Abstract
Background With the aim of populating the Lives Saved Tool (LiST) with parameters of effectiveness of existing interventions, we conducted a systematic review of the literature assessing the effect of Haemophilus influenzae type b (Hib) and pneumococcal (PC) conjugate vaccines on incidence, severe morbidity and mortality from childhood pneumonia.
Methods We summarized cluster randomized controlled trials (cRCTs) and case–control studies of Hib conjugate vaccines and RCTs of 9- and 11-valent PC conjugate vaccines conducted in developing countries across outcome measures using standard meta-analysis methods. We used a set of standardized rules developed for the purpose of populating the LiST tool with required parameters to promote comparability across reviews of interventions against the major causes of childhood mortality. The estimates could be adjusted further to account for factors such as PC vaccine serotype content, PC serotype distribution and human immunodeficiency virus prevalence but this was not included as part of the LiST model approach.
Results The available evidence from published data points to a summary effect of the Hib conjugate vaccine on clinical pneumonia of 4%, on clinical severe pneumonia of 6% and on radiologically confirmed pneumonia of 18%. Respective effectiveness estimates for PC vaccines (all valent) on clinical pneumonia is 7%, clinical severe pneumonia is 7% and radiologically confirmed pneumonia is 26%.
Conclusions The findings indicated that radiologically confirmed pneumonia, as a severe morbidity proxy for mortality, provided better estimates for the LiST model of effect of interventions on mortality reduction than did other outcomes evaluated. The LiST model will use this to estimate the pneumonia mortality reduction which might be observed when scaling up Hib and PC conjugate vaccination in the context of an overall package of child health interventions.
Keywords: childhood pneumonia, pneumococcal conjugate vaccine, Haemophilus influenzae type b vaccine, developing countries
Background
According to a UNICEF-WHO report from 2006, over two million children die from pneumonia each year, accounting for almost one in five under-5 deaths worldwide.1 The estimated incidence of clinical pneumonia in children aged <5 years in developing countries is 0.28 episodes per child-year, whereas in developed countries is 0.05 episodes per child-year.2,3 WHO recommends routine immunization programmes including measles, pertussis, Haemophilus influenzae type b (Hib) conjugate and pneumococcal (PC) conjugate vaccines, in order to prevent pneumonia.
Hib conjugate vaccines were developed during the late 1980s and involved conjugation of polyribistol-phoshate (PRP) to carrier proteins such as diphtheria toxoid conjugate (PRP-D). Other HibCV formulations include PRP covalently conjugated to other proteins carriers. These included mutant diphtheria toxin conjugate (PRP-CRM197), meningococcal outer membrane protein conjugate (PRP-OMP) and tetanus-toxoid protein (PRP-T).4 The seven-valent PC conjugate vaccine (PCV7), which includes the seven most common pneumococcal serotypes found in industrialized countries and uses CRM197 as a protein carrier, was first licensed in the USA (2000) and today is licensed in ∼90 countries and adopted in immunization programmes in 26 countries.5
Thanks to the efforts of Global Alliance for Vaccines and Immunization (GAVI Alliance), The Hib Initiative and The Pneumococcal vaccines Accelerated Development and Introduction Plan (PneumoADIP), the majority of GAVI Alliance eligible countries have now introduced Hib vaccine (http://www.hibaction.org). The coverage of the PC vaccine is still very limited in the areas where it is needed most6; however, efforts to improve its coverage are underway (http://www.preventpneumo.org). As of early 2009, GAVI Alliance has officially approved support for PC vaccine introduction in 11 developing countries, most of them in Africa, but 72 countries are eligible for GAVI Alliance funding and additional approvals are expected.
This report reviews data from several studies and presents evidence of the effectiveness of the Hib and PC conjugate vaccines in order to provide parameters needed for the Lives Saved Tool (LiST) software to model the preventable deaths of childhood pneumonia and to transparently document all steps of this process, thus assisting to the transparency and wider acceptance of LiST tool. The reviews of effectives of the interventions are shaped in large part by the needs of the LiST model. In that model, increases in coverage of an intervention results in a reduction of one or more cause-specific deaths or in reduction of a risk factor. Therefore, the reviews and the grade process used were designed to develop estimates of the effect of an intervention in reducing either a risk factor or a death due to specific cause. For more details of the review methods, the adapted grade approach or the LiST model see other articles in this supplement.
In addition, we used an approach that was carefully standardized across all the groups of investigators, who addressed the main causes of childhood deaths. The approach focuses on published results which were not then subject to any subsequent detailed modelling (i.e. there was no attempt to account for population differences such as differing national or regional serotype distributions or varying zinc deficiency prevalence; or for differences in interventions such as vaccine valency or zinc dose). This systematically applied and objective approach facilitates the standard presentation across all child health interventions of tables giving key details of the studies reviewed and of the outcome data reported.
Methods
Identification and selection of studies
We attempted to identify all randomized controlled trials (RCTs), quasi-RCTs or observational studies investigating the effect of PC or Hib conjugate vaccines on pneumonia related outcomes in children less than 5 years old. Trials were identified from the following databases: Medline (1970 to August 2008); EMBASE (1970 to August 2008) and the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, Issue 3, 2008). Search terms included various combinations of: Pneumococcal vaccines, Haemophilus vaccines, and pneumonia. In addition, relevant studies were identified by searching the references of the selected studies and the PneumoADIP (http://www.preventpneumo.org/index.cfm) and Hib initiative (http://www.hibaction.org/) websites.
Eligible studies were selected according to the pre-determined inclusion criteria (7). In particular: (i) included studies: (a) were RCTs, quasi-RCTs or observational studies, (b) had a control arm of placebo or no treatment and (c) were conducted in a developing country; (ii) children of included studies were (a) <5 years old and (b) were followed up until ≥2 years of age (not applicable for the case–control studies); (iii) the main types of outcome measures were: (a) pneumonia-specific mortality, (b) all-cause mortality, (c) WHO-defined or predefined radiologically confirmed pneumonia, (d) clinical severe pneumonia and (e) clinical pneumonia. There were no language or publication restrictions. In addition, a parallel review was conducted by two independent investigators (SJ and AJ) and results from the two searches and study selections were compared and merged.
Abstraction, quality assessment and meta-analyses
Data from all studies that met final inclusion and exclusion criteria were abstracted into a standardized form for each outcome of interest. We abstracted key variables with regard to the study identifiers and context, study design and limitations, intervention specifics, and outcome effects. The quality of each study was assessed and graded according to the Child Health Epidemiology Reference Group (CHERG) adaptation of the GRADE technique (‘GRADE Profiler version 3.2’ scoring system) (Supplementary Table a).
We summarized the evidence by outcome including qualitative assessment of the quality of each specific outcome (Supplementary Table b). In addition, for any outcome with more than one study a meta-analysis was conducted and reported pooled relative risk (RR) and corresponding 95% confidence interval (CI) using the fixed effect model (Mantel-Haenszel method).8 In case of heterogeneity (P < 0.1) the random effect model (DerSimonian-Laird method) was applied (although it is recognized that due to the variation in precise interventions, study methods and outcome definitions the meta-estimates should be interpreted cautiously). All analyses were conducted using STATA 10.0 statistical software.
For the outcome of interest, namely the effect of PC or Hib on pneumonia mortality, we applied the CHERG Rules for Evidence Review to the collective pneumonia morbidity and mortality outcomes, to generate a final estimate for reduction in pneumonia mortality (Supplementary Table c). The final version of the paper was presented and peer-reviewed by the CHERG committee.
Results
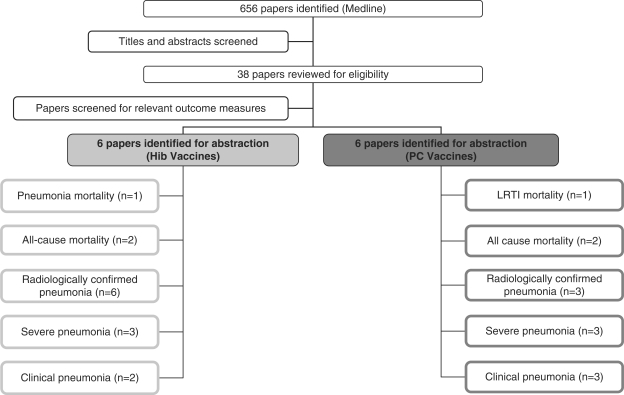
We identified 323 titles from the search conducted in Medline, 333 from Embase and 98 from CENTRAL. After elimination of duplicates, studies dealing only with safety and/or immunogenicity, studies with alternative outcome parameters (e.g. otitis media, meningitis), studies conducted in industrialized countries, review articles and studies which did not fit the inclusion criteria, a total of 13 studies were extracted from the bibliographic databases (Figure 1). One RCT on the effect of seven-valent PC vaccines was excluded from the analysis, because it was not conducted in a developing country.9
Figure 1.
Synthesis of study identification in review of the effects of Hib or PC vaccines on pneumonia mortality, all-cause mortality, radiologically confirmed pneumonia, clinical pneumonia, invasive Hib disease and invasive pneumococcal disease
The characteristics of the studies that were identified to estimate the effect of Hib vaccines on pneumonia mortality are presented in Supplementary Table 1. A summary of the identified outcomes as well as their exact definitions are presented in Supplementary Table 2a. In particular for Hib conjugate vaccines, the following outcomes were identified:
one cluster randomized controlled trials (cRCT)10 reporting pneumonia mortality outcomes;
one cRCT10 and one RCT11 reporting all-cause mortality outcome;
two cRCTs10,12 one RCT11 and three case–control studies13–15 reporting radiologically confirmed pneumonia,
two cRCTs10,12 and one RCT11 reporting clinical severe pneumonia and
The characteristics of the studies that were identified to estimate the effect of PC vaccines on pneumonia mortality are presented in Supplementary Table 1. A summary of the identified outcomes as well as their exact definitions are presented in Supplementary Table 2b. In particular for PC conjugate vaccines, the following outcomes were identified:
one 9-valent RCT reporting lower respiratory tract infection (LRTI) specific mortality,16
two 9-valent17,18 and one 11-valent RCT19 reporting radiologically confirmed pneumonia,
two 9-valent RCTs16,17 and one 11-valent RCT19reporting clinical severe pneumonia and
two 9-valent17,18 and one 11-valent RCT19 on clinical pneumonia.
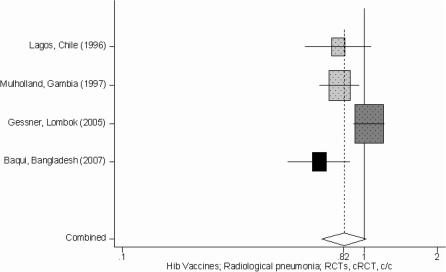
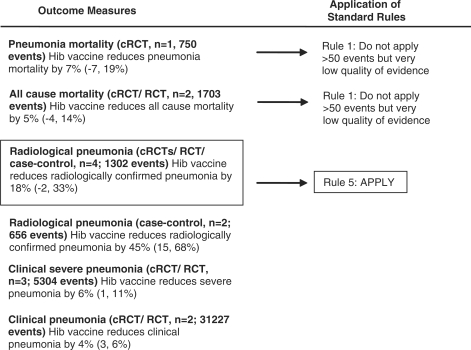
In Tables 1 and 2 we report the quality assessment of studies by outcome and results from corresponding meta-analyses for the Hib and PC vaccines, respectively. With respect to the Hib vaccines, although there was an estimate of pneumonia mortality [clinical or verbal autopsy defined; 7% (95% CI –7, 19%)], the specific outcome quality of evidence was very low (since the estimate was based on one study which was not adequately powered to address this outcome) (Table 1). Therefore, the effect of Hib vaccines on pneumonia mortality was estimated by using the radiologically confirmed pneumonia summary effect (according to the CHERG Rules 1 and 5 for Evidence Review) (Table 1, Figures 2 and 3) based on the rationale that effect on a relatively severe pneumonia outcome might serve as a proxy for an effect on pneumonia mortality in the absence of high quality evidence on the latter. The summary effect of the Hib conjugate vaccine on radiologically confirmed pneumonia was 18% (95% CI –2, 33%) estimated by combining one RCT, two cRCTs and one case–control study that used systematic vaccine allocation and therefore included in the analysis. Regarding the other pneumonia related outcomes, the effect of Hib conjugate vaccines on all cause-mortality was 5% (95% CI –4, 14%), on clinical severe pneumonia was 6% (95% CI 1, 11%) and on clinical pneumonia was 4% (95% CI 3, 6%) (Table 1, Figure 3).
Table 1.
Quality assessment of studies of Hib conjugate vaccines on pneumonia related outcomes
| Hib vaccines |
Quality assessment |
|||||||
|---|---|---|---|---|---|---|---|---|
| Directness |
No of events |
|||||||
| No of studies (ref.) | Design | Limitationsa | Consistencyb | Generalizability to population of interestc | Generalizability to intervention of interestd | Intervention | Control | RR (95% CI) |
| Pneumonia mortalitye: Very low outcome-specific quality of evidencef | ||||||||
| 1 (10) | cRCT | Eligible number of children wasn’t based on census counts, not designed to look at effect on mortality | n/a | Only 1 study | Hib conjugate vaccine (DTP-PRP-T) | 370 | 380 | 0.93 (0.81, 1.07)g |
| All cause mortalitye: Very low outcome-specific quality of evidence | ||||||||
| 2 (10,11) | RCT/ cRCT | Not designed to look at effect on mortality | Both studies show benefit | Africa and Asia | Hib conjugate vaccine (DTP-PRP-T) | 844 | 859 | 0.95 (0.86, 1.04)h |
| Radiologically confirmed pneumoniae: Moderate outcome specific quality of evidence | ||||||||
| 4 (10,11,13,45),j | RCTs/ Crct case–control | Mainly no major limitations; for 1 study per protocol analysis and for 1 study there was no blinding | Heterogeneity from meta-analysis (P = 0.01); 3 of 4 studies show benefit | S. America, Africa, Asia | Hib conjugate vaccine (DTP-PRP-T) | 633k | 669k | 0.82 (0.67, 1.02)l |
| 2 (14,15) | case–control | Mainly no major limitations; for 1 study there was no blinding | Heterogeneity from meta-analysis (P = 0.09); both studies show benefit | S. America, Asia | PRF-Hib vaccine conjugated with tetanus protein; Hib CRM-197 conjugate vaccine | 472 | 184 | 0.53 (0.32, 0.85)h |
| Clinical severe pneumonia: Moderate outcome specific quality of evidence | ||||||||
| 3 (10,11,45) | cRCT/ RCT | Mainly no major limitations; for 1 study per protocol analysis | 2 of 3 studies show benefit | S. America, Africa, Asia | Hib conjugate vaccine (DTP-PRP-T) | 2623 | 2681 | 0.94 (0.89, 0.99)h |
| Clinical pneumoniae: Low outcome specific quality of evidence | ||||||||
| 2 (10,11) | cRCT/ RCT | No major | Both studies show benefit | Africa, Asia | Hib conjugate vaccine (DTP-PRP-T) | 15595 | 15632 | 0.96 (0.94, 0.97)h |
aLimitations include comments on such aspects as blinding, placebo, how valid is the measure (i.e. self reported incidence vs. active case detection) for each study included.
bConsistency is a summary measure of the heterogeneity of the meta-analysis and a short description applying judgment based on the overall directionality of the effect.
cGeneralizability to population of interest examines the age of the children and the regions the studies were conducted in for each meta-analysis.
dGeneralizability to intervention of interest examines how direct the intervention is measured.
eOutcome definition for each study described in Supplementary Table 2a.
fMore information about the quality grades is presented in Supplementary Table b.
gDirectly calculated from the study results.
hMantel-Haenszel pooled RR, fixed effect meta-analysis.
iPneumonia outcomes of the Lagos trial were published in the study of Lavine et al. 1999.
jBaqui AH study was a case control study with systematic vaccine allocation (staggering introduction of vaccine) and therefore included in the analysis of the cRCTs; Hospital controls were included in the analysis.
kBaqui AH study was included in the meta-analysis estimate, however the exact number of radiologically confirmed cases of the intervention and control arm could not be estimated and therefore were not included in the total number of events.
lDerSimonian-Laird pooled RR, random effect meta-analysis. The bold value indicates the fact that this outcome was used to infer the effect of Hib vaccines on pneumonia mortality.
Table 2.
Quality assessment of studies of PC conjugate vaccines on pneumonia related outcomes
| PC vaccines |
Quality assessment |
|||||||
|---|---|---|---|---|---|---|---|---|
| Directness |
No of events |
|||||||
| No of studies (ref.) | Design | Limitationsa | Consistencyb | Generalizability to population of interestc | Generalizability to intervention of interestd | Intervention | Control | RR (95% CI) |
| LRTI specific mortalitye: Very low outcome specific quality of evidencef | ||||||||
| 1 (HIV- children from Klugman et al.18) | RCT | Not designed to look at effect on mortality | n/a | Only 1 study | 9-valent pneumococcal conjugate CRM-197 | 18 | 22 | 0.82 (0.44, 1.52) |
| All cause mortalitye: Very low outcome specific quality of evidence | ||||||||
| 2 (HIV- children from Cutts FT et al.17 and Klugman KP et al.18) | RCT | Not designed to look at effect on mortality and for 1 study per protocol analysis | Both studies show benefit | Only Africa | 9-valent pneumococcal conjugate CRM-197 | 366 | 425 | 0.85 (0.74, 0.98)g |
| Radiologically confirmed pneumoniae: Moderate outcome specific quality of evidence | ||||||||
| 2 (HIV- children from Cutts FT et al.17 and Klugman KP et al.18) | RCT | No major | Heterogeneity from meta-analysis (P = 0.09); both studies show benefit | Only Africa | 9-valent pneumococcal conjugate CRM-197 | 557 | 802 | 0.71 (0.58, 0.87)h |
| 1(19) | RCT | No major | n/a | Only 1 study | 11-valent pneumococcal sanofi pasteur | 119 | 141 | 0.84 (0.66, 1.07) |
| 3 (Cuts et al.17, HIV- children from Klugman et al.18, Lucero et al.19) | RCT | See above | Heterogeneity from meta-analysis (P = 0.09); all studies show benefit | Africa, Asia | All valent | 676 | 943 | 0.74 (0.63, 0.88) |
| Clinical severe pneumoniae: Moderate outcome specific quality of evidence | ||||||||
| 2 (HIV-children from Cutts et al.17 and Klugman et al.18) | RCT | No major, for one study per protocol analysis | Both studies show benefit | Only Africa | 9-valent pneumococcal conjugate CRM-197 | 763 | 854 | 0.89 (0.81, 0.98) |
| 1(19) | RCT | No major | n/a | Only 1 study | 11-valent pneumococcal sanofi pasteur | 397 | 383 | 1.03 (0.90, 1.19) |
| 3 (HIV-children from Cutts et al.17 and Klugman et al.18, Lucero et al.19) | RCT | See above | 2 of 3 studies show benefit | Africa, Asia | All valent | 1160 | 1237 | 0.93 (0.86, 1.01) |
| Clinical pneumoniae: Moderate outcome specific quality of evidence | ||||||||
| 2 (HIV-children from Cutts et al.17 and Klugman et al.18) | RCT | No major, for one study per protocol analysis | Heterogeneity from meta-analysis (P = 0.08); both studies show benefit | Only Africa | 9-valent pneumococcal conjugate CRM-197 | 2738 | 2965 | 0.89 (0.80, 0.99) |
| 1(19) | RCT | No major | n/a | Only 1 study | 11-valent pneumococcal sanofi pasteur | 1093 | 1080 | 1.08 (0.93, 1.10) |
| 3 (Cutts et al.17, HIV- children from Klugman et al.18, Lucero et al.19) | RCT | See above | Heterogeneity from meta-analysis (P = 0.03); 2 of 3 studies show benefit | Africa, Asia | All valent | 3831 | 4045 | 0.93 (0.85, 1.02) |
aLimitations include comments on such aspects as blinding, placebo, how valid is the measure (i.e. self reported incidence vs. active case detection) for each study included.
bConsistency is a summary measure of the heterogeneity of the meta-analysis and a short description applying judgment based on the overall directionality of the effect.
cGeneralizability to population of interest examines the age of the children and the regions the studies were conducted in for each meta-analysis.
dGeneralizability to intervention of interest examines how direct the intervention is measured.
eOutcome definition for each study described in Supplementary Table 2b.
fMore information about the quality grades is presented in Supplementary Table b.
gMantel-Haenszel pooled RR, fixed effect meta-analysis.
hDerSimonian-Laird pooled RR, random effect meta-analysis. The bold value indicates the fact that this outcome was used to infer the effect of Hib vaccines on pneumonia mortality.
Figure 2.
Forest plot for the effect of Hib conjugate vaccines on radiologically confirmed pneumonia; RCTs (light grey), cRCT (dark grey), c/c (black)
Figure 3.
Application of standardized rules for choice of final outcome to estimate effect of Hib conjugate vaccines on the reduction of pneumonia mortality
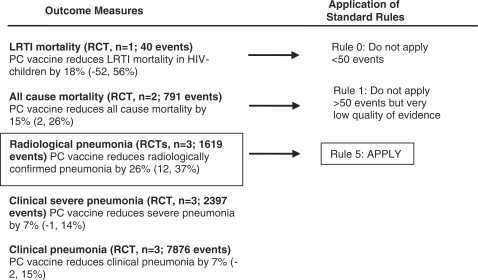
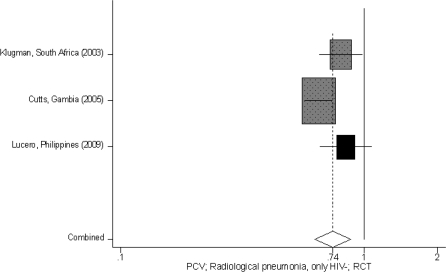
For the PC conjugate vaccines, although there was an estimate of LRTI specific mortality [18% (95% CI −52, 56%)], the specific outcome quality was very low (since the estimate was based on one study with less than 50 events) (Table 2). Therefore according to the CHERG Rules 0, 1 and 5 for Evidence Review in order to estimate the effect on pneumonia mortality, we used the effect of PC vaccines on radiologically confirmed pneumonia, which was 26% (95% CI 12, 37%) (Tables 1 and 2, Figure 4). Regarding the other pneumonia related outcomes, the effect of PC vaccines [all valent) on all-cause mortality was 15% (95% CI 2, 26%)], on clinical severe pneumonia was 7% (95% CI –1, 14%) and on clinical pneumonia was 7% (95% CI –2, 15%). One study (ref. for Klugman) reported outcomes separately for human immunodeficiency virus (HIV) positive and HIV negative children. Outcomes for HIV positive children were examined separately and are summarized here: LRTI specific mortality: –3% (95% CI –23, 20%); all cause mortality: 6% (95% CI –16, 24%); radiologically confirmed pneumonia: 13% (95% CI –7, 29%); clinical severe pneumonia: 17% (95% CI 5, 27%); clinical pneumonia: 15% (95% CI 5, 24%).
Figure 5.
Application of standardized rules for choice of final outcome to estimate effect of PC conjugate vaccines on the reduction of pneumonia mortality
Figure 4.
Forest plot for the effect of PC conjugate vaccines on radiologically confirmed pneumonia; 9-valent (dark grey) and 11-valent (black) RCTs
Finally, we undertook the jacknife approach, where we serially removed one study and then re-run the meta-analysis for the effect of the Hib or PC vaccines on radiologically confirmed pneumonia to indicate whether the meta-analysis results are influenced by the findings of one study. The results of this approach are presented in Supplementary Table 3.
Discussion
Estimation of direct vaccine effect on pneumonia mortality
The identified studies were grouped according to the specific outcomes and were given an overall quality judgment assessed by applying the GRADE approach (Supplementary Table b). To translate the evidence of the collective pneumonia morbidity and mortality outcomes into a final estimate of effectiveness of reducing cause-specific mortality, we applied the CHERG Rules for Evidence Review (Supplementary Table c). In particular for Hib vaccines, the pneumonia mortality and all cause mortality outcome were dropped out, since the initial quality of evidence of these outcomes was very low since these trials were not designed to measure mortality (Rule 1). Therefore, to estimate the effect of Hib vaccines on pneumonia mortality we used the estimate of the effect on radiologically confirmed pneumonia (Rule 5; combined RR 0.82, 95% CI 0.67, 1.02). To estimate the effect of PC vaccines on pneumonia mortality we used the estimate on radiologically confirmed pneumonia (Rule 5; combined RR 0.74, 95% CI 0.63, 0.88) since the estimate of the effect on pneumonia mortality (for HIV– children) was based on less than 50 events (Rule 0), the quality of the estimate on pneumonia mortality (for HIV+ children) was very low (Rule 1) and the outcome all-cause mortality was initially very low and therefore had to dropped out after the quality adjustment (Rule 1).
The question of how to estimate best the pneumonia mortality effects of Hib and PC vaccines from outcomes observed and reported in the published randomized controlled trials (e.g. morbidity, radiological confirmed pneumonia, severe morbidity) is unresolved. We have elected to base this on effects on radiological confirmed pneumonia but the degree to which this decision is valid is uncertain. The Gambian study showed that even though the case fatality ratio was greater for radiological confirmed pneumonia, the number of pneumonia deaths for non-radiologically confirmed pneumonia was the same for that of radiologically confirmed pneumonia, dispelling the idea that all severe pneumonia would fulfil the criteria of ‘radiologically-confirmed’20 There is also evidence to show that not all bacterial pneumonia presents as radiological pneumonia. In the South Africa study, only 36% of the prevented PC pneumonia cases were radiologically confirmed. In The Gambia, this percentage was closer to 85%.
Outcome definitions
The definitions of radiologically confirmed pneumonia, clinical severe pneumonia and clinical pneumonia are not always clear or defined in the same way across the publications we included in the meta-analysis. In particular, definitions of pneumonia morbidity outcomes were not standardized between the Hib vaccine clinical trials, whereas they were standardized between the PC vaccine trials. In addition, radiologically positive cases detected when admitted to hospital rather than in the community may be biased to ward including children who may have failed first line therapy targeting pneumococcus and Hib and therefore in whom the aetiology of pneumonia is less likely to be pneumococcus or Hib. Standardization in definitions of radiological pneumonia between studies was carried out but not all factors which influence radiographic were standardized. For example, more intense community surveillance (and related treatment) may paradoxically reduce the likelihood of pneumococcus (or Hib) presenting as radiological pneumonia. Also, issues such as access to antibiotic treatment and access to health care may all impact on the radiographic presentation of PC and Hib pneumonia. These issues may explain some of the differences for Hib effectiveness estimates for radiologically confirmed pneumonia between Indonesia and The Gambia, as well as for PC effectiveness estimates between The Gambia setting and the settings in South Africa and Philippines.20 The latter differences in the PCV trials might also be explained by the different background epidemiology of these settings (high child mortality—low income in The Gambia setting and lower child mortality—middle income in the South Africa and Philippines settings). However, we would not consider segmenting the effectiveness estimate and apply different estimates in different sets of countries, mainly because there are only few data-points.
Regarding the severe clinical pneumonia outcome, if severe pneumonia were identified when admitted to the hospital, then these cases would be consistent with the current CHERG published estimates of severe childhood pneumonia episodes (incidence rate of severe clinical pneumonia: 0.02–0.04 episodes/child-years).2,3 However, if these cases are defined on signs such as indrawing only at the community level this would likely represent a more diverse group of pneumonia cases. The definition of ‘clinical pneumonia’ in these studies was consistent with the definition which was most frequently used in epidemiological studies estimating pneumonia incidence and also with and the current estimate of the number of pneumonia cases globally (incidence rate of clinical pneumonia: 0.29 episodes/child-years).2,3
Parameters influencing estimates of direct vaccine effect on pneumonia mortality
Although it is not included in the standardized review process for the LiST model, it is be possible to adjust the vaccine effectiveness summary estimate for the valency of the PC vaccine studied, for the age specific serotype distribution of the study population and for the HIV prevalence in the study population. This has been attempted and reported by other groups.22
For pneumococcal vaccine, effectiveness depends on serotype distributions across the world and is likely to provide different levels of effectiveness for different regions. The distribution of serotypes in the local population is important, and estimates of the direct effect of the vaccine should ideally be adjusted to account for the proportion of circulating pneumococci that are vaccine type. However, data on serotype distribution are currently incomplete and estimates of the proportion of vaccine serotypes among circulating PC by region of the world vary.23,24 Increased availability of data on aetiological spectrum of childhood pneumonia at the community level and on serotype distributions in particular will provide much needed evidence base for modelling the effect of the vaccines (http://www.preventpneumo.org). As new vaccines become available, the coverage of serotypes will need to be adjusted accordingly to cover the wider range of serotypes included in these vaccines. For Hib conjugate vaccine regional differences may also exist if Hib is a less important problem in Asia than in Africa.
Vaccine effectiveness in areas where HIV is a major problem may substantially differ from the estimates in regions where HIV is not such a problem. More published data on this issue is required to be able to address this properly, because good quality data is currently restricted to one source—a study in Soweto in South Africa18 Thus, more evidence is required to model the differential impact in HIV-affected regions.
As noted above, the LiST approach has decided not to employ such adjustments of published trial data for any of the child health interventions reviewed and, in any case, any modelling process would be challenging due to the sparse evidence base.
Other vaccine effects (not included in the current review)
Positive indirect effects
The Hib and PC vaccines have an important impact on reducing carriage of the pathogenic organisms in vaccinated children and by reducing the pool of infectious children in the community, and are thus expected to confer a significant degree of indirect protection by protecting unvaccinated children, including young infants. This has been indirectly shown by the reduction in invasive pneumococcal disease in children less than 2 months of age in the USA as well as among only partially vaccinated infants.25
To measure the positive indirect effect for conjugate Hib vaccination, one approach has been to compare the reduction in invasive Hib disease observed several years after vaccine introduction, with the reported coverage of the third dose. Using this approach, the overall effect on invasive Hib disease was estimated to be 15–30% points higher than the reported coverage of three doses, with a herd immunity threshold (i.e. elimination) at around 85% coverage of the third dose.26 However, a case control study conducted 5 years after vaccine introduction in the Gambia has demonstrated a similar effect for Hib pneumonia, with short-term elimination of all invasive Hib disease following 68% coverage of three doses. This was achieved despite a significant number of the doses being administered later than the peak age of invasive Hib disease,27 although cases of both Hib pneumonia and Hib meningitis had started to re-emerge a few years later,28
For pneumococcal conjugate vaccine, the most robust information on positive indirect effects is based on a comparison of pneumonia hospital admissions in the pre-vaccine era (1997–1999) and post-vaccine era (2001–2004) in the USA.29 In this study, clear herd immunity benefits were observed in older unvaccinated children and adults with the following reductions estimated for ‘all-cause’ pneumonia admissions: 18% (5–17 years), 26% (18–39 years), 19% (40–64 years) and 15% (65+ years). In the age group targeted by the vaccination programme (<2 years), the reduction in ‘all-cause’ pneumonia was 39% but it is not clear how much of this benefit can be attributed to the herd effect. One approach is to use the reported 65% reduction in pneumonia admissions caused by pneumococcal, and to derive the direct effect by assuming: (i) reported vaccination coverage of 73% (68–83%); (ii) 80% prevalence of pneumcoocci that are vaccine type30; and (iii) 94% vaccine efficacy (9). This rather crude back-calculation gives a direct effect in the region of 55% (51–62%) and suggests that indirect effects may have contributed ∼15% (5–22%) of the total benefit in children aged <2 years. A separate analysis of all invasive pneumococcal disease from eight states in the USA 5 years after PCV7 introduction estimated the contribution of indirect effects to be around 20% of the total benefit in children aged <5 years.31
For both Hib and SP vaccines, the size of the herd effect will be influenced by the number of doses and intervals between them32 the coverage and timeliness of vaccination programmes33,34 and the underlying patterns of close contact within the local population.35 Positive indirect vaccine effects are important and have to be addressed in some way in any models that estimate their potential impact, however, the dynamics of S. pneumonia in developing countries needs further study to delineate whether it is the same as in industrialized countries. The LiST model will reflect this in an ‘effective vaccine coverage’ estimate which will be based on empiric data on the indirect effect from published sources.
Negative indirect effects
Pneumococcal conjugate vaccines may also be associated with a negative indirect effect of serotype replacement. This has been seen most notably among Alaskan native and aboriginal populations36,37 where a negligible long-term effect on ‘all-cause’ pneumonia has been observed. Some increases in invasive pneumococcal disease attributed to non-vaccine types (19 A) have also been observed coincided with the routine immunization programme in the USA. This replacement was significantly outweighed by reductions in other serotypes,38 but the long-term impact of serotype replacement remains uncertain, and the impressive results of PCV7 in the USA should be transferred to other settings with some caution. Indeed, the size of these indirect vaccine effects (both positive and negative) is likely to be specific to the local epidemiological and geographical circumstances.
Antibiotic resistance
An additional long term benefit of the implementation of Hib and PC vaccination programmes is their expected contribution to the reduction of antibiotic resistance by reducing both the number of respiratory illnesses and therefore the number of antibiotic prescriptions.39 Results from two studies conducted in California (1995–1998) and the USA (1997–2004) have demonstrated that administration of 7 valent PC vaccines reduced the number of antibiotic prescriptions by 35 and 42% respectively,40,41 which is potentially helpful to contain drug-resistant Streptococcus pneumoniae since the level of antibiotic use drives the rise in antibiotic resistance.42
More directly and immediately, five of seven serotypes in the seven valent PC vaccines, introduced for infants in the USA in 2000, are responsible for most penicillin-resistant infections43 and the rate of antibiotic-resistant invasive pneumococcal infections decreased in young children (and older persons) after the introduction of PC vaccines.43 However, continued exposure of non-PCV7 serotypes to antibiotic pressure may reduce the overall impact of PCVs on drug resistance.42 In addition, the gain from this vaccine effect will be partially eroded over time as vaccine-included serotypes are replaced by resistant clones of non vaccine types.44
Future vaccine developments
GlaxoSmithKline’s 10-valent pneumococcal conjugate vaccine has just received license in the European Union. In addition, newer vaccine formulations currently being evaluated and licensed include formulations consisting of 10 or 13 PC serotypes, which will broaden coverage of the selected pneumococcal serotypes commonly identified in developing countries. (http://www.preventpneumo.org). It is probable that the 13-valent 2010 will have a larger effect than that of the 9- and 11-valent vaccines. Thus, this review may under-estimate the impact of any future conjugate vaccines, which have a better coverage of the main serotypes that account for mortality.
Conclusion
The estimates presented in this paper represent only the first step in thinking about vaccine effectiveness against childhood pneumonia and they should be looked in parallel with estimates published from other groups. As noted above the LiST approach for review of effect of interventions on childhood mortality has not introduced scaling of estimates to take account of varying populations or interventions—in this case varying vaccine serotype distributions, varying prevalence of HIV, varying vaccine serotype compositions, and due to the effect of concurrent Hib immunisation in PCV trials. In addition, it could be argued that any pneumonia mortality reduction estimates should ideally be considered along with information on overall child mortality rates in the setting in which the study was undertaken. For example, the vaccine may have less impact on mortality where access to care is high and overall child mortality rates are low, but it may still substantially reduce the incidence of chest X-ray positive pneumonia. Bearing these points in mind, we can conclude at this stage that the available evidence points to the summary effect of the Hib conjugate vaccine on clinical pneumonia of 4%, on clinical severe pneumonia of 6% and on radiologically confirmed pneumonia of 18%. Respective effectiveness estimates for PC vaccines (all valent) on clinical pneumonia is 7%, on clinical severe pneumonia is 7% and on radiologically confirmed pneumonia is 26%. Therefore, the inferred effectiveness of Hib and PC vaccines against pneumonia mortality (based on the effectiveness against radiologically confirmed pneumonia) is estimated to be 18 and 26%, respectively. These estimates will be adjusted further in List to account for the indirect positive effects due to herd immunity.
Supplementary data
Supplementary data are available at IJE online.
Funding
US Fund for UNICEF from the Bill & Melinda Gates Foundation (grant 43386) to ’Promote evidence-based decision making in designing maternal, neonatal and child health interventions in low- and middle-income countries’.
Acknowledgements
The authors thank our colleagues at WHO and UNICEF for their review of the manuscript and valuable feedback.
Conflict of interest: None declared.
KEY MESSAGES.
Findings of this review indicated that the summary effect of Hib and PC conjugate vaccines on radiologically confirmed pneumonia was 18% and 26% respectively.
Radiologically confirmed pneumonia will be used as a severe morbidity proxy for pneumonia mortality in the LiST model.
References
- 1. [(Accessed October 26, 2009)]. UNICEF, World Health Organisation. Pneumonia; The forgotten killer of children. 2008. http://www.unicef.org/ publications/files/Pneumonia_The_Forgotten_Killer_of_Children.pdf. [Google Scholar]
- 2.Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland K, Campbell H. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ. 2008;86:408–16. doi: 10.2471/BLT.07.048769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudan I, Tomaskovic L, Boschi-Pinto C, Campbell H. Global estimate of the incidence of clinical pneumonia among children under five years of age. Bull World Health Organ. 2004;82:895–903. [PMC free article] [PubMed] [Google Scholar]
- 4.Swingler G, Fransman D, Hussey G. Conjugate vaccines for preventing Haemophilus influenzae type b infections. Cochrane Database Syst Rev. 2003;4:CD001729. doi: 10.1002/14651858.CD001729. [DOI] [PubMed] [Google Scholar]
- 5.Progress in introduction of pneumococcal conjugate vaccine–worldwide, 2000-2008. MMWR Morb Mortal Wkly Rep. 2008;57:1148–51. [PubMed] [Google Scholar]
- 6.Rudan I, El AS, Black RE, Campbell H. Childhood pneumonia and diarrhoea: setting our priorities right. Lancet Infect Dis. 2007;7:56–61. doi: 10.1016/S1473-3099(06)70687-9. [DOI] [PubMed] [Google Scholar]
- 7.Rudan I, Lawn J, Cousens S, et al. Gaps in policy-relevant information on burden of disease in children: a systematic review. Lancet. 2005;365:2031–40. doi: 10.1016/S0140-6736(05)66697-4. [DOI] [PubMed] [Google Scholar]
- 8.Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ. 1997;315:1533–7. doi: 10.1136/bmj.315.7121.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J. 2000;19:187–95. doi: 10.1097/00006454-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Gessner BD, Sutanto A, Linehan M, et al. Incidences of vaccine-preventable Haemophilus influenzae type b pneumonia and meningitis in Indonesian children: hamlet-randomised vaccine-probe trial. Lancet. 2005;365:43–52. doi: 10.1016/s0140-6736(04)17664-2. [DOI] [PubMed] [Google Scholar]
- 11.Mulholland K, Hilton S, Adegbola R, et al. Randomised trial of Haemophilus influenzae type-b tetanus protein conjugate vaccine [corrected] for prevention of pneumonia and meningitis in Gambian infants. Lancet. 1997;349:1191–7. doi: 10.1016/s0140-6736(96)09267-7. [DOI] [PubMed] [Google Scholar]
- 12.Levine OS, Lagos R, Munoz A, et al. Defining the burden of pneumonia in children preventable by vaccination against Haemophilus influenzae type b. Pediatr Infect Dis J. 1999;18:1060–64. doi: 10.1097/00006454-199912000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Baqui AH, El AS, Saha SK, et al. Effectiveness of Haemophilus influenzae type B conjugate vaccine on prevention of pneumonia and meningitis in Bangladeshi children: a case-control study. Pediatr Infect Dis J. 2007;26:565–71. doi: 10.1097/INF.0b013e31806166a0. [DOI] [PubMed] [Google Scholar]
- 14.de Andrade AL, de Andrade JG, Martelli CM, et al. Effectiveness of Haemophilus influenzae b conjugate vaccine on childhood pneumonia: a case-control study in Brazil. Int J Epidemiol. 2004;33:173–81. doi: 10.1093/ije/dyh025. [DOI] [PubMed] [Google Scholar]
- 15.de la Hoz F, Higuera AB, Di Fabio JL, et al. Effectiveness of Haemophilus influenzae type b vaccination against bacterial pneumonia in Colombia. Vaccine. 2004;23:36–42. doi: 10.1016/j.vaccine.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 16.Madhi SA, Kuwanda L, Cutland C, Klugman KP. The impact of a 9-valent pneumococcal conjugate vaccine on the public health burden of pneumonia in HIV-infected and -uninfected children. Clin Infect Dis. 2005;40:1511–8. doi: 10.1086/429828. [DOI] [PubMed] [Google Scholar]
- 17.Cutts FT, Zaman SM, Enwere G, et al. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet. 2005;365:1139–46. doi: 10.1016/S0140-6736(05)71876-6. [DOI] [PubMed] [Google Scholar]
- 18.Klugman KP, Madhi SA, Huebner RE, Kohberger R, Mbelle N, Pierce N. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med. 2003;349:1341–48. doi: 10.1056/NEJMoa035060. [DOI] [PubMed] [Google Scholar]
- 19.Lucero MG, Nohynek H, Williams G, et al. Efficacy of an 11-valent pneumococcal conjugate vaccine against radiologically confirmed pneumonia among children less than 2 years of age in the Philippines: a randomized, double-blind, placebo-controlled trial. Pediatr Infect Dis J. 2009;28:455–62. doi: 10.1097/INF.0b013e31819637af. [DOI] [PubMed] [Google Scholar]
- 20.Enwere G, Cheung YB, Zaman SM, et al. Epidemiology and clinical features of pneumonia according to radiographic findings in Gambian children. Trop Med Int Health. 2007;12:1377–85. doi: 10.1111/j.1365-3156.2007.01922.x. [DOI] [PubMed] [Google Scholar]
- 21.Madhi SA, Klugman KP. World Health Organisation definition of “radiologically-confirmed pneumonia” may under-estimate the true public health value of conjugate pneumococcal vaccines. Vaccine. 2007;25:2413–19. doi: 10.1016/j.vaccine.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 22.O'Brien KL, Wolfson LJ, Watt JP, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 23.Constenla D, Gomez E, Pio de la Hoz F, et al. 2007. [(Accessed July 3, 2009)]. The Burden of Pneumococcal Disease and Cost-Effectiveness of a Pneumococcal Vaccine in Latin America and the Caribbean. A Review of the Evidence and a Preliminary Economic Analysis. Available at: http://www.sabin.org. [Google Scholar]
- 24.PneumoADIP. GSP Summary Report (Stage 1; Version 1) for SAGE Meeting. Geneva: WHO. SAGE Meeting; 2007. [Google Scholar]
- 25.Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737–46. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 26.Watt JP, Wolfson LJ, O'Brien KL, et al. Burden of disease caused by Haemophilus influenzae type b in children younger than 5 years: global estimates. Lancet. 2009;374:903–11. doi: 10.1016/S0140-6736(09)61203-4. [DOI] [PubMed] [Google Scholar]
- 27.Adegbola RA, Secka O, Lahai G, et al. Elimination of Haemophilus influenzae type b (Hib) disease from The Gambia after the introduction of routine immunisation with a Hib conjugate vaccine: a prospective study. Lancet. 2005;366:144–50. doi: 10.1016/S0140-6736(05)66788-8. [DOI] [PubMed] [Google Scholar]
- 28.Howie SR, Antonio M, Akisanya A, et al. Re-emergence of Haemophilus influenzae type b (Hib) disease in The Gambia following successful elimination with conjugate Hib vaccine. Vaccine. 2007;25:6305–9. doi: 10.1016/j.vaccine.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 29.Grijalva CG, Nuorti JP, Arbogast PG, Martin SW, Edwards KM, Griffin MR. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet. 2007;369:1179–86. doi: 10.1016/S0140-6736(07)60564-9. [DOI] [PubMed] [Google Scholar]
- 30.Preventing pneumococcal disease among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2000;49(RR-9):1–35. [PubMed] [Google Scholar]
- 31.Invasive pneumococcal disease in children 5 years after conjugate vaccine introduction–eight states, 1998-2005. MMWR Morb Mortal Wkly Rep. 2008;57:144–48. [PubMed] [Google Scholar]
- 32.Trotter CL, McVernon J, Ramsay ME, et al. Optimising the use of conjugate vaccines to prevent disease caused by Haemophilus influenzae type b, Neisseria meningitidis and Streptococcus pneumoniae. Vaccine. 2008;26:4434–45. doi: 10.1016/j.vaccine.2008.05.073. [DOI] [PubMed] [Google Scholar]
- 33.Cowgill KD, Ndiritu M, Nyiro J, et al. Effectiveness of Haemophilus influenzae type b Conjugate vaccine introduction into routine childhood immunization in Kenya. JAMA. 2006;296:671–78. doi: 10.1001/jama.296.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clark A, Sanderson C. Timing of children's vaccinations in 45 low-income and middle-income countries: an analysis of survey data. Lancet. 2009;373:1543–49. doi: 10.1016/S0140-6736(09)60317-2. [DOI] [PubMed] [Google Scholar]
- 35.Mossong J, Hens N, Jit M, et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 2008;5:e74. doi: 10.1371/journal.pmed.0050074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singleton RJ, Hennessy TW, Bulkow LR, et al. Invasive pneumococcal disease caused by nonvaccine serotypes among alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA. 2007;297:1784–92. doi: 10.1001/jama.297.16.1784. [DOI] [PubMed] [Google Scholar]
- 37.O'Grady K-A, Taylor-Thomson D, Chang A, et al. The burden of hospitalized, radiologically diagnosed pneumonia in Northern Territory Indigenous children in Australia. Proceedings of the 6th International Symposium on Pneumococci & Pneumococcal Diseases. 2008. [Google Scholar]
- 38.Isaacman DJ, Fletcher MA, Fritzell B, Ciuryla V, Schranz J. Indirect effects associated with widespread vaccination of infants with heptavalent pneumococcal conjugate vaccine (PCV7; Prevnar) Vaccine. 2007;25:2420–27. doi: 10.1016/j.vaccine.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 39.Cohen R. The need for prudent use of antibiotics and routine use of vaccines. Clin Microbiol Infect. 2009;15(Suppl 3):21–23. doi: 10.1111/j.1469-0691.2009.02727.x. [DOI] [PubMed] [Google Scholar]
- 40.Fireman B, Black SB, Shinefield HR, Lee J, Lewis E, Ray P. Impact of the pneumococcal conjugate vaccine on otitis media. Pediatr Infect Dis J. 2003;22:10–16. doi: 10.1097/00006454-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Zhou F, Shefer A, Kong Y, Nuorti JP. Trends in acute otitis media-related health care utilization by privately insured young children in the United States, 1997-2004. Pediatrics. 2008;121:253–60. doi: 10.1542/peds.2007-0619. [DOI] [PubMed] [Google Scholar]
- 42.Dagan R, Klugman KP. Impact of conjugate pneumococcal vaccines on antibiotic resistance. Lancet Infect Dis. 2008;8:785–95. doi: 10.1016/S1473-3099(08)70281-0. [DOI] [PubMed] [Google Scholar]
- 43.Kyaw MH, Lynfield R, Schaffner W, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med. 2006;354:1455–63. doi: 10.1056/NEJMoa051642. [DOI] [PubMed] [Google Scholar]
- 44.Hanage WP, Huang SS, Lipsitch M, et al. Diversity and antibiotic resistance among nonvaccine serotypes of Streptococcus pneumoniae carriage isolates in the post-heptavalent conjugate vaccine era. J Infect Dis. 2007;195:347–52. doi: 10.1086/510249. [DOI] [PubMed] [Google Scholar]
- 45.Lagos R, Horwitz I, Toro J, et al. Large scale, postlicensure, selective vaccination of Chilean infants with PRP-T conjugate vaccine: practicality and effectiveness in preventing invasive Haemophilus influenzae type b infections. Pediatr Infect Dis J. 15:216–22. doi: 10.1097/00006454-199603000-00008. [DOI] [PubMed] [Google Scholar]