Abstract
Background In the absence of planned efforts to target the poor, child survival programs often favour the rich. Further evidence is needed urgently about which interventions and programme approaches are most effective in addressing inequities. The Lives Saved Tool (LiST) is available and can be used to model mortality levels across economic groups based on coverage levels for child survival interventions.
Methods We used LiST to model neonatal and under-5 mortality levels among the highest and the lowest wealth quintiles in Bangladesh based on national and wealth-quintile-specific coverage of child survival interventions. The cause-of-death structure among children under-5 was also modelled using the coverage levels. Modelled rates were compared to the rates measured directly from the 2004 Bangladesh Demographic and Health Survey and associated verbal autopsies.
Results Modelled estimates of mortality within wealth quintiles fell within the 95% confidence intervals of measured mortality for both neonatal and post-neonatal mortality. LiST also performed well in predicting the cause-of-death structure for these two age groups for the poorest quintile of the population, but less well for the richest quintile.
Conclusions LiST holds promise as a useful tool for assessing socio-economic inequities in child survival in low-income countries.
Keywords: Child survival, Neonatal mortality, Under-five mortality, Modelling, Causes of death, Wealth quintiles, LiST
Introduction
Evidence continues to show that poor children have higher mortality than their wealthier peers, especially in low- and middle-income countries,1 and there are increasing calls for ‘pro-poor’ programming.2 With few exceptions,3 studies demonstrating higher mortality among poor children are based on cross-sectional designs that provide little guidance about which interventions and program approaches are most effective in reducing these inequities.4 More evidence is needed to help Ministries of Health and their partners develop programs that will redress socioeconomic inequities in maternal and child health programs.
The Lives Saved Tool (LiST), as described earlier in this volume,5 supports program decision making by estimating the lives that can be saved by increasing coverage for proven maternal and child health interventions, alone or in combination, for user-defined populations and time frames. Our aim in this study was to determine whether LiST produces valid estimates for wealth subgroups within a population, allowing users to compare alternative program scenarios based on the extent to which they would differentially prevent child deaths among the poorest populations.
Methods
Our original design for this study was to identify large population-based household surveys that collected data on deaths by cause, intervention coverage and household wealth at two points in time (say, 2000 and 2005) in a single setting. We would then have used LiST to model changes in the distributions of deaths by cause from 2000 to 2005 using the baseline (2000) cause-of-death distribution and changes in intervention coverage between 2000 and 2005 as inputs. We reviewed all Demographic and Health Survey (DHS) data sets and were not able to identify a single country with two surveys that included measurement of under-5 deaths by cause.
We therefore revised the design to allow us to pose the research question using a single data set: How well can LiST predict under-5 mortality and child deaths by cause using input data on intervention coverage from a single wealth quintile, using national measured results as baseline? In two countries, Bangladesh and Pakistan, the DHS survey included data on intervention coverage and household wealth, and was accompanied by a verbal autopsy study in which trained surveyors visited households reporting a child death to determine the cause of death. The verbal autopsy data were not yet available in Pakistan; in Bangladesh the 2004 Bangladesh Demographic and Health survey (2004 BDHS) met these criteria and the investigators who conducted the verbal autopsy agreed to work with us on this analysis.
We used the wealth index as defined in the 2004 BDHS, which uses standard procedures1,6 and principal components analysis to categorize the population into one of five equal-sized groups from the lowest (poorest) to the highest (wealthiest). We reanalysed the 2004 BDHS data to obtain coverage estimates by wealth quintile for all interventions for which data were available (Table 1). Coverage estimates were adjusted using the sampling weights provided by DHS.
Table 1.
Intervention coverage from 2004 BDHS data
| Intervention | Coverage indicator |
|---|---|
| Antenatal interventions | |
| Case management of pregnancy | Percentage of pregnant women with at least four antenatal care visits* |
| Syphilis detection and treatment | Percentage of pregnant women with at least four antenatal care visits* |
| Tetanus toxoid vaccination | Percentage of pregnant women who received two or more doses of tetanus toxoid during pregnancy or ever |
| Multiple micronutrient supplementation | Percentage of women who bought or received iron supplementation during pregnancy |
| Childbirth care interventions | |
| Antenatal corticosteroids for preterm labour | Percentage of infants born in a facility* |
| Antibiotics for prevention of premature rupture of membranes | Percentage of infants born in a facility* |
| Labour monitoring, skilled delivery and access to emergency obstetric care | Percentage of infants born in a facility* |
| Newborn resuscitation | Percentage of infants born in a facility* |
| Clean delivery kit | Percentage of infants delivered with a skilled attendant, among those delivering at home |
| Postnatal preventive interventions | |
| Infant postnatal care | Percentage of infants delivered at home with a postnatal health contact/visit within six weeks of birth |
| Water connection in the home | Percentage of households with water piped into home or yard |
| Improved water source | Percentage of households with access to either piped water or a tubewell |
| Improved excreta disposal | Percentage of homes with access to an improved latrine or flush toilet |
| Vitamin A supplementation | Percentage of children aged 0–59 months receiving at least one dose of vitamin A in the past six months |
| Vaccinations | |
| Measles vaccine | Percentage of infants aged 12–23 months having received one dose of measles-containing vaccine |
| Diphtheria Pertussis Tetanus (DPT) vaccine | Percentage of infants aged 12–23 months having received three doses of DPT vaccine |
| Postnatal curative interventions | |
| Case management of serious neonatal illness | Percentage of children delivering in a facility* |
| Oral rehydration salt (ORS) for diarrhoea | Percentage of children with diarrhoea given ORS |
| Case management of pneumonia | Medical care sought among children with fever/cough in previous 2 weeks |
| Antibiotics for dysentery | Medical care sought among children with fever/cough in previous 2 weeks |
| Vitamin A for measles treatment | Percentage of children aged 0–59 months receiving at least one dose of vitamin A in the past 6 months |
*A standard fraction built into LiST. The exact formula used for each indicator can be found in the LiST manual at http://software.futuresgroup.com/Spectrum/CSManual.pdf, page 53 (accessed on February 7, 2010).
Data on the cause of child death were obtained from the verbal autopsy study conducted in association with the 2004 BDHS.7 When a child death in the previous 5 years was identified in the 2004 BDHS, a trained verbal autopsy data collector visited the household to administer the survey. The primary cause of death was assigned using a hierarchical process, in which diagnoses that are more specific are given greater priority than less certain diagnoses. We grouped the deaths by age (neonatal = age ≤ 28 days; post-neonatal = age > 28 days) and cause to facilitate analysis. Neonatal deaths included those assigned causes of sepsis pneumonia, asphyxia, prematurity, diarrhoea, tetanus, congenital anomalies, and other. Post-neonatal deaths included those assigned causes of pneumonia, diarrhoea, measles, and injury/other. Deaths assigned to the dual-cause category of ‘diarrhoea and acute respiratory infection’ (1.8%) were considered as diarrhoea deaths. Post-neonatal deaths assigned to the triple-cause category of ‘measles and diarrhoea or acute respiratory infection’ (0.6%) were considered as measles deaths.
The verbal autopsy data were then merged with the 2004 BDHS household data. The resulting data set included the full set of child deaths by cause in each of the five wealth quintiles.
Neonatal and under-5 mortality rates were calculated for the 5 years preceding the survey for the total sample and by wealth quintiles using DHS methods.8 Typically, DHS uses women’s birth history data to estimate directly childhood mortality using a synthetic cohort life table approach. Probability of death is calculated for small age segments of children up to 59 months of age and then combined into under-5 mortality using life table approach. We estimated standard errors using the jack-knife method9 and computed 95% confidence intervals (CIs).
LiST modelling
Comprehensive information about LiST is described elsewhere in this volume.5 We describe here how 2004 BDHS data were used to meet the requirements for LiST data inputs for the lowest and highest quintiles.
National BDHS data were used to calculate estimates by quintile for neonatal, infant and under-5 mortality rates, stunting (height for age z-score < –2) percent by age, and the percent breastfed exclusively, predominantly, partially, and not at all. The percent intrauterine growth retardation (IUGR) was derived using data from UNICEF report on low birth weight10 and the prediction formula developed by De Onis and colleagues.11
Table 1 shows the interventions for which coverage data from the 2004 BDHS were used in the LiST scenarios. Coverage estimates for several nutritional interventions included in LiST were not available in the 2004 BDHS. Coverage values for balanced energy supplementation and complementary feeding education and supplementation were imputed to generate corresponding modeled stunting rates that closely approximate the observed rates reported in the BDHS. However, there was a discrepancy in the observed and modeled stunting rates among children under one month because we assumed, at the national level, the same stunting rate for children under 1 month and those aged 1–5 months. Prevalence of IUGR was estimated based on surveys in India and Pakistan showing rates of IUGR as 20% lower and 20% higher than the national mean in the lowest and highest quintiles, respectively.12,13 The version of LiST used for this analysis calls for coverage of face-to-face counselling as an intervention to increase exclusive breastfeeding to 6 months, for which data were not available in the 2004 BDHS. We therefore used the prevalence of exclusive breastfeeding among children up to 6 months of age as reported in the survey as a basis for imputing coverage rates for the counselling intervention.
LiST generates estimates of deaths averted based on changes in coverage over time. We used LiST to model mortality rates in the lowest and highest wealth quintiles by assuming changes in coverage from the national level to the levels measured in each of the wealth subgroups. To do this, we assumed a 5-year time period to allow interventions to achieve their full effect by using 2004 as the baseline year and modelling the results that would occur in mortality given the measured coverage levels for each wealth quintile. We assumed that the entire change in coverage between the national estimate and the estimates for the lowest and highest quintiles occurred in the first year. We used LiST to produce predictions of mortality levels for two age groups (neonatal and post-neonatal) and two wealth quintiles (lowest and highest).
The BDHS 2004 estimated that 47.3% of all under-5 deaths in the data set occurred in the neonatal period, compared to 55.7% of deaths based on the verbal autopsy data set. We investigated this by comparing the child deaths in the 2004 BDHS and verbal autopsy data sets by matching each death on cluster, household number, mother line number and birth year, and found that among 587 deaths in the verbal autopsy dataset, only 474 matched children in the 2004 BDHS data. The reasons for this are unknown. We performed the analyses using both the full and limited data sets and the results were similar; here we report results from analyses using the full verbal autopsy dataset (n = 587).
Results
Table 2 shows coverage levels, national and by wealth quintile, for the interventions included in the LiST analyses. These results, which have been reported elsewhere,7,14 show the expected overall trend towards higher coverage as household wealth increases, but levels of inequality vary by intervention. There are relatively small differences between the lowest and highest quintiles in coverage for improved water source, vaccinations, vitamin A supplementation, and oral rehydration salt solution and much larger differences in coverage for antenatal care, facility-based births, infant post-natal care, and water connection in the home with coverage rates in the wealthiest quintile being more than seven times higher than coverage in the poorest quintile. Inequalities were moderate for iron supplementation, case management of pneumonia and improved excreta disposal.
Table 2.
Reported coverage for LiST interventions, 2004 BDHS
| Indicators | National | Wealth quintiles |
Ratio highest/poorest | ||||
|---|---|---|---|---|---|---|---|
| Lowest | Second | Middle | Fourth | Highest | |||
| Antenatal care | 15.9 | 3.9 | 7.1 | 12.0 | 18.2 | 44.9 | 11.5 |
| Tetanus toxoid immunization | 63.7 | 56.0 | 61.3 | 63.5 | 69.7 | 70.9 | 1.3 |
| Iron supplementation | 50.0 | 31.6 | 44.6 | 48.1 | 57.4 | 76.1 | 2.4 |
| Facility-based birth | 9.9 | 2.3 | 3.3 | 6.3 | 12.3 | 31.9 | 13.9 |
| Skilled birth attendance | 13.4 | 3.4 | 4.5 | 10.5 | 17.4 | 39.6 | 11.6 |
| Infant postnatal care | 18.6 | 6.8 | 9.2 | 14.3 | 21.3 | 47.9 | 7.0 |
| Water connection in the home | 6.2 | 0.2 | 0.5 | 2.3 | 5.1 | 28.5 | 142.5 |
| Improved water source | 97.8 | 96.9 | 97.4 | 97.8 | 98.4 | 99.0 | 1.0 |
| Improved excreta disposal | 56.2 | 24.3 | 46.0 | 61.2 | 77.9 | 89.4 | 3.7 |
| Vitamin A supplementation | 71.9 | 69.1 | 72.4 | 69.7 | 72.7 | 77.5 | 1.1 |
| Measles vaccination | 75.7 | 59.5 | 79.6 | 76.3 | 80.6 | 90.5 | 1.5 |
| DPT vaccination | 81.0 | 70.7 | 80.9 | 82.4 | 84.9 | 91.0 | 1.3 |
| Case management of pneumonia | 16.6 | 8.2 | 11.2 | 16.1 | 21.1 | 33.8 | 4.1 |
| ORS for diarrhoea | 67.9 | 56.0 | 62.0 | 69.4 | 86.4 | 77.3 | 1.4 |
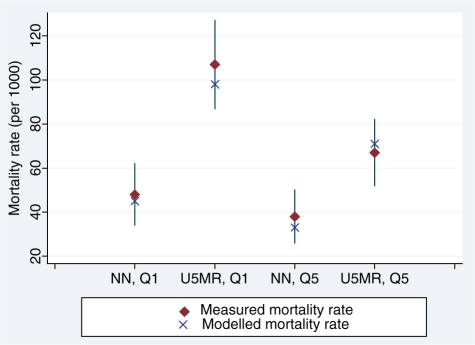
Figure 1 shows the results of using these coverage levels to model mortality rates for the neonatal and post-neonatal age groups, assuming a change in coverage from national level to the level observed in lowest and highest wealth quintiles. In all four comparisons the modelled estimates rates fell within the 95% CIs of the measured mortality. Modelled estimates were lower than measured estimates in three of the four groups.
Figure 1.
Measured neonatal (NN) and under-5 mortality rates (U5MR), 95% CIs and modelled rates for lowest (Q1) and highest (Q5) wealth quintiles
Table 3 shows the causes of deaths in the neonatal period for the poorest and richest quintiles as measured and as modelled by LiST. Agreement between measured and modelled results for the lowest quintile was good, with no single cause showing a difference of greater than 6 percentage points and all estimates falling within the CIs of the measured estimate. Agreement for the wealthiest quintile was less good, with the modelled estimate of deaths due to asphyxia falling 17.1 percentage points below the measured estimate, and the modelled estimates for deaths due to prematurity and other causes falling 6.3 and 8.6 percentage points above the measured estimates, respectively.
Table 3.
Neonatal deaths in 2004 BDHS verbal autopsy data by cause, observed and modelled using LiST
| Causes | National | Lowest wealth quintile |
Highest wealth quintile |
||||
|---|---|---|---|---|---|---|---|
| Observed (95% CI) | Modelled | Difference | Observed (95% CI) | Modelled | Difference | ||
| Diarrhoea | 1.8 | 1.3 (0.0–3.9) | 1.9 | −0.6 | 5.4 (0.0–12.9) | 1.9 | 3.5 |
| Sepsis Pneumonia | 43.6 | 50.0 (38.4–61.6) | 44.8 | 5.2 | 37.8 (24.2–51.4) | 39.6 | −1.8 |
| Asphyxia | 21.1 | 17.1 (7.7–26.5) | 21.3 | −4.2 | 37.8 (23.0–52.7) | 20.7 | 17.1 |
| Prematurity | 11.0 | 9.5 (2.9–16.0) | 10.8 | −1.3 | 4.7 (0.0–9.9) | 11.0 | −6.3 |
| Tetanus | 4.0 | 5.4 (0.1–10.7) | 4.3 | 1.1 | 2.6 (0.0–8.0) | 3.9 | −1.3 |
| Congenital anomalies | 5.1 | 4.1 (0.0–8.5) | 4.6 | −0.5 | 3.7 (0.0–9.9) | 6.3 | −2.6 |
| Other | 13.5 | 12.6 (4.1–21.1) | 12.4 | 0.3 | 8.0 (1.4–14.5) | 16.6 | −8.6 |
| Total | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | ||
| N | 338 | 104 | 51 | ||||
Table 4 shows similar results for the post-neonatal period. Again the agreement between modelled and measured results was good for the poorest quintile and less good for the wealthiest quintile, with LiST predicting 17.1 percentage points more pneumonia deaths than measured and 14.5 percentage points fewer diarrhoea deaths.
Table 4.
Post-neonatal deaths in 2004 BDHS verbal autopsy data by cause, observed and modelled using LiST
| Causes | National | Lowest wealth quintile |
Highest wealth quintile |
||||
|---|---|---|---|---|---|---|---|
| Observed (95% CI) | Modelled | Difference | Observed (95% CI) | Modelled | Difference | ||
| Diarrhoea | 13.3 | 13.4 (5.0–21.7) | 16.6 | −3.2 | 24.2 (6.4–42.1) | 9.7 | 14.5 |
| Pneumonia | 63.2 | 66.4 (55.9–76.8) | 62.5 | 3.9 | 44.7 (27.8–61.6) | 61.8 | −17.1 |
| Measles | 1.3 | 1.6 (0.0–3.8) | 1.6 | −0.01 | 0.0a | 1.1 | −1.1 |
| Injury/other | 22.1 | 18.7 (9.9–27.5) | 19.3 | −0.6 | 31.1 (14.5–47.6) | 27.4 | 3.7 |
| Total | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | ||
| N | 270 | 92 | 33 | ||||
aNo cases in the numerator.
Discussion
This first effort to assess how well LiST predicts mortality within wealth quintiles found promising results, with modelled estimates falling within the 95% CIs of measured mortality for both neonatal and post-neonatal deaths in the lowest and highest quintiles. These results suggest that LiST can capture the impact of coverage inequities on neonatal and under-5 mortality rates in this context. Limitations in the results reflect the quality of the verbal autopsy and coverage data used. Small sample size for the verbal autopsy data resulted in lower precision for the estimates of proportions of deaths by cause. Similarly, estimates of all cause mortality for the lowest and highest quintiles suffered from small sample size in each quintile group. Furthermore, coverage estimates for some indicators—for example improved water source—were not sensitive enough to discriminate across quintiles.
LiST performed well in predicting the cause-of-death profile for these two age groups for the poorest quintile of the population, but less well for the richest quintile. These findings merit further investigation through a closer examination of the categorization of deaths in the original verbal autopsy results and in this analysis. Cause of death was assigned through a hierarchical process, which is sensitive to the order of assignment. The lower predictive ability for deaths among the rich is not surprising, because one might expect LiST to perform less well as mortality levels drop and other interventions not taken into account in LiST (e.g. hospital-based care) become more important. Characteristics specific to Bangladesh may also have affected the predictions of deaths by cause. Of the sixty-eight countries that accounted for 97% of maternal and child deaths worldwide in 2006 identified by the Countdown to 2015, Bangladesh is among those with the lowest rate of skilled deliveries.15 Only 20% of deliveries in Bangladesh are attended by a skilled professional compared with a median of 32% among the 68 Countdown countries. In addition, an estimated 85% of deliveries occur at home. Large socioeconomic inequities in access to delivery care may explain why LiST underestimated the percentage of deaths due to asphyxia in the wealthiest quintile. The more recent BDHS, in 2007, reported 51% of deliveries attended by a medically trained professional among the wealthiest quintile compared to only 5% among the lowest quintile, and 43% vs 4% for health facility deliveries.16 Effects of contextual factors on the prediction of neonatal and post-neonatal cause-of-death profiles using LiST can only be accurately assessed when cause-of-death data become readily available for several countries with different contextual characteristics.
Further validation of LiST predictions of cause-specific deaths can be done only in settings where recent data on the causes of child deaths are available. This is one reason why verbal autopsies should be considered for inclusion in future DHS surveys. Sufficient sample sizes would be needed in these surveys to allow stable estimates of deaths by cause at national level and among specific subpopulations such as wealth subgroups.
To further illustrate the possible programmatic applications of LiST, we used the data reported here to assess the number of child lives that could be saved if coverage for these proven interventions was increased among households in the poorest quintile of the population to the mean coverage levels reported for the national population. The results showed that in Bangladesh, ∼8000 child deaths (10% of child deaths in the poorest 20% of the population) could be averted, or 3% of all deaths nationally, through this strategy. Increasing coverage levels in the poorest quintile to that of the wealthiest quintile would avert >20 000 child deaths each year, or almost 10% of all child deaths in the country.
Conclusions
LiST holds promise as a useful tool for those planning maternal and child health programs in low-income countries with high levels of socioeconomic inequity. Program planners can consider alternative scenarios and use LiST to assess the extent to which they are ‘pro-poor’ and will contribute to redressing inequities in mortality by wealth status.
Funding
This work was supported in part by a grant to the US Fund for UNICEF from the Bill & Melinda Gates Foundation (grant 43386) to Promote evidence-based decision making in designing maternal, neonatal and child health interventions in low- and middle-income countries , and a grant to the Institute for International Programs at the Johns Hopkins Bloomberg School of Public Health from the Canadian International Development Agency (grant 7052335) for Real-time monitoring of child mortality.
Supplementary data
Supplementary data are available at IJE online.
KEY MESSAGES.
LiST performed well in predicting inequities in under-5 mortality (overall and by cause of death) for the poorest and least-poor population quintiles in Bangladesh.
LiST can contribute to the design and evaluation of programs that are ‘pro-poor’, but only if large-scale surveys in low-income countries measure intervention coverage, cause of death and household assets using adequate sample sizes.
LiST produces accurate estimates for poor populations that are unable to access tertiary care; children in wealthier households are more likely to receive advanced biomedical interventions that are not included in LiST.
References
- 1.Gwatkin D, Rutstein S, Johnson K, Suliman E, Wagstaff A, Amouzou A. Socio-economic Differences in Health, Nutrition and Population Within Developing Countries: An Overview. Washington, DC: HNP The World Bank; 2007. [PubMed] [Google Scholar]
- 2.Gwatkin D, Bhuiya A, Victora C. Making health systems more equitable. Lancet. 2004;364:1273–80. doi: 10.1016/S0140-6736(04)17145-6. [DOI] [PubMed] [Google Scholar]
- 3.Masanja H, Schellenberg J, de Savigny D, Mshinda H, Victora C. Impact of integrated management of childhood illness on child health inequities in rural Tanzania. Health Policy Planning. 2005:i77–i84. doi: 10.1093/heapol/czi054. [DOI] [PubMed] [Google Scholar]
- 4.Victora C, Vaughan PJ, Barros F, Silva A, Tomasi E. Explaining trends in inequities: evidence from Brazilian child health studies. Lancet. 2000;356:1093–98. doi: 10.1016/S0140-6736(00)02741-0. [DOI] [PubMed] [Google Scholar]
- 5.Boschi-Pinto C, Black R, Young M. The Child health epidemiology reference group reviews of the effectiveness of interventions to reduce maternal, neonatal and child mortality. Int J Epidemiol. 2009;39(Suppl 1):i3–6. doi: 10.1093/ije/dyq018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filmer D, Pritchett L. Estimating wealth effects without expenditure data – or tears: An application to educational enrollments in states of India. Demography. 2001;38:115–32. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- 7.National Institute of Population and Training (NIPORT) Bangladesh Demographic and Health Survey 2004. Dhaka Bangladesh; Calverton, Maryland [USA]: National Institute of Population and Training (NIPORT), Mitra and Associates, and ORC Macro; 2005. Mitra and Associates, ORC Macro. [Google Scholar]
- 8.Rutstein S, Rojas G. Guide to DHS Statistics. Calverton, MD: ORC Macro; 2006. [Google Scholar]
- 9.Kish L. Survey Sampling. New York: Wiley Interscience; 1965. [Google Scholar]
- 10.United Nations Children’s Fund and World Health Organization. . Low Birthweight: Country, Regional and Global Estimates. New York: UNICEF; 2004. [Google Scholar]
- 11.De Onis M, Blossner M, Villar J. Levels and patterns of intrauterine growth retardation in developing countries. European Journal of Clinical Nutrition. 1998;52:S5–15. [PubMed] [Google Scholar]
- 12.National Family Health Survey (NFHS. 3), 2005–06. Mumbai: IIPS and Macro International Inc; 2007. International Institute for Population Sciences (IIPS), Macro International Inc. [Google Scholar]
- 13.Pakistan Demographic and Health Survey 2006–07. Islamabad: National Institute of Population Studies and Macro International Inc; 2008. National Institute of Population Studies (NIPS) [Pakistan], Macro International Inc. [Google Scholar]
- 14.Bryce J, Daelmans B, Dwivedi A, et al. Countdown to 2015 for maternal, newborn, and child survival: the 2008 report on tracking coverage of interventions. Lancet. 2008;371:1247–58. doi: 10.1016/S0140-6736(08)60559-0. [DOI] [PubMed] [Google Scholar]
- 15.Bryce J, Requejo J. Countdown to 2015: Tracking Progress in Maternal, Newborn and Child Survival: The 2008 Report. New York: UNICEF; 2008. [Google Scholar]
- 16.Bangladesh Demographic and Health Survey 2007. Dakha, Bangladesh; Calverton, MD: National Institute of Population and Training (NIPORT), Mitra and Associates, and Macro International Inc; 2009. National Institute of Population and Training (NIPORT), Mitra and Associates, Macro International Inc. [Google Scholar]