Abstract
Background & Aims
Invasive pancreatic ductal adenocarcinoma is thought to originate from duct-like lesions called pancreatic intraepithelial neoplasia (PanIN). PanINs progress from low grade (PanIN-1) to high grade (PanIN-3) as the cells attain molecular alterations to key regulatory genes, including activating mutations in the KRAS protooncogene. Despite a well documented progression model, our knowledge of the initiator cells of PanINs and the transcriptional networks and signaling pathways that impact PanIN formation remains incomplete.
Methods
In this study, we examined the importance of the acinar-restricted transcription factor Mist1 to KrasG12D-induced mouse PanIN (mPanIN) formation in three different mouse models of pancreatic cancer.
Results
In the absence of Mist1 (Mist1KO), KrasG12D expressing mice exhibited severe exocrine pancreatic defects that were rescued by ectopic expression of Mist1 in acinar cells. Mouse PanIN development was greatly accelerated in Mist1KO/KrasG12D/+ pancreata and in vitro assays revealed that Mist1KO acinar cells were predisposed to convert to a ductal phenotype and activate EGFR and Notch signaling pathways.
Conclusions
We propose that convergence of EGFR, Notch and Kras pathways in acinar cells lacking Mist1 leads to enhanced mPanIN formation.
Introduction
With a five-year survival rate of less than 5%, pancreatic ductal adenocarcinoma (PDA) is among the most lethal of all human malignancies 1, 2. The factors responsible for this frightening statistic include the resistance of pancreatic cancers to conventional chemotherapy and radiotherapy and the absence of early warning signs and symptoms. Despite a number of advances in basic and clinical pancreas biology, our understanding of the pathogenesis and the molecular mechanisms underlying PDA remain incomplete.
Three different ductal precursor lesions have been identified which give rise to invasive PDA - pancreatic intraepithelial neoplasia (PanIN), intraductal papillary mucinous neoplasia (IPMN), and mucinous cystic neoplasia (MCN) 3-5. Of these, PanINs are the best characterized, being classified from low grade (PanIN-1) to high grade (PanIN-3) based on a number of histological criteria including the degree of architectural and nuclear atypia. Activating mutations in the KRAS2 protooncogene are thought to be the initiating mutations responsible for PanIN-1 lesions 4. Additional genetic modifications include telomere shortening, inactivation of the p16INK4a locus (PanIN-2), and inactivation of the TP53, SMAD4/DPC4 genes (PanIN-3) 6-9. Alterations in these oncogenes and tumor suppressor genes cause pleiotropic effects that lead to the deregulation of signaling pathways controlling cell proliferation, survival, adhesion and migration 2, 10.
In an effort to model PDA, a number of mutant mouse strains have been developed that overexpress an activated Kras oncogene within specific pancreatic cellular compartments 11-14 or that conditionally activate a mutant Kras allele from it’s own endogenous locus 15-18. In many instances, KrasG12D expressing mice develop PanINs (referred to as mPanINs in mice) that on rare occasions progress to PDA after a long latency period (>1 year). While PanINs can be modeled in mice, the identity of the initial target cell remains controversial. Although the histological features of PanINs suggest a duct cell origin, several studies have proposed that acinar cells also may participate in PanIN development 10, 14, 17, 19-21.
The successful establishment of mouse models that mimic human disease has been a significant breakthrough in pancreatic oncology, although the role of individual transcription networks in PanIN development has not been fully studied. As a first step, we examined the importance of Mist1, a basic helix-loop-helix transcription factor that is expressed in pancreatic acinar cells but not in duct, islet, or centroacinar cells 20, 22, 23. To investigate the importance of Mist1 and acinar cells to mPanIN initiation, we focused on the early events of mPanIN development in three different mutant KrasG12D model systems - Mist1Kras/+ mice in which a KrasG12D coding region was targeted to the Mist1 locus 13, LSL-KrasG12D/+/ptf1aCre/+ mice in which an endogenous KrasG12D allele was expressed upon “pan” pancreas Cre expression 16, 24, and LSL-KrasG12D/+/Mist1Cre-ER/+ mice in which the endogenous KrasG12D allele was expressed in adult acinar cells upon tamoxifen addition. Our studies revealed that in the absence of Mist1, Mist1Kras/LacZ mice exhibited gross pancreatic defects that could be rescued by ectopic expression of Mist1 in acinar cells. Similarly, mPanIN formation in LSL-KrasG12D/+/ptf1aCre/+ and LSL-KrasG12D/+/Mist1Cre-ER/+ mice was greatly accelerated in the absence of Mist1, suggesting that Mist1 null acinar cells either locally influenced duct cells or directly converted to mPanINs. Indeed, lineage tracing studies confirmed that mPanINs from LSL-KrasG12D/+/Mist1Cre-ER/Cre-ER mice were derived from adult acinar cells. Interestingly, in vitro assays demonstrated that Mist1LacZ/LacZ cells were predisposed to convert to a ductal phenotype and activate key EGFR and Notch signaling pathways that cooperated with KrasG12D. We conclude that loss of Mist1 leads to enhanced KrasG12D-induced mPanIN formation in these mouse models.
Materials and Methods
Mouse strains
Mist1KrasG12D/+ (Mist1Kras/+) mice 13 were crossed to Mist1LacZ/LacZ mice 25 to generate Mist1Kras/LacZ mice. The elastasepr-Mist1myc (Elpr-Mist1myc) construct driving acinar-specific expression of a myc-tagged Mist1 protein was used to produce Elpr-Mist1myc transgenic mice as described previously 26. Mist1Cre-ER/+ mice were generated by standard ES cell targeting in which the complete Mist1 coding region was replaced with the Cre-ERT2 coding region 27. LSL-KrasG12D/+ and ptf1aCre/+ strains were crossed to Mist1LacZ/LacZ mice to generate Mist1LacZ/LacZ/LSL-KrasG12D/+/ptf1aCre/+ mice. Similarly, LSL-KrasG12D/+/Mist1Cre-ER/Cre-ER mice were generated by standard crosses. Induction of Cre-ERT2 activity was accomplished by providing adult mice (>6 weeks) tamoxifen (4 mg/mouse/day) for 3 consecutive days. All studies were conducted in compliance with NIH and the Purdue University IACUC guidelines.
Histology and immunohistochemistry
Mouse pancreas tissues were processed as previously described 20. Primary antibodies included rabbit amylase (Calbiochem, San Diego, CA), mouse β-gal, mouse myc (9E10), and rat K19 (TROMA-3) (Developmental Studies Hybridoma Bank, Iowa City, IA), rabbit Hes1 (gift of Tetsuo Sudo), mouse Ki67 (Novocastra, Newcastle Upon Tyne, UK), mouse phospho-Stat3 (Upstate, Lake Placid, NY), rabbit insulin (Linco Research, St. Louis, MO), and rabbit Mist1 22, 25.
Protein immunoblot assays
20 μg of whole cell protein extracts were separated on 12% acrylamide gels, transferred to PVDF membranes and incubated with primary antibodies (1:1000) against rabbit Mist1 22, 25, mouse myc (9E10, Developmental Hybridoma Bank), rabbit ErbB1, rabbit phospho-MEK1/2 (Ser217/221), rabbit phospho-ERK (Thr202/204), rabbit phospho-Akt (Ser473) (Cell Signaling, Danvers, MA), and mouse phospho-Stat3 (Upstate, Lake Placid, NY). Detection of Hsp90 with rabbit Hsp 90a/b (1:2000, Santa Cruz, Santa Cruz, CA) was used as a loading control. Immunoblots were developed using an ECL kit (Pierce, Rockford, IL) as per manufacturer’s instructions.
RNA expression analysis
Pancreas RNA was isolated using the RNeasy isolation system (QIAGEN, Valencia, CA) and reverse transcribed using the iScript™ cDNA synthesis kit (Bio-Rad, Hercules, CA). PCR conditions are described in Supplemental Data.
Acinar cell cultures
Primary acinar cells were isolated from wild type or Mist1LacZ/LacZ mice as described by Means et al. 28. After plating in 1.35 mg/ml collagen (BD biosciences, San Jose, CA), the cells were supported with RPMI medium (Invitrogen, Carlsbad, CA) supplemented with 1% FBS, 0.1 mg/ml soybean trypsin inhibitor, 1 μg/ml dexamethasone, 100 units/ml penicillin and 100 μg/ml streptomycin. TGFα (10 ng/ml or 50 ng/ml) (R&D systems, Minneapolis, MN) was added to the medium and the efficiency of acinar-to-ductal conversion was measured after 5 days.
Results
Mist1 is essential for survival of Mist1Kras/+ mice
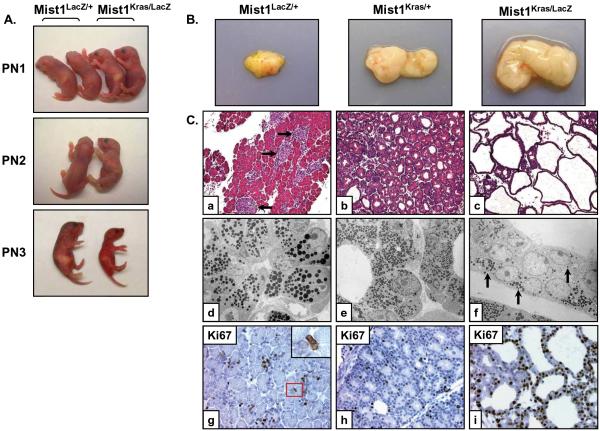
Previous studies showed that pancreata from Mist1Kras/+ mice had significantly lower levels of the acinar-restricted transcription factor Mist1 13. In order to establish if loss of Mist1 protein was critical to the Mist1Kras/+ phenotype, we generated Mist1Kras/LacZ animals that expressed KrasG12D and β-gal from the Mist1 locus but lacked Mist1 protein. Although Mist1Kras/LacZ mice appeared normal at birth, they rapidly lost body weight, became dehydrated, and died within three days (Figure 1A). Newborn animals had a significantly enlarged pancreas that was ~10-fold larger than Mist1LacZ/+ mice and ~2.5-fold larger than Mist1Kras/+ mice (Figure 1B). As expected, control newborn Mist1LacZ/+ animals exhibited normal acini and islet organization (Figure 1C, a). Mist1Kras/+ pancreata also retained relatively normal exocrine organization with the exception that the lumina of the acini were often dilated, revealing early signs of acinar metaplasia (Figure 1C, b) 13. In older animals (2-3 months), the presence of acinar/duct biphenotypic cells was readily observed as acinar cells transitioned to a duct-like cell (Supplemental Figure S1) 13, 20. In contrast to Mist1Kras/+ mice, Mist1Kras/LacZ pancreata were grossly distorted at birth, consisting of extensive epithelial tubular networks in which the normal apical-basal polarity of secretory granules, nuclei, and endoplasmic reticulum was lost and individual cell boundaries were difficult to distinguish (Figure 1C, c-f). The zymogen-containing tubular cells also co-expressed keratin 19 (K19) (Supplemental Figure S1), suggesting a greatly accelerated acinar-ductal conversion for Mist1Kras/LacZ acinar cells. Despite the dramatic exocrine pancreas phenotype, insulin-positive islets appeared normal in Mist1Kras/LacZ animals (Supplemental Figure S2).
Figure 1. Mist1Kras/LacZ pancreas structure is grossly distorted at postnatal day 1.
(A) Mist1LacZ/+ and Mist1Kras/LacZ littermates in the first three days following birth. (B) Gross anatomy of the Mist1LacZ/+, Mist1Kras/+, and Mist1Kras/LacZ mouse pancreata at PN1. (C) (a-c) H&E sections from PN1 Mist1LacZ/+, Mist1Kras/+, and Mist1Kras/LacZ pancreata (x200). Arrows in (a) indicate islets. (d-f) Transmission electron microscopy of PN1 pancreas samples. The tubular networks that form in the Mist1Kras/LacZ samples exhibit greatly reduced zymogen granules (arrows) and crowding of nuclei (x1,100). (g-i) Immunohistochemistry labeling with Ki67 reveals a higher proliferation index in the Mist1Kras/LacZ mice at PN1 (x400). Note that most Ki67 positive cells in (g) are interstitial cells that lie between acini (inset).
The highly disrupted organization of Mist1Kras/LacZ pancreata prompted us to investigate if alterations in cellular proliferation were associated with constitutive Kras signaling in the absence of Mist1. Analysis of newborn Mist1+/+ or Mist1LacZ/+ pancreata revealed a substantial number of Ki67 positive cells, but the vast majority were either duct cells or stellate cells that were located within the interstitial spaces surrounding acinar structures (Figure 1C, g). Very few (6%) proliferating acinar cells were detected at this age. In contrast, 35% of Mist1Kras/+ acinar cells were Ki67 positive (Figure 1C, h). Interestingly, Mist1Kras/LacZ pancreata exhibited an even greater increase in cell proliferation where >80% of the cells expressed Ki67 (Figure 1C, i). TUNEL assays failed to reveal any significant apoptotic activity (data not shown), suggesting that the extensive proliferation of Mist1Kras/LacZ cells likely accounts for the dramatic expansion of the duct-like lesions in this model.
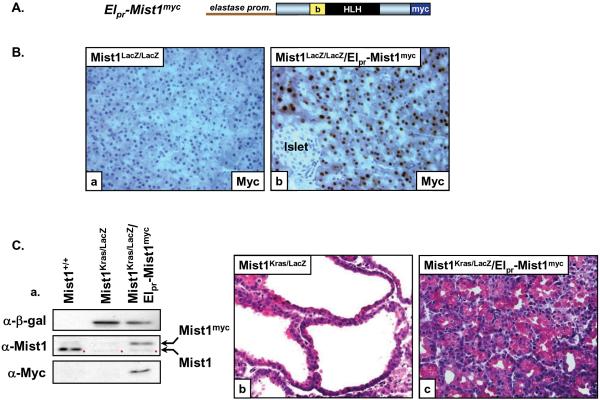
Finally, to determine if ectopic Mist1 expression from a different acinar-specific promoter could rescue Mist1Kras/LacZ mice, we crossed Mist1Kras/+ mice to Mist1LacZ/LacZ mice containing an elastase promoter-Mist1myc transgene (Elpr-Mist1myc) (Figure 2A). As predicted, Mist1myc protein was detected only in acinar cells in Mist1LacZ/LacZ/Elpr-Mist1myc samples (Figure 2B, a-b). Islet and duct cells remained Mist1myc negative. Similarly, Mist1Kras/LacZ pancreata were Mist1 negative whereas the Elpr-Mist1myc transgene was expressed in Mist1Kras/LacZ/Elpr-Mist1myc acinar cells (Figure 2C, a). Unlike the severe phenotype of Mist1Kras/LacZ pancreata, Mist1Kras/LacZ/Elpr-Mist1myc pancreata displayed a phenotype that was virtually identical to Mist1Kras/+ mice (Figure 2C, b-c). Mist1Kras/LacZ/Elpr-Mist1myc mice survived for months and acinar organization was largely restored, although areas of acinar metaplasia were similarly observed as in Mist1Kras/+ animals. As predicted, the number of Ki67-positive cells also was significantly reduced (~40%) in Mist1Kras/LacZ/Elpr-Mist1myc mice. These results confirm that the absence of Mist1 promotes KrasG12D-induced cell proliferation and dramatically enhances pancreas transformation.
Figure 2. Ectopic expression of Mist1myc rescues the Mist1Kras/LacZ phenotype.
(A) Elpr-Mist1myc transgene. b, basic domain; HLH, helix-loop-helix domain; myc, epitope tag. (B) Immunohistochemistry using anti-myc on Mist1LacZ/LacZ and Mist1LacZ/LacZ/Elpr-Mist1myc pancreas sections showing acinar-specific expression of the Elpr-Mist1myc transgene (x400). (C) (a) Immunoblot analysis of extracts from Mist1+/+, Mist1Kras/LacZ, and Mist1Kras/LacZ/Elpr-Mist1myc pancreata. β-gal protein is expressed from the Mist1 locus in the Mist1Kras/LacZ and Mist1Kras/LacZ/Elpr-Mist1myc pancreata. The Mist1myc protein is detected only in the Mist1Kras/LacZ/Elpr-Mist1myc samples. Red dots - nonspecific bands from the Mist1 antibody. (b, c) Elpr-Mist1myc expression rescues the severe Mist1Kras/LacZ phenotype (x400).
Loss of Mist1 accelerates mPanIN initiation in LSL-KrasG12D/+/ptf1aCre/+ mice
Mist1Kras/+ mice undergo acinar-ductal metaplasia and develop invasive and metastatic pancreatic cancer 13, but in the absence of Mist1 protein the mice die shortly after birth, preventing a detailed analysis of early precursor lesions. To examine the importance of Mist1 to mPanIN initiation events, we turned to the Cre-activated LoxP-STOP-LoxP(LSL)-KrasG12D/+ model which produces mPanINs when pancreas-restricted Cre expression is provided by pdx1pr-Cre or ptf1aCre/+ mouse lines 16, 20. Mist1LacZ/LacZ/LSL-KrasG12D/+/ptf1aCre/+ mice were generated by crossing LSL-KrasG12D/+/ptf1aCre/+ mice to Mist1LacZ/LacZ mice. At 6 weeks of age, control LSL-KrasG12D/+/ptf1a+/+ mice (lacking Cre) exhibited average sized pancreata with normal histological features (Figure 3A). As previously reported 16, Mist1+/+/LSL-KrasG12D/+/ptf1aCre/+ mice developed slightly enlarged pancreata that otherwise had normal gross structure. In contrast, Mist1LacZ/LacZ/LSL-KrasG12D/+/ptf1aCre/+ pancreata were significantly larger than pancreata from control littermates, paralleling the increased size phenotype of Mist1Kras/LacZ mice (Figure 3A).
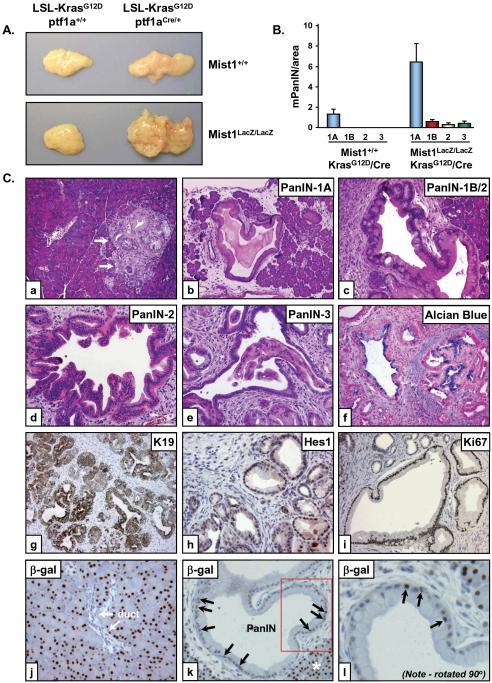
Figure 3. Mist1LacZ/LacZ/LSL-KrasG12D/+/ptf1aCre/+ pancreata display accelerated histological progression of mPanIN lesions at early stages.
(A) Gross anatomy of the Mist1LacZ/LacZ/LSL-KrasG12D/+/ptf1aCre/+ versus Mist1+/+/LSL-KrasG12D/+/ptf1aCre/+ pancreata at 6 weeks. (B) Quantitative analysis of mPanIN lesions in 6 week Mist1+/+/LSL-KrasG12D/+/ptf1aCre/+ and Mist1LacZ/LacZ/LSL-KrasG12D/+/ptf1aCre/+ samples (n=4). Note that high-grade mPanIN-1B, mPanIN-2 and mPanIN-3 are never observed in the Mist1+/+/LSL-KrasG12D/+/ptf1aCre/+ mice at this age. (C) High-grade mPanIN lesions rapidly develop in Mist1LacZ/LacZ/LSL-KrasG12D/+/ptf1aCre/+ pancreata. (a) 6-week Mist1+/+/LSL-KrasG12D/+/ptf1aCre/+ pancreas showing a rare focus of mPanIN-1A (arrows) (x100). (b-l) Representative images from Mist1LacZ/LacZ/LSL-KrasG12D/+/ptf1aCre/+ samples (6 week). mPanIN-1A lesions are broadly distributed (b, x100) with early appearance of higher grade mPanIN-1B (c, x200), mPanIN-2 (d, x200) and mPanIN-3 (e, x200) lesions. (f) Alcian blue staining reveals abundant mucin content in the mPanIN lesions. K19 (g), Hes1 (h) and Ki67 (i) are highly expressed in the Mist1LacZ/LacZ/LSL-KrasG12D/+/ptf1aCre/+ mPanIN lesions. (j) β-gal immunohistochemistry on Mist1LacZ/LacZ/LSL-KrasG12D/+ control samples revealing nuclear β-gal only in acinar cells. (k,l) β-gal positive nuclei are found within large PanINs from Mist1LacZ/LacZ/LSL-KrasG12D/+/ptf1aCre/+ samples (arrows). Boxed area in (k) is shown at higher magnification in (l). Asterisk in (k) denotes normal acinar tissue.
We next compared mPanIN initiation and progression between LSL-KrasG12D/+/ptf1aCre/+ mice maintained on a Mist1+/+ or Mist1LacZ/LacZ genetic background. Animals of both genotypes showed rare mPanIN-1A lesions surrounded by normal acinar structures at 3 weeks of age (data not shown). However, mPanIN lesions developed much more rapidly in Mist1LacZ/LacZ/LSL-KrasG12D/+/ptf1aCre/+ mice where mPanIN-1A lesions were commonly seen at 4 weeks and by 6 weeks mPanIN-1A distribution was extensive throughout the entire pancreas (Figure 3B,C, b-e). Contrary to these results, mPanIN formation in 6 week Mist1+/+/LSL-KrasG12D/+/ptf1aCre/+ animals remained rare and focally localized (Figure 3C, a). Significantly, high grade mPanIN-2 and mPanIN-3 lesions were also much more abundant in Mist1LacZ/LacZ/LSL-KrasG12D/+/ptf1aCre/+ mice (Figure 3B,C, c-e). As predicted, Mist1LacZ/LacZ/LSL-KrasG12D/+/ptf1aCre/+ mPanINs displayed features common to human PanINs, including elevated levels of mucin, expression of the duct-restricted marker K19, and activation of Notch downstream signaling targets such as Hes1 (Figure 3C, f-h). Cells within Mist1LacZ/LacZ/LSL-KrasG12D/+/ptf1aCre/+ mPanINs were also highly proliferative (Figure 3C, i). Interestingly, real-time RT-PCR revealed a dramatic change from acinar gene products (amylase) to duct gene products (K19) in 6 week Mist1LacZ/LacZ/LSL-KrasG12D/+/ptf1aCre/+ pancreata (Supplemental Figure S3), suggesting a switch from predominantly acinar to predominantly ductal cell types in young mice expressing KrasG12D but lacking Mist1. Whether this reflects a conversion of acinar cells to ductal cells is not known, but acinar cells clearly contribute to mPanIN formation in this model since β-gal positive cells (expressed from the Mist1 locus) were readily observed in mPanINs of Mist1LacZ/LacZ/LSL-KrasG12D/+/ptf1aCre/+ pancreata (Figure 3C, j-l) 20.
LSL-KrasG12D/+/Mist1Cre-ER/Cre-ER mice reveal that Mist1 null acinar cells readily generate advanced mPanINs upon KrasG12D expression
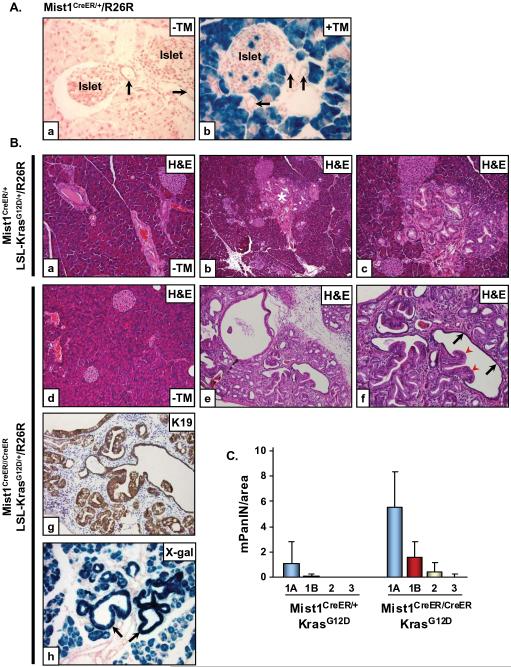
The presence of β-gal+ cells in mPanINs from Mist1LacZ/+/LSL-KrasG12D/+/ptf1aCre/+ and Mist1LacZ/LacZ/LSL-KrasG12D/+/ptf1aCre/+ mice suggested that KrasG12D expressing acinar cells are the major cellular source of mPanINs in this model. In order to examine this in greater detail, we generated Mist1Cre-ER/+ mice in which the complete Mist1 coding region was replaced with a Cre-ERT2 coding region. Using the R26R reporter line, tamoxifen-induced Cre activity was observed primarily in Mist1Cre-ER/+ acinar cells although a small percentage (<3%) of islet cells also exhibited β-gal activity (Figure 4A, a-b). Importantly, all cells from small and large ducts remained β-gal negative, confirming that the Mist1 locus is not expressed in this pancreas compartment. Administering tamoxifen to 6 week LSL-KrasG12D/+/Mist1Cre-ER/+ mice lead to mPanIN-1A formation that mimicked the pattern observed with LSL-KrasG12D/+/ptf1aCre/+ mice (Figure 4B, a-c). Similarly, tamoxifen induction in adult LSL-KrasG12D/+/Mist1Cre-ER/Cre-ER mice (lacking Mist1) also readily generated mPanINs, but in this instance the number and grade of mPanINs was greatly accelerated, paralleling the results obtained with Mist1LacZ/LacZ/LSL-KrasG12D/+/ptf1aCre/+ mice. mPanIN-2 and mPanIN-3 lesions with atypical nuclei and large papillary extensions were often observed fusing to normal ductal epithelial (Figure 4B, d-f, 4C). As predicted, the mPanINs from LSL-KrasG12D/+/Mist1Cre-ER/Cre-ER mice exhibited duct cell characteristics, including expression of K19 (Figure 4B, g). R26R lineage tracing of LSL-KrasG12D/+/Mist1Cre-ER/Cre-ER mice confirmed the acinar cell origin of the advanced mPanINs in which virtually all expressed β-gal whereas duct cells remained β-gal negative (Figure 4B, h). Based on these results, we conclude that the absence of Mist1 promotes KrasG12D-expressing acinar cells to form ductal mPanINs.
Figure 4. LSL-KrasG12D/+/Mist1Cre-ER/+ mice reveal that acinar cells generate mPanIN lesions.
(A) Mist1Cre-ER/+/R26R adult mice were given a single dose of corn oil (a) or tamoxifen (TM) (b) for three consecutive days and then pancreata were harvested and processed for β-gal detection 2 weeks later. Acinar cells and rare islet cells are β-gal+ whereas all duct cells (arrows) remain β-gal negative (x400). (B) Adult 6 week LSL-KrasG12D/+/Mist1Cre-ER/+/R26R (a-c) and LSL-KrasG12D/+/Mist1Cre-ER/Cre-ER/R26R (d-f) mice were administered corn oil (a,d, x200) or tamoxifen (b,c, x100; e,f, x200) and pancreata were analyzed at 3 months of age. LSL-KrasG12D/+/Mist1Cre-ER/Cre-ER/R26R pancreata develop advanced mPanINs which express high levels of K19 (g, x200). (h) β-gal+ mPanINs (arrows) confirm the acinar cell origin of these lesions (x200). Arrows in (f) indicate mPanIN extensions (red) and fusion with normal ductal epithelial (black). (C) Quantitative analysis of mPanIN lesions in 6 week LSL-KrasG12D/+/Mist1Cre-ER/+ and LSL-KrasG12D/+/Mist1Cre-ER/Cre-ER mice treated with tamoxifen (n=4). Note the large increase in all mPanIN grades in the LSL-KrasG12D/+/Mist1Cre-ER/Cre-ER (Mist1KO) mice.
EGFR signaling is hyperactive in KrasG12D expressing cells lacking Mist1
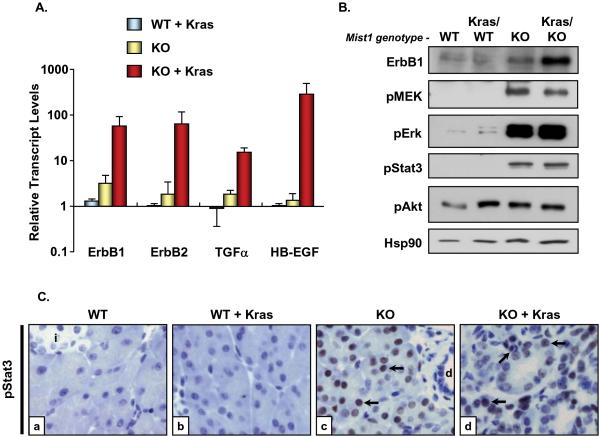
Several studies have shown that EGFR signaling can induce acinar cells to transition to duct-like cells in a number of pancreatic cancer mouse models 20, 21, 29, 30. The increased propensity by which loss of Mist1 and Kras signaling generates mPanIN lesions also suggests that Mist1LacZ/LacZ cells are primed to influence mPanIN formation, possibly through activation of EGFR downstream signaling pathways. To evaluate if EGFR signaling components are differentially active in Mist1LacZ/LacZ and LSL-KrasG12D/+/ptf1aCre/+ animals, 6 week pancreata from different mouse genotypes were isolated and processed for real-time RT-PCR, immunoblot, and immunohistochemistry analyses. As shown in Figure 5A, transcript levels for the EGFR family members ErbB1 and ErbB2, as well as the target ligands TGFα and HB-EGF, were not significantly different between control Mist1+/+/LSL-KrasG12D/+ (WT) and Mist1+/+/LSL-KrasG12D/+/ptf1aCre/+ (WT + Kras) animals. In contrast, moderate (2-3-fold) increases in ErbB1 and TGFα transcript levels were always observed in Mist1LacZ/LacZ/LSL-KrasG12D/+ (KO) animals (Figure 5A). However, when the Mist1LacZ/LacZ genotype was combined with active KrasG12D (KO + Kras) large (30-500-fold) increases in ErbB1, ErbB2, TGFα, and HB-EGF transcripts were produced (Figure 5A).
Figure 5. Mist1LacZ/LacZ and Mist1LacZ/LacZ/LSL-KrasG12D/+/ptf1aCre/+ pancreata exhibit elevated levels of EGFR signaling components and activation of downstream signaling pathways.
(A) Quantitative RT-PCR to detect ErbB1, ErbB2, TGFα, and HB-EGF transcript levels from the indicated pancreata samples. All values were normalized to Mist1+/+ (WT) samples which were set to 1.0. (B) Immunoblots for a number of EGFR signaling components. In all cases, Mist1LacZ/LacZ (KO) and Mist1LacZ/LacZ/LSL-KrasG12D/+/ptf1aCre/+ (KO + Kras) samples exhibited activation of the downstream pathways, with the exception of pAKT, which did not significantly change with different genotypes. Hsp90 was used as a loading control. (C) Immunohistochemistry with anti-pStat3 reveals that acinar cells in the Mist1KO background are pStat3 positive (arrows). Duct (d) and islet (i) cells remain pStat3 negative, as do acinar cells in a Mist1WT background (x800).
Immunoblot analyses on additional animals confirmed that the levels of ErbB1 were increased in the Mist1LacZ/LacZ/LSL-KrasG12D/+/ptf1aCre/+ pancreata (Figure 5B). As predicted, components of the MAPK pathway were also activated, including elevated levels of phospho-MEK1/2 and phospho-Erk1/2 (Figure 5B). Interestingly, these pathways were similarly active in control Mist1LacZ/LacZ/LSL-KrasG12D/+ samples lacking Mist1 and KrasG12D protein (KO). Stat3, another downstream effector of the ErbB pathway 31, was also activated in the Mist1LacZ/LacZ samples. Indeed, immunohistochemistry confirmed that the increase in pStat3 levels was exclusively due to expression in acinar cells (Figure 5C). These results reveal that loss of Mist1 triggers MAPK and Stat3 signaling in acinar cells and suggest that the extreme phenotype associated with Mist1LacZ/LacZ/LSL-KrasG12D/+/ptf1aCre/+ and LSL-KrasG12D/+/Mist1Cre-ER/Cre-ER animals is due to a convergence of signaling components involving KrasG12D expression and hyperactivity of the EGFR pathway in the absence of Mist1.
Mist1LacZ/LacZ acinar cells rapidly convert to duct-like cells in 3D cultures
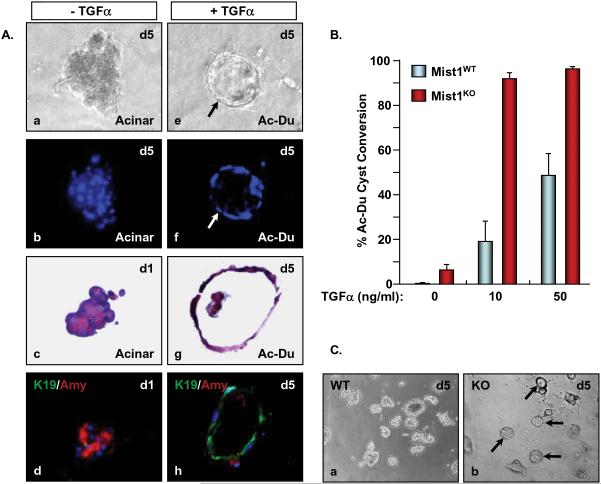
The large increase in mPanINs in very young Mist1LacZ/LacZ/LSL-KrasG12D/+/ptf1aCre/+ and LSL-KrasG12D/+/Mist1Cre-ER/Cre-ER animals suggested that Mist1KO pancreata are highly sensitive to KrasG12D signaling events and that the response to KrasG12D expression may induce acinar cells to develop duct-like properties, including expression of K19, formation of tubular complexes, and participation in mPanIN formation. Indeed, Mist1LacZ/LacZ pancreata exhibit hyperactive EGFR signaling, suggesting that this pathway may accelerate conversion of acinar cells to mPanINs. To determine if Mist1LacZ/LacZ acinar cells were “primed” to attain duct cell properties, we turned to a 3D in vitro culture system to evaluate how acinar cells from Mist1+/+ and Mist1LacZ/LacZ animals responded to EGFR signaling pathways. As previously reported 28, acinar cells from control Mist1+/+ mice placed in 3D collagen gels remained as acinar cells over a 5 day period (Figure 6A, a-d; 6B,C). However, when the cultures were supplemented with 10 ng/ml TGFα, a small percentage (~20%) of cells acquired duct-like properties, forming ductal cysts and expressing the duct cell product K19 (Figure 6A, e-h; 6B). Increasing TGFα to 50 ng/ml led to a maximum 45% cell conversion. In contrast, Mist1LacZ/LacZ cells were much more efficient in ductal conversion. The majority (>90%) of Mist1LacZ/LacZ acinar cells readily formed ductal cysts when placed in collagen gels containing as low as 10 ng/ml TGFα (Figure 6B,C). Indeed, even in the absence of TGFα, small (~5%) but reproducible numbers of Mist1LacZ/LacZ acinar cells spontaneously converted to ductal cysts (Figure 6B). Thus, the absence of Mist1 primes cells for ductal conversion, mimicking the accelerated mPanIN formation in Mist1LacZ/LacZ/LSL-KrasG12D/+ and LSL-KrasG12D/+/Mist1Cre-ER/Cre-ER mice.
Figure 6. Mist1LacZ/LacZ acinar cells rapidly convert to ductal cysts in 3D collagen cultures.
(A) Acinar cells were isolated from Mist1+/+ pancreata and cultured in collagen gels in the presence or absence of TGFα. Cells in control medium without TGFα (a-d, x800) maintain a normal amylase positive acinar cell phenotype while cells supplied TGFα (e-h, x800) convert to K19 positive ductal cysts (arrow) (b,f - Dapi fluorescence; c.g - H&E sections; d,h - K19 and amylase co-immunofluorescence). (B) Mist1LacZ/LacZ acinar cells exhibit a propensity to convert to ductal cysts, even in the absence of TGFα. (C) Nearly all Mist1LacZ/LacZ acinar cells convert to ductal cysts after 5 days in 10 ng/ml TGFα whereas Mist1+/+ cells remain as acinar cell clusters (x200).
Discussion
Identifying the earliest events that initiate PanIN formation is critical to fully understanding the origins of pancreatic cancer. Defining PanIN development has been difficult from a clinical perspective because patients often present with advanced disease. Thus, despite some progress in disease management, there remains uncertainty about which cell types contribute to PanIN development and which intracellular pathways are critical to PanIN progression.
To examine if alterations to the acinar cell transcriptional network could affect KrasG12D-induced early events, we evaluated the importance of Mist1, an acinar-restricted bHLH transcription factor, to mPanIN formation. Our studies demonstrated that mice lacking Mist1 were highly sensitive to KrasG12D expression, exhibited early acinar-ductal metaplasia (Mist1Kras/+ model), and showed greatly accelerated mPanIN initiation and progression (LSL-KrasG12D/+ model). Since Mist1 is not expressed in duct cells, these results reveal that altering the acinar Mist1 transcriptional network has a profound effect on the development of mPanIN lesions.
At this time, it is unclear why loss of Mist1 generates KrasG12D sensitivity. Mist1LacZ/LacZ pancreata exhibit alterations in acinar cell organization where cells acquire defects in apical-basal polarity 25, intercellular communication 32, and regulated exocytosis 33. Mist1LacZ/LacZ mice also are susceptible to caerulein-induced pancreatitis 34, suggesting that dysplasia of Mist1LacZ/LacZ acinar cells may provide a sufficient epigenetic environment where KrasG12D expression efficiently initiates mPanIN development. Indeed, patients with chronic pancreatitis are more susceptible to developing PDA 35 and studies from Guerra et al. 17 have confirmed that PanIN formation is greatly enhanced in mice with chronic pancreatitis. Nonetheless, loss of Mist1 is an intrinsic event restricted to acinar cells - centroacinar cells and duct cells do not express Mist1 20. Thus, either dysplastic acinar cells influence the local environment to allow duct cells to develop into proliferative mPanINs or acinar cells directly participate in mPanIN formation. Although both scenarios are possible, we favor the latter for a number of reasons. First, Mist1Kras/+ mice express KrasG12D exclusively from the Mist1 locus and yet develop rare mPanIN lesions and several different pancreatic tumor types with ductal features 13. Second, LSL-KrasG12D/+/ptf1aCre/+ mice develop acinar metaplastic units that contain biphenotypic cells, suggesting that acinar cells contribute to mPanINs in this model 20. Indeed, Mist1 (or β-gal) positive cells are readily identified in early mPanIN lesions in LSL-KrasG12D/+/ptf1aCre/+ samples. Finally, lineage tracing of LSL-KrasG12D/+/Mist1Cre-ER/+ mice revealed that KrasG12D expressing acinar cells directly give rise to mPanIN lesions.
The transcriptional network through which Mist1 operates has yet to be defined, although several Mist1 target genes have been identified, including the gap junction gene connexin32 32 and the cyclin-dependent kinase inhibitor gene p21CIP1/WAF1 36. Loss of both proteins is known to lead to cellular hyperplasia and it is possible that upon KrasG12D expression the absence of these key regulators in Mist1LacZ/LacZ mice accelerates acinar cells to acquire a duct-like phenotype towards mPanIN formation. The possibility also exists that loss of Mist1 during Kras activation is not simply a consequence of acinar metaplasia, but rather is a defined molecular pathway that is intimately associated with KrasG12D-induced pancreas transformation. Indeed, preliminary data supports a model where the human Mist1 locus is silenced as acinar cells undergo acinar-ductal metaplasia and PanIN formation in diseased tissues (Supplemental Figure S4) 20.
Despite a deficiency in understanding the transcriptional networks involved in tumor promotion, the signaling pathways through which activated Kras induces PDA have been well characterized, with many studies showing that the Notch and EGFR pathways are instrumental in complementing Kras signaling 21, 37. The EGFR pathway also has been implicated in acinar-ductal metaplasia 19, 28-30, suggesting that events controlling conversion of acinar cells to duct-like mPanINs share overlapping regulatory pathways with tumor promotion. In this current study, we showed that Mist1LacZ/LacZ acinar cells precociously activate the EGFR signaling pathway and are primed to convert to ductal cysts in vitro. Preliminary studies also have revealed that the Notch signaling pathway is active in Mist1LacZ/LacZ pancreata where Hes1 expressing acinar cells are readily identified (Supplemental Figure S5). This is a significant finding since induction of Notch signaling is thought to be an early event in PanIN initiation and pancreatic tumorigenesis 21, 38. Despite precocious activation of the EGFR (pMEK1/2, pErk1/2, pStat3) and Notch (Hes1) signaling pathways in Mist1LacZ/LacZ acinar cells, activation of these pathways alone is not sufficient to generate PanIN lesions. KrasG12D activity is still required. Nonetheless, acinar cells deficient in Mist1 are highly susceptible to KrasG12D-induced events and show a greatly increased, accelerated rate of mPanIN formation. These results suggest that the EGFR and Notch pathways likely cooperate with a distinct KrasG12D downstream pathway (e.g., PI3 Kinase, RalGDS, p120-GAP) to increase the efficiency by which individual acinar cells generate PanIN lesions. Whether identical pathways converge in the human disease will have to await a more thorough analysis of PanIN progression in patients. Continued studies of the molecular pathways and transcriptional networks that operate in both normal and neoplastic pancreatic cells will be critical to delineating the individual components that lead to the generation of this deadly disease.
Supplementary Material
Acknowledgments
Sincere thanks go to Chris Wright for generously providing ptf1aCre/+ mice, David Tuveson for the LSL-KrasG12D/+ mice and helpful discussions, Ray MacDonald for the Elastase promoter constructs, Pierre Chambon for the Cre-ERT2 cDNA, and Tetsuo Sudo for the anti-Hes1 antibody. We also thank Anna Means for assistance with the 3D acinar cell cultures, Judy Hallett and the Purdue Cancer Center TMCF for generating the mouse lines used in this study, and Michael Collingwood for assistance in generating the Mist1Cre-ER targeting construct.
Grant Support: This work was supported by grants to S.F.K. from the National Institutes of Health (DK55489, CA124586), the Phi Beta Psi Sorority Cancer Fund, and from the Lustgarten Foundation for Pancreatic Cancer. R.H.H. was supported by a NCI Specialized Program of Research Excellence grant (P50CA62924).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflicts of interest exist
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Schneider G, Siveke JT, Eckel F, Schmid RM. Pancreatic cancer: basic and clinical aspects. Gastroenterology. 2005;128:1606–25. doi: 10.1053/j.gastro.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Brugge WR, Lauwers GY, Sahani D, Fernandez-del Castillo C, Warshaw AL. Cystic neoplasms of the pancreas. N Engl J Med. 2004;351:1218–26. doi: 10.1056/NEJMra031623. [DOI] [PubMed] [Google Scholar]
- 4.Maitra A, Fukushima N, Takaori K, Hruban RH. Precursors to invasive pancreatic cancer. Adv Anat Pathol. 2005;12:81–91. doi: 10.1097/01.pap.0000155055.14238.25. [DOI] [PubMed] [Google Scholar]
- 5.Maitra A, Kern SE, Hruban RH. Molecular pathogenesis of pancreatic cancer. Best Pract Res Clin Gastroenterol. 2006;20:211–26. doi: 10.1016/j.bpg.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Hruban RH, Goggins M, Parsons J, Kern SE. Progression model for pancreatic cancer. Clin Cancer Res. 2000;6:2969–72. [PubMed] [Google Scholar]
- 7.Hruban RH, Adsay NV, Albores-Saavedra J, Compton C, Garrett ES, Goodman SN, Kern SE, Klimstra DS, Kloppel G, Longnecker DS, Luttges J, Offerhaus GJ. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001;25:579–86. doi: 10.1097/00000478-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Hruban RH, Adsay NV, Albores-Saavedra J, Anver MR, Biankin AV, Boivin GP, Furth EE, Furukawa T, Klein A, Klimstra DS, Kloppel G, Lauwers GY, Longnecker DS, Luttges J, Maitra A, Offerhaus GJ, Perez-Gallego L, Redston M, Tuveson DA. Pathology of genetically engineered mouse models of pancreatic exocrine cancer: consensus report and recommendations. Cancer Res. 2006;66:95–106. doi: 10.1158/0008-5472.CAN-05-2168. [DOI] [PubMed] [Google Scholar]
- 9.Kojima K, Vickers SM, Adsay NV, Jhala NC, Kim HG, Schoeb TR, Grizzle WE, Klug CA. Inactivation of Smad4 accelerates Kras(G12D)-mediated pancreatic neoplasia. Cancer Res. 2007;67:8121–30. doi: 10.1158/0008-5472.CAN-06-4167. [DOI] [PubMed] [Google Scholar]
- 10.Prasad NB, Biankin AV, Fukushima N, Maitra A, Dhara S, Elkahloun AG, Hruban RH, Goggins M, Leach SD. Gene expression profiles in pancreatic intraepithelial neoplasia reflect the effects of Hedgehog signaling on pancreatic ductal epithelial cells. Cancer Res. 2005;65:1619–26. doi: 10.1158/0008-5472.CAN-04-1413. [DOI] [PubMed] [Google Scholar]
- 11.Brembeck FH, Schreiber FS, Deramaudt TB, Craig L, Rhoades B, Swain G, Grippo P, Stoffers DA, Silberg DG, Rustgi AK. The mutant K-ras oncogene causes pancreatic periductal lymphocytic infiltration and gastric mucous neck cell hyperplasia in transgenic mice. Cancer Res. 2003;63:2005–9. [PubMed] [Google Scholar]
- 12.Grippo PJ, Nowlin PS, Demeure MJ, Longnecker DS, Sandgren EP. Preinvasive pancreatic neoplasia of ductal phenotype induced by acinar cell targeting of mutant Kras in transgenic mice. Cancer Res. 2003;63:2016–9. [PubMed] [Google Scholar]
- 13.Tuveson DA, Zhu L, Gopinathan A, Willis NA, Kachatrian L, Grochow R, Pin CL, Mitin NY, Taparowsky EJ, Gimotty PA, Hruban RH, Jacks T, Konieczny SF. Mist1-KrasG12D Knock-In Mice Develop Mixed Differentiation Metastatic Exocrine Pancreatic Carcinoma and Hepatocellular Carcinoma. Cancer Res. 2006;66:242–7. doi: 10.1158/0008-5472.CAN-05-2305. [DOI] [PubMed] [Google Scholar]
- 14.Carriere C, Seeley ES, Goetze T, Longnecker DS, Korc M. The Nestin progenitor lineage is the compartment of origin for pancreatic intraepithelial neoplasia. Proc Natl Acad Sci U S A. 2007;104:4437–42. doi: 10.1073/pnas.0701117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R, Jacks T, Tuveson DA. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15:3243–8. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, Kawaguchi Y, Johann D, Liotta LA, Crawford HC, Putt ME, Jacks T, Wright CV, Hruban RH, Lowy AM, Tuveson DA. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–50. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 17.Guerra C, Schuhmacher AJ, Canamero M, Grippo PJ, Verdaguer L, Perez-Gallego L, Dubus P, Sandgren EP, Barbacid M. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 18.Guerra C, Mijimolle N, Dhawahir A, Dubus P, Barradas M, Serrano M, Campuzano V, Barbacid M. Tumor induction by an endogenous K-ras oncogene is highly dependent on cellular context. Cancer Cell. 2003;4:111–20. doi: 10.1016/s1535-6108(03)00191-0. [DOI] [PubMed] [Google Scholar]
- 19.Strobel O, Dor Y, Alsina J, Stirman A, Lauwers G, Trainor A, Castillo CF, Warshaw AL, Thayer SP. In vivo lineage tracing defines the role of acinar-to-ductal transdifferentiation in inflammatory ductal metaplasia. Gastroenterology. 2007;133:1999–2009. doi: 10.1053/j.gastro.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu L, Shi G, Schmidt CM, Hruban RH, Konieczny SF. Acinar cells contribute to the molecular heterogeneity of pancreatic intraepithelial neoplasia. Am J Pathol. 2007;171:263–73. doi: 10.2353/ajpath.2007.061176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyamoto Y, Maitra A, Ghosh B, Zechner U, Argani P, Iacobuzio-Donahue CA, Sriuranpong V, Iso T, Meszoely IM, Wolfe MS, Hruban RH, Ball DW, Schmid RM, Leach SD. Notch mediates TGF alpha-induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell. 2003;3:565–76. doi: 10.1016/s1535-6108(03)00140-5. [DOI] [PubMed] [Google Scholar]
- 22.Pin CL, Bonvissuto AC, Konieczny SF. Mist1 expression is a common link among serous exocrine cells exhibiting regulated exocytosis. Anat Rec. 2000;259:157–67. doi: 10.1002/(SICI)1097-0185(20000601)259:2<157::AID-AR6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Johnson CL, Kowalik AS, Rajakumar N, Pin CL. Mist1 is necessary for the establishment of granule organization in serous exocrine cells of the gastrointestinal tract. Mech Dev. 2004;121:261–72. doi: 10.1016/j.mod.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–83. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 25.Pin CL, Rukstalis JM, Johnson C, Konieczny SF. The bHLH transcription factor Mist1 is required to maintain exocrine pancreas cell organization and acinar cell identity. J Cell Biol. 2001;155:519–30. doi: 10.1083/jcb.200105060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu L, Tran T, Rukstalis JM, Sun P, Damsz B, Konieczny SF. Inhibition of Mist1 homodimer formation induces pancreatic acinar-to-ductal metaplasia. Mol Cell Biol. 2004;24:2673–81. doi: 10.1128/MCB.24.7.2673-2681.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feil R, Wagner J, Metzger D, Chambon P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun. 1997;237:752–7. doi: 10.1006/bbrc.1997.7124. [DOI] [PubMed] [Google Scholar]
- 28.Means AL, Meszoely IM, Suzuki K, Miyamoto Y, Rustgi AK, Coffey RJ, Jr, Wright CV, Stoffers DA, Leach SD. Pancreatic epithelial plasticity mediated by acinar cell transdifferentiation and generation of nestin-positive intermediates. Development. 2005 doi: 10.1242/dev.01925. [DOI] [PubMed] [Google Scholar]
- 29.Sandgren EP, Luetteke NC, Palmiter RD, Brinster RL, Lee DC. Overexpression of TGF alpha in transgenic mice: induction of epithelial hyperplasia, pancreatic metaplasia, and carcinoma of the breast. Cell. 1990;61:1121–35. doi: 10.1016/0092-8674(90)90075-p. [DOI] [PubMed] [Google Scholar]
- 30.Wagner M, Greten FR, Weber CK, Koschnick S, Mattfeldt T, Deppert W, Kern H, Adler G, Schmid RM. A murine tumor progression model for pancreatic cancer recapitulating the genetic alterations of the human disease. Genes Dev. 2001;15:286–93. doi: 10.1101/gad.184701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siveke JT, Einwachter H, Sipos B, Lubeseder-Martellato C, Kloppel G, Schmid RM. Concomitant Pancreatic Activation of Kras(G12D) and Tgfa Results in Cystic Papillary Neoplasms Reminiscent of Human IPMN. Cancer Cell. 2007;12:266–79. doi: 10.1016/j.ccr.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Rukstalis JM, Kowalik A, Zhu L, Lidington D, Pin CL, Konieczny SF. Exocrine specific expression of Connexin32 is dependent on the basic helix-loop-helix transcription factor Mist1. J Cell Sci. 2003;116:3315–25. doi: 10.1242/jcs.00631. [DOI] [PubMed] [Google Scholar]
- 33.Luo X, Shin DM, Wang X, Konieczny SF, Muallem S. Aberrant localization of intracellular organelles, Ca2+ signaling, and exocytosis in Mist1 null mice. J Biol Chem. 2005;280:12668–75. doi: 10.1074/jbc.M411973200. [DOI] [PubMed] [Google Scholar]
- 34.Kowalik AS, Johnson CL, Chadi SA, Weston JY, Fazio EN, Pin CL. Mice lacking the transcription factor Mist1 exhibit an altered stress response and increased sensitivity to caerulein-induced pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1123–32. doi: 10.1152/ajpgi.00512.2006. [DOI] [PubMed] [Google Scholar]
- 35.Malka D, Hammel P, Maire F, Rufat P, Madeira I, Pessione F, Levy P, Ruszniewski P. Risk of pancreatic adenocarcinoma in chronic pancreatitis. Gut. 2002;51:849–52. doi: 10.1136/gut.51.6.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jia D, Sun Y, Konieczny SF. Mist1 regulates pancreatic acinar cell proliferation through p21 CIP1/WAF1. Gastroenterology. 2008;135:1687–97. doi: 10.1053/j.gastro.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maitra A, Hruban RH. Pancreatic cancer. Annu Rev Pathol. 2008;3:157–88. doi: 10.1146/annurev.pathmechdis.3.121806.154305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De La OJ-P, Emerson LL, Goodman JL, Froebe SC, Illum BE, Curtis AB, Murtaugh LC. Notch and Kras reprogram pancreatic acinar cells to ductal intraepithelial neoplasia. Proc Natl Acad Sci U S A. 2008;105:18907–912. doi: 10.1073/pnas.0810111105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.