Abstract
BACKGROUND
Corticosteroids are commonly used to treat infantile hemangioma, but the mechanism of action of this therapy is unknown. We investigated the effect of corticosteroids in a previously described in vivo model of infantile hemangioma and in cultured hemangioma-derived cells.
METHODS
We tested hemangioma-derived stem cells for vasculogenic activity in vivo after implantation into immune-deficient (nude) mice. We studied dexamethasone treatment of both the cells before implantation and the mice after implantation. We also tested hemangioma-derived stem cells for expression of vascular endothelial growth factor A (VEGF-A) in vitro and studied the inhibition of VEGF-A expression, using short hairpin RNA (shRNA) in vivo and in vitro.
RESULTS
Systemic treatment with dexamethasone led to dose-dependent inhibition of tumor vasculogenesis in the murine model. Pretreatment of hemangioma-derived stem cells in vitro before implantation also inhibited vasculogenesis. Dexamethasone suppressed VEGF-A production by hemangioma-derived stem cells in vitro but not by hemangioma-derived endothelial cells or human umbilical-vein endothelial cells. Silencing VEGF-A in hemangioma-derived stem cells reduced vasculogenesis in vivo. VEGF-A was detected in hemangioma specimens in the proliferating phase but not in the involuting phase and was shown by immunostaining to reside outside of vessels. Corticosteroid treatment suppressed other proangiogenic factors in hemangioma-derived stem cells, including urokinase plasminogen activator receptor, interleukin-6, monocyte chemoattractant protein 1, and matrix metalloproteinase 1.
CONCLUSIONS
In a murine model, dexamethasone inhibited the vasculogenic potential of stem cells derived from human infantile hemangioma. The corticosteroid also inhibited the expression of VEGF-A by hemangioma-derived stem cells, and silencing of VEGF-A expression in these cells inhibited vasculogenesis in vivo.
Infantile hemangioma is the most common tumor of infancy, affecting about 10% of infants of mixed European descent by the age of 1 year.1–3 Infantile hemangioma occurs more frequently in female infants and in premature and low-birth-weight newborns.3,4 These tumors can be solitary or multiple; they appear early in post-natal life, grow rapidly during infancy, and involute spontaneously in early childhood. Infantile hemangioma is usually harmless; however, about 10% of hemangiomas are destructive, disfiguring, and even vision- or life-threatening. Corticosteroids given orally or by intralesional injection have been the first-line treatment for problematic hemangiomas since the 1960s. Nevertheless, the mechanism by which corticosteroids stabilize or seem to accelerate regression of this tumor is unknown.
We recently identified and isolated hemangioma-derived multipotential stem cells from specimens of proliferating infantile hemangiomas. 5 These cells display a mesenchymal morphology, robust proliferation, and multilineage differentiation in vitro and form human blood vessels with features of infantile hemangioma when injected subcutaneously into nude mice.5 This vasculogenic activity (i.e., the de novo formation of blood vessels) is unique to hemangioma-derived stem cells; hemangioma-derived endothelial cells6 and hemangioma-derived endothelial progenitor cells,7 which are phenotypically similar to normal human endothelial cells when cultured in vitro, do not form blood vessels in this murine model. Our findings suggest that hemangioma-derived stem cells are the cellular origin of infantile hemangiomas. In this study, we set out to determine how corticosteroids affect hemangioma-derived stem cells and endothelial cells.
METHODS
CELL ISOLATION AND CULTURE
We obtained surgical specimens of proliferating infantile hemangiomas under a human-subject protocol approved by the Committee on Clinical Investigation at Children’s Hospital Boston. The clinical diagnosis was confirmed by histologic evaluation in the hospital’s pathology department. Written informed consent was obtained for use of the infantile hemangioma specimens, according to the provisions of the Declaration of Helsinki. The derivation, sources, and culture conditions for the hemangioma-derived stem cells and other cells used in this study have been detailed previously.5,7,8 Additional information is also provided in the Supplementary Appendix, available with the full text of this article at NEJM.org.
MURINE HEMANGIOMA MODEL
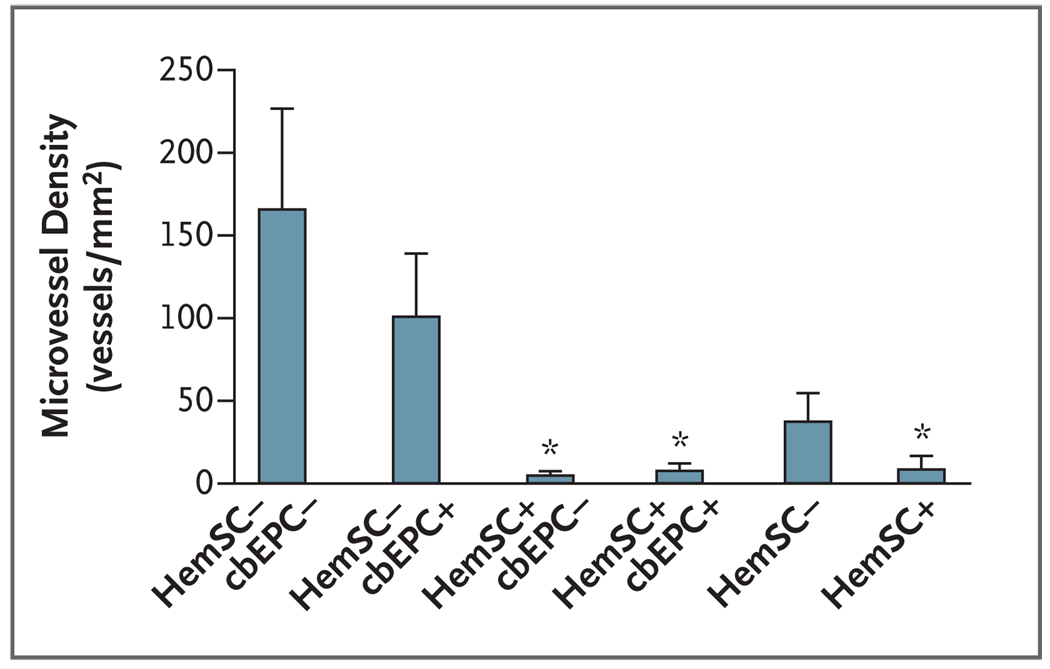
The murine model of infantile hemangioma was produced, as described previously,5 with the addition of endothelial progenitor cells isolated from human umbilical-cord blood7 as follows: 1×106 hemangioma-derived stem cells and 7×105 cord-blood endothelial progenitor cells per mouse were mixed, sedimented, resuspended in Matrigel (BD Biosciences), and injected subcutaneously into the backs of 6- to 8-week-old male athymic nu/nu mice (Massachusetts General Hospital). When cord-blood endothelial progenitor cells were included with hemangioma-derived stem cells in the Matrigel implant, the formation of microvessels was enhanced. However, cord-blood endothelial progenitor cells that were implanted alone did not form vessels in this model.8 Microvessel density was quantified as described previously.8 Values that are reported for each experimental condition correspond to the average values obtained from all the individual mice.
IMMUNOHISTOCHEMICAL AND IMMUNOFLUORESCENCE ANALYSES
Specific immunostaining for human CD31 was performed, as described previously.8 Double immunofluorescence for CD31 and vascular endothelial growth factor A (VEGF-A) was performed on cryosections as follows: slides were fixed with acetone, blocked with 5% serum, and incubated with mouse antihuman CD31 monoclonal antibody (Dako) at a titer of 1:300, followed by biotinylated antimouse IgG and streptavidin–Texas Red (Vector Laboratories) at a titer of 1:200. Subsequently, sections were incubated with rabbit anti-VEGF polyclonal antibody (Labvision) at a titer of 1:100 overnight at 4°C, followed by Alexa-Fluor 488–conjugated antirabbit IgG.
VEGF-A MESSENGER RNA AND PROTEIN
A quantitative real-time reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay was used to quantify VEGF-A messenger RNA (mRNA).9 An enzyme-linked immunosorbent assay (ELISA) was performed with the use of Quantikine Human VEGF (R&D Systems). For Western blots, infantile hemangioma tissues were homogenized and lysed, and 30 µg of protein was separated by sodium dodecyl sulfate–polyacrylamide-gel electrophoresis (SDS–PAGE) and transferred to a membrane. The membrane was blotted with rabbit antihuman VEGF-A polyclonal antibody (Labvision) at a titer of 1:1000 and with goat anti-CD31 polyclonal antibody (Santa Cruz Biotechnology).
VEGF-A DOWN-REGULATION BY SHORT HAIRPIN RNA
Short hairpin RNA (shRNA) lentiviral particles for VEGF-A targeting (NM_003376) and nontargeting shRNA (control) were purchased from Sigma. Hemangioma-derived stem cells that stably expressed VEGF-A shRNA were produced by infection and puromycin selection, according to the manufacturer’s instructions. The clone used for the in vivo experiments was selected after testing five different shRNA sequences for silencing efficacy.
CELL VIABILITY AND MIGRATION ASSAYS
Hemangioma-derived stem cells, normal human dermal fibroblasts, and hemangioma-derived endothelial progenitor cells were automatically plated in 384-well plates at 1500 cells per well with the use of Matrix WellMate (Thermo Fisher Scientific). Twenty-four hours later, the medium was removed and replaced with media containing various concentrations of dexamethasone. Seventy-two hours later, cells were fixed and stained with Hoechst 33258. After image capture by automated laser microscopy, the cell number was determined with the use of MetaXpress software. The migration of endothelial cells was assayed as described previously.10
ANGIOGENESIS ANTIBODY ARRAY
Conditioned media from hemangioma-derived stem cells, either treated or untreated with dexamethasone, were analyzed for protein expression with the use of RayBio Human Angiogenesis Antibody Array C Series 1000 (RayBiotech; www.raybiotech.com/map/human_angio_1000_^t map.pdf), according to the manufacturer’s instructions. Blots were analyzed by means of ImageJ software (National Institutes of Health). Results of interest in the angiogenesis antibody array were verified by quantitative RT-PCR (for details, see the Supplementary Appendix).
RESULTS
EFFECT OF DEXAMETHASONE
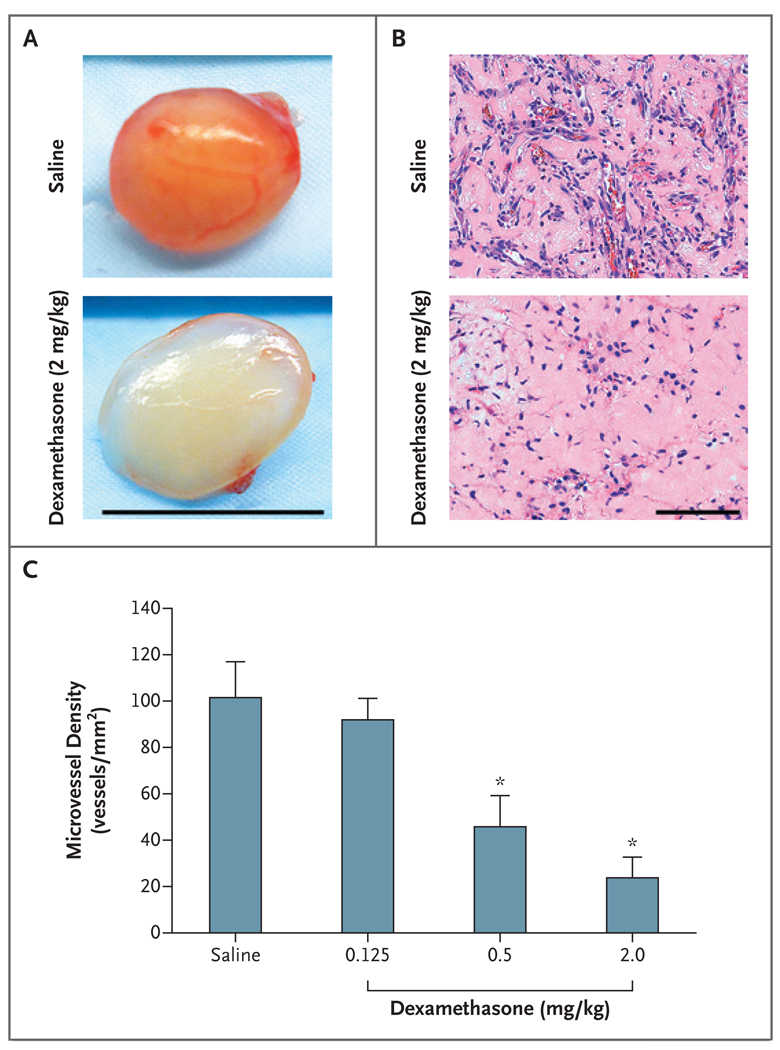
To test whether our murine model of infantile hemangioma would respond to the systemic administration of a corticosteroid, we injected immune-deficient (nu/nu) mice with hemangioma-derived stem cells and human cord-blood endothelial progenitor cells suspended in Matrigel. Dexamethasone was then administered by intra-peritoneal injection each day for 7 days. As compared with mice injected with saline, dexamethasone suppressed blood-vessel formation in a dose-dependent manner (Fig. 1A, 1B, and 1C, and Fig. 1 in the Supplementary Appendix).
Figure 1. Dose-Dependent Effect of Systemic Dexamethasone on a Murine Model of Infantile Hemangioma.
Hemangioma-derived stem cells and endothelial progenitor cells isolated from human umbilical-cord blood were suspended in Matrigel and injected subcutaneously into nude mice. Dexamethasone, at the indicated doses, was injected intraperitoneally for 7 consecutive days. Panel A shows cell–Matrigel explants from mice that were treated with either dexamethasone (2 mg per kilogram of body weight) or saline at day 7 (scale bar, 1 cm). Panel B shows sections from paraffin-embedded explants shown in Panel A (scale bar, 100 µm; hematoxylin and eosin). Panel C shows the microvessel density of samples from mice that were treated with dexamethasone (in doses of 0.125, 0.5, or 2.0 mg per kilogram of body weight) or saline, according to the number of erythrocyte-filled vessels in the sections (microvessel density). Bars represent the mean microvessel density determined from all the explants, with five samples in each subgroup. The T bars indicate standard errors. Asterisks indicate that the comparison with the control group was significant. For details regarding the experimental design, see Figure 1A in the Supplementary Appendix.
In order to isolate the effect of dexamethasone on hemangioma-derived stem cells from the effect on cord-blood endothelial progenitor cells, as well as to rule out possible indirect effects (such as antiinflammatory or metabolic effects), we treated each of the two cell types separately with dexamethasone for 3 days. We then washed out the dexamethasone, suspended the cells in Matrigel, and injected the cells into mice (Fig. 2A in the Supplementary Appendix). Pretreatment of hemangioma-derived stem cells with dexamethasone led to a significant decrease in the number of blood vessels in the murine tumors. In contrast, pretreatment of cord-blood endothelial progenitor cells with dexamethasone did not lead to such a decrease (Fig. 2, and Fig. 2B and 2C in the Supplementary Appendix).
Figure 2. Inhibition of Vasculogenesis by Pretreatment of Hemangioma-Derived Stem Cells with Dexamethasone.
Hemangioma-derived stem cells (HemSC), cord-blood endothelial progenitor cells (cbEPC), or both types of cells were treated with 0.2 µM dexamethasone for 3 days. Dexamethasone was washed out, and the cells were implanted in nude mice. Pretreatment of hemangioma-derived stem cells with dexamethasone led to a significant decrease in the number of blood vessels in the murine tumors, as compared with no significant decrease in cord-blood endothelial progenitor cells. Plus symbols indicate cells that were treated with dexamethasone. The bars represent the mean microvessel density determined from all the explants, with four to six samples in each subgroup. The T bars indicate standard errors. Asterisks indicate that the comparison with the control group was significant. This experiment was repeated twice with similar results. For details regarding the experimental design, see Figure 2A in the Supplementary Appendix.
To differentiate de novo formation of blood vessels (vasculogenesis) from in-growth of murine vessels from preexisting host vessels (angiogenesis), we stained Matrigel explant sections with human-specific anti-CD31 antibody. Human CD31-positive cells were organized into vascular structures in the absence of dexamethasone. In implants containing dexamethasone-treated hemangioma-derived stem cells, CD31-positive cells were present but were not assembled into vascular structures (Fig. 2C and 2D in the Supplementary Appendix).
VEGF-A PRODUCTION
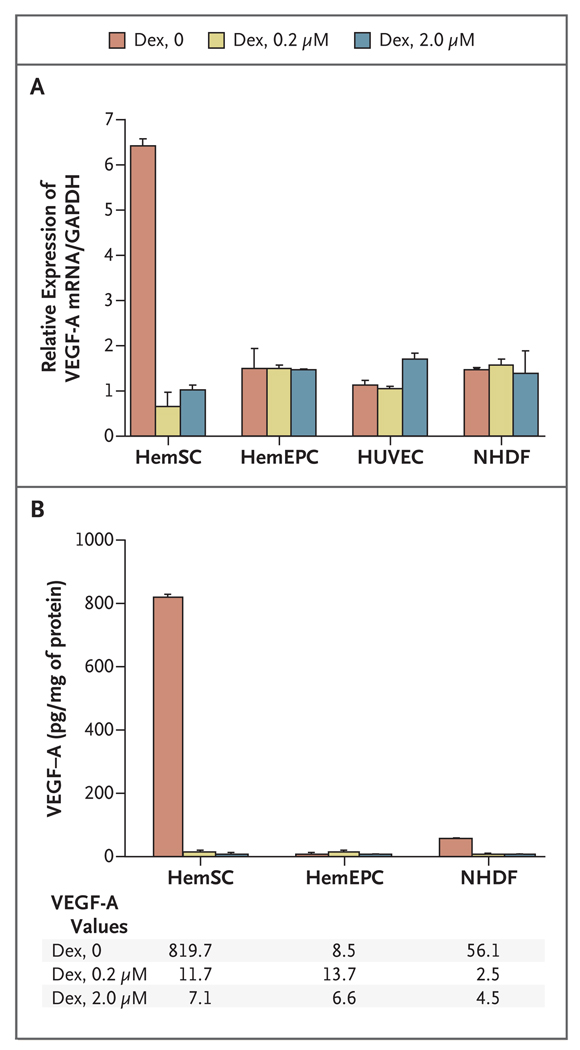
We next tested whether dexamethasone interferes with the expression of VEGF-A, a critical proangiogenic and provasculogenic factor. Hemangioma-derived stem cells expressed more VEGF-A mRNA and protein than did normal human dermal fibroblasts, human umbilical-vein endothelial cells, or hemangioma-derived endothelial progenitor cells. We also observed significant down-regulation of VEGF-A mRNA and protein when the hemangioma-derived stem cells were treated with dexamethasone (Fig. 3A and 3B). The suppression was dose-dependent from 0 to 200 nM (Fig. 3A in the Supplementary Appendix). Mifepristone (RU-486), a glucocorticoid receptor antagonist,11 blocked the suppressive effect of dexamethasone (Fig. 3B in the Supplementary Appendix). When dexamethasone was removed from the cultures of hemangioma-derived stem cells, the suppressive effect of dexamethasone persisted for up to 8 days (Fig. 3C in the Supplementary Appendix). A similar effect on VEGF-A expression was seen with other types of corticosteroids, including prednisone, prednisolone, methylprednisolone, and hydro-cortisone (Fig. 3D and 3E in the Supplementary Appendix).
Figure 3. Down-Regulation of VEGF-A Production in Hemangioma-Derived Stem Cells Treated with Dexamethasone.
Hemangioma-derived stem cells (HemSC) and hemangioma-derived endothelial progenitor cells (HemEPC) that were isolated from the same infantile hemangioma specimen, as well as normal human fibroblasts (NHDF) and human umbilical-vein endothelial cells (HUVEC), were treated with 0.2 µM or 2.0 µM dexamethasone (Dex) for 3 days in culture. Panel A shows the results of quantitative reverse-transcriptase–polymerase-chain-reaction assays for vascular endothelial growth factor A (VEGF-A) messenger RNA (mRNA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the normalization control. Panel B shows VEGF-A protein levels, as measured by enzyme-linked immunosorbent assay, in the conditioned medium of the cells. Results are representative of two different experiments performed with cells isolated from two different specimens of infantile hemangioma. The bars represent the mean values, and the T bars indicate standard deviations.
To assess the functional significance of VEGF-A production, we tested the ability of conditioned media from cultures of hemangioma-derived stem cells to stimulate the migration of cord-blood endothelial progenitor cells, an activity that would be expected if VEGF-A was present. We detected endothelial-cell chemotactic activity in the conditioned media, which was abolished with dexamethasone (Fig. 3F in the Supplementary Appendix).
We also investigated whether the decreased vasculogenic potential observed in the hemangioma-derived stem cells resulted from apoptosis triggered by dexamethasone. Dexamethasone did not cause reduced cell viability of hemangioma-derived stem cells or hemangioma-derived endothelial progenitor cells in concentrations of up to 1000 times those used for our in vitro or in vivo experiments (Fig. 3G in the Supplementary Appendix). In concentrations above 400 µM, viability was reduced, and disarrangement of the actin filaments and apoptosis were detected (Fig. 3H and 3I in the Supplementary Appendix).
EFFECT OF VEGF-A SILENCING BY SHORT HAIRPIN RNA
To test our hypothesis that the activity of dexamethasone on the vasculogenic potential of hemangioma-derived stem cells is mediated by its ability to suppress VEGF-A, we down-regulated VEGF-A, using shRNA silencing. Hemangioma-derived stem cells that were stably infected with VEGF-A shRNA expressed 80% less VEGF-A mRNA and 57% less protein (Fig. 4A and 4B in the Supplementary Appendix). VEGF-A shRNA-infected cells proliferated more slowly than the control nontargeting shRNA-infected cells (Fig. 4C in the Supplementary Appendix). However, increased cell death was not observed (data not shown).
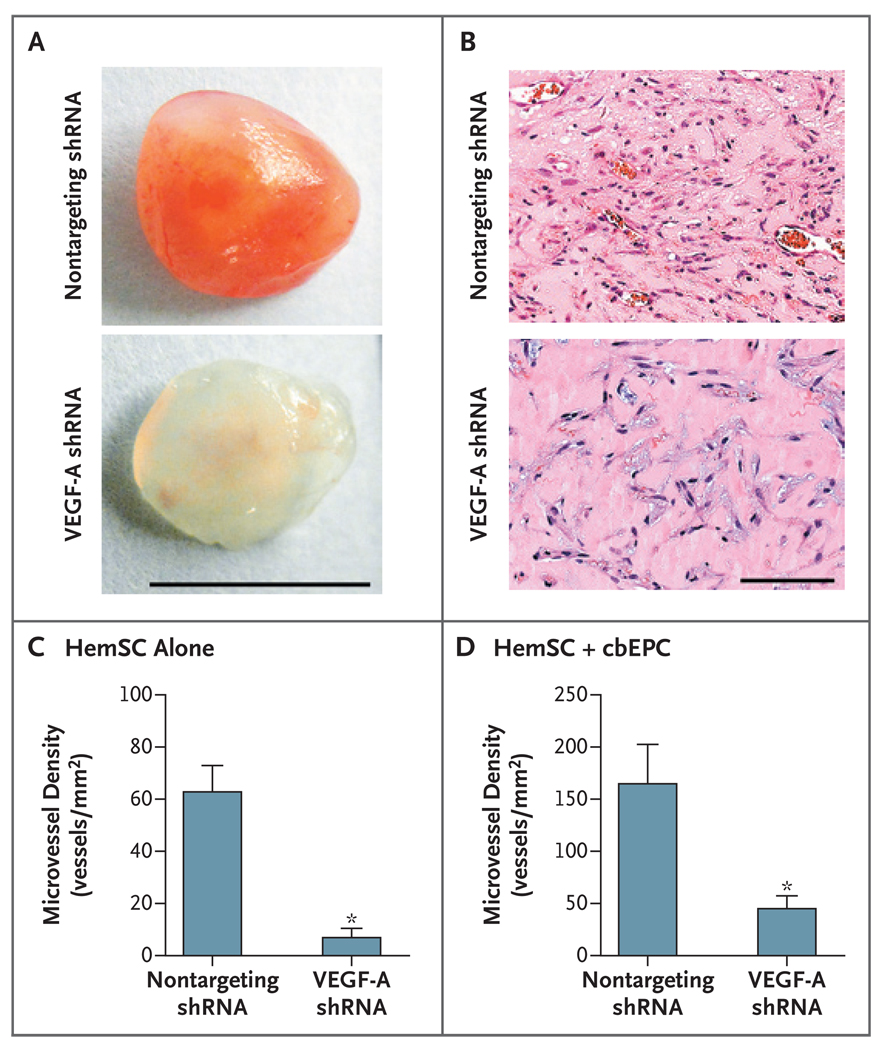
In initial in vivo experiments with shRNA-infected cells, we implanted infected hemangioma-derived stem cells alone to eliminate potential compensatory factors that might be supplied by cord-blood endothelial progenitor cells. VEGF-A silencing led to an 89% decrease in microvessel density in the explants (P<0.001) (Fig. 4A, 4B, and 4C).
Figure 4. Inhibition of Vasculogenesis through Silencing of VEGF-A in Hemangioma-Derived Stem Cells.
Hemangioma-derived stem cells (HemSC) were stably infected with short hairpin RNA (shRNA) from vascular endothelial growth factor A (VEGF-A) or with a nontargeting shRNA (control). Hemangioma-derived stem cells (4×106) that were infected with either VEGF-A shRNA or nontargeting shRNA were suspended in Matrigel and implanted subcutaneously in nude mice. Panel A shows representative Matrigel explants at day 7, with 7 to 10 samples in each subgroup. Panel B shows sections from representative explants from the VEGF-A shRNA group and the nontargeting shRNA group (hematoxylin and eosin). Panel C shows microvessel-density analysis of explants containing VEGF-A shRNA or nontargeting shRNA after 7 days in vivo. Panel D shows microvessel-density analysis of explants with the use of the two-cell model, in which either VEGF-A shRNA cells or nontargeting shRNA cells were combined with cord-blood endothelial progenitor cells (cbEPC), with eight samples in each subgroup. In Panels C and D, asterisks indicate that the comparison with the control group was significant. The T bars indicate standard errors. For both one-cell and two-cell models, the experiment was repeated twice with similar results.
We repeated this experiment in the two-cell-type model. Hemangioma-derived stem cells that were infected with VEGF-A shRNA combined with cord-blood endothelial progenitor cells were implanted, and the resulting Matrigel explants were evaluated for microvessel density. VEGF-A silencing caused a 70% decrease in microvessel density, as compared with hemangioma-derived stem cells infected with a nontargeting shRNA vector (P = 0.01) (Fig. 4D).
VEGF-A EXPRESSION IN INFANTILE HEMANGIOMAS
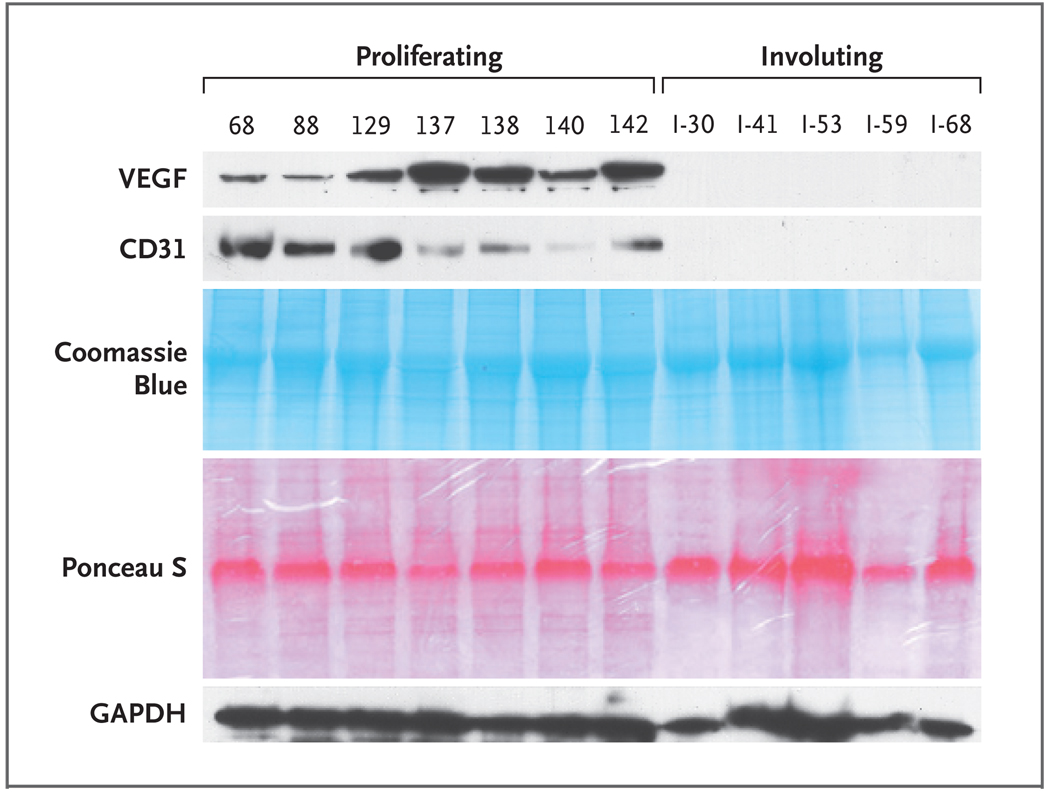
To determine whether VEGF-A expression in infantile hemangiomas is correlated with the clinical course of these tumors, we analyzed tissue specimens from proliferating and involuting hemangiomas by Western blot and by immunofluorescence staining. VEGF-A was detected by Western blot in all 15 proliferating infantile hemangioma specimens that were tested but was usually undetectable in involuting specimens (1 positive result of 11 tested) (Fig. 5, and Table 1 in the Supplementary Appendix).
Figure 5. VEGF-A Expression in Infantile Hemangiomas in the Proliferating Phase but Not the Involuting Phase.
In Panel A, tissue lysates from seven proliferating and five involuting hemangiomas were analyzed by Western blot with the use of antihuman VEGF-A antibody, antihuman CD31 antibody, and anti–glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (protein-loading control). CD31, an endothelial-cell adhesion molecule, was reduced in the involuting-phase samples to a level below detection by Western blot. Coomassie blue staining of the sodium dodecyl sulfate–polyacrylamide-gel electrophoresis analysis shows equivalent protein in each tissue lysate, and Ponceau S staining of the Western blot membrane verifies equivalent protein transfer. Proliferating hemangiomas are listed according to the coded number (e.g., 68, 88, etc.), and involuting hemangiomas are designated with “I” and the coded number (I-30, I-41, and so forth). Clinical data for patients from whom the individual specimens were obtained are provided in Table 1 in the Supplementary Appendix.
Infantile hemangioma tissue sections were immunostained for VEGF-A and for CD31 to examine the localization of VEGF-A–positive cells in relation to the hemangioma blood vessels. VEGF-A was detected in infantile hemangiomas only in the proliferating phase, consistent with the Western blot data, and was localized in cells outside the blood vessels (Fig. 5 in the Supplementary Appendix).
EFFECT OF DEXAMETHASONE ON OTHER PROANGIOGENIC FACTORS
We used a human angiogenesis antibody array that enabled detection of the expression of 43 different proangiogenic proteins to investigate these factors in hemangioma-derived stem cells with and without dexamethasone treatment. We found suppression of urokinase plasminogen activator receptor (uPAR), monocyte chemoattractant protein 1 (MCP-1), interleukin-6, and matrix metalloproteinase 1 (MMP-1) (Fig. 6A and 6B in the Supplementary Appendix). The expression of these factors in hemangioma-derived stem cells, as well as the suppressive effect of dexamethasone, was verified by quantitative RT-PCR (Fig. 6C in the Supplementary Appendix). Suppression of mRNA expression for each of these factors was achieved with 0.2 µM of dexamethasone, the same dose that suppressed VEGF-A (Fig. 3, and Fig. 3 and 6A in the Supplementary Appendix).
DISCUSSION
To investigate the effect of corticosteroid treatment on vasculogenesis in infantile hemangioma, we used stem-cell isolation techniques and a murine model that have been reported previously.5 We found that dexamethasone inhibits vasculogenesis induced by hemangioma-derived stem cells in vivo. We also found that dexamethasone and other corticosteroids inhibit the secretion of VEGF-A from hemangioma-derived stem cells in vitro. VEGF-A secretion from hemangioma-derived endothelial cells and human umbilical-vein endothelial cells was not affected by dexamethasone. Furthermore, we found that the suppression of VEGF-A is sufficient to block the vasculogenic potential of hemangioma-derived stem cells in vivo. VEGF-A protein was detected in infantile hemangiomas in the proliferating phase but not in the involuting phase, consistent with previous studies,12–16 but was found localized outside the endothelial-lined vessels. Finally, we identified uPAR, interleukin-6, MCP-1, and MMP-1 as other proangiogenic factors that are down-regulated by dexamethasone.
We concluded that dexamethasone specifically targets the multipotential hemangioma stem cells in infantile hemangiomas by suppressing their vasculogenic capability and that this effect is mediated, at least in part, by down-regulation of VEGF-A. More broadly, we believe that this model may provide a useful approach for the investigation of the mechanisms of current treatment regimens for infantile hemangioma and potentially for the testing and development of new therapies.
Histologically, hemangiomas consist of plump endothelial cells that form compact sinusoidal capillary channels.17,18 Sparsely distributed CD133-positive stem or progenitor cells exist in proliferating infantile hemangiomas and reside in clusters among the vascular channels.19,20 It is intriguing to find that corticosteroid treatment of hemangioma-derived stem cells, but not of endothelial cells, suppressed vasculogenesis. Corticosteroids are known to be more effective in the early proliferating phase of infantile hemangioma than in the late proliferating phase.21 This finding raises the question of whether the clinical response to corticosteroid treatment is determined by the ratio of immature stem cells to mature endothelial cells in the tumor. If this were true, future pharmacotherapeutic strategies might be tailored to have anti–stem-cell (antivasculogenic) effects in immature lesions in which stem cells are prominent while also having anti-endothelial (antiangiogenic) effects in tumors with high vascularity but fewer stem cells. Combination therapies targeting both stem- and endothelial-cell compartments might prove to be highly effective.
Two lines of investigation in this study expose VEGF-A as a major target of corticosteroid-mediated inhibition of vasculogenesis associated with infantile hemangioma. First, dexamethasone down-regulates VEGF-A in hemangioma-derived stem cells, and second, silencing of VEGF-A blocks the vasculogenic activity of hemangioma-derived stem cells in vivo. Previous studies have also supported the role of VEGF-A in this disease. A recent report showed an imbalance in the expression of VEGF receptor 1 (VEGFR-1) and the function of VEGF receptor 2 (VEGFR-2) in hemangioma-derived endothelial cells and hemangioma tissue, leading to aberrant VEGF-A signaling.22,23 In addition, serum VEGF-A levels have been shown to be higher in infants with tumors in the proliferating phase than in the involuting phase and to be reduced during corticosteroid therapy.14,24 Corticosteroid inhibition of the secretion of VEGF has been shown in a variety of cell types, both normal and tumoral.25–28 The fact that infantile hemangioma responds to corticosteroids as a sole treatment suggests that dependence of this tumor on VEGF-A for growth is greater than that in many other tumors.
Corticosteroids are known to induce apoptosis in some cells, such as thymocytes and lymphocytes, 29,30 and inhibit apoptosis in other cells, such as human neutrophils, hepatoma cells, and primary human and rat hepatocytes.31,32 In our study, growth inhibition or the induction of apoptosis in hemangioma-derived stem cells or hemangioma-derived endothelial progenitor cells by dexamethasone occurred only at concentrations that were 1000 times those that caused antivasculogenic activity. Hence, our study suggests that triggering of the death of stem cells or endothelial cells is an unlikely mechanism of corticosteroid therapy in this disease.
We suspected that dexamethasone would affect the expression of a broad array of factors that might be important in hemangiogenesis. Hemangioma-derived stem cells that were treated with dexamethasone for 3 days in vitro were shown to have reduced expression of MCP-1, which has been reported to be overexpressed in proliferating versus involuting infantile hemangioma. 33 Interleukin-6, which was detected in in vitro hemangioma models,34 was also suppressed, as were MMP-1 and uPAR, which have not previously been reported in infantile hemangioma. Future knock-down or overexpression studies will be required to shed light on the function of uPAR, interleukin-6, MCP-1, and MMP-1 in the life cycle of infantile hemangioma.
There are some limitations to our use of the murine model. First, although the dissection of infantile hemangioma into purified cellular components enabled the exploration of their specific roles, it prevented us from studying the tumor as a complex of multiple cell types, tumoral and normal. Second, because the model is xenogenic and carried out in nude mice, the role of immune-system cells cannot be investigated. Third, the implant microenvironment is composed of Matrigel, a commercially prepared murine basement membrane extract that could alter the behavior of hemangioma-derived stem cells. We used dexamethasone as a prototype corticosteroid, but we confirmed that other corticosteroids that are commonly used to treat infantile hemangioma (including prednisone, prednisolone, and methylprednisolone) also suppressed VEGF-A mRNA and protein in hemangioma-derived stem cells.
In summary, although corticosteroids are commonly used to treat infantile hemangioma, little is known about the mechanism of action. We showed that dexamethasone suppresses the vasculogenic potential of hemangioma-derived stem cells in a murine model. We also showed that dexamethasone suppresses VEGF-A expression by hemangioma-derived stem cells in vitro and that silencing of VEGF-A expression inhibits the vasculogenic potential of these cells in vivo. These findings provide insight into the mechanism of action of corticosteroid therapy in this condition.
Supplementary Material
Acknowledgments
Supported by the National Institutes of Health (P01-AR48564 and R01-HL096384); the Translational Research Program at Children’s Hospital Boston; a Harvard Skin Diseases Pilot Study Grant (to Dr. Greenberger); the Talpiot Medical Leadership Program, Sheba Medical Center, Israel (to Dr. Greenberger); and the John Butler Mulliken Foundation.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank the Institute for Chemistry and Cell Biology, Harvard Medical School, for use of the robotic cell-plating equipment and scanning fluorescence microscope for in vitro studies; Zia Khan, Ph.D., for initially testing the combination of hemangioma-derived stem cells and cord-blood endothelial progenitor cells in nude mice; Siming Yuan, M.D., for helping with cellular image analyses; Juan Melero-Martin, Ph.D., and Patrick Allen, B.S., for helping with confocal microscopy; Sarah Short, Ph.D., for helping with the migration assay; Sandra Smith for initial help with the VEGF-A ELISA; and Keren Levanon, M.D., Ph.D., for a critical reading of the manuscript.
REFERENCES
- 1.Mulliken JB. Cutaneous vascular anomalies. Semin Vasc Surg. 1993;6:204–218. [PubMed] [Google Scholar]
- 2.Mulliken JB, Fishman SJ, Burrows PE. Vascular anomalies. Curr Probl Surg. 2000;37:517–584. doi: 10.1016/s0011-3840(00)80013-1. [DOI] [PubMed] [Google Scholar]
- 3.Drolet BA, Esterly NB, Frieden IJ. Hemangiomas in children. N Engl J Med. 1999;341:173–181. doi: 10.1056/NEJM199907153410307. [DOI] [PubMed] [Google Scholar]
- 4.Drolet BA, Swanson EA, Frieden IJ. Infantile hemangiomas: an emerging health issue linked to an increased rate of low birth weight infants. J Pediatr. 2008;153:712–715. doi: 10.1016/j.jpeds.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 5.Khan ZA, Boscolo E, Picard A, et al. Multipotential stem cells recapitulate human infantile hemangioma in immunodeficient mice. J Clin Invest. 2008;118:2592–2599. doi: 10.1172/JCI33493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boye E, Yu Y, Paranya G, Mulliken JB, Olsen BR, Bischoff J. Clonality and altered behavior of endothelial cells from hemangiomas. J Clin Invest. 2001;107:745–752. doi: 10.1172/JCI11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan ZA, Melero-Martin JM, Wu X, et al. Endothelial progenitor cells from infantile hemangioma and umbilical cord blood display unique cellular responses to endostatin. Blood. 2006;108:915–921. doi: 10.1182/blood-2006-03-006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melero-Martin JM, Khan ZA, Picard A, Wu X, Paruchuri S, Bischoff J. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007;109:4761–4768. doi: 10.1182/blood-2006-12-062471. [DOI] [PubMed] [Google Scholar]
- 9.Zins K, Abraham D, Sioud M, Aharinejad S. Colon cancer cell-derived tumor necrosis factor-alpha mediates the tumor growth-promoting response in macrophages by up-regulating the colony-stimulating factor-1 pathway. Cancer Res. 2007;67:1038–1045. doi: 10.1158/0008-5472.CAN-06-2295. [DOI] [PubMed] [Google Scholar]
- 10.Short SM, Derrien A, Narsimhan RP, Lawler J, Ingber DE, Zetter BR. Inhibition of endothelial cell migration by throm-bospondin-1 type-1 repeats is mediated by beta1 integrins. J Cell Biol. 2005;168:643–653. doi: 10.1083/jcb.200407060. [Erratum, J Cell Biol 2005;169:541.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaillard RC, Poffet D, Riondel AM, Saurat JH. RU 486 inhibits peripheral effects of glucocorticoids in humans. J Clin Endocrinol Metab. 1985;61:1009–1011. doi: 10.1210/jcem-61-6-1009. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi K, Mulliken JB, Kozakewich HP, Rogers RA, Folkman J, Ezekowitz RA. Cellular markers that distinguish the phases of hemangioma during infancy and childhood. J Clin Invest. 1994;93:2357–2364. doi: 10.1172/JCI117241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang J, Most D, Bresnick S, et al. Proliferative hemangiomas: analysis of cytokine gene expression and angiogenesis. Plast Reconstr Surg. 1999;103:1–9. doi: 10.1097/00006534-199901000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Kleinman ME, Greives MR, Churgin SS, et al. Hypoxia-induced mediators of stem/progenitor cell trafficking are increased in children with hemangioma. Arterioscler Thromb Vasc Biol. 2007;27:2664–2670. doi: 10.1161/ATVBAHA.107.150284. [DOI] [PubMed] [Google Scholar]
- 15.Yu Y, Varughese J, Brown LF, Mulliken JB, Bischoff J. Increased Tie2 expression, enhanced response to angiopoietin-1, and dysregulated angiopoietin-2 expression in hemangioma-derived endothelial cells. Am J Pathol. 2001;159:2271–2280. doi: 10.1016/S0002-9440(10)63077-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ritter MR, Dorrell MI, Edmonds J, Friedlander SF, Friedlander M. Insulin-like growth factor 2 and potential regulators of hemangioma growth and involution identified by large-scale expression analysis. Proc Natl Acad Sci U S A. 2002;99:7455–7460. doi: 10.1073/pnas.102185799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg. 1982;69:412–422. doi: 10.1097/00006534-198203000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Enjolras O, Mulliken JB. Vascular tumors and vascular malformations (new issues) Adv Dermatol. 1997;13:375–423. [PubMed] [Google Scholar]
- 19.Smoller BR, Apfelberg DB. Infantile (juvenile) capillary hemangioma: a tumor of heterogeneous cellular elements. J Cutan Pathol. 1993;20:330–336. doi: 10.1111/j.1600-0560.1993.tb01271.x. [DOI] [PubMed] [Google Scholar]
- 20.Yu Y, Flint AF, Mulliken JB, Wu JK, Bischoff J. Endothelial progenitor cells in infantile hemangioma. Blood. 2004;103:1373–1375. doi: 10.1182/blood-2003-08-2859. [DOI] [PubMed] [Google Scholar]
- 21.Edgerton MT. The treatment of hemangiomas: with special reference to the role of steroid therapy. Ann Surg. 1976;183:517–532. doi: 10.1097/00000658-197605000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jinnin M, Medici D, Park L, et al. Suppressed NFAT-dependent VEGFR1 expression and constitutive VEGFR2 signaling in infantile hemangioma. Nat Med. 2008;14:1236–1246. doi: 10.1038/nm.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Claesson-Welsh L. Healing hemangiomas. Nat Med. 2008;14:1147–1148. doi: 10.1038/nm1108-1147. [DOI] [PubMed] [Google Scholar]
- 24.Zhang L, Lin X, Wang W, et al. Circulating level of vascular endothelial growth factor in differentiating hemangioma from vascular malformation patients. Plast Reconstr Surg. 2005;116:200–204. doi: 10.1097/01.prs.0000170804.80834.5f. [DOI] [PubMed] [Google Scholar]
- 25.Wu WS, Wang FS, Yang KD, Huang CC, Kuo YR. Dexamethasone induction of keloid regression through effective suppression of VEGF expression and keloid fibroblast proliferation. J Invest Dermatol. 2006;126:1264–1271. doi: 10.1038/sj.jid.5700274. [DOI] [PubMed] [Google Scholar]
- 26.Wilson C, Scullin P, Worthington J, et al. Dexamethasone potentiates the antiangiogenic activity of docetaxel in castration-resistant prostate cancer. Br J Cancer. 2008;99:2054–2064. doi: 10.1038/sj.bjc.6604804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hata Y, Sassa Y, Kita T, et al. Vascular endothelial growth factor expression by hyalocytes and its regulation by glucocorticoid. Br J Ophthalmol. 2008;92:1540–1544. doi: 10.1136/bjo.2008.141002. [DOI] [PubMed] [Google Scholar]
- 28.Nauck M, Karakiulakis G, Perruchoud AP, Papakonstantinou E, Roth M. Corticosteroids inhibit the expression of the vascular endothelial growth factor gene in human vascular smooth muscle cells. Eur J Pharmacol. 1998;341:309–315. doi: 10.1016/s0014-2999(97)01464-7. [DOI] [PubMed] [Google Scholar]
- 29.Cifone MG, Migliorati G, Parroni R, et al. Dexamethasone-induced thymocyte apoptosis: apoptotic signal involves the sequential activation of phosphoinositide-specific phospholipase C, acidic sphingo-myelinase, and caspases. Blood. 1999;93:2282–2296. [PubMed] [Google Scholar]
- 30.Zacharchuk CM, Merćep M, Chakraborti PK, Simons SS, Jr, Ashwell JD. Programmed T lymphocyte death: cell activation-and steroid-induced pathways are mutually antagonistic. J Immunol. 1990;145:4037–4045. [PubMed] [Google Scholar]
- 31.Yamamoto M, Fukuda K, Miura N, Suzuki R, Kido T, Komatsu Y. Inhibition by dexamethasone of transforming growth factor beta1-induced apoptosis in rat hepatoma cells: a possible association with Bcl-xL induction. Hepatology. 1998;27:959–966. doi: 10.1002/hep.510270410. [DOI] [PubMed] [Google Scholar]
- 32.Oh HY, Namkoong S, Lee SJ, et al. Dexamethasone protects primary cultured hepatocytes from death receptor-mediated apoptosis by upregulation of cFLIP. Cell Death Differ. 2006;13:512–523. doi: 10.1038/sj.cdd.4401771. [DOI] [PubMed] [Google Scholar]
- 33.Isik FF, Rand RP, Gruss JS, Benjamin D, Alpers CE. Monocyte chemoattractant protein-1 mRNA expression in hemangiomas and vascular malformations. J Surg Res. 1996;61:71–76. doi: 10.1006/jsre.1996.0083. [DOI] [PubMed] [Google Scholar]
- 34.Hasan Q, Tan ST, Xu B, Davis PF. Effects of five commonly used glucocorticoids on haemangioma in vitro. Clin Exp Pharmacol Physiol. 2003;30:140–144. doi: 10.1046/j.1440-1681.2003.03815.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.