Abstract
A large proportion of recessive non-syndromic hearing loss is due to mutations in the GJB2 gene encoding connexin 26 (Cx26), a component of a gap junction. Within different ethnic groups there are specific common recessive mutations each with a relatively high carrier frequency suggesting a possibility of heterozygous advantage. Carriers of the R143W GJB2 allele, the most prevalent in the African population, present with a thicker epidermis than non-carriers. In this study, we show (R143W)Cx26 expressing keratinocytes form a significantly thicker epidermis in an organotypic co-culture skin model. In addition, we show increased migration of cells expressing (R143W)Cx26 compared to (WT)Cx26 overexpressing cells. We also demonstrate that cells expressing (R143W)Cx26 are significantly less susceptible to cellular invasion by the enteric pathogen Shigella flexneri than (WT)Cx26 expressing cells. These in vitro studies suggest an advantageous effect of (R143W)Cx26 in epithelial cells.
Introduction
It has been suggested that intercellular communication via gap junctions play a role in keratinocyte differentiation and wound healing by regulating the passage of ions, small metabolites and second messenger molecules (< 1 kDa) (Goliger & Paul, 1995). Specific connexins are expressed at different layers (or strata) of the normal human epidermis. One of the predominant connexins is connexin 43 (Cx43) which is expressed throughout the suprabasal layers. Extremely low levels of Cx26 are expressed in the basal layer and similarly Cx30 is sparsely expressed in the basal and granular layer (Di et al., 2001; Lucke et al., 1999). The expression and distribution of these epidermal connexins alter during various stages of wound healing. After wounding rat epidermis in vivo, Cx26 expression was upregulated in the differentiated cells proximal to the wound but was downregulated in the cells at the wound edge. Using dye transfer analysis the changes in connexin expression corresponded to changes in junctional communication (Goliger & Paul, 1995). In addition, the enteric pathogen Shigella flexneri can open Cx26 hemichannels allowing the release of ATP (Tran Van Nhieu et al., 2003) suggesting Cx26 may play a role in bacterial cell entry.
Distinct dominant mutations in GJB2 encoding Cx26 can produce an array of ectodermal phenotypes including hearing loss, neuropathy, hair growth abnormalities and hyperkeratosis (Richard, 2005). These disease associations support an important function for this gap junction protein in epidermal differentiation. Although dominant GJB2 mutations can result in syndromic disease, recessive GJB2 mutations only cause hearing loss (Kelsell et al., 1997). Subsequently numerous studies have shown that GJB2 mutations account for a significant proportion of recessive genetic deafness (Hutchin et al., 2005; Kenneson, Van Naarden Braun & Boyle, 2002; Snoeckx et al., 2005). Within different ethnic groups there are specific common recessive mutations that account for the majority of GJB2-related hearing loss, for example 35delG, 235delC and R143W in the European, Japanese and African populations respectively (Brobby, Muller-Myhsok & Horstmann, 1998; Denoyelle et al., 1997; Kudo et al., 2000). Each ethnic or geographic population has a carrier frequency of between 1-3 % suggesting a possibility of heterozygous advantage. Although there is no clinically defined dermatological signs, data generated from skin biopsies has shown that heterozygotes for the R143W mutation in GJB2 have a thicker epidermis than wild-type (WT) GJB2 homozygotes (Meyer et al., 2002). Enhanced cellular viability of HeLa cells overexpressing (R143W)Cx26 compared to those overexpressing (WT)Cx26 has also been shown (Common et al., 2004).
In this study, we demonstrate in vitro an extended keratinocyte differentiation programme and increased migration of (R143W)Cx26 expressing cells compared to the wild-type counterpart. We also demonstrate that HeLa cells expressing this Cx26 mutant do not increase cellular invasion by the enteric pathogen S. flexneri unlike that of (WT)Cx26 expressing cells.
Materials and Methods
All reagents were supplied from Sigma (Poole, Dorset, UK) unless otherwise stated.
Antibodies
Monoclonal keratin 10 (LH2), involucrin (Sy5), keratin 14 (LL002), keratin 6 (LHK6) and keratin 16 (LL025) antibodies were all generated by CRUK, United Kingdom. Transglutaminase 1 (TG1) (Biogenesis), keratin 2e (Abcam), loricrin (Covance Research Products) and Ki67 (DAKO) antibodies were obtained commercially.
Plasmid constructs
Full length cDNA of (WT)Cx26, (R143W)Cx26, (WT)Cx30 and (WT)Cx31 fused with EGFP, together with EGFP alone, was cloned and inserted into pSIN retroviral vector (Deng, Lin & Khavari, 1997). All clones were verified by automated sequencing using ABI 3700 (Genome Centre, Queen Mary University, London).
Culture of nTERT keratinocytes and HeLa cells
The human cell line nTERT was derived from primary keratinocytes immortalised by telomerase (Dickson et al., 2000). The culture medium consisted of E4 Ham’s medium containing 10 % (v/v) foetal calf serum, 2mM glutamine, 1 % (v/v) RM + supplement (final concentration of 400 ng/ml hydrocortisone, 5 μg/ml insulin, 10 ng/ml EGF, 8.4 ng/ml cholera toxin, 5 μg/ml transferrin, 13 ng/ml lyothyronine), 50 units/ml penicillin-G and 50 μg/ml streptomycin sulphate. HeLa cells (ATCC) were cultured in DMEM containing 10 % (v/v) foetal calf serum, 2mM glutamine, 50 units/ml penicillin-G and 50μg/ml streptomycin sulphate.
Retroviral transduction
A retroviral transduction protocol was carried out in order to create nTERT or HeLa cells that overexpress (WT)Cx26-EGFP, (R143W)Cx26-EGFP, (WT)Cx30-EGFP, (WT)Cx31-EGFP or EGFP alone. Phoenix 293 viral packaging cells were cultured in DMEM medium as described above. They were transfected with the plasmid constructs using a 1:3 ratio of DNA:Fugene 6 reagent (Roche). After 24 hours, cells were selected with 1 μg/ml puromycin for 48 hours at 37°C. The media was replaced and the cells incubated at 32°C for 24 hours before the virus-containing supernatant was collected, filter-sterilised and snap frozen in liquid nitrogen. All retrovirus collections were stored at −80°C until ready for use.
For retroviral transduction, the virus was prepared in a glass container whereby 5 μg/ml (final concentration) polybrene reagent was added and incubated at room temperature for 15 minutes. Keratinocytes were also treated with polybrene by adding a final concentration of 5 μg/ml to the normal culture medium. These cells were transferred to 37°C. After 15 minutes incubation, the polybrene-containing media was replaced with polybrene-treated virus. Cells were centrifuged at 350 × g, 32°C for 1 hour. Upon completion of the centrifugation step, the virus was replaced with fresh normal culture media and returned to 37°C/10 % CO2. After 24-48 hours, cells were viewed under a UV lamp microscope to confirm expression. In all cases, transduction efficiency was comparable between constructs.
Connexin Localisation
Transduced nTERT cells were plated onto coverslips at a suitable density, and after 24-hours fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 30-minutes. The cells were washed in PBS, and mounted using Immu-Mount (Thermo Electron Corporation) containing 10 μg/ml DAPI, before being visualised by laser scanning confocal microscopy (LSM510, Carl Zeiss).
Organotypic co-culture
Organotypic co-culture was carried out following the protocol described by Ojeh et al. (2001).
Dye injection
The growing epidermis was removed from the dermis raft using fine forceps, and suspended in low melting point agarose. Small blocks of tissue in agarose were mounted on a vibratome and sections cut at 200μm. Lucifer yellow (LY; 4% in 3M LiCl) was microinjected into cells using microelectrodes with resistance ~100MΩ. Following LY injection slices were fixed in 4% paraformaldehyde in PBS for 30 minutes. Slices were then permeabilized (0.1% Triton-X) and blocked (10% normal goat serum), and subsequently incubated in an anti-LY antibody overnight at 4°C. Following several washes in PBS, the anti-LY antibody was detected using an anti-rabbit FITC-conjugated secondary antibody. Slices were mounted in an anti-fade medium containing DAPI (Vectashield, Vector Laboratories). Immunofluorescence was detected by laser scanning confocal microscopy (LSM510, Carl Zeiss). The number of cells containing LY were counted for each injection site. Data from tissue transfected with (WT)Cx26 were compared to that from (R143W)Cx26 transfected tissue using Student’s unpaired T-test (Prism v4; Graphpad Software Inc.).
Co-culture staining
Each tissue was cut in half such that one half was snap frozen whilst embedded in Cryo-M-Bed™ (Bright) and was stored at −80°C. The other half was fixed in 4 % (v/v) paraformaldehyde and embedded in paraffin. Sections were cut at 5-6 μm in thickness.
Haematoxylin and eosin staining
Haematoxylin and eosin staining was performed on the paraffin sections following the standard protocol. Briefly, sections were dewaxed in xylene and rehydrated in sequential washes of 100 %, 90 % and 70 % alcohol. Sections were stained in haematoxylin for 3 minutes, followed by eosin for 2 minutes, dehydrated in 70 %, 90 % and 100 % alcohol and mounted with DePeX (VWR).
The thickness of the cornified envelope (green arrows) and the epidermal/dermal junctions (orange arrows) were measured across three representative areas of three independent sections per sample. This was repeated for three independently grown organotypic co-cultures.
Immunofluorescent staining
Frozen sections were air-dried for 30 minutes and cells were permeabilised in 0.1 % (v/v) Triton X-100 in PBS for 15 minutes. Sections were washed in PBS, blocked in 0.2 % (v/v) gelatin from cold water fish skin for 15 minutes and incubated with primary antibody for 1 hour. All antibodies mentioned in this paper were used at a 1/200 dilution. After three PBS washes, fluorescent secondary antibody (donkey anti-rabbit or donkey anti-mouse Alexa Fluor 568, Molecular Probes) was added at a 1/1000 dilution and incubated for 1 hour protected from light exposure. Sections were washed three times in PBS, incubated with DAPI (0.125 μg/ml) for five minutes and washed three more times. Sections were mounted with immunomount reagent (ThermoShandon, Pittsburg, CA) and viewed under a Nikon Eclipse TE2000-S microscope.
Scratch migration assay
Cells were seeded at 5 × 105cells per well in 6-well plate culture dishes. Each cell sample was seeded in duplicate and incubated at 37°C for 24 hours. After culturing in serum-free medium (Ham’s DMEM) for 24 hours, cells were treated with mitomycin C at 10 mg/ml for 1 hour at 37°C to inhibit proliferation. The media was removed and a “cross” was scratched across the dish using a P1000 pipette tip leaving “scratched” areas with no cells (Boyer et al., 1989). Cells were washed three times in PBS. An area of the “cross” was imaged at 5 × magnification at 0 hours and 48 hours after the initial scratch to ensure the same part of the scratch was imaged each time. Cells were incubated at 37°C/5 % CO2 in between time points. Images were analysed using ImageJ software program (downloaded from website http://rsb.info.nih.gov/ij/) whereby the cell-free area was recorded at each time point. The data was presented as average percentage area migrated.
Proliferation assay
Cellular proliferation was analysed using the alamarBlue assay (Biosource). Cells (1 × 104) were seeded in triplicate in 12-well plates and incubated for 20 hours at 37°C to allow the cells to attach onto the culture dish. Cells were washed with PBS and 10 % (v/v) alamarBlue dye in normal culture media was added. Cells were incubated for 6 hours at 37°C. 150 μl from each sample was removed from each well for fluorescence detection and the remaining dye was washed from the cells before normal culture media was replaced. The assay was performed over three consecutive days, with fresh alamarBlue added 6 hours prior to assay.
Each sample was measured in duplicate by recording the optical density at 570 nm (OD570). The fold change (i.e. average OD570 each day / average OD570 on day 1) per sample was calculated and plotted against time (days).
Bacterial invasion assay
Shigella flexneri serotype 2a (2457T) (ATCC) was grown in LB broth overnight at 37°C in an orbital shaker. The optical density of the culture was measured at 600 nm. Retrovirally transduced HeLa cells were seeded into 24-well plate dishes at 2 × 105 cells per well. The medium was removed and replaced with antibiotic-free DMEM containing 10 % (v/v) foetal calf serum and 2mM glutamine. S. flexneri was added to each well of transduced HeLa cells (0.93 OD600 units per well, corresponding to 3 × 107 CFUs). The plate was centrifuged at 15 × g for two minutes and incubated at 37°C/10 % CO2 for 4 hours. Cells were washed three times with PBS and media was added containing 50 μg/ml of gentamycin and incubated at 37°C for 1 hour. Following three washes in PBS, cells were lysed with 0.1 % (v/v) Triton X-100 in PBS. Lysates were serially diluted in PBS and were plated onto LB agar. Bacteria were propagated overnight at 37°C. The number of colonies was counted and statistical analysis was carried out using two-tailed, unpaired t-test.
Statistical analysis
All statistical analysis was carried out either as a one or two-tailed, unpaired t-test as stated depending on the type of experiment performed. p-values equal to or less than 0.05 were considered as statistically significant.
Results
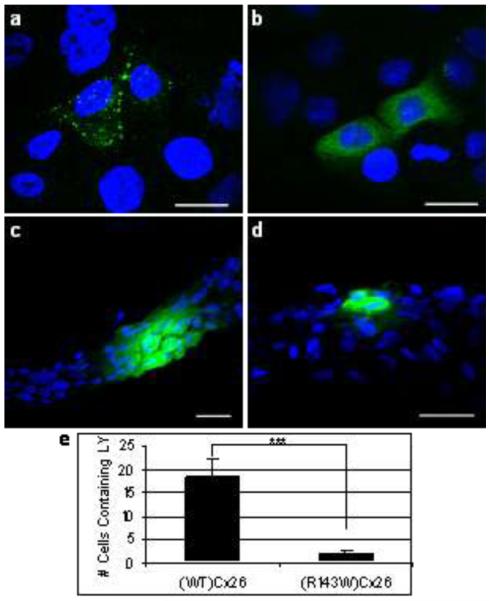
To confirm expression of the EGFP-tagged connexin proteins, cells transduced with the constructs were first visualised by confocal microscopy (fig 1). Aggregations were observed in cells transduced with (WT)Cx26 at the cell periphery which are indicative of gap junction plaques. No such aggregations were observed in the (R143W)Cx26-expressing cells. Organotypic co-cultures expressing (WT)Cx26 or (R143W)Cx26 were subjected to LY dye transfer assays. Whilst (WT)Cx26 allowed dye transfer to adjoining cells, in the (R143W)Cx26 co-cultures the amount of dye transfer was significantly reduced by eight-fold.
Figure 1.
Localisation of (WT)Cx26 (a) and (R143W)Cx26 (b) (green). Note the aggregations of (WT)Cx26 at the cell periphery which are indicative of gap junction plaques are absent in the cells expressing (R143W)Cx26. LY (green) transfer following microinjection of organotypic co-cultures expressing (WT)Cx26 (c), or (R143W)Cx26 (d). Quantification of number of cells containing LY after microinjection of (WT)Cx26 and (R143W)Cx26 expressing co-cultures (e). There is statistical difference between the two (*** represents p<0.0001; unpaired t-test). (a-d): Scale bars represent 20 μm. Nuclei are shown with blue DAPI staining.
Morphological characterisation of organotypic co-cultures overexpressing connexins
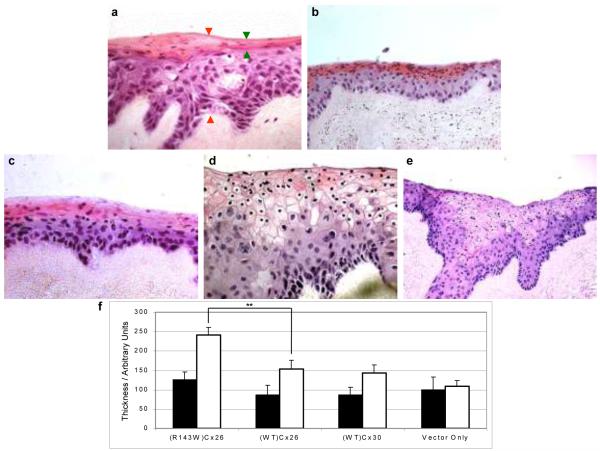
The morphological characteristics of keratinocytes on a three-dimensional basis were examined by organotypic co-cultures (Ojeh, Frame & Navsaria, 2001). Haematoxylin and eosin staining showed little difference between the co-cultures using cells transduced with (WT)Cx26-EGFP, (WT)Cx30-EGFP and pSIN-EGFP (vector-only), (fig 2a-c). These co-cultures all exhibited typical histological features of stratified squamous epidermis i.e. nucleated basal cells eventually differentiating into flatter, anucleated cells towards the top of the epidermal layer. (R143W)Cx26 co-cultures showed evidence of a significantly thicker epidermis compared to the other samples despite there being equal numbers of keratinocytes seeded at the start of each organotypic co-culture (fig 2d-e). Observationally, the (R143W)Cx26 co-culture had more densely packed basal keratinocytes compared to the control suggesting an increased number of proliferative cells. Some suprabasal keratinocytes in the spinous layer appeared bigger in size and an eosin-positive layer (stained pink) was also visible. In the latter, some cells appeared to lose their nuclei, whilst others retained very small nuclei. The (R143W) co-culture lacked a clearly defined cornified envelope at day 14, compared to control co-cultures where it had formed by this stage. However, the (R143W) co-cultures have formed a cornified envelope by day 16 (data not shown) indicating delayed terminal differentiation.
Figure 2.
Histological features of organotypic co-cultures. Haematoxylin and eosin stained sections of a) pSIN-GFP (vector-only); b) (WT)Cx30; c) (WT)Cx26; d) (R143W)Cx26 co-cultures at 400 × magnification and e) (R143W)Cx26 at 200 × magnification. Green and orange arrows shown on a) represent the distance at which the eosin-positive layer and full thickness of the epidermal equivalent were measured, respectively. f) Graph representing the average thickness of the eosin-positive layer (black bars) and average full thickness of the epidermal equivalent (white bars). Unpaired, two-tailed t-test showed that the total thickness of the epidermal equivalent of the (R143W)Cx26 co-culture was statistically different from (WT)Cx26 (** represents p < 0.01). Error bars represent the standard error of the mean (SEM), n= 3.
The average epidermal thickness measurement of (R143W)Cx26 was 1.6 times higher than that corresponding to the (WT)Cx26 co-cultures (p=0.003) (fig 2f). The average measurements of the eosin-positive (pink) layer remained fairly constant in all the co-cultures. Similarly, no differences in the epidermal thickness measurements were found between (WT)Cx26 and (WT)Cx30 co-cultures compared to the vector-only control. This data is comparable to an in vivo study (Meyer et al., 2002).
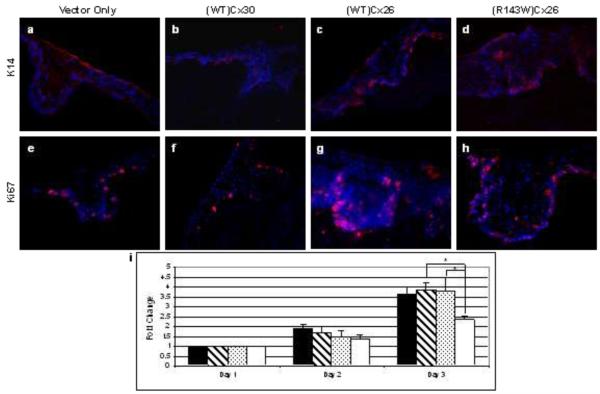
Immunohistochemistry was performed to assess any molecular changes in the differentiation programme of the transduced keratinocytes on organotypic co-cultures using differentiation markers. Normal expression patterns were found for keratin 10, transglutaminase 1, involucrin, keratin 2e and loricrin between all co-cultures (data not shown). This suggests that the onset of late differentiation is similar between all the organotypic models. In contrast a larger number of keratin 14 positive cells were observed in (R143W)Cx26 co-cultures compared to the other co-cultures (fig 3a-d). Keratin 14, a basal cell marker of the epidermis, was not only expressed in the basal layer but was also evident in (R143W)Cx26 expressing cells in the higher layers of the three-dimensional culture.
Figure 3.
Characterisation of nTERTs overexpressing wild-type (WT)Cx26, (WT) Cx30 and (R143W)Cx26. Keratin 14 immunofluorescent staining (red) of a) vector-only control; b) (WT)Cx30; c) (WT)Cx26 and d) (R143W)Cx26 co-cultures. Ki67 immunofluorescence (red) of; e) vector-only control f) (WT)Cx30 g) (WT)Cx26 and h) (R143W)Cx26 co-cultures. Nuclei are shown with blue DAPI staining. i) Graph showing the average fold-change in proliferation over a 3-day time course. The black, striped, speckled and white bars represent nTERTS overexpressing (R143W)Cx26, (WT)Cx26, (WT)Cx30 and vector-only control, respectively. The data represent mean ± the standard error of the mean (SEM) (n=3). * represents p ≤ 0.05.
Proliferative features of organotypic co-cultures and nTERT keratinocytes
No evidence of hyperproliferation was observed, with only weak staining of keratin 6 and keratin 16 observed in all co-cultures (data not shown). The proliferation status was further assessed by the cellular pattern of Ki67 expression in the organotypic co-cultures (fig 3e-h). Ki67 is expressed in the nuclei of actively proliferating cells normally exclusive to those in the basal layer of the epidermis. This expression pattern was observed in the (WT)Cx30 culture (fig 3f) and in the vector-only control (fig 3e). More Ki67 positive keratinocytes were present along the basal layer of the (R143W)Cx26 co-culture (fig 3h) compared to the vector-only control. Similarly, in (WT)Cx26 co-cultures, Ki67 was present at an increased level in the basal layer relative to control co-cultures. These Ki67-positive cells also appeared in the early spinous layer (fig 3g). The increase in number of proliferating (Ki67-expressing) cells in the (WT)Cx26 and (R143W)Cx26 organotypic co-cultures was confirmed by a blind scoring analysis (data not shown). The proliferative status of the cells was also analysed using the alamarBlue assay (fig 3i). The data revealed that keratinocytes overexpressing (R143W)Cx26, (WT)Cx26 and (WT)Cx30 proliferated faster than the vector only control. There was no difference in proliferation between (R143W)Cx26 and (WT)Cx26 transduced cells.
Migratory characteristics of keratinocytes
A scratch assay was performed to assess cell migration (fig 4). Keratinocytes overexpressing (R143W)Cx26 showed an increase in migration by 1.5-fold compared to the (WT)Cx26 equivalent (p = 0.025). (WT)Cx26 and (WT)Cx30 cells exhibited an increased migration compared to vector-only control by 3.5-fold (p = 0.0013) and 2.7-fold (p = 0.013), respectively.
Figure 4.
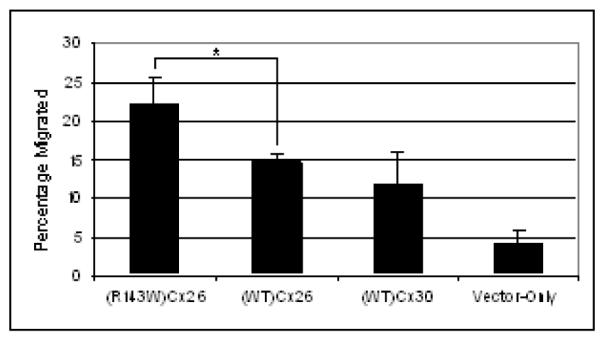
Keratinocyte cellular migration analysis. A monolayer of NTERTS overexpressing (R143W)Cx26, (WT)Cx26, (WT)Cx30, or vector-only control were scratched. Graph shows the percentage of cells migrated after 48 hours. The data represent mean ± the standard error of the mean (SEM). Comparison was carried out using unpaired, one tailed t-test where a significant difference was found between (WT)Cx26 and (R143W)Cx26. * represents p ≤ 0.05.
The increase in bacterial invasion seen in (WT)Cx26 expressing HeLa cells is not seen in the (R143W)Cx26 counterpart
Previously, it has been shown that cellular invasion of the gut pathogen S. flexneri is associated with opening Cx26 hemichannels in an actin-phospholipase-C-dependent manner (Tran Van Nhieu et al., 2003). We have confirmed and extended these findings (Figure 5). The total number of S. flexneri bacteria which invaded into (WT)Cx26 overexpressing HeLa cells was higher than in cells expressing vector-only control. Interestingly two other epidermally expressed connexins, (WT)Cx30 and (WT)Cx31, showed no evidence for enhancing the potential for S. flexneri to invade. HeLa cells expressing (R143W)Cx26 do not have the increased levels of bacterial invasion observed in (WT)Cx26 transduced cells (4670 ± 324.8 bacteria invaded in (WT)Cx26, and 3069 ± 238.3 bacteria invaded in (R143W)Cx26. A significant difference (p = 0.044) in bacterial invasion was illustrated between (R143W)Cx26 and (WT)Cx26.
Figure 5.
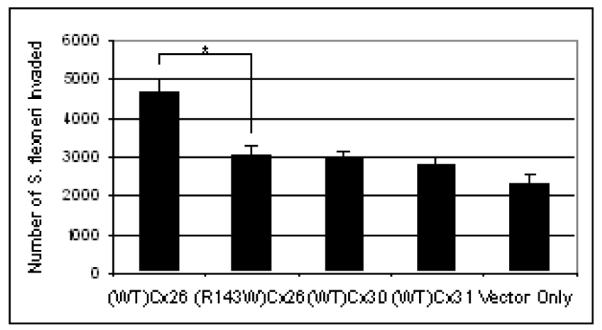
Bacterial invasion via Cx26 hemichannels. Histogram showing invasion of Shigella flexneri bacteria in HeLa cells overexpressing (WT)Cx26, (R143W)Cx26, (WT)Cx30, (WT)Cx31 and vector-only control. The data represent mean ± the standard error of the mean (SEM) n = 15. Comparison was carried out using unpaired, two-tailed t-test where a significant difference was found between (WT)Cx26 and (R143W)Cx26. * represents p ≤ 0.05.
Discussion
Multiple differences were observed between keratinocytes overexpressing human (WT)Cx26 compared to those overexpressing (R143W)Cx26. (WT)Cx26 formed aggregates indicative of gap junction plaques, whereas cells expressing (R143W)Cx26 were devoid of plaque formation. Palmada et al (2006) observed the (R143W)Cx26 mutant in the 10,000×g pellet which is consistent with an internal membranous structure. However other studies (Wang et al., 2003) report a plasma membrane localisation. Differences in cell type and expression conditions could explain these contrasting results. However, regardless of the mutant protein’s cellular localisation, our dye conductance experiments herein confirm that the (R143W)Cx26 is unable to form functional channels (Mese et al., 2004; Palmada et al., 2006). The dye transfer observed was most likely due to endogenous nTERT connexin expression.
The human (R143W)Cx26 model presented with thickening of the epidermal equivalent, increased number of cells expressing basal marker K14 and increased cellular mobility. Coincidentally, the overall increase in thickness was similar in both our model and in an in vivo study (Meyer et al., 2002). This might suggest that there is a limit to the overall thickness of the epidermis before it becomes pathological. Furthermore we provide evidence that, unlike (WT)Cx26, (R143W)Cx26 did not increase S. flexneri invasion.
From our data in this study, the increase in cell numbers seen in the (R143W)Cx26 organotypic co-culture showed little difference in proliferation compared to (WT)Cx26 co-cultures, a conclusion further confirmed by the proliferation assay. Since increased proliferation cannot explain the thickened epidermis in (R143W)Cx26 co-cultures, the phenotypic effect may be due to an increased number of undifferentiated basal cells as demonstrated by the Ki67 and K14 immunostaining data, respectively. It is possible that the overall proliferation rate is unaltered but because more undifferentiated cells are present more daughter cells are generated, giving rise to epidermal thickening.
Additionally, skin-thickening conditions such as psoriasis and palmoplantar keratoderma (PPK) display an increase in K6 and K16 expression, markers of hyperproliferation (Leigh et al., 1995). Although the organotypic co-culture model of (R143W)Cx26 showed significant epidermal thickening, no significant changes were seen in K6 expression compared to the control, supporting the notion that the phenotype is not associated with any pathological changes. The very weak staining observed in all the co-cultures is most likely due to the keratinocytes adopting a slight hyperproliferative state – a finding consistent with previous descriptions of organotypic co-cultures (Stark et al., 2004). If the observations were not of a pathological nature, (R143W)Cx26 may exert beneficial properties to the skin.
One way R143W(Cx26) may benefit the skin is by decreasing the ability of bacterial pathogens to invade. As a preliminary experimental model we used the gut invading pathogen S. flexneri in HeLa cells as HeLa cells express low endogenous levels of connexins and were previously used to show that Cx26 enhances S. flexneri invasion (4). Our data shows that two other epidermally expressed connexins, Cx30 and Cx31, do not have a similar effect. The mechanism behind relative specificity of Cx26 to elicit the invasion increase is yet to be explored. (R143W)Cx26 expressing cells showed reduced bacterial invasion compared with (WT)Cx26 expressing cells. This may be due to the functional disruption of the Cx26 hemichannel since the wild-type hemichannel is reported to allow S. flexneri invasion through the actin-phospholipase-C-dependant pathway in HeLa cells (Tran Van Nhieu et al., 2003). Patients who carry the (R143W)Cx26 variant have increased levels of sodium and chloride in their eccrine sweat glands compared to normal individuals. This physiological finding was postulated to create an osmotic environment that is difficult for microbial colonisation (Meyer et al., 2002). Our model shows that (R143W)Cx26 expressing cells are protected against this gut pathogen compared to (WT)Cx26 counterpart. It could be possible that a similar effect is seen in the keratinocytes expressing (R134W)Cx26. It is interesting to note that Cx26 expression is limited to the basal layer in interfollicular human epidermis and to the epidermal eccrine sweat glands. This specific cellular localisation of Cx26 is consistent with protecting the epidermis from bacterial infection. Similarly, in human colon tissue, Cx26 expression levels appeared to be greatest around the goblet cells – cells that interact closely with the contents (bacterial or otherwise) of the gut (data not shown).
In human cutaneous wounding Cx26 is expressed 24–48 hours post-injury. It is detected near the wound margin and eventually as the wound heals, normal low expression of Cx26 resumes (Brandner et al., 2004). The role of this connexin at times of injury remains elusive. It has been suggested that Cx26 (and Cx30) is massively upregulated in psoriasis to control differentiation (Labarthe et al., 1998; Rivas et al., 1997; Wiszniewski et al., 2000). However, we did not observe any obvious differentiation changes in our Cx26 overexpression model. We postulate that Cx26 regulates the wound healing process, not by promoting it but by controlling and maybe even dampening the process. Our data demonstrated that overexpression of (WT)Cx26 did not promote an increase in migration whereas inhibition of Cx26 (by the R143W mutation) appeared to lead to an effect on keratinocyte migration. In support of our hypothesis, an in vivo study in human skin has shown an upregulation of Cx26 and Cx30 at wound margins of non-healing wounds, whereas during spontaneous wound repair these connexins are absent at the leading edge of the regenerating epidermis but are overexpressed behind the wound margin (Brandner et al., 2004). This expression pattern may reflect the proliferative capacity of Cx26 expressing cells which correlates well in our study herein. It has been reported that cells at this location relative to the wound undergo a proliferative burst which possibly provide a pool of extra cells to replace those lost during injury (Hertle et al., 1992; Matoltsy & Viziam, 1970).
Whilst this paper was in preparation, Segre and colleagues reported that transgenic mice heterozygous for involucrin-Cx26 had persistent Cx26 expression in the epidermis (Djalilian et al., 2006). The wounded epidermis was infiltrated with immune cells and the wound remained in a hyperproliferative state with the remodelling phase blocked, resulting in wound healing failure. This in vivo data is consistent with our findings and supports the hypothesis that Cx26 suppresses wound healing. In summary, these studies suggest that a non-functional Cx26 channel due to a GJB2 hearing loss associated mutation confers beneficial properties to the epidermis and possibly other Cx26 expressing epithelium resulting in an improved barrier, accelerated wound repair and protection from infection. As also recently suggested (Djalilian et al., 2006), knock-down of Cx26 expression may have a therapeutic application in wound healing and also as an anti-infective.
Acknoledgements
This work has been funded by the Research Advisory Board of Barts and the London, the BBSRC and the Wellcome Trust to Professor David P. Kelsell. With special thanks to Dr. Jash Vyas for advice and help with the migration assays and Professor Tom MacDonald for the normal human colon and help with interpretation of Cx26 localisation
References
- Boyer B, Tucker GC, Valles AM, Franke WW, Thiery JP. Rearrangements of desmosomal and cytoskeletal proteins during the transition from epithelial to fibroblastoid organization in cultured rat bladder carcinoma cells. J Cell Biol. 1989;109:1495–509. doi: 10.1083/jcb.109.4.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandner JM, Houdek P, Husing B, Kaiser C, Moll I. Connexins 26, 30, and 43: differences among spontaneous, chronic, and accelerated human wound healing. J Invest Dermatol. 2004;122:1310–20. doi: 10.1111/j.0022-202X.2004.22529.x. [DOI] [PubMed] [Google Scholar]
- Brobby GW, Muller-Myhsok B, Horstmann RD. Connexin 26 R143W mutation associated with recessive nonsyndromic sensorineural deafness in Africa [letter] New England Journal of Medicine. 1998;338:548–50. doi: 10.1056/NEJM199802193380813. [DOI] [PubMed] [Google Scholar]
- Common JE, Di WL, Davies D, Kelsell DP. Further evidence for heterozygote advantage of GJB2 deafness mutations: a link with cell survival. J Med Genet. 2004;41:573–5. doi: 10.1136/jmg.2003.017632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H, Lin Q, Khavari PA. Sustainable cutaneous gene delivery. Nat Biotechnol. 1997;15:1388–91. doi: 10.1038/nbt1297-1388. [DOI] [PubMed] [Google Scholar]
- Denoyelle F, Weil D, Maw MA, Wilcox SA, Lench NJ, Allen-Powell DR, Osborn AH, Dahl HH, Middleton A, Houseman MJ, Dode C, Marlin S, Boulila-ElGaied A, Grati M, Ayadi H, BenArab S, Bitoun P, Lina-Granade G, Godet J, Mustapha M, Loiselet J, El-Zir E, Aubois A, Joannard A, Petit C, et al. Prelingual deafness: high prevalence of a 30delG mutation in the connexin 26 gene. Human Molecular Genetics. 1997;6:2173–7. doi: 10.1093/hmg/6.12.2173. [DOI] [PubMed] [Google Scholar]
- Di WL, Rugg EL, Leigh IM, Kelsell DP. Multiple epidermal connexins are expressed in different keratinocyte subpopulations including connexin 31. J Invest Dermatol. 2001;117:958–64. doi: 10.1046/j.0022-202x.2001.01468.x. [DOI] [PubMed] [Google Scholar]
- Dickson MA, Hahn WC, Ino Y, Ronfard V, Wu JY, Weinberg RA, Louis DN, Li FP, Rheinwald JG. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol Cell Biol. 2000;20:1436–47. doi: 10.1128/mcb.20.4.1436-1447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djalilian AR, McGaughey D, Patel S, Seo EY, Yang C, Cheng J, Tomic M, Sinha S, Ishida-Yamamoto A, Segre JA. Connexin 26 regulates epidermal barrier and wound remodeling and promotes psoriasiform response. J Clin Invest. 2006;116:1243–53. doi: 10.1172/JCI27186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goliger JA, Paul DL. Wounding alters epidermal connexin expression and gap junction-mediated intercellular communication. Mol Biol Cell. 1995;6:1491–501. doi: 10.1091/mbc.6.11.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertle M, Kubler M-D, Leigh IM, Watt FM. Aberrant integrin expression during epidermal wound healing and in psoriatic epidermis. J Clin Invest. 1992;89:1892–1901. doi: 10.1172/JCI115794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchin T, Coy NN, Conlon H, Telford E, Bromelow K, Blaydon D, Taylor G, Coghill E, Brown S, Trembath R, Liu XZ, Bitner-Glindzicz M, Mueller R. Assessment of the genetic causes of recessive childhood non-syndromic deafness in the UK - implications for genetic testing. Clin Genet. 2005;68:506–12. doi: 10.1111/j.1399-0004.2005.00539.x. [DOI] [PubMed] [Google Scholar]
- Kelsell DP, Dunlop J, Stevens HP, Lench NJ, Liang JN, Parry G, Mueller RF, Leigh IM. Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature. 1997;387:80–3. doi: 10.1038/387080a0. [DOI] [PubMed] [Google Scholar]
- Kenneson A, Van Naarden Braun K, Boyle C. GJB2 (connexin 26) variants and nonsyndromic sensorineural hearing loss: a HuGE review. Genet Med. 2002;4:258–74. doi: 10.1097/00125817-200207000-00004. [DOI] [PubMed] [Google Scholar]
- Kudo T, Ikeda K, Kure S, Matsubara Y, Oshima T, Watanabe K, Kawase T, Narisawa K, Takasaka T. Novel mutations in the connexin 26 gene (GJB2) responsible for childhood deafness in the Japanese population. Am J Med Genet. 2000;90:141–5. doi: 10.1002/(sici)1096-8628(20000117)90:2<141::aid-ajmg10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Labarthe MP, Bosco D, Saurat JH, Meda P, Salomon D. Upregulation of connexin 26 between keratinocytes of psoriatic lesions. Journal of Investigative Dermatology. 1998;111:72–6. doi: 10.1046/j.1523-1747.1998.00248.x. [DOI] [PubMed] [Google Scholar]
- Leigh IM, Navsaria H, Purkis PE, McKay IA, Bowden PE, Riddle PN. Keratins (K16 and K17) as markers of keratinocyte hyperproliferation in psoriasis in vivo and in vitro. Br J Dermatol. 1995;133:501–11. doi: 10.1111/j.1365-2133.1995.tb02696.x. [DOI] [PubMed] [Google Scholar]
- Lucke T, Choudhry R, Thom R, Selmer IS, Burden AD, Hodgins MB. Upregulation of connexin 26 is a feature of keratinocyte differentiation in hyperproliferative epidermis, vaginal epithelium, and buccal epithelium. J Invest Dermatol. 1999;112:354–61. doi: 10.1046/j.1523-1747.1999.00512.x. [DOI] [PubMed] [Google Scholar]
- Matoltsy AG, Viziam CB. Further observations on epithelialization of small wounds: an autoradiographic study of incorporation and distribution of 3H-thymidine in the epithelium covering skin wounds. J Invest Dermatol. 1970;55:20–5. doi: 10.1111/1523-1747.ep12290488. [DOI] [PubMed] [Google Scholar]
- Mese G, Londin E, Mui R, Brink PR, White TW. Altered gating properties of functional Cx26 mutants associated with recessive non-syndromic hearing loss. Hum Genet. 2004;115:191–9. doi: 10.1007/s00439-004-1142-6. [DOI] [PubMed] [Google Scholar]
- Meyer CG, Amedofu GK, Brandner JM, Pohland D, Timmann C, Horstmann RD. Selection for deafness? Nat Med. 2002;8:1332–3. doi: 10.1038/nm1202-1332. [DOI] [PubMed] [Google Scholar]
- Ojeh NO, Frame JD, Navsaria HA. In vitro characterization of an artificial dermal scaffold. Tissue Eng. 2001;7:457–72. doi: 10.1089/10763270152436508. [DOI] [PubMed] [Google Scholar]
- Palmada M, Schmalisch K, Bohmer C, Schug N, Pfister M, Lang F, Blin N. Loss of function mutations of the GJB2 gene detected in patients with DFNB1-associated hearing impairment. Neurobiol Dis. 2006;22:112–8. doi: 10.1016/j.nbd.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Richard G. Connexin disorders of the skin. Clin Dermatol. 2005;23:23–32. doi: 10.1016/j.clindermatol.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Rivas MV, Jarvis ED, Morisaki S, Carbonaro H, Gottlieb AB, Krueger JG. Identification of aberrantly regulated genes in diseased skin using the cDNA differential display technique. Journal of Investigative Dermatology. 1997;108:188–94. doi: 10.1111/1523-1747.ep12334217. [DOI] [PubMed] [Google Scholar]
- Snoeckx RL, Huygen PL, Feldmann D, Marlin S, Denoyelle F, Waligora J, Mueller-Malesinska M, Pollak A, Ploski R, Murgia A, Orzan E, Castorina P, Ambrosetti U, Nowakowska-Szyrwinska E, Bal J, Wiszniewski W, Janecke AR, Nekahm-Heis D, Seeman P, Bendova O, Kenna MA, Frangulov A, Rehm HL, Tekin M, Incesulu A, Dahl HH, du Sart D, Jenkins L, Lucas D, Bitner-Glindzicz M, Avraham KB, Brownstein Z, del Castillo I, Moreno F, Blin N, Pfister M, Sziklai I, Toth T, Kelley PM, Cohn ES, Van Maldergem L, Hilbert P, Roux AF, Mondain M, Hoefsloot LH, Cremers CW, Lopponen T, Lopponen H, Parving A, Gronskov K, Schrijver I, Roberson J, Gualandi F, Martini A, Lina-Granade G, Pallares-Ruiz N, Correia C, Fialho G, Cryns K, Hilgert N, Van de Heyning P, Nishimura CJ, Smith RJ, Van Camp G. GJB2 mutations and degree of hearing loss: a multicenter study. Am J Hum Genet. 2005;77:945–57. doi: 10.1086/497996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark HJ, Willhauck MJ, Mirancea N, Boehnke K, Nord I, Breitkreutz D, Pavesio A, Boukamp P, Fusenig NE. Authentic fibroblast matrix in dermal equivalents normalises epidermal histogenesis and dermoepidermal junction in organotypic co-culture. Eur J Cell Biol. 2004;83:631–45. doi: 10.1078/0171-9335-00435. [DOI] [PubMed] [Google Scholar]
- Tran Van Nhieu G, Clair C, Bruzzone R, Mesnil M, Sansonetti P, Combettes L. Connexin-dependent inter-cellular communication increases invasion and dissemination of Shigella in epithelial cells. Nat Cell Biol. 2003;5:720–6. doi: 10.1038/ncb1021. [DOI] [PubMed] [Google Scholar]
- Wang HL, Chang WT, Li AH, Yeh TH, Wu CY, Chen MS, Huang PC. Functional analysis of connexin-26 mutants associated with hereditary recessive deafness. J Neurochem. 2003;84:735–42. doi: 10.1046/j.1471-4159.2003.01555.x. [DOI] [PubMed] [Google Scholar]
- Wiszniewski L, Limat A, Saurat JH, Meda P, Salomon D. Differential expression of connexins during stratification of human keratinocytes. J Invest Dermatol. 2000;115:278–85. doi: 10.1046/j.1523-1747.2000.00043.x. [DOI] [PubMed] [Google Scholar]