Abstract
It is becoming increasingly evident that discrete genetic alterations in neoplastic cells alone cannot explain multistep carcinogenesis whereby tumor cells are able to express diverse phenotypes during the complex phases of tumor development and progression. The epigenetic model posits that the host microenvironment exerts an initial, inhibitory constraint on tumor growth that is followed by acceleration of tumor progression through complex cell–matrix interactions. This review emphasizes the epigenetic aspects of breast cancer development in light of such interactions between epithelial cells (“seed”) and the tumor microenvironment (“soil”). Our recent research findings suggest that epigenetic perturbations induced by the tumor microenvironment may play a causal role in promoting breast cancer development. It is believed that abrogation of these initiators could offer a promising therapeutic strategy.
Keywords: Breast cancer, Tumor microenvironment, Stromal fibroblast, Epigenetics, DNA methylation, Chromatin remodeling
1. Introduction
Breast cancer is a common malignancy among females in most western countries, where women have an overall lifetime risk of >10% for developing invasive breast cancer. It is not a single disease, but rather is composed of distinct subtypes associated with different clinical outcomes and is highly heterogeneous at both the molecular and clinical levels [1]. Although tumor initiation and progression are predominantly driven by acquired genetic alterations, our recent data suggest that microenvironment-mediated epigenetic perturbations may play a role in neoplasm development [2].
The origin of breast carcinomas lies in the epithelial linings of glands or ducts, rather than in the adjacent stroma. Stroma, or the supportive platform, is composed of fibroblasts, endothelial cells, smooth muscle cells, adipocytes, inflammatory cells, and nerve cells. This complicated network of various cell types, as well as the associated factors that are secreted, is collectively known as the microenvironment. Whether and how the tumor microenvironment influences breast neoplasms by altering epigenomes is increasingly gaining the attention and interest of researchers.
Epigenetics is the study of DNA modifications and its associated histone proteins, which do not change the primary DNA sequence, but serve a critical role in regulating the dynamics of gene expression (review in [3,4]). To date, one of the best-characterized epigenetic alterations is DNA methylation, which converts the cytosine of CpG residues to 5-methylcytosine. This chemical occurrence is mediated by DNA methyltransferases and occurs in CpG-rich stretch sequences, known as CpG islands, which exist in 60–70% of the promoter or in the first exon of known genes (review in [3,4]).
Besides promoter methylation, regional modifications of chromatin also exert a similar effect of controlling gene activity in cancer cells. N-terminal tails of histones undergo post-translational modifications, including acetylation, phosphorylation, ubiquitination, and methylation [5]. With a few exceptions [6,7], acetylated lysine is commonly associated with active gene transcription, while methylation of histone H3 on lysine 27 frequently instructs the target genes to undergo silencing [8–10], an event mediated by polycomb repressors which recruit DNA methyltransferases [11]. The subsequent acquisition of DNA methylation may then warrant an irrevocable suppression of the target gene. As a result, the interplay between chromatin remodeling and DNA methylation conveys combinatorial alterations and imprints differential degrees of gene silencing. Most importantly, epigenetic markings are mitotically heritable in progeny cells [12], suggesting that they can exert long-term repression without the genetic mutations that are traditionally required.
As aberrations within the epigenome have been proven to play important roles in breast tumorigenesis, there is an urgent need for a better understanding of possible mechanistic causes leading to epigenomic shifts, particularly from the cancer-associated stroma (“soil”). This article delves into the prospect of approaching a potential treatment regimen by targeting the tumor microenvironment. Having highlighted the potential advantages of taking this approach, we believe that more efficient and valuable therapeutic strategies can be further developed along with a whole array of advancement possibilities lying ahead, in order to combat the effects of tumor microenvironment.
2. Epigenetic perturbations in breast cancer
In human cancers, aberrant epigenomes are known to contribute to various phases of neoplastic development, including initiation, invasion, metastasis, and chemotherapy resistance (review in [13,14]). Epigenome-controlled loci include repair genes (MLH1, GST3), cell cycle inhibitors (p16INK4a, p15, p14ARF), tumor suppressor genes (VHL, BRCA1), tissue remodeling enzymes and structures (TIMP3, E-cadherin), and receptors (estrogen receptor) (reviewed in [15,16]).
Epigenetic silencing has been shown to augment various pre-neoplastic and malignant phenotypes. For example, using an unbiased global screen for aberrant CpG island methylation, Tlsty et al. have identified a non-randomized pattern of DNA hypermethylation in p16INK4a-deprived cells [17]. Hypermethylation of a p16INK4a promoter was observed in a subpopulation of primary human mammary epithelial cells that would have undergone senescence after long-term cultivation [18,19]. Lack of p16INK4a activity, however, conveyed growth capabilities extending past the normal proliferation barriers. Furthermore, depletion of p16INK4a resulted in the upregulation of polycomb repressors (EZH2 and SUZ12) which subsequently recruit DNA methyltransferases to a target gene, HOXA9, leading to hyper-methylation of its promoter [20]. Interestingly, HOXA9 is expressed during normal breast development but is commonly silenced in breast cancers by an epigenetic control. Moreover, in the absence of p16INK4a expression but with continued proliferation, cells hold great potential for acquiring eroding telomeric sequences and centrosomal dysfunction. As a result, this subpopulation of cells is allowed to freely accumulate chromosomal abnormalities and mutations. Depletion of p16INK4a, therefore, allows for the acquisition of multiple genetic changes necessary to oncogenic evolution and triggers the initiating steps of carcinogenesis. These results depict a causal role for p16INK4a disruption in modulating DNA hypermethylation, and identify a dynamic and active process whereby epigenetic modulation of gene expression appears to be an early event in breast tumor progression [17].
In addition to regional allele-specific hypermethylation, DNA in cancer cells experiences genome-wide hypomethylation. The perturbation of which is associated with gene reactivation, chromosomal instabilities, upregulation or overexpression of proto-oncogene transcription, increased recombination and mutations, loss of imprinting, and reactivation of transposable elements [21,22]. Interestingly enough, hypomethylation was observed in colorectal carcinoma cells that underwent hypoxia treatments [23]. Likewise, both hypomethylated and hypermethylated loci co-exist in colorectal and breast cancers (review in [24]). To this end, methylation-mediated E-cadherin loss in human breast cancer has been shown to be heterogeneous and unstable, characterized by coexistence of methylated and unmethylated entities at this promoter, throughout various stages of breast neoplasm [25]. Together, data indicate that epigenetic plasticity may contribute to this heterogeneity and drive metastatic progression of breast cancer in response to the dynamic tumor microenvironment.
On the other hand, a mounting body of evidence has demonstrated that histone modification alone, or its synergistic interactions with aberrant promoter methylation, can regulate gene activity. In the former occurrence, epigenomic silencing was attained solely by histone modification without detectable promoter methylation [6,7]. For example, Hinshelwood et al. observed that suppression of TGF-β-regulated downstream target genes was not associated with DNA methylation, but with chromatin remodeling involving a decrease in histone H3 lysine 27 trimethylation and an increase in histone H3 lysine 9 dimethylation and deacetylation [26]. However, in a separate incidence, Dumont et al. demonstrated that histone remodeling not only preceded but also persisted throughout the duration of promoter methylation of E-cadherin in breast epithelial cells undergoing epithelial to mesenchymal transition, induced by over-expression of oncogene ras and cultured in serum-rich media [27].
To date, the causal mechanism that initiates epigenomic alterations remains poorly understood. Yet we recently observed that epigenetic perturbations in breast epithelial cells could be induced by the surrounding tumor stromal fibroblasts, via an AKT1 kinase-induced mechanism ([2] and section 6 of this review).
3. Roles of tumor microenvironment in breast neoplasm
Over the past 2 decades, the majority of cancer-related studies have focused on examining the functional consequences of activating and/or inactivating mutations in critical genes involved in cell cycle control or apoptotic regulation. These studies have provided great insight into the functions of oncogenes and tumor suppressor genes and the signaling pathways that regulate cell proliferation and/or cell death. Nevertheless, they have largely ignored the fact that cancers are heterogeneous cellular entities whose growth are dependent upon reciprocal interactions between genetically altered initiated cells (“seed”) and the dynamic stromal microenvironment (“soil”) in which they reside.
3.1. Disrupted tumor microenvironment promotes tumor growth
Decades ago, using skin [28] or bladder [29] tissues, investigators observed that enhanced tumor formation could be induced if carcinogen-treated stroma was heterotypically grafted with untreated epithelial cells. Likewise, in an animal model, neoplastic transformation was achieved only when stromal fibroblasts were previously exposed to the carcinogen N-nitrosomethylurea [30]. Furthermore, in the irradiated stroma (with cleared fat pads devoid of epithelial cells), mammary epithelial cells were able to fully develop malignant phenotypes and progressed into fast-growing tumors with a size greater than the same cells transplanted into un-irradiated counterpart stroma [31,32].
3.2. Aberrant extracellular matrix (ECM) affects breast tumorigenesis
Among microenvironment constituents, the ECM is a key component secreted by stromal cells and is situated in a position of close contact with tumor cells. It functions as a critical source for growth, survival, motility, and angiogenic factors that significantly affects tumor behavior and progression. Perturbations in the production, deposition and degradation of the ECM present during neoplastic transformation and progression have been reported to arise from alterations in the stromal response [33]. Recently, the contribution of ECM alterations to tumor development and growth was examined. Using a three-dimensional culture assay developed with reconstituted basement membrane, Weaver and colleagues [34,35] demonstrated that the malignant phenotype of human breast cancer cells could be reversed by correcting ECM-integrin signaling. When this integrin switching was reversed, proliferation was controlled, morphogenesis was restored, and tumorigenesis was dramatically reduced, despite the fact that genetic abnormalities persisted [35]. These data suggest that appropriate integrin signaling is a critical microenvironmental effector that can act dominantly by overriding genetic constraints in the epithelium, to suppress the expression of the malignant phenotype.
3.3. Stromal cells influences breast tumorigenesis
Besides the ECM, there is increasing evidence indicating that tumors actively recruit stromal cells including inflammatory cells, vascular cells, and fibroblasts [36–40], into tumor masses. This recruitment is essential for the generation of a tumor microenvironment that actively fosters tumor growth.
The fibroblast is the major cell type of the stromal compartment that is closely involved in orchestrating the stromal portion of the dialog with the epithelium in maintaining tissue homeostasis [41]. Fibroblasts are responsible for the elaboration of most connective tissue components in the ECM, including collagens and structural proteoglycans, as well as various classes of proteolytic enzymes, their inhibitors, and a variety of growth factors. As each organ has specialized requirements, fibroblasts from different organs demonstrate organ-specific variations in the classes of biologically active molecules that they express [42].
Historically, fibroblasts were thought to be passive participants in the neoplastic programming of tissues. However, alterations in stromal fibroblasts adjacent to transformed epithelial cells have been documented in several tumor systems [43–45]. These include aberrations in growth characteristics, migratory potential, and altered expression of growth factors [43–45]. Cancer associated fibroblasts (CAFs) isolated from malignant tissues exhibit altered characteristics, most notably the enhanced production of collagens, hyaluronate, epithelial growth factors, and disorganized patterns of growth as well as enhanced proliferation [46].
The tumor-supportive role that fibroblasts play has recently been proven. For instance, deletion of the type II TGF-β receptor in fibroblasts in mice [39,47] and carcinogen treatment of mammary fat pad stroma in rats promoted tumor initiation and progression [30]. Likewise, Ulrich, et al. implicated microenvironmental changes in tumorigenesis, as inflammation is primarily a stromal reaction [48]. In support of these observations are the findings that irradiated, senescent, cancer-associated, or inflammatory fibroblasts promote tumor growth more effectively than normal fibroblasts [49–51]. Similarly, low-dose ionizing radiation-induced senescence-like fibroblasts significantly perturbed the mammary stromal microenvironment and sustained full expression of malignant potential in the resident breast carcinoma cells in vitro [32].
That fibroblasts exert an active role in tumorigenesis has been observed in multiple systems [52]. In combination with inflammatory cells, CAFs can promote neoplastic programming of tissues [53]. Likewise, when CAFs were grafted with immortalized (but non-tumorigenic) human prostatic epithelial cells, the interaction resulted in tumors that exceeded the weight of control grafts by five hundred-fold [36]. Interestingly enough, isolation of resultant human epithelial cell populations from these tumors (devoid of fibroblasts) and subsequent grafting into animals demonstrated that the epithelial cells were then able to form tumors in which the contributing activity from CAFs was no longer necessary [36]. In that instance, oncogenic signals from CAFs conferred nonrandom chromosomal changes and subsequently promoted nontumorigenic cells toward a malignant state.
The molecular mechanism revealing how CAFs play the tumor-promoting role has begun to be uncovered. Orimo et al. showed that CAFs promote the growth of oncogene-expressing breast cells in mice far more effectively than normal mammary fibroblasts derived from the same patients [40]. This growth-promoting effect is attributed to the secretion of stromal cell-derived factor 1 (SDF-1) that acts on the cognate receptor (CXCR4) expressed by carcinoma cells and subsequently promotes angiogenesis by recruiting endothelial progenitor cells into carcinomas [40]. Taken together, these findings suggest that oncogenic signals from CAFs can stimulate development of neoplastic properties and establish an active role in turmorigenic processes, rather than acting merely as passive participants.
4. Aberrant microenvironment influences cell plasticity and conveys epigenomic perturbations
The importance of the microenvironment and context in control of cellular differentiation and tissue polarity has been illustrated [54–56]. An in vitro model of differentiation that encompasses human normal mammary epithelial cells can form polarized and hollow tissue structures (acini) when cultured in the presence of basement membrane components, a technology known as a three-dimensional culture [54,57–59]. Acinar morphogenesis is accompanied by chromatin remodeling, along with an increase in expression of MeCP2 (a mediator of DNA-methylation-induced gene silencing), suggesting that DNA methylation is a mechanism by which mammary epithelial differentiation is coordinated at both the cellular and tissue levels [55]. In addition to DNA methylation, chromatin remodeling was evidenced by sensitivity to AluI digestion, in which the malignant cells resisted digestion relative to nonmalignant cells. Treatment of T4-2 breast cancer cells in a three-dimensional culture with cAMP analogs or with a phosphatidylinositol 3-kinase inhibitor not only reverted their phenotype from nonpolar to polar acinar-like structures, but also enhanced chromatin sensitivity to AluI [60]. Introduction of cAMP analogs or inhibitory antibody sequestering fibronectin resulted in phenotypic reversion, polarization, and a shift in DNA organization acting through a cAMP-dependent protein-kinase A-coupled signaling pathway.
A similar observation revealing epigenome can be influenced by microenvironment was recently reported [27]. In that report, immortalized human mammary epithelial cells with repressed p16INK4A but excessively expressed oncogenic ras (known as vHMEC-ras) was subjected to epigenomic perturbations if cells were grown in serum-rich media, a condition that can induce a gene expression pattern similar to that of a wound response. Here, the resultant cells experienced histone modification, gained de novo DNA hypermethylation at targeted genes frequently silenced in basal-like breast cancer cells, became more motile, and underwent phenotypic changes indicative of epithelial to mesenchymal transition [27]. Together, these findings demonstrate that the architecture of epigenome is highly plastic and reveal the concept that modifying the tumor microenvironment, such as specified growth in serum-rich media, can alter the epigenome organization and render aberrant DNA methylation in tumor cells.
5. Epigenomic perturbations in stroma, in relation to cancer cells
How the microenvironment differs between normal and cancer tissues is attracting growing research attention. Recently, Kurose et al. observed that genetic alterations occurred in the tumor stroma without an equivalent change in cancer cells [61]. Similarly, Hu et al. showed distinct epigenetic changes in cultured epithelial and myoepithelial cells and in stromal fibroblasts from normal breast tissue and breast carcinomas [62], suggesting that aberrant epigenomes in stroma are unique and discrete from their associated carcinoma cells. However, in HER-2/neu-positive cancers, aberrant DNA methylation is found not only in the stroma, but also in the associated cancer cells [63]. Of the five genes methylated in carcinoma cells, two loci were concordantly methylated in the stroma. Both genes are involved in estrogen metabolism: (a) estrogen receptor PGR, and (b) 17-β-estradiol metabolizing enzymes HSD17B4 [63]. Silencing of these two loci may account for inhibition of the anti-tumor activities intrinsic to tamoxifen. This implies that HER-2/neu cancer cells interact with the surrounding stroma and subsequently result in a “memory” by means of epigenetic imprints. However, the question of which cell type initiated the aberrant methylation remains unresolved.
6. Tumor stromal fibroblasts conveyed epigenetic silencing in breast epithelial cells
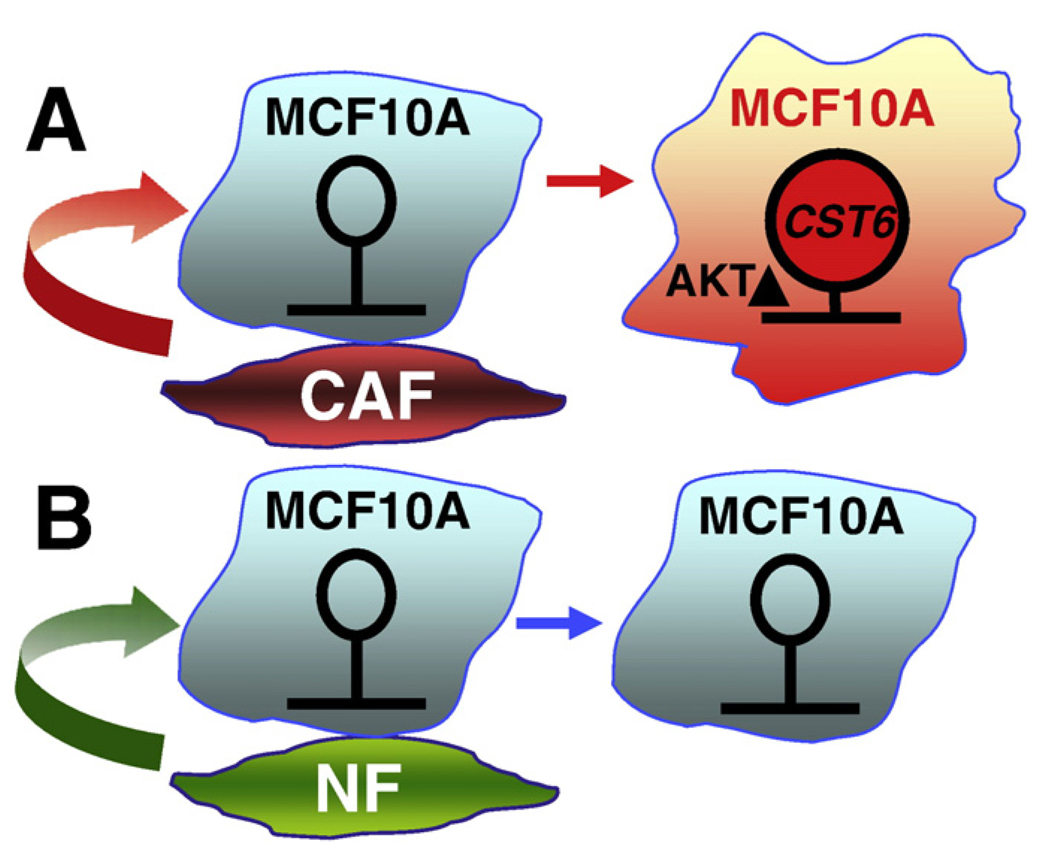
The initiator commanding the acquisition of promoter hyper-methylation in breast carcinoma cells remains poorly studied. Identifying these originators is critically important and should provide a better understanding of how the tumor microenvironment controls gene silencing in the surrounding pre-neoplastic and malignant cells. To this regard, our laboratory has developed a two-dimensional in vitro coculture system to decipher whether and how the tumor microenvironment conveys epigenetic gene silencing. We have observed that, by augmenting DNA methylation, fibroblastic signals exerted from CAFs can convey epigenetic silencing of tumor suppressor Cystatin M (known as CST6) and other genes, in the neighboring normal epithelial cells, namely MCF10A (Fig. 1) [2]. CST6 was recently characterized as a tumor suppressor gene for breast cancer [64] and its epigenetic silencing was observed in breast cancer cell lines as well as distally metastasized lesions [65,66]. Our data, therefore, provides a proof-of-principle, depicting that CAFs induce hypermethylation of tumor suppressor loci and subsequently lead to gene silencing in the contacted breast epithelial cells. As a result, loss of CST6 activity advances breast tumorigenesis and/or progression to metastasis.
Fig. 1.
An epigenetic model depicting the influence of breast cancer-associated fibroblasts on the non-cancerous breast epithelial cells (MCF10A). (A) After cocultured with cancer-associated fibroblasts (CAF), CST-6 (and perhaps other breast cancer-associated genes) became hypermethylated and silenced (marked in red). Epigenetic perturbation was mediated by an activation of AKT1 signaling pathway. (B) In contrast, exposure to normal fibroblasts (NF) confers negligible levels of epigenetic perturbations and AKT1 kinase activation in the same MCF10A cells (marked in light blue).
Furthermore, we reported that the signaling pathway leading to hypermethylation of CST6 is induced by the activated serine/threonine kinase AKT1/PKB pathway. Activation of AKT1 signaling not only conveyed DNA hypermethylation but also recruited DNA methyltransferase and repressive histone marks to the promoter of CST6, events which together contribute to epigenetic silencing [2]. The AKT1 kinase pathway in cocultured MCF10A cells can be aberrantly activated by being placed in contact with cancer-associated fibroblasts for a period as short as 1 week (Lin et al., unpublished data). In fact, elevated AKT1 kinase signaling pathway remarkably correlated with hypermethylation at the CST6 promoter in primary breast tumors, demonstrating that our in vitro coculture model is able to closely simulate the in vivo occurrences observed in tumors [2].
Our findings are in agreement with those of others. It has been reported that normal fibroblasts impede or prevent tumor formation while the CAFs promote tumorigenesis [37,67]. Likewise, coculture of premalignant breast cells with normal fibroblasts resulted in only weak induction of epithelial growth and morphogenesis, but similar cocultures with benign or tumor-derived fibroblasts conveyed an induction of highly proliferative ductal-alveolar morphogenesis [68]. Interestingly, besides inhibiting morphologic transformation of pre-malignant breast cells, reduction mammoplasty-derived fibroblasts were also found to have the ability to suppress estrogen responsiveness of premalignant breast cells [68]. In that respect, breast fibroblasts derived from normal or tumor tissues have the ability to override and accentuate the genetic constraints imposed by the epithelial cells [68].
7. Future clinical applications
In conclusion, these intriguing findings not only indicate that genetic and epigenetic alterations in the stroma significantly contribute to neoplastic phenotypes, but also present the novel concept that stromal–epithelial interactions play important roles in the development and progression of breast tumorigenesis. Although the current observations merely uncover the tip of the iceberg, they hold the potential to evolve novel therapeutic regimens that antagonize the tumor-promoting effect provoked from stromal cells or from ECM. For example, as we have observed that the tumor microenvironmental niche from CAFs activates the AKT/PKB pathway and confers epigenetic imprinting within the breast epithelial cells [2], development of a therapeutic strategy by abrogating this signaling pathway may reverse hypermethylation of CST6 and prevent metastatic spread. Clinical benefits resulting from anti-tumor microenvironment therapy may not be too far away from being a practical occurrence.
Acknowledgements
This work was supported in part by NIH grants (U54CA113001 and R01CA069065) (to T. H.-M. Huang) and by funds from American Cancer Society, the Department of Defense (BC073892), and the Susan G. Komen Breast Cancer Foundation (KG081123) (to H.-J. L. Lin).
References
- 1.Bloushtain-Qimron N, Yao J, Snyder EL, Shipitsin M, Campbell LL, Mani SA, Hu M, Chen H, Ustyansky V, Antosiewicz JE, Argani P, Halushka MK, Thomson JA, Pharoah P, Porgador A, Sukumar S, Parsons R, Richardson AL, Stampfer MR, Gelman RS, Nikolskaya T, Nikolsky Y, Polyak K. Cell type-specific DNA methylation patterns in the human breast. Proc. Natl. Acad. Sci. U. S. A. 2008;105:14076–14081. doi: 10.1073/pnas.0805206105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin H-JL, Zuo T, Lin C-H, Kuo CT, Liyanarachchi S, Sun S, Shen R, Deatherage DE, Potter D, Asamoto L, Lin S, Yan PS, Cheng A-L, Ostrowski MC, Huang THM. Breast cancer-associated fibroblasts confer AKT1-mediated epigenetic silencing of cystatin M in epithelial cells. Cancer Res. 2008;68:10257–10266. doi: 10.1158/0008-5472.CAN-08-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Callinan PA, Feinberg AP. The emerging science of epigenomics. Hum. Mol. Genet. 2006;15(Spec No 1):R95–R101. doi: 10.1093/hmg/ddl095. [DOI] [PubMed] [Google Scholar]
- 4.Fuks F. DNA methylation and histone modifications: teaming up to silence genes. Curr. Opin. Genet. Dev. 2005;15:490–495. doi: 10.1016/j.gde.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Ting AH, McGarvey KM, Baylin SB. The cancer epigenome—components and functional correlates. Genes Dev. 2006;20:3215–3231. doi: 10.1101/gad.1464906. [DOI] [PubMed] [Google Scholar]
- 6.Kondo Y, Shen L, Cheng AS, Ahmed S, Boumber Y, Charo C, Yamochi T, Urano T, Furukawa K, Kwabi-Addo B, Gold DL, Sekido Y, Huang TH, Issa JP. Gene silencing in cancer by histone H3 lysine 27 trimethylation independent of promoter DNA methylation. Nat. Genet. 2008;40:741–750. doi: 10.1038/ng.159. [DOI] [PubMed] [Google Scholar]
- 7.Chan MW, Huang YW, Hartman-Frey C, Kuo CT, Deatherage D, Qin H, Cheng AS, Yan PS, Davuluri RV, Huang TH, Nephew KP, Lin HJ. Aberrant transforming growth factor beta1 signaling and SMAD4 nuclear translocation confer epigenetic repression of ADAM19 in ovarian cancer. Neoplasia. 2008;10:908–919. doi: 10.1593/neo.08540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lachner M, Sullivan RJO, Jenuwein T. An epigenetic road map for histone lysine methylation. J. Cell Sci. 2003;116:2117–2124. doi: 10.1242/jcs.00493. [DOI] [PubMed] [Google Scholar]
- 9.Gibbons RJ. Histone modifying and chromatin remodelling enzymes in cancer and dysplastic syndromes. Hum. Mol. Genet. 2005;14(Spec No 1):R85–R92. doi: 10.1093/hmg/ddi106. [DOI] [PubMed] [Google Scholar]
- 10.McGarvey KM, Greene E, Fahrner JA, Jenuwein T, Baylin SB. DNA methylation and complete transcriptional silencing of cancer genes persist after depletion of EZH2. Cancer Res. 2007;67:5097–5102. doi: 10.1158/0008-5472.CAN-06-2029. [DOI] [PubMed] [Google Scholar]
- 11.Vire E, Brenner C, Deplus R, Blanchon L, Fraga M, Didelot C, Morey L, Van Eynde A, Bernard D, Vanderwinden JM, Bollen M, Esteller M, Di Croce L, de Launoit Y, Fuks F. The polycomb group protein EZH2 directly controls DNA methylation. Nature. 2006;439:871–874. doi: 10.1038/nature04431. [DOI] [PubMed] [Google Scholar]
- 12.Antequera F, Bird A. CpG islands as genomic footprints of promoters that are associated with replication origins. Curr. Biol. 1999;9:R661–R667. doi: 10.1016/s0960-9822(99)80418-7. [DOI] [PubMed] [Google Scholar]
- 13.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat. Rev. Genet. 2002;3:415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 14.Feinberg AP, Tycko B. The history of cancer epigenetics. Nat. Rev. Cancer. 2004;4:143–153. doi: 10.1038/nrc1279. [DOI] [PubMed] [Google Scholar]
- 15.Baylin SB, Ohm JE. Epigenetic gene silencing in cancer—a mechanism for early oncogenic pathway addiction? Nat. Rev. Cancer. 2006;6:107–116. doi: 10.1038/nrc1799. [DOI] [PubMed] [Google Scholar]
- 16.Baylin SB, Herman JG. DNA hypermethylation in tumorigenesis: epigenetics joins genetics. Trends Genet. 2000;16:168–174. doi: 10.1016/s0168-9525(99)01971-x. [DOI] [PubMed] [Google Scholar]
- 17.Tlsty TD, Crawford YG, Holst CR, Fordyce CA, Zhang J, McDermott K, Kozakiewicz K, Gauthier ML. Genetic and epigenetic changes in mammary epithelial cells may mimic early events in carcinogenesis. J. Mammary Gland Biol. Neoplasia. 2004;9:263–274. doi: 10.1023/B:JOMG.0000048773.95897.5f. [DOI] [PubMed] [Google Scholar]
- 18.Romanov SR, Kozakiewicz BK, Holst CR, Stampfer MR, Haupt LM, Tlsty TD. Normal human mammary epithelial cells spontaneously escape senescence and acquire genomic changes. Nature. 2001;409:633–637. doi: 10.1038/35054579. [DOI] [PubMed] [Google Scholar]
- 19.Tlsty TD, Romanov SR, Kozakiewicz BK, Holst CR, Haupt LM, Crawford YG. Loss of chromosomal integrity in human mammary epithelial cells subsequent to escape from senescence. J. Mammary Gland Biol. Neoplasia. 2001;6:235–243. doi: 10.1023/a:1011369026168. [DOI] [PubMed] [Google Scholar]
- 20.Reynolds PA, Sigaroudinia M, Zardo G, Wilson MB, Benton GM, Miller CJ, Hong C, Fridlyand J, Costello JF, Tlsty TD. Tumor suppressor p16INK4A regulates polycomb-mediated DNA hypermethylation in human mammary epithelial cells. J. Biol. Chem. 2006;281:24790–24802. doi: 10.1074/jbc.M604175200. [DOI] [PubMed] [Google Scholar]
- 21.Esteller M, Herman JG. Cancer as an epigenetic disease: DNA methylation and chromatin alterations in human tumours. J. Pathol. 2002;196:1–7. doi: 10.1002/path.1024. [DOI] [PubMed] [Google Scholar]
- 22.Van Zee KJ, Calvano JE, Bisogna M. Hypomethylation and increased gene expression of p16INK4a in primary and metastatic breast carcinoma as compared to normal breast tissue. Oncogene. 1998;16:2723–2727. doi: 10.1038/sj.onc.1201794. [DOI] [PubMed] [Google Scholar]
- 23.Shahrzad S, Bertrand K, Minhas K, Coomber BL. Induction of DNA hypomethylation by tumor hypoxia. Epigenetics. 2007;2:119–125. doi: 10.4161/epi.2.2.4613. [DOI] [PubMed] [Google Scholar]
- 24.Agrawal A, Murphy RF, Agrawal DK. DNA methylation in breast and colorectal cancers. Mod. Pathol. 2007;20:711–721. doi: 10.1038/modpathol.3800822. [DOI] [PubMed] [Google Scholar]
- 25.Graff JR, Gabrielson E, Fujii H, Baylin SB, Herman JG. Methylation patterns of the E-cadherin 5′ CpG island are unstable and reflect the dynamic, heterogeneous loss of E-cadherin expression during metastatic progression. J. Biol. Chem. 2000;275:2727–2732. doi: 10.1074/jbc.275.4.2727. [DOI] [PubMed] [Google Scholar]
- 26.Hinshelwood RA, Huschtscha LI, Melki J, Stirzaker C, Abdipranoto A, Vissel B, Ravasi T, Wells CA, Hume DA, Reddel RR, Clark SJ. Concordant epigenetic silencing of transforming growth factor-signaling pathway genes occurs early in breast carcinogenesis. Cancer Res. 2007;67:11517–11527. doi: 10.1158/0008-5472.CAN-07-1284. [DOI] [PubMed] [Google Scholar]
- 27.Dumont N, Wilson MB, Crawford YG, Reynolds PA, Sigaroudinia M, Tlsty TD. Sustained induction of epithelial to mesenchymal transition activates DNA methylation of genes silenced in basal-like breast cancers. Proc. Natl. Acad. Sci. 2008;105:14867–14872. doi: 10.1073/pnas.0807146105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Billingham RE, Orr JW, Woodhouse DL. Transplantation of skin components during chemical carcinogenesis with 20-methylcholanthrene. Br. J. Cancer. 1951;5:417–432. doi: 10.1038/bjc.1951.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uchida K, Samma S, Momose H, Kashihara N, Rademaker A, Oyasu R. Stimulation of urinary bladder tumorigenesis by carcinogen-exposed stroma. J. Urol. 1990;143:618–621. doi: 10.1016/s0022-5347(17)40041-3. [DOI] [PubMed] [Google Scholar]
- 30.Maffini MV, Soto AM, Calabro JM, Ucci AA, Sonnenschein C. The stroma as a crucial target in ratmammary gland carcinogenesis. J.Cell. Sci. 2004;117:1495–1502. doi: 10.1242/jcs.01000. [DOI] [PubMed] [Google Scholar]
- 31.Barcellos-Hoff MH, Ravani SA. Irradiated mammary gland stroma promotes the expression of tumorigenic potential by unirradiated epithelial cells. Cancer Res. 2000;60:1254–1260. [PubMed] [Google Scholar]
- 32.Tsai KK, Chuang EY, Little JB, Yuan ZM. Cellular mechanisms for low-dose ionizing radiation-induced perturbation of the breast tissue microenvironment. Cancer Res. 2005;65:6734–6744. doi: 10.1158/0008-5472.CAN-05-0703. [DOI] [PubMed] [Google Scholar]
- 33.Nelson CM, Bissell MJ. Modeling dynamic reciprocity: engineering three-dimensional culture models of breast architecture, function, and neoplastic transformation. Semin. Cancer Biol. 2005;15:342–352. doi: 10.1016/j.semcancer.2005.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weaver VM, Howlett AR, Langton-Webster B, Petersen OW, Bissell MJ. The development of a functionally relevant cell culture model of progressive human breast cancer. Semin. Cancer Biol. 1995;6:175–184. doi: 10.1006/scbi.1995.0021. [DOI] [PubMed] [Google Scholar]
- 35.Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J. Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tlsty TD, Hein PW. Know thy neighbor: stromal cells can contribute oncogenic signals. Curr. Opin. Genet. Dev. 2001;11:54–59. doi: 10.1016/s0959-437x(00)00156-8. [DOI] [PubMed] [Google Scholar]
- 38.Cunha GR, Hayward SW, Wang YZ, Ricke WA. Role of the stromal microenvironment in carcinogenesis of the prostate. Int. J. Cancer. 2003;107:1–10. doi: 10.1002/ijc.11335. [DOI] [PubMed] [Google Scholar]
- 39.Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 40.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 41.Cunha GR, Young P, Hom YK, Cooke PS, Taylor JA, Lubahn DB. Elucidation of a role for stromal steroid hormone receptors in mammary gland growth and development using tissue recombinants. J. Mammary Gland Biol. Neoplasia. 1997;2:393–402. doi: 10.1023/a:1026303630843. [DOI] [PubMed] [Google Scholar]
- 42.Chang HY, Sneddon JB, Alizadeh AA, Sood R, West RB, Montgomery K, Chi JT, van de Rijn M, Botstein D, Brown PO. Gene expression signature of fibroblast serum response predicts human cancer progression: similarities between tumors and wounds. PLoS Biol. 2004;2:E7. doi: 10.1371/journal.pbio.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chiquet-Ehrismann R, Mackie EJ, Pearson CA, Sakakura T. Tenascin: an extracellular matrix protein involved in tissue interactions during fetal development and oncogenesis. Cell. 1986;47:131–139. doi: 10.1016/0092-8674(86)90374-0. [DOI] [PubMed] [Google Scholar]
- 44.Singer C, Rasmussen A, Smith HS, Lippman ME, Lynch HT, Cullen KJ. Malignant breast epithelium selects for insulin-like growth factor II expression in breast stroma: evidence for paracrine function. Cancer Res. 1995;55:2448–2454. [PubMed] [Google Scholar]
- 45.Yee D, Rosen N, Favoni RE, Cullen KJ. The insulin-like growth factors, their receptors, and their binding proteins in human breast cancer. Cancer Treat. Res. 1991;53:93–106. doi: 10.1007/978-1-4615-3940-7_5. [DOI] [PubMed] [Google Scholar]
- 46.Rasmussen AA, Cullen KJ. Paracrine/autocrine regulation of breast cancer by the insulin-like growth factors. Breast Cancer Res. Treat. 1998;47:219–233. doi: 10.1023/a:1005903000777. [DOI] [PubMed] [Google Scholar]
- 47.Cheng N, Bhowmick NA, Chytil A, Gorksa AE, Brown KA, Muraoka R, Arteaga CL, Neilson EG, Hayward SW, Moses HL. Loss of TGF-[beta] type II receptor in fibroblasts promotes mammary carcinoma growth and invasion through upregulation of TGF-[alpha]-, MSP- and HGF-mediated signaling networks. Oncogene. 2005;24:5053. doi: 10.1038/sj.onc.1208685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ulrich CM, Bigler J, Potter JD. Non-steroidal anti-inflammatory drugs for cancer prevention: promise, perils and pharmacogenetics. Nat. Rev. Cancer. 2006;6:130–140. doi: 10.1038/nrc1801. [DOI] [PubMed] [Google Scholar]
- 49.Barcellos-Hoff MH. The potential influence of radiation-induced microenvironments in neoplastic progression. J. Mammary Gland Biol. Neoplasia. 1998;3:165–175. doi: 10.1023/a:1018794806635. [DOI] [PubMed] [Google Scholar]
- 50.Krtolica A, Campisi J. Cancer and aging: a model for the cancer promoting effects of the aging stroma. Int. J. Biochem. Cell Biol. 2002;34:1401–1414. doi: 10.1016/s1357-2725(02)00053-5. [DOI] [PubMed] [Google Scholar]
- 51.Liu D, Hornsby PJ. Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion. Cancer Res. 2007;67:3117–3126. doi: 10.1158/0008-5472.CAN-06-3452. [DOI] [PubMed] [Google Scholar]
- 52.Tlsty TD. Stromal cells can contribute oncogenic signals. Semin. Cancer Biol. 2001;11:97–104. doi: 10.1006/scbi.2000.0361. [DOI] [PubMed] [Google Scholar]
- 53.Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H, Porter D, Hu M, Chin L, Richardson A, Schnitt S, Sellers WR, Polyak K. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 54.Bissell MJ, Radisky D. Putting tumours in context. Nat. Rev. Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Plachot C, Lelievre SA. DNA methylation control of tissue polarity and cellular differentiation in the mammary epithelium. Exp. Cell Res. 2004;298:122–132. doi: 10.1016/j.yexcr.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 56.Bissell MJ, Labarge MA. Context, tissue plasticity, and cancer: are tumor stem cells also regulated by the microenvironment? Cancer Cell. 2005;7:17–23. doi: 10.1016/j.ccr.2004.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roskelley CD, Bissell MJ. The dominance of the microenvironment in breast and ovarian cancer. Semin. Cancer Biol. 2002;12:97–104. doi: 10.1006/scbi.2001.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bissell MJ, Radisky DC, Rizki A, Weaver VM, Petersen OW. The organizing principle: microenvironmental influences in the normal and malignant breast. Differentiation. 2002;70:537–546. doi: 10.1046/j.1432-0436.2002.700907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bissell MJ, Rizki A, Mian IS. Tissue architecture: the ultimate regulator of breast epithelial function. Curr. Opin. Cell Biol. 2003;15:753–762. doi: 10.1016/j.ceb.2003.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sandal T, Valyi-Nagy K, Spencer VA, Folberg R, Bissell MJ, Maniotis AJ. Epigenetic reversion of breast carcinoma phenotype is accompanied by changes in DNA sequestration as measured by AluI restriction enzyme. Am. J. Pathol. 2007;170:1739–1749. doi: 10.2353/ajpath.2007.060922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kurose K, Gilley K, Matsumoto S, Watson PH, Zhou XP, Eng C. Frequent somatic mutations in PTEN and TP53 are mutually exclusive in the stroma of breast carcinomas. Nat. Genet. 2002;32:355–357. doi: 10.1038/ng1013. [DOI] [PubMed] [Google Scholar]
- 62.Hu M, Yao J, Cai L, Bachman KE, van den Brule F, Velculescu V, Polyak K. Distinct epigenetic changes in the stromal cells of breast cancers. Nat. Genet. 2005;37:899–905. doi: 10.1038/ng1596. [DOI] [PubMed] [Google Scholar]
- 63.Fiegl H, Millinger S, Goebel G, Muller-Holzner E, Marth C, Laird PW, Widschwendter M. Breast cancer DNA methylation profiles in cancer cells and tumor stroma: association with HER-2/neu status in primary breast cancer. Cancer Res. 2006;66:29–33. doi: 10.1158/0008-5472.CAN-05-2508. [DOI] [PubMed] [Google Scholar]
- 64.Zhang J, Shridhar R, Dai Q, Song J, Barlow SC, Yin L, Sloane BF, Miller FR, Meschonat C, Li BD, Abreo F, Keppler D. Cystatin m: a novel candidate tumor suppressor gene for breast cancer. Cancer Res. 2004;64:6957–6964. doi: 10.1158/0008-5472.CAN-04-0819. [DOI] [PubMed] [Google Scholar]
- 65.Ai L, Kim WJ, Kim TY, Fields CR, Massoll NA, Robertson KD, Brown KD. Epigenetic silencing of the tumor suppressor cystatin M occurs during breast cancer progression. Cancer Res. 2006;66:7899–7909. doi: 10.1158/0008-5472.CAN-06-0576. [DOI] [PubMed] [Google Scholar]
- 66.Rivenbark AG, Livasy CA, Boyd CE, Keppler D, Coleman WB. Methylation-dependent silencing of CST6 in primary human breast tumors and metastatic lesions. Exp. Mol. Pathol. 2007;83:188–197. doi: 10.1016/j.yexmp.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dong-Le Bourhis X, Berthois Y, Millot G, Degeorges A, Sylvi M, Martin PM, Calvo F. Effect of stromal and epithelial cells derived from normal and tumorous breast tissue on the proliferation of human breast cancer cell lines in co-culture. Int. J. Cancer. 1997;71:42–48. doi: 10.1002/(sici)1097-0215(19970328)71:1<42::aid-ijc9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 68.Shekhar MP, Werdell J, Santner SJ, Pauley RJ, Tait L. Breast stroma plays a dominant regulatory role in breast epithelial growth and differentiation: implications for tumor development and progression. Cancer Res. 2001;61:1320–1326. [PubMed] [Google Scholar]