaps include studies of community level initiation of KMC as well as follow up of facility KMC initiation with early discharge in low income countries.
References
- 1.Lawn JE, Cousens S, Zupan J. 4 million neonatal deaths: when? Where? Why? Lancet. 2005;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 2.Lawn JE, Wilczynska-Ketende K, Cousens SN. Estimating the causes of 4 million neonatal deaths in the year 2000. Int J Epidemiol. 2006;35:706–18. doi: 10.1093/ije/dyl043. [DOI] [PubMed] [Google Scholar]
- 3.Darmstadt GL, Bhutta ZA, Cousens S, Adam T, Walker N, De Bernis L. Evidence-based, cost-effective interventions: how many newborn babies can we save? Lancet. 2005;365:977–88. doi: 10.1016/S0140-6736(05)71088-6. [DOI] [PubMed] [Google Scholar]
- 4.Institute of Medicine. Preterm Birth. . Causes, Consequences, and Prevention. Washington, DC: National Academies Press; 2007. [PubMed] [Google Scholar]
- 5.Ruiz-Peláez JG, Charpak N, Cuervo LG. Kangaroo Mother Care, an example to follow from developing countries. BMJ. 2004;329:1179–81. doi: 10.1136/bmj.329.7475.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rey E, Martinez H. Manejio racional del Nino Prematuro. Bogota, Colombia: Universidad Nacional, Cursode Medicina Fetal; 1983. [Google Scholar]
- 7.Charpak N, Riuz JG, Zupan J, et al. Kangaroo mother care: 25 years after. Acta Paediatrica. 2005;94:514–22. doi: 10.1111/j.1651-2227.2005.tb01930.x. [DOI] [PubMed] [Google Scholar]
- 8.Charpak N, Riuz-Pelaez JG, Figueroa Z, Charpak Y. Kangaroo mother versus traditional care for newborn infants less than or equal to 2000 grams: a randomised controlled trial. Pediatrics. 1997;100:682–89. doi: 10.1542/peds.100.4.682. [DOI] [PubMed] [Google Scholar]
- 9.Charpak N, Riuz-Pelaez JG, Figueroa Z, Charpak Y. A randomised controlled trial of Kangaroo mother care: results of follow-up at one year of corrected age. Pediatrics. 2001;108:1072–79. doi: 10.1542/peds.108.5.1072. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organisation. Kangaroo Mother Care: A Practical Guide. Geneva: WHO; 2003. [Google Scholar]
- 11.Conde-Agudelo A, az-Rossello JL, Belizan JM. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst Rev. 2003;2:CD002771. doi: 10.1002/14651858.CD002771. [DOI] [PubMed] [Google Scholar]
- 12.Schunemann HJ, Oxman AD, Brozek J, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ (Clinical research ed) 2008;336:1106–10. doi: 10.1136/bmj.39500.677199.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.STATA/IC 10.1. . Statistical Program. College Station, TX: STATA Corporation; 2008. [Google Scholar]
- 14.Sloan NL, Ahmed S, Mitra SN, et al. Community-based kangaroo mother care to prevent neonatal and infant mortality: a randomized, controlled cluster trial. Pediatrics. 2008;121:e1047–59. doi: 10.1542/peds.2007-0076. [DOI] [PubMed] [Google Scholar]
- 15.Suman RP, Udani R, Nanavati R. Kangaroo mother care for low birthweight infants: a randomized controlled trial. Indian Pediatr. 2008;45:17–23. [PubMed] [Google Scholar]
- 16.Worku B, Kassie A. Kangaroo mother care: a randomized controlled trial on effectiveness of early kangaroo mother care for the low birthweight infants in Addis Ababa, Ethiopia. J Trop Pediatr. 2005;51:93–7. doi: 10.1093/tropej/fmh085. [DOI] [PubMed] [Google Scholar]
- 17.Sloan NL, Camacho LW, Rojas EP, Stern C. Kangaroo mother method: randomised controlled trial of an alternative method of care for stabilised low-birthweight infants. Maternidad Isidro Ayora Study Team. Lancet. 1994;344:782–85. doi: 10.1016/s0140-6736(94)92341-8. [DOI] [PubMed] [Google Scholar]
- 18.Cattaneo A, Davanzo R, Worku B, et al. Kangaroo mother care for low birthweight infants: a randomized controlled trial in different settings. Acta Paediatrica. 1998;87:976–85. doi: 10.1080/080352598750031653. [DOI] [PubMed] [Google Scholar]
- 19.Udani. [(September 2009, date last accessed)]. Innovation: KEM Kangaroo Bag - For Kangaroo Mother Care. R. http://kangaroo.javeriana.edu.co/encuentros/7encuentro/posters/Rekha%20Udani%204.pdf. [Google Scholar]
- 20.Ramanathan K, Paul VK, Deorari AK, Taneja U, George G. Kangaroo Mother Care in very low birthweight infants. Indian J Pediatr. 2001;68:1019–23. doi: 10.1007/BF02722345. [DOI] [PubMed] [Google Scholar]
- 21.Roberts KL, Paynter C, McEwan B. A comparison of kangaroo mother care and conventional cuddling care. Neonatal Netw. 2000;19:31–35. doi: 10.1891/0730-0832.19.4.31. [DOI] [PubMed] [Google Scholar]
- 22.Charpak N, Ruiz Pelaez JG, Charpak Y. Rey-Martinez. Kangaroo mother program: an alternative way of caring for low birthweight infants? One year mortality in a two cohort study. Pediatrics. 1994;94:804–10. [PubMed] [Google Scholar]
- 23.Kambarami RA, Chidede O, Kowo DT. Kangaroo care versus incubator care in the management of well preterm infants. A pilot study. Ann Trop Paediatr. 1998;18:81–86. doi: 10.1080/02724936.1998.11747932. [DOI] [PubMed] [Google Scholar]
- 24.Chwo MJ, Anderson GC, Good M, Dowling DA, Shiau SH, Chu DM. A randomized controlled trial of early kangaroo care for preterm infants: effects on temperature, weight, behavior, and acuity. J Nurs Res. 2002;10:129–42. doi: 10.1097/01.jnr.0000347592.43768.46. [DOI] [PubMed] [Google Scholar]
- 25.Lincetto O, Nazir AI, Cattaneo A. Kangaroo mother care with limited resources. J Trop Pediatr. 2000;46:293–95. doi: 10.1093/tropej/46.5.293. [DOI] [PubMed] [Google Scholar]
- 26.Rodrigues MAG, Cano MAT. Estudo do ganho de peso e duração da internação do recém-nascido pré-termo de baixo peso com a utilização do método canguru. Revista Eletrônica de Enfermagem. 2006;8:185–91. [Google Scholar]
- 27.Pattinson RC, Bergh A-M, Malan AF, Prinsloo R. Does kangaroo mother care save lives? J Trop Pediatr. 2006;52:438–41. doi: 10.1093/tropej/fml032. [DOI] [PubMed] [Google Scholar]
- 28.Bergh AM, Arsalo I, Malan AF, Patrick M, Pattinson RC, Phillips N. Measuring implementation progress in kangaroo mother care. Acta Paediatr. 2005;94:1102–8. doi: 10.1111/j.1651-2227.2005.tb02052.x. [DOI] [PubMed] [Google Scholar]
- 29.Bergh AM, van Rooyen E, Lawn J, Zimba E, Ligowe R, Chiunda G. Retrospective Evaluation of KMC in Malawi. Malawi Save the Children Country Office: MRC South Africa and University of Pretoria; 2007. [Google Scholar]