Abstract
Integrins contribute to lymphopoiesis, whereas Toll-like receptors (TLRs) facilitate the myeloid replenishment during inflammation. The combined role of TLRs and integrin on hematopoiesis remains unclear. gp96 (grp94, HSP90b1) is an endoplasmic reticulum master chaperone for multiple TLRs. We report herein that gp96 is also essential for expression of 14 hematopoietic system-specific integrins. Genetic deletion of gp96 thus enables us to determine the collective roles of gp96, integrins, and TLRs in hematopoiesis. We found that gp96-null hematopoietic stem cells could support long-term myelopoiesis. B- and T-cell development, however, was severely compromised with transitional block from pro-B to pre-B cells and the inability of thymocytes to develop beyond the CD4−CD8− stage. These defects were cell-intrinsic and could be recapitulated on bone marrow stromal cell culture. Furthermore, defective lymphopoiesis correlated strongly with failure of hematopoietic progenitors to form close contact with stromal cell niche and was not the result of the defect in the assembly of antigen receptor or interleukin-7 signaling. These findings define gp96 as the only known molecular chaperone to specifically regulate T- and B-cell development.
Introduction
Integrins are a family of 24 αβ heterodimers in vertebrates formed noncovalently by 18 α and 8 β integrins, of which 17 integrins are expressed in the hematopoietic system.1,2 Known best for their adhesion properties, integrins also orchestrate signals between extracellular matrix and intracellular cytoskeletons in regulating diverse functions of cells, including proliferation and differentiation. However, despite the expression of integrins on hematopoietic stem cells (HSCs) and the role of integrins in HSC homing to the bone marrow (BM) niche, their function in hematopoiesis remains controversial. For example, although α4 integrin has been implicated in both T and B lymphopoiesis from fetal HSCs,3,4 it appears to play a less significant role in adult hematopoiesis.5,6 Furthermore, combined deletion of both β1 and β7 integrins, which are the only known partners of α4 integrin, causes no defect in either lymphopoiesis or myelopoiesis.7 Genetic β2 integrin deficiency causes myeloid hyperplasia, including profound granulocytosis and splenomegaly, but no significant problems in hematopoiesis.8 Clearly, both α4 and β2 integrins are involved in homing of HSCs in the BM and recruitment of leukocytes to sites of inflammation.5,9,10 Although pan-integrin deficient system is now available,11 no resolution of the roles of integrin in hematopoiesis has emerged.
Toll-like receptors (TLRs) are pattern recognition receptors that play important roles in sensing pathogen-associated molecular patterns from microbes, which are critical for host immune response.12 More than 10 TLRs have been described in vertebrates, recognizing a spectrum of microbial moieties, such as endotoxin, flagellin, dsRNA, and DNA. In the steady state, TLRs do not contribute significantly to hematopoiesis, although TLRs on HSCs have been implicated in the replenishment/recruitment of myeloid cells in response to inflammation.13,14 TLRs and integrins do not share significant structural homology. Nevertheless, the folding and proper expression of many TLR and integrin family members are dependent on gp96, the heat shock protein 90 (HSP90) paralogue in the endoplasmic reticulum (ER). Deletion of gp96 leads to posttranslational loss of multiple TLRs (TLR1, TLR2, TLR4, TLR5, TLR6, TLR7, and TLR9) and several integrins (β2, α4, and αV integrins),15–17 although no study has probed the entire hematopoietic system-specific integrins for their dependence on gp96.
As a major ER luminal protein whose expression can be further induced by accumulation of misfolded proteins, gp96 is also thought to participate in the ER-unfolded protein response (UPR)18 and ER-associated protein degradation,19 and has been implicated to play a major “housekeeping” function to maintain protein homeostasis in the secretory pathway.20 The discovery that gp96 seems to selectively fold TLRs and integrins15–17 was unexpected, which raises the intriguing possibility that gp96 is evolved to play more specialized function in the multicellular organism.
In this study, we used tamoxifen (TAM)–inducible gp96 knockout (KO) mice to further map the clientele of gp96 and to study the roles of gp96 in hematopoiesis. Hematopoiesis is an attractive model system to elucidate the function of gp96, not only because of the possible roles of integrins and TLRs in the process, but also because of the importance of UPR in regulating several aspects of the immune system, such as B-cell development,21 plasma cell differentiation,22,23 and dendritic-cell (DC) development and function.24 We demonstrated that gp96 is essential for the expression of a majority of hematopoietic system-specific integrins. Unexpectedly, we found that gp96 null HSCs were able to engraft and initiate long-term hematopoiesis. We further revealed that deletion of gp96 led to a selective and stage-specific loss in both T and B lymphopoiesis, but not NK-cell development or myelopoiesis, underscoring that gp96 plays a specialized rather than a general role in hematopoiesis. Furthermore, because of the complete loss of multiple TLRs and integrins in gp96 null cells, we also conclude that the functional requirement of TLRs and integrins in hematopoieis is highly lineage-specific.
Methods
Mice
C57BL/6 (CD45.2) mice were obtained from The Jackson Laboratory. C57BL/6 (CD45.1) mice were purchased from NCI-Frederick. hsp90b1 floxed mice were crossed to R26R-creERT2 mice (kindly provided by James Y. H. Li, University of Connecticut Health Center [UCHC]) and further backcrossed to C57BL/6 background for 6 to 10 generations. Control mice were hsp90b1flox/flox-creER− littermates (designated as wild-type [WT]) of hsp90b1flox/flox-creER+ KO mice. All mice were maintained by the Center for Laboratory Animal Care of UCHC (Farmington, CT) on an Institutional Animal Care and Use Committee–approved animal care protocol.
Cell lines
WT and gp96 mutant 70Z/3 pre-B cells were a gift from Brian Seed (Harvard University),15 which were cultured in RPMI medium (Sigma-Aldrich) supplemented with 10% heat-inactivated fetal calf serum (Atlas Biologicals), 55μM 2-mercaptoethanol (Invitrogen), and penicillin-streptomycin (Invitrogen). OP9 and OP9-DL1 cells were cultured in α-minimum essential medium containing l-glutamine and ribonucleotides (Invitrogen) supplemented with 20% fetal calf serum, 1mM sodlium pyruvate (Invitrogen), 10mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (Invitrogen), 55μM 2-mercaptoenthanol, and penicillin-streptomycin. All cells were cultured in 5% CO2 incubator.
Tamoxifen-inducible gp96 deletion
Tamoxifen (Sigma-Aldrich; 100 μg/20 g body weight) in peanut oil (Sigma-Aldrich) was administered by intraperitoneal injection daily for 12 to 14 consecutive days to both experimental and control mice, and deletion of gp96 was monitored by loss of CD11b on Gr1+ cells in the peripheral blood leukocytes. Deletion of gp96 was also confirmed by immunoblot and by intracellular stain for gp96.
Flow cytometry
After Fc-receptor blocking, cells were stained for surface lineage markers. For Lin−Scal-1+c-Kit+ (LSK) population, lineage-negative (Lin−) cells (CD3, CD4, CD8, CD5, B220, NK1.1, Gr1, F4/80, and Ter119) were analyzed for expression of Sca-1 and c-Kit. For gp96 intracellular staining, cells were stained for surface markers, then fixed in 4% formalin/phosphate-buffered saline, permeabilized using ice-cold MeOH. Nonspecific binding was blocked with 10% goat serum, and gp96 was stained intracellularly with rabbit IgG isotype control or SPA-851 polyclonal gp96 antibody (Stressgen), followed by anti–rabbit secondary antibody. All antibodies were purchased from eBioscience, except where indicated. Fluorescence-activated cell sorter data were analyzed on FlowJo software (TreeStar) before being imported to ACD Systems Canvas.
Immunoblot
Single-cell suspension of BM cells, thymocytes, and splenocytes was lysed in RIPA buffer supplemented with proteinase inhibitor cocktail (Sigma-Aldrich) as described.17 For all other organs, tissue was minced in lysis buffer and briefly sonicated. Bradford assay was used to quantitate protein concentration, and equal amounts of lysate were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. gp96 was detected using 9G-10 primary antibody (Stressgen) followed by horseradish peroxidase–conjugated anti–rat secondary antibody (Sigma-Aldrich). β-Actin was blotted (AC-74; Sigma-Aldrich) as a loading control.
BM transplantation
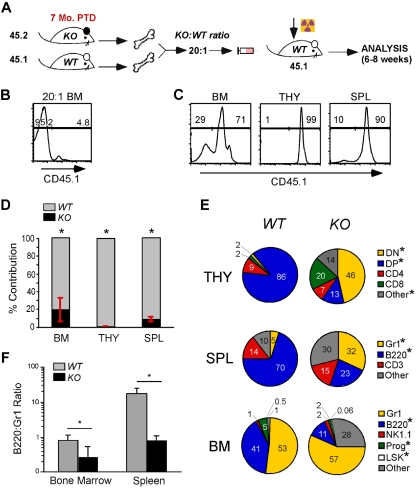
BM cells were isolated from both tibia and femur using aseptic technique. Red blood cells were lysed using sterile ammonium chloride lysis buffer, and BM cells were resuspended in sterile phosphate-buffered saline, pH 7.2, at 10 million cells/mL. A total of 2 million BM cells were injected via tail vein to irradiated C57BL/6 mice (550 cGy ×2, 4 hours apart, 1 day before BM transplantation). Recipients were monitored for hematopoietic reconstitution in peripheral blood leukocytes using CD3, B220, Gr1, and CD11b markers. Similarly, for 1:1 transplantation experiments, 2 million WT (CD45.1/CD45.2) and KO (CD45.2) BM was injected intravenously to lethally irradiated WT C57BL/6 (CD45.1) recipients. Six weeks after transplantation, TAM was administered intraperitoneally to induce gp96 deletion. For 20:1 transplantation experiments, 7 months post-TAM deletion (PTD), 10 million gp96 null BM cells (CD45.2) and 0.5 million WT C57BL/6 (CD45.1) BM cells were injected to lethally irradiated WT C57BL/6 (CD45.1) mice.
Enrichment of Lin−c-Kit+ progenitors
BM cells from WT and KO chimeras were isolated, stained using biotinylated lineage cocktail, and Lin+ cells were depleted using antibiotin MACs beads (Miltenyi Biotec). Subsequently, Lin− cells were stained with c-Kit–phycoerythrin (PE) antibody followed by positive selection using anti-PE MACS beads. Enrichment of progenitors was routinely verified by flow cytometry after staining with biotinylated lineage-specific antibody cocktail followed by streptavidin-allophycocyanin and Sca-1–fluorescein isothiocyanate. c-Kit–PE staining was maintained after positive selection. Purity was more than 85%.
OP9 and OP9-DL1 coculture
A total of 10 000 OP9 and OP9-DL1 were plated the day before to a 6-well plate. When the cells reached approximately 70% confluence, 1 × 105 Lin−c-Kit+ progenitors were seeded on top in media supplemented with interleukin-7 (IL-7; 5 ng/mL; Biovision) and FLT3L (5 ng/mL; Biovision). Cocultures were refed every third day and split to a fresh stromal layer weekly. A small aliquot of cells was reserved for analysis of differentiation by flow cytometry.
Statistics
Numbers in all bar graphs represent mean plus or minus SD. The Student t test was used to determine whether the difference between 2 groups is statistically significant (P < .05).
Results
gp96 is essential for cell-surface expression of most hematopoietic system-specific integrins
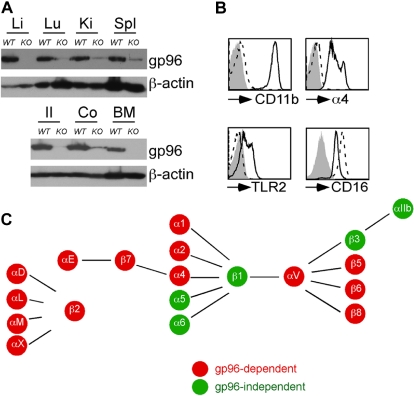
Loss of gp96 is embryonic lethal (e5.5).25 To study the roles of gp96 in hematopoiesis, we generated TAM-inducible gp96 KO mice by crossing hsp90b1flox/flox mice with Rosa26-creERT2 mice. After 14 days of daily injection with TAM, Cre-mediated deletion of gp96 from all adult tissues of KO mice was accomplished (Figure 1A; and data not shown). As expected, loss of gp96 led to abrogation of surface integrins CD11b, α4, and TLR2, but not a nonclient CD16/32 on hematopoietic cells (Figure 1B), consistent with the known chaperone functions of gp96.15–17 Next, we systematically examined and compared the expression of all integrins in the hematopoietic system of KO mice and control littermates by flow cytometry. We found that gp96 is essential for cell surface expression of 14 of 17 integrin pairs (summarized in Figure 1C). KO cells failed to express a majority of integrins except α5β1, α6β1, and αIIbβ3 integrins.
Figure 1.
Inducible deletion of gp96 reveals its critical role for expression of multiple integrins. WT or gp96 KO mice were injected with tamoxifen (TAM) intraperitoneally for 14 days followed by analysis. (A) Immunoblot for gp96 and β-actin (a loading control) from multiple organs: Li indicates liver; Lu, lung; Ki, kidney; Spl, spleen; Il, ileum; and Co, colon. (B) Flow cytometric analysis of BM Gr1+ cells for cell-surface expression of indicated molecules. Dotted histogram represents KO cells; solid line indicates WT cells. (C) Summary of gp96-dependent and independent integrins on the hematopoietic system. These data are based on numerous experiments.
General lymphopoiesis, but not myelopoiesis, is dependent on gp96
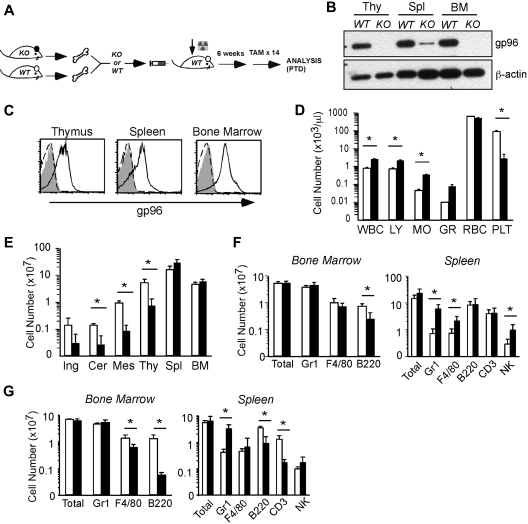
gp96 is ubiquitously expressed in all tissues. To study the hematopoietic system-autonomous roles of gp96 in hematopoiesis, we used a BM chimera approach to generate chimeric mice that were deficient of gp96 only in the hematopoietic system (Figure 2A). WT recipient mice were lethally irradiated, followed by transplantation with either KO or WT BM cells. Chimeric mice were allowed to first establish multilineage reconstitution (6-8 weeks) before TAM treatment and deletion of gp96.
Figure 2.
Global consequence of gp96 deletion on hematopoiesis. (A) Experimental scheme. (B) Immunoblot of various hematopoietic tissues from WT and KO mice 12 days post-TAM deletion (PTD). Thy indicates thymus. β-Actin serves as loading control. (C) Intracellular staining for gp96 in various hematopoietic tissues from WT (solid line) or KO (dotted line) bone marrow (BM) chimeras using gp96 antibody (open) or isotype control (shaded). (D) Peripheral blood cell counts of WT (n = 3, □) and KO (n = 4, ■) BM chimeras 1 month PTD. (E) Organ cellularity of WT (n = 3, □) and KO (n = 4, ■) BM chimeras 1 month PTD. Ing indicates inguinal; Cer, cervical; and Mes, mesenteric. (F-G) Absolute cell numbers of indicated lineages in BM and spleen of WT (n = 5, □) and KO (n = 6, ■) BM chimeras 3 to 4 weeks PTD (F) and 7 months PTD (G). Data are representative of at least 3 independent experiments. WBC indicates white blood cell; LY, lymphocyte; MO, monocyte; GR, granulocyte; RBC, red blood cell; and PLT, platelet. *P < .05.
One month PTD, we confirmed the gp96 knockdown by immunoblot (Figure 2B) and intracellular stain (Figure 2C). There was a general leukocytosis at this point in the peripheral blood (Figure 2D). Red blood cell count was normal, although there was a evidence for thrombocytopenia (Figure 2D); the etiology of the latter is unclear and is being pursued in a separate study (M.S. and J.L., unpublished observation, May 2007). Noticeably, KO chimeras had markedly decreased cellularities in thymus and peripheral lymph nodes with modestly increased cellularities in the spleen and BM (Figure 2E). Although the absolute numbers of myeloid cells were well maintained over time in both the BM and spleen (Figure 2F-G), there were severe T- and B-cell developmental blocks (discussed in Figure 5) that contributed to a significant reduction in mature peripheral B and T cells 7 months PTD (Figure 2G; and data not shown). Gr1+ cells and other myeloid cells were increased (5- to 10-fold) at all time points in KO mice (Figure 2F-G). In addition, at 2 months PTD, immunofluorescent staining of KO spleen sections confirmed a notable increase in Gr1+ cells in the red pulp, and the reduced size/organization of T-cell zone and B-cell follicles (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
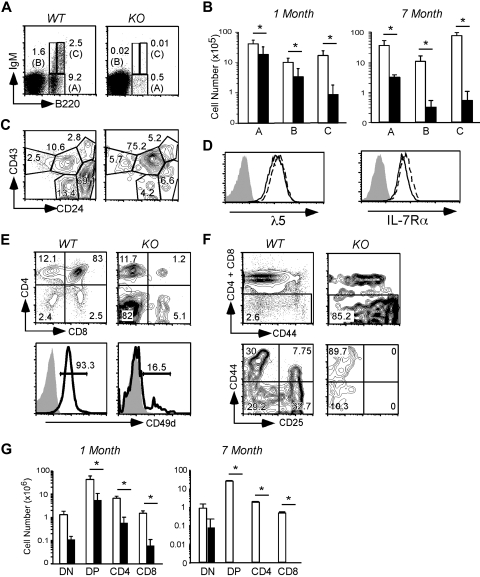
Figure 5.
B- and T-cell lymphopoiesis is dependent on gp96 at critical transitional stages. (A) B220/IgM surface staining of BM cells from WT and KO BM chimeras 1 month PTD. (B) Absolute numbers of BM B cells in various fractions indicated in panel A from WT (n = 5, □) and KO (n = 6, ■) BM chimeras 3 to 4 weeks PTD. Data are pooled from 2 independent experiments. (C) CD43/CD24 surface staining on B220+ cells in BM. (D) Expression of λ5 pre-BCR and IL-7Rα on the surface of WT (solid line) and KO (dotted line) pre–B-cell line. Shaded histograms represent isotype control. (E) CD4/CD8 (top) surface staining and surface expression of CD49d (bottom) on thymocytes from WT and KO BM chimeras 1 month PTD. (F) DN gating (top) and CD44/CD25 surface staining on DN thymocytes from WT and KO BM chimeras 1 month PTD. (G) Absolute cell numbers of thymocytes from the indicated stages from WT (n = 3, □) and KO (n = 4, ■) BM chimeras 1 month and 7 months PTD. Data are representative of at least 2 independent experiments. *P < .05.
We also examined the impact of gp96 loss on the development of DCs.26 By costaining BM cells with c-Kit and c-FMS, we found that gp96 loss did not significantly alter the macrophage-DC progenitors in the BM (supplemental Figure 2A).27 Because of loss of CD11c, we used lineage gating to enumerate mature DCs. We noted that the splenic B220−CD3−CD5−NK1.1−F4/80−MHCII+ (Lin−MHCII+) population that contains myeloid DCs (> 70% CD11c+) was similar between KO and controls (data not shown); likewise, the number and percentage of Lin−MHCII+PDCA-1+ plasmacytoid DCs were comparable (data not shown). Furthermore, both macrophage and DCs could be differentiated successfully in vitro from KO progenitors (supplemental Figure 2B-C), although KO macrophage clearly failed to adhere to the culture dish (supplemental Figure 2D). Taken together, we conclude that myeloid lineage differentiation is surprisingly robust despite the disappearance of almost all integrins, multiple TLRs, and gp96 itself.
Hematopoietic progenitors persist in the absence of gp96, integrins, and TLRs
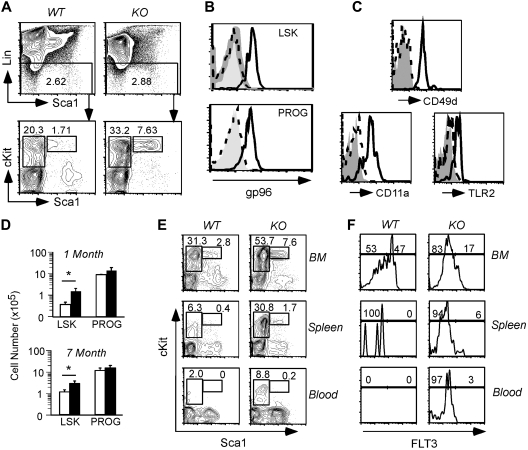
Normal myelopoiesis in the absence of gp96 suggests strongly that HSCs are able to self-renew and differentiate in a manner that is independent of gp96. This point was further validated experimentally. We first confirmed the loss of gp96 in the LSK population, which contains HSCs (long-term and short-term) and early lymphoid progenitors, and Lin−Sca-1−c-Kit+ progenitors (PROG), which include mostly myeloid progenitors.28 Loss of gp96 was observed in both these populations and was concomitant with the loss of α4 (CD49d), αL (CD11a) integrin and TLR2 expression (Figure 3A-C). We observed a significant expansion in both percentage and numbers of LSK and, to a lesser extent, PROG in KO BM (Figure 3D). Consistent with the robust myelopoiesis of KO cells in vivo, colony-forming unit cultures in semisolid methylcellulose demonstrated no differences in overall myeloid/erythroid differentiation potential between WT and KO BM cells (data not shown).
Figure 3.
Multipotent hematopoietic progenitor persists in the absence of gp96. (A top) Dump gating of BM cells from WT and KO BM chimeras 1 month PTD. (Bottom) Gating of LSK (L) and PROG (P) populations. Numbers represent percentage of cells in the gated population. (B) Intracellular staining of gp96 on BM LSK and PROG populations from WT (solid line) and KO (dotted line) BM chimeras 2 months PTD using polyclonal gp96 antibody or isotype control (shaded). (C) CD49d, CD11a, and TLR2 expression on BM LSK populations from WT (solid line) and KO (dotted line) BM chimeras 1 month PTD. (D) Absolute numbers of BM LSK and PROG from WT (n = 3, □) and KO (n = 4, ■) BM chimeras 1 month and 7 months PTD. *P < .05. (E) LSK and PROG analysis from WT and KO BM chimeras 2 months PTD in the indicated tissues. (F) FLT3 expression on LSK from WT and KO BM chimeras 2 months PTD in the indicated tissues. Data are representative of at least 2 independent experiments.
We next sought to more closely examine the effects of loss of gp96 on progenitor populations in the BM and thymus. We focused primarily on the early lymphoid progenitor (Lin−Sca-1+c-Kit+FLT3+) and early thymic progenitor populations (Lin−CD25−c-Kit+) that are derived from FLT3+LSK.29–33 We found that LSK/PROG was increased in the peripheral blood and spleen of gp96 KO chimeras (Figure 3E), which were maintained upwards 7 months PTD (Figure 3D; and data not shown). Interestingly, the increase in both LSK percentage and number in KO chimeras was predominately of FLT3−/lo that contains HSCs and multipotent progenitors (Figure 3F).30 However, although the percentage of BM FLT3+LSK was reduced, the absolute numbers of FLT3+LSK were comparable because of an increase in the overall numbers of BM KO LSK. In addition, the percentage of BM common lymphoid progenitors (CLPs; Lin−Sca-1−c-KitloIL-7Rα+) was also comparable between WT and KO 1 month PTD (supplemental Figure 3A). Consistent with the persistence and function of BM CLPs, we found that NK cell developed normally in the absence of gp96 (supplemental Figure 3B-C).
gp96 KO BM cells were unable to rescue lethally irradiated mice (supplemental Figure 4), which could be the result of poor engraftment. To circumvent this problem and to further address the impact of gp96 loss on long-term hematopoiesis, we performed secondary BM transplantation with a mixture of KO and WT BMs in a 20:1 ratio (Figure 4A). WT (CD45.1) and KO (CD45.2) donor cells were congenically marked for easy distinction (Figure 4B). We found that gp96 KO BM cells were able to engraft, albeit not as efficiently as WT BM cells (∼ 80-fold less efficient based on BM cellularity; Figure 4C-F). Consistent with single donor chimera experiments, KO cells were restricted in their hematopoietic potential and favored myelopoiesis over lymphopoiesis (Figure 4E-F). Cells in the “other” category in KO BM and spleen were probably of myeloid origin, such as F4/80+ cells, which develop normally in the absence of gp96 (Figure 2F). We therefore conclude that the maintenance or survival of HSCs in vivo is independent of gp96 and its client network including integrins and TLRs.
Figure 4.
De novo lymphopoiesis, but not myelopoiesis, is dependent on gp96. (A) Diagram of experimental design: BM cells from KO (7 months PTD BM chimera, 45.2) and WT (45.1) mice were transplanted in 20:1 ratio to lethally irradiated recipients (45.1) and analyzed 4 to 6 weeks PTD. (B) Representative congenic analysis of donor BM after 20:1 mix of WT and KO cells. (C) Congenic analysis of a representative recipient for relative contribution by WT (n = 4) and KO (n = 4) cells to the various hematopoietic tissues 4 to 6 weeks after BM reconstitution. (D-E) Congenic gated WT (gray) and KO (black) cells were analyzed for their relative contribution (number indicates percentage) to various lineages and hematopoietic tissues. (F) Percentage of B220+ cells over Gr1+ cells in BM and spleen of WT and KO mice. Data are representative of 2 independent experiments. *P < .05.
gp96 is critical for pro-pre–B-cell transition
We next focused our attention on defining the critical developmental stages of B and T lymphocytes that were controlled by gp96. gp96 KO chimeras were found to have a profound block in B lymphopoiesis at B220loIgM− stage, as evidenced by a marked reduction in the percentage and number of B220loIgM+ cells and a complete absence of B220hiIgM+ recirculating B cells at 1 month and 7 months PTD (Figure 5A-B). More specifically, we noted a significant developmental blockade at the B220+CD43+CD24lo stage at the transition from c-Kit+ to CD25+ corresponding to a defect in late pro-B to early pre–B-cell transition (Figure 5C; supplemental Figure 5A).34 Of note, splenic B-cell numbers were maintained in gp96 KO BM chimeras early PTD, which indicated that once B cells matured, they no longer require gp96 for maintaining homeostasis, which was in line with our previous study.17 However, B-cell number did decline significantly by 7 months PTD (Figure 2G). Consistent with earlier work,17,35 KO mice had additional loss of the splenic marginal zone B cells resulting from integrin-mediated retention defect (supplemental Figure 5B-C).
Before acquisition of B-cell receptor (BCR), developing B cells express a pre-BCR, composed of Vpre-B and λ5 that comprise the surrogate light chain on a μH backbone.36 Expression of pre-BCR further defines the transition between pro– and pre–B-cell stages and has been shown to be an important checkpoint in pre-B proliferation and differentiation.37,38 λ5 expression on a gp96 null pre-B cell line was, however, equivalent to WT cells (Figure 5D). Comparable expression of λ5 was also confirmed on B220loIgM− pre-B cells in WT and KO chimeras (data not shown), thus excluding a role for gp96 in pre-BCR assembly.
Given the important role of IL-7R in B- and T-cell development, we next examined the role of gp96 on IL-7Rα expression and signaling. IL-7Rα was uncompromised on gp96 null pre-B cells (Figure 5D). Furthermore, IL-7 stimulation of gp96 null and control pre-B cells led to equal phosphorylation of Stat-5 (supplemental Figure 6). In addition, IL-7Rα expression on gp96 null leukocytes and on CLPs was normal (data not shown).
gp96 is essential for effective thymopoiesis
Analogous to B-cell development blockade with gp96 KO chimeras, at 1 month PTD, we observed a marked reduction of thymic cellularity (Figure 2D). Thymopoiesis begins at the CD4−CD8− double-negative (DN) stage that can be defined by differences in expression of CD44 and CD25 surface markers, where the earliest stage DN1 population (CD44+CD25−) also encompasses c-kithi early thymic progenitors (ETPs),39 followed by DN2 (CD44+CD25+), DN3 (CD44−CD25+), and DN4 (CD44−CD25−) stages.40 gp96 KO thymocytes have a developmental blockade at the DN1 stage (Figure 5E-F), which strongly correlated with thymic atrophy (supplemental Figure 7A). By 7 months PTD, thymopoiesis was virtually nonexistent in KO mice (Figure 5G).
DN3 thymocytes, similar to pre-B cells, express a pre–T-cell receptor (pre-Tα), which corresponds to transition through DN3 stage. We were able to analyze pre-Tα expression in KO thymocytes early after gp96 deletion and before the loss of DN3 thymocytes; we found that these KO cells express normal levels of pre-Tα (data not shown), arguing against a defect in pre–T-cell receptor assembly in KO mice. In addition, gp96 KO mice crossed to T-cell receptor transgenic OT-I Rag1−/− mice were not able to rescue thymic development (data not shown). Consistent with the role of α4 and β2 integrins in ETP homing to the thymus,41 the percentage and absolute numbers of ETPs (Lin−CD25−c-kit+) were reduced in KO mice (supplemental Figure 7B). Immunofluorescence microscopy of c-Kit+CD25+ DN thymocytes42 confirmed their near-complete absence from KO thymus (supplemental Figure 7C). The reduction but persistence of other DN cells such as c-Kit− population 1 to 2 months after gp96 deletion was probably the result of DN1-DN2 transitional block. Thus, the systematic loss of thymopoiesis involves both the DN1 to DN2 transitional block and a decrease in the migration/seeding of KO ETPs in the thymus over time.
Loss of B and Tymphopoiesis in gp96 null mice is cell intrinsic
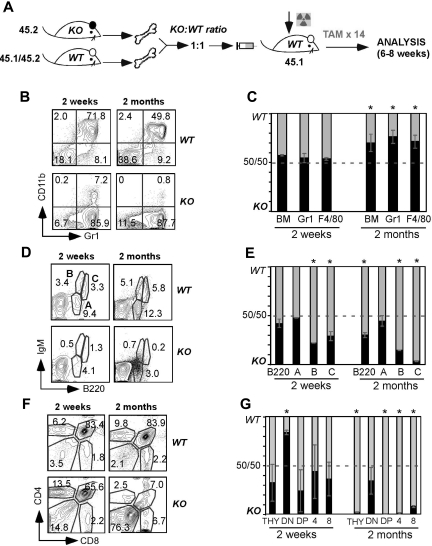
Our previous mixed chimera experiment using 20:1 combination of KO to WT BM donors suggests that the lymphopoietic defect after gp96 loss is cell intrinsic (Figure 4). However, those data were confounded by the poor homing or engraftment of gp96 null BM precursors. To further determine whether the developmental defects in T and B lymphopoiesis were cell intrinsic, we made 1:1 chimeras in which we cotransplanted BM from congenic WT and KO mice (Figure 6A). In contrast to 20:1 experiments using gp96 null BM cells, we waited for a full reconstitution of recipient mice before inducing gp96 deletion. If the developmental block of KO cells was the result of loss of soluble cell-extrinsic factors but not a cell-intrinsic mechanism, the presence of WT cells should rescue the defect. At 2 weeks PTD, KO and WT cells constituted similar percentage contributions to total BM cellularity and commitment to the Gr1+ and F4/80+ populations; however, 2 months PTD, the balance in total BM cellularity and myeloid populations clearly favored KO donors (Figure 6B-C). This was in contrast to the BM B220+IgM+ B cell pool in which we observed a competitive disadvantage by KO cells at all time points (Figure 6D-E; and data not shown). Likewise, we observed that KO thymocytes arrested at the DN stage, 2 weeks PTD, reaffirming an early intrathymic developmental defect and a comprehensive loss in thymocytes in KO mice over time (Figure 6F-G). Total DN thymocytes of KO origin also progressively declined to less than 5% of the entire DN thymocytes 4 months PTD (data not shown), which was consistent with the additional homing defect of KO ETPs. Thus, gp96 regulates B and T lymphopoiesis in a cell-intrinsic fashion. In addition, despite the presence of a WT competitor, gp96 LSK and progenitors were well sustained in the BM, and their percentage and number were consistently increased. Moreover, gp96 LSK and PROG were capable of long-term myelopoiesis upward 9 months PTD even in this competitive setting (data not shown).
Figure 6.
Competitive BM reconstitution reveals cell-intrinsic role for gp96 in B and T lymphopoiesis, but not myelopoiesis. (A) Diagram of experimental design. (B,D,F) Surface staining of the indicated lineages on gated KO (45.2) and WT (45.1/45.2) BM cells from 1:1 BM chimeras 2 weeks and 2 months PTD. (C,E,G) Percentage of KO and WT contribution to total BM myeloid cells (C), BM B-cell subsets (E), and thymocytes (G) at 2 weeks and 2 months PTD (DN: CD4−CD8−; double-positive: CD4+CD8+; 4: CD4+CD8−; 8: CD4−CD8+). Data are representative of 3 mice per group. *P < .05.
gp96 null hematopoietic progenitors proliferate poorly and fail to differentiate into B and T cells on stromal cells in vitro
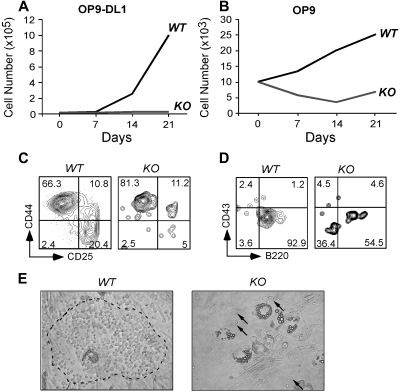
The failure of gp96 KO cells to differentiate along T- and B-cell lineages in vivo could be the result of a defect in the intrinsic developmental program or to the inability of progenitors to migrate into the proper niche, or both. To examine these possibilities, we performed an in vitro differentiation assay using a well-described B- and T-cell developmental coculture system. The stromal cell line OP9, and OP9-DL1, which expresses notch ligand Delta-like 1 (DL1), have been used to effectively drive B- and T-cell differentiation, respectively.43 We purified Lin−c-Kit+ progenitors from WT and KO BM (supplemental Figure 8) and plated equal numbers of cells onto OP-9 and OP9-DL1 in culture. Cells were then enumerated and analyzed by flow cytometry at weekly intervals. We found that KO cells proliferated poorly on both OP-9 and OP9-DL1 (Figure 7A-B), with little evidence of B- and T-cell differentiation (Figure 7C-D). Moreover, gp96 KO progenitors were unable to transmigrate beneath stromal cells to form prototypical “cobblestone” colonies (Figure 7E), a sign of an early differentiation program.4 The inability of KO cells to transmigrate through BM stromal layers was also recapitulated quantitatively using a gp96 mutant pre–B-cell line (supplemental Figure 9).
Figure 7.
gp96 null hematopoietic progenitors fail to proliferate and differentiate in BM stromal cell cultures. Purified WT and gp96 KO BM Lin−c-kit+ progenitor cells were cultured on BM stromal cell OP9 or OP9-DL1 for 3 weeks and analyzed for cell growth and differentiation. (A-B) Kinetic analysis of cell proliferation. (C-D) Flow cytometric analysis of T- and B-cell development after 3 weeks of coculture. (E) Bright field image of day 5 coculture of WT or KO Lin−c-kit+ progenitors on OP9 (original magnification ×100). Dotted circle denotes “cobblestone” colony. A few KO progenitors were indicated by. Three experiments were performed with similar results.
Discussion
gp96 is a ubiquitously expressed and evolutionarily conserved ER-resident molecular chaperone belonging to the HSP90 family.20 Genetic and biochemical approaches have demonstrated the pivotal role of gp96 in folding and maturation of TLRs and integrins.15–17 In addition, as a canonical member of the HSP90 family, gp96 participates in a variety of molecular and biochemical processes to maintain protein homeostasis in the ER.19 Thus, the functional importance of gp96 in hematopoiesis could be at least 2-fold, insomuch as it participates in the folding/maturation of 2 very important classes of immunologically relevant molecules, and it also serves a second perhaps more general housekeeping role in ER protein homeostasis.
Full appreciation of the function of gp96 requires knowledge of gp96 client proteins, whose expression is dependent on gp96. In this study, we significantly expanded on the gp96 client network by determining the differential expression of integrins by WT and gp96 KO hematopoietic cells. We found that gp96 is essential for the expression of a majority of integrins, including α1, α2, α4, αD, αE, αL, αM, αV, and αX of the hematopoietic system. Because α integrin must heterodimerize with its appropriate β integrin partner for cell surface expression, we conclude that gp96 is crucial for the expression of 14 of 17 integrin pairs expressed by the hematopoietic system. The requirement for gp96 in folding these integrins is absolute and cannot be compensated by any other ER chaperones. Although the structural basis for selective dependence on gp96 by integrins is unclear, it is intriguing to point out that the loss of all β integrins can be explained by the loss of their correspondingα partners, but not the converse. By deduction, we postulate that gp96 is critical for chaperoning α, but not β, subunits of integrins.
The loss of multiple TLRs and integrins in gp96 null mice created an unprecedented opportunity to address their combinational roles in hematopoiesis. Using a TAM-inducible gp96 deletion system coupled with competitive BM reconstitution, we found that gp96 is surprisingly not required by HSCs to self-renew and to initiate long-term hematopoiesis along the myeloid lineage. gp96 is, however, required at defined critical stages of early T- and B-cell development, which correlated with the inability of gp96 KO progenitors to transmigrate BM stromal cell layers.
The underlying mechanism by which gp96 selectively regulates lymphopoiesis is not entirely clear. TLRs have been suggested to function in replenishment of the myeloid compartment after bacterial infection.13 Furthermore, although TLRs were not directly examined, inflammation can affect lymphoid versus myeloid distribution in favor of the latter.44 However, the role of TLRs in hematopoiesis in the steady state is limited, evidenced by uncompromised B-cell development in MyD88 and Trif double KO mice, which lack all known TLR downstream signaling.45 It is thus unlikely that the loss of TLRs contributes significantly to the lymphopoietic defect in gp96 KO mice.
In contrast, the role for integrins in hematopoiesis is extensive, particularly related to the function of α4 and β2 integrins, both of which are bona fide clientele of gp96. Like gp96, α4 integrin is essential for embryogenesis and is also required for both early B- and T-cell development in fetal liver chimeric mice.3,4 It is required for stromal cell adhesion by both B- and T-cell progenitors,4,46–48 correlating with its probable role in lymphopoiesis. However, transplantation of adult α4 null BM cells to irradiated Rag2−/− mice revealed only partial defect in thymopoiesis and a decrease in certain subsets of BM B cells.6 Conditional ablation of α4 or β1/β7 integrin from adult mice had little infringement on lymphopoiesis.5,7,49 These studies highlight that the roles of gp96 in early lymphopoiesis probably extend beyond chaperoning α4 and β2 integrins, and implicate that other members of integrins or/and yet unidentified gp96 clients also play a role in regulating lymphopoiesis.
In gp96 KO BM chimeras, B- and T-cell differentiation is arrested at developmentally analogous stages, pro-B–pre-B and DN1-DN2 transitions, respectively. Two obvious possibilities have been ruled out: the roles of gp96 in the assembly/function of antigen receptor and IL-7R. In addition, annexin V and propidium iodide staining of DN thymocytes subsets did not reveal any significant increase in apoptosis of gp96 null leukocytes (data not shown). Rather, KO cells appear to lose their tight interaction with developmental niche in the BM stromal cells, a scenario that can be explained by the role of gp96 in chaperoning integrins. Consistent with this idea, we observed an increase in LSKs, progenitors, and pro-B cells (B220+CD43+) in the peripheral blood and spleen of gp96 KO chimeras. However, despite increased BM precursors in the periphery, we found no evidence of significant extramedullary hematopoiesis in KO chimeras.
Finally, our BM stromal cell cultures allowed us to conclude that the lymphopoietic defect of gp96 KO HSCs is not simply the result of poor seeding of progenitors in the BM and the thymus. However, gp96 KO progenitors clearly had difficulty in transmigrating the stromal cell layer, arguing strongly that KO hematopoietic precursors might be unable to form an optimal niche locally to initiate lymphopoiesis at an early stage, despite appropriate differentiation cytokines and other cues. More work is necessary to define the critical downstream signals that are missing in gp96 null cells at the pre–B- and pre–T-cell stages and the coordinate function of integrins in these processes, although it is tempting to speculate that stromal contact and signals in general are a prerequisite for development of lymphoid but not myeloid compartments; such a claim warrants further investigation.
There have been increased interests in the roles of protein folding chaperones in hematopoiesis, a highly regulated process with simultaneous proliferation and multilineage differentiation that are probably dependent on robust protein chaperone machinery. However, deletion of individual molecular chaperone has not resulted in catastrophic loss of hematopoiesis. For example, in mice with loss of critical UPR regulator XBP1 or ATF6, hematopoiesis proceeds normally. Only plasma differentiation was affected with loss of XBP-1 and ATF6,21–23 which may be related in part to the importance of downstream effector chaperone GRP78 in immunologlobulin assembly.50 Loss of heat shock factor-1, a major transcription factor for HSPs, resulted in ablation of induction of HSPs51 but no noticeable compromise of hematopoiesis.52 Deletion of calreticulin causes heightened immune response resulting from aberrant activation of peripheral T cells.53 Although genetic deletion of GRP78 and calnexin has been done, and it has revealed the critical importance of these 2 molecules in embryogenesis54 and postnatal survival,55 no specific roles of either molecule on hematopoiesis have been reported. Likewise, cytosolic HSP70 also appeared to be dispensable for hematopoiesis.56 Thus, with the exception of lymphopoiesis defect in gp96 null mice, hematopoiesis and in particular myelopoiesis does not subject to tight regulation by the protein chaperone machinery, underscoring the possibility that myelopoiesis is vital to the survival of high vertebrates and is hardwired to withstand environmental and intracellular stress. These arguments also once again highlight the uniqueness of gp96 in hematopoiesis.
In conclusion, we have demonstrated that the expression of a majority of the hematopoietic system-specific integrins is controlled by gp96; thus, gp96 is a master chaperone for both TLRs and integrins. Yet, gp96 and its client network are dispensable for self-renewal and differentiation of HSCs. We further uncovered the dramatic difference between lymphopoiesis and myelopoiesis in their requirement for gp96. Our study thus illustrates that the developmental requirement for gp96 and its client network in hematopoiesis is both lineage- and stage-specific, highlighting that gp96 plays more specialized rather than general roles in regulating protein homeostasis in the ER and the secretory pathway. High resolution mapping of the gp96 client network is the essential next step to understand the function/mechanism of gp96 in hematopoiesis and to identify key downstream molecules in regulating lineage decision of lymphopoiesis versus myelopoiesis.
Acknowledgments
The authors thank past and present members of the laboratory of Z.L. for helpful discussions, Sierra Root for assistance with the methylcellulose assay, and Drs Lynn Puddington, Pramod Srivastava, and Leo Lefrancois for their invaluable input and support.
This work was supported by the National Institutes of Health (grants AI070603, AI077283, and RC1HL100556; Z.L.). M.S. is supported in part by the National Institutes of Health (training grant 5T32AI007080).
Z.L. is a Clinical Scholar of the Leukemia & Lymphoma Society of the United States.
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.S. conceived the idea, designed the research, performed all the experiments except Figure 1C, and wrote the manuscript; Y.Y. produced the hsp90b1flox/flox-Rosa26creER+ mouse line; B.L., J.L., and Y.S. generated Figure 1C; J.C.Z.-P. generated Figure 7 and critically read the manuscript; H.L.A. generated supplemental Figure 3; I.G. critically read the manuscript; Z.L. conceived the idea, designed the research, and wrote the manuscript; and all authors contributed to the analysis of the data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current address of Y.Y. is Kimmel Center for Biology and Medicine of the Skirball Institute, New York University, New York, NY. The current address of J.L. is Massachusetts Institute of Technology, Cambridge, MA.
Correspondence: Zihai Li, MC 1601, University of Connecticut School of Medicine, 263 Farmington Ave, Farmington, CT 06030-1601; e-mail: zihai@uchc.edu.
References
- 1.Miranti CK, Brugge JS. Sensing the environment: a historical perspective on integrin signal transduction. Nat Cell Biol. 2002;4(4):E83–E90. doi: 10.1038/ncb0402-e83. [DOI] [PubMed] [Google Scholar]
- 2.Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arroyo AG, Yang JT, Rayburn H, Hynes RO. Differential requirements for alpha4 integrins during fetal and adult hematopoiesis. Cell. 1996;85(7):997–1008. doi: 10.1016/s0092-8674(00)81301-x. [DOI] [PubMed] [Google Scholar]
- 4.Arroyo AG, Yang JT, Rayburn H, Hynes RO. Alpha4 integrins regulate the proliferation/differentiation balance of multilineage hematopoietic progenitors in vivo. Immunity. 1999;11(5):555–566. doi: 10.1016/s1074-7613(00)80131-4. [DOI] [PubMed] [Google Scholar]
- 5.Scott LM, Priestley GV, Papayannopoulou T. Deletion of alpha4 integrins from adult hematopoietic cells reveals roles in homeostasis, regeneration, and homing. Mol Cell Biol. 2003;23(24):9349–9360. doi: 10.1128/MCB.23.24.9349-9360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banerjee ER, Latchman YE, Jiang Y, Priestley GV, Papayannopoulou T. Distinct changes in adult lymphopoiesis in Rag2−/− mice fully reconstituted by alpha4-deficient adult bone marrow cells. Exp Hematol. 2008;36(8):1004–1013. doi: 10.1016/j.exphem.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bungartz G, Stiller S, Bauer M, et al. Adult murine hematopoiesis can proceed without beta1 and beta7 integrins. Blood. 2006;108(6):1857–1864. doi: 10.1182/blood-2005-10-007658. [DOI] [PubMed] [Google Scholar]
- 8.Horwitz BH, Mizgerd JP, Scott ML, Doerschuk CM. Mechanisms of granulocytosis in the absence of CD18. Blood. 2001;97(6):1578–1583. doi: 10.1182/blood.v97.6.1578. [DOI] [PubMed] [Google Scholar]
- 9.Papayannopoulou T, Priestley GV, Nakamoto B, Zafiropoulos V, Scott LM. Molecular pathways in bone marrow homing: dominant role of alpha(4)beta(1) over beta(2)-integrins and selectins. Blood. 2001;98(8):2403–2411. doi: 10.1182/blood.v98.8.2403. [DOI] [PubMed] [Google Scholar]
- 10.Ulyanova T, Priestley GV, Banerjee ER, Papayannopoulou T. Unique and redundant roles of alpha4 and beta2 integrins in kinetics of recruitment of lymphoid vs myeloid cell subsets to the inflamed peritoneum revealed by studies of genetically deficient mice. Exp Hematol. 2007;35(8):1256–1265. doi: 10.1016/j.exphem.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lämmermann T, Bader BL, Monkley SJ, et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453(7191):51–55. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- 12.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449(7164):819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 13.Nagai Y, Garrett KP, Ohta S, et al. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24(6):801–812. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massberg S, Schaerli P, Knezevic-Maramica I, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131(5):994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Randow F, Seed B. Endoplasmic reticulum chaperone gp96 is required for innate immunity but not cell viability. Nat Cell Biol. 2001;3(10):891–896. doi: 10.1038/ncb1001-891. [DOI] [PubMed] [Google Scholar]
- 16.Yang Y, Liu B, Dai J, et al. Heat shock protein gp96 is a master chaperone for toll-like receptors and is important in the innate function of macrophages. Immunity. 2007;26(2):215–226. doi: 10.1016/j.immuni.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu B, Li Z. Endoplasmic reticulum HSP90b1 (gp96, grp94) optimizes B-cell function via chaperoning integrin and TLR but not immunoglobulin. Blood. 2008;112(4):1223–1230. doi: 10.1182/blood-2008-03-143107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol. 2007;18(6):716–731. doi: 10.1016/j.semcdb.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christianson JC, Shaler TA, Tyler RE, Kopito RR. OS-9 and GRP94 deliver mutant alpha1-antitrypsin to the Hrd1-SEL1L ubiquitin ligase complex for ERAD. Nat Cell Biol. 2008;10(3):272–282. doi: 10.1038/ncb1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Y, Li Z. Roles of heat shock protein gp96 in the ER quality control: redundant or unique function? Mol Cells. 2005;20(2):173–182. [PubMed] [Google Scholar]
- 21.Zhang K, Wong HN, Song B, Miller CN, Scheuner D, Kaufman RJ. The unfolded protein response sensor IRE1alpha is required at 2 distinct steps in B cell lymphopoiesis. J Clin Invest. 2005;115(2):268–281. doi: 10.1172/JCI21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwakoshi NN, Lee AH, Vallabhajosyula P, Otipoby KL, Rajewsky K, Glimcher LH. Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nat Immunol. 2003;4(4):321–329. doi: 10.1038/ni907. [DOI] [PubMed] [Google Scholar]
- 23.Reimold AM, Iwakoshi NN, Manis J, et al. Plasma cell differentiation requires the transcription factor XBP-1. Nature. 2001;412(6844):300–307. doi: 10.1038/35085509. [DOI] [PubMed] [Google Scholar]
- 24.Iwakoshi NN, Pypaert M, Glimcher LH. The transcription factor XBP-1 is essential for the development and survival of dendritic cells. J Exp Med. 2007;204(10):2267–2275. doi: 10.1084/jem.20070525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wanderling S, Simen BB, Ostrovsky O, et al. GRP94 is essential for mesoderm induction and muscle development because it regulates insulin-like growth factor secretion. Mol Biol Cell. 2007;18(10):3764–3775. doi: 10.1091/mbc.E07-03-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu L, Liu YJ. Development of dendritic-cell lineages. Immunity. 2007;26(6):741–750. doi: 10.1016/j.immuni.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Waskow C, Liu K, Darrasse-Jeze G, et al. The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues. Nat Immunol. 2008;9(6):676–683. doi: 10.1038/ni.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhandoola A, von Boehmer H, Petrie HT, Zuniga-Pflucker JC. Commitment and developmental potential of extrathymic and intrathymic T cell precursors: plenty to choose from. Immunity. 2007;26(6):678–689. doi: 10.1016/j.immuni.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc Natl Acad Sci U S A. 2001;98(25):14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adolfsson J, Borge OJ, Bryder D, et al. Upregulation of Flt3 expression within the bone marrow Lin(-)Sca1(+)c-kit(+) stem cell compartment is accompanied by loss of self-renewal capacity. Immunity. 2001;15(4):659–669. doi: 10.1016/s1074-7613(01)00220-5. [DOI] [PubMed] [Google Scholar]
- 31.Sitnicka E, Bryder D, Theilgaard-Monch K, Buza-Vidas N, Adolfsson J, Jacobsen SE. Key role of flt3 ligand in regulation of the common lymphoid progenitor but not in maintenance of the hematopoietic stem cell pool. Immunity. 2002;17(4):463–472. doi: 10.1016/s1074-7613(02)00419-3. [DOI] [PubMed] [Google Scholar]
- 32.Benz C, Bleul CC. A multipotent precursor in the thymus maps to the branching point of the T versus B lineage decision. J Exp Med. 2005;202(1):21–31. doi: 10.1084/jem.20050146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benz C, Martins VC, Radtke F, Bleul CC. The stream of precursors that colonizes the thymus proceeds selectively through the early T lineage precursor stage of T cell development. J Exp Med. 2008;205(5):1187–1199. doi: 10.1084/jem.20072168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rolink A, Grawunder U, Winkler TH, Karasuyama H, Melchers F. IL-2 receptor alpha chain (CD25, TAC) expression defines a crucial stage in pre-B cell development. Int Immunol. 1994;6(8):1257–1264. doi: 10.1093/intimm/6.8.1257. [DOI] [PubMed] [Google Scholar]
- 35.Lu TT, Cyster JG. Integrin-mediated long-term B cell retention in the splenic marginal zone. Science. 2002;297(5580):409–412. doi: 10.1126/science.1071632. [DOI] [PubMed] [Google Scholar]
- 36.Karasuyama H, Rolink A, Shinkai Y, Young F, Alt FW, Melchers F. The expression of Vpre-B/lambda 5 surrogate light chain in early bone marrow precursor B cells of normal and B cell-deficient mutant mice. Cell. 1994;77(1):133–143. doi: 10.1016/0092-8674(94)90241-0. [DOI] [PubMed] [Google Scholar]
- 37.Hess J, Werner A, Wirth T, Melchers F, Jack HM, Winkler TH. Induction of pre-B cell proliferation after de novo synthesis of the pre-B cell receptor. Proc Natl Acad Sci U S A. 2001;98(4):1745–1750. doi: 10.1073/pnas.041492098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kitamura D, Kudo A, Schaal S, Muller W, Melchers F, Rajewsky K. A critical role of lambda 5 protein in B cell development. Cell. 1992;69(5):823–831. doi: 10.1016/0092-8674(92)90293-l. [DOI] [PubMed] [Google Scholar]
- 39.Porritt HE, Rumfelt LL, Tabrizifard S, Schmitt TM, Zuniga-Pflucker JC, Petrie HT. Heterogeneity among DN1 prothymocytes reveals multiple progenitors with different capacities to generate T cell and non-T cell lineages. Immunity. 2004;20(6):735–745. doi: 10.1016/j.immuni.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Ceredig R, Rolink T. A positive look at double-negative thymocytes. Nat Rev Immun. 2002;2(11):888–897. doi: 10.1038/nri937. [DOI] [PubMed] [Google Scholar]
- 41.Scimone ML, Alfantis I, Apostolou I, von Boehmer H, von Andrian UH. A multistep adhesion cascade for lymphoid progenitor cell homing to the thymus. Proc Natl Acad Sci U S A. 2006;103(18):7006–7011. doi: 10.1073/pnas.0602024103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lind E, Prockop S, Porrit H, Petrie H. Mapping precursor movement through the postnatal thymus reveals specific microenvironments supporting defined stages of early lymphoid development. J Exp Med. 2001;194(2):127–134. doi: 10.1084/jem.194.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Pooter R, Zuniga-Pflucker JC. T-cell potential and development in vitro: the OP9-DL1 approach. Curr Opin Immunol. 2007;19(2):163–168. doi: 10.1016/j.coi.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 44.Ueda Y, Kondo M, Kelsoe G. Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J Exp Med. 2005;201(11):1771–1780. doi: 10.1084/jem.20041419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gavin AL, Hoebe K, Duong B, et al. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314(5807):1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ryan DH, Nuccie BL, Abboud CN, Winslow JM. Vascular cell adhesion molecule-1 and the integrin VLA-4 mediate adhesion of human B cell precursors to cultured bone marrow adherent cells. J Clin Invest. 1991;88(3):995–1004. doi: 10.1172/JCI115403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miyake K, Weissman IL, Greenberger JS, Kincade PW. Evidence for a role of the integrin VLA-4 in lympho-hemopoiesis. J Exp Med. 1991;173(3):599–607. doi: 10.1084/jem.173.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crisa L, Cirulli V, Ellisman MH, Ishii JK, Elices MJ, Salomon DR. Cell adhesion and migration are regulated at distinct stages of thymic T cell development: the roles of fibronectin, VLA4, and VLA5. J Exp Med. 1996;184(1):215–228. doi: 10.1084/jem.184.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gribi R, Hook L, Ure J, Medvinsky A. The differentiation program of embryonic definitive hematopoietic stem cells is largely alpha4 integrin independent. Blood. 2006;108(2):501–509. doi: 10.1182/blood-2005-10-4209. [DOI] [PubMed] [Google Scholar]
- 50.Haas IG. BiP (GRP78), an essential hsp70 resident protein in the endoplasmic reticulum. Experientia. 1994;50(11):1012–1020. doi: 10.1007/BF01923455. [DOI] [PubMed] [Google Scholar]
- 51.Xiao X, Zuo X, Davis AA, et al. HSF1 is required for extra-embryonic development, postnatal growth and protection during inflammatory responses in mice. EMBO J. 1999;18(21):5943–5952. doi: 10.1093/emboj/18.21.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng H, Li Z. Cutting edge: cross-presentation of cell-associated antigens to MHC class I molecule is regulated by a major transcription factor for heat shock proteins. J Immunol. 2004;173(10):5929–5933. doi: 10.4049/jimmunol.173.10.5929. [DOI] [PubMed] [Google Scholar]
- 53.Porcellini S, Traggiai E, Schenk U, et al. Regulation of peripheral T cell activation by calreticulin. J Exp Med. 2006;203(2):461–471. doi: 10.1084/jem.20051519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Luo S, Mao C, Lee B, Lee AS. GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development. Mol Cell Biol. 2006;26(15):5688–5697. doi: 10.1128/MCB.00779-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Denzel A, Molinari M, Trigueros C, et al. Early postnatal death and motor disorders in mice congenitally deficient in calnexin expression. Mol Cell Biol. 2002;22(21):7398–7404. doi: 10.1128/MCB.22.21.7398-7404.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Molle W, Wielockx B, Mahieu T, et al. HSP70 protects against TNF-induced lethal inflammatory shock. Immunity. 2002;16(5):685–695. doi: 10.1016/s1074-7613(02)00310-2. [DOI] [PubMed] [Google Scholar]