Abstract
Rhodococcal cocaine esterase (CocE) is an attractive potential treatment for both cocaine overdose and cocaine addiction. CocE directly degrades cocaine into inactive products, whereas traditional small-molecule approaches require blockade of the inhibitory action of cocaine on a diverse array of monoamine transporters and ion channels. The usefulness of wild-type (wt) cocaine esterase is hampered by its inactivation at 37°C. Herein, we characterize the most thermostable form of this enzyme to date, CocE-L169K/G173Q. In vitro kinetic analyses reveal that CocE-L169K/G173Q displays a half-life of 2.9 days at 37°C, which represents a 340-fold improvement over wt and is 15-fold greater than previously reported mutants. Crystallographic analyses of CocE-L169K/G173Q, determined at 1.6-Å resolution, suggest that stabilization involves enhanced domain-domain interactions involving van der Waals interactions and hydrogen bonding. In vivo rodent studies reveal that intravenous pretreatment with CocE-L169K/G173Q in mice provides protection from cocaine-induced lethality for longer time periods before cocaine administration than wt CocE. Furthermore, intravenous administration (pretreatment) of CocE-L169K/G173Q prevents self-administration of cocaine in a time-dependent manner. Termination of the in vivo effects of CoCE seems to be dependent on, but not proportional to, its clearance from plasma as its half-life is approximately 2.3 h and similar to that of wt CocE (2.2 h). Taken together these data suggest that CocE-L169K/G173Q possesses many of the properties of a biological therapeutic for treating cocaine abuse but requires additional development to improve its serum half-life.
Cocaine abuse affects over 1.7 million Americans (Substance Abuse and Mental Health Services Administration, 2007). Long-term use causes multiple health problems including heart arrhythmias, high blood pressure, liver necrosis, mental illness, and death (Benowitz, 1993). The economic cost of health care and lost wages attributable to cocaine use accounts for a large portion of the $180.7 billion spent on drug abuse treatment in the United States (Office of National Drug Control Policy, 2004). Despite these tragic personal and financial costs, there is no FDA-approved pharmacotherapy for cocaine abuse.
Cocaine blocks monoamine transporters (Johanson and Fischman, 1989; Benowitz, 1993; Uhl et al., 2002) accounting for the drug's psychotropic effects. The enhanced adrenergic stimulation and direct blockade of sodium channel also leads to profound cardiac disturbances (Billman, 1990; Crumb and Clarkson, 1990). Inhibitors that block cocaine's action at dopamine and serotonin transporters (Carroll et al., 2006; Daniels et al., 2006; Rothman et al., 2007; Negus et al., 2009) cannot eliminate the cardiovascular effects of cocaine or enhance in vivo degradation.
An alternate pharmacological approach is to enhance cocaine metabolism. The endogenous enzyme butyrylcholinesterase (BChE) cleaves cocaine to the physiologically inert products ecgonine methyl ester and benzoic acid (Vmax = 3.9 min−1). Large doses of BChE can protect animals from cocaine-induced lethality (Hoffman et al., 1996; Gorelick, 1997; Lynch et al., 1997; Mattes et al., 1997; Browne et al., 1998). Albumin-fused engineered mutants of BChE displaying enhanced kinetics against cocaine (Vmax = 2700 min−1) (Xie et al., 1999; Duysen et al., 2002; Gao and Brimijoin, 2004; Pan et al., 2005; Zheng et al., 2008) increase the plasma half-life of BChE (Brimijoin et al., 2008; Gao et al., 2008) and block both the lethal effects of cocaine and reinstatement of cocaine-seeking behavior in a rat cocaine self-administration model (Brimijoin et al., 2008). Commercial production of this enzyme, however, may be challenging because of its low expression levels in mammalian expression systems and complex post-translational processing.
Our approach to treating cocaine abuse and toxicity has been the use of a bacterial cocaine esterase (CocE). Isolated from the MB1 strain of Rhodococcus sp. found in the Rhizosphere soil surrounding the coca plant, CocE has a high Vmax toward cocaine (Vmax = 2300 min−1) (Gao et al., 2009) and produces the same products as BchE (Bresler et al., 2000). CocE has previously been shown to block cocaine-induced cardiac disturbance, neurological changes, and lethality in rodents when administered before or after cocaine (Cooper et al., 2006; Jutkiewicz et al., 2009; Ko et al., 2007; Wood et al., 2010).
The wild-type (wt) CocE cannot be used as a pharmacotherapy for cocaine abuse because of its 13.7-min half-life at 37°C (Cooper et al., 2006; Ko et al., 2007; Gao et al., 2009). We have previously identified a mutant of CocE (T172R and G173Q) that extends the half-life of CocE to ∼4.5 h, as assessed by in vitro kinetic assays (at 37°C) or in vivo by protection against cocaine-induced lethality in mice (Gao et al., 2009). These mutations are thought to improve stability by burying additional surface area and/or through the formation of additional hydrogen bonds between domains I and II of CocE (Gao et al., 2009; D. Narasimhan and R. K. Sunahara, unpublished observations).
The increased stability of CocE-T172R/G173Q allowed the enzyme to block the reinforcing effects of cocaine during 1-h cocaine self-administration sessions after pretreatment with CocE-T172R/G173Q (Collins et al., 2009). This behavioral effect of CocE was specific to cocaine-reinforced behavior and did not block responding for food or WIN-35065-2, a cocaine analog not hydrolyzed by CocE. These data support the hypothesis that CocE hydrolyzes cocaine rapidly enough to prevent its reinforcing effects. Although CocE-T172R/G173Q is capable of blocking the reinforcing effects of cocaine after an immediate pretreatment and can protect against cocaine toxicity for up to 4.5 h, its actions are still too short in duration to make the enzyme a feasible candidate to treat cocaine abuse (although this duration would be sufficient to cocaine toxicity indication).
Given that rapid cocaine hydrolysis is sufficient to block the reinforcing properties of cocaine (Collins et al., 2009) and the reinstatement of cocaine-reinforced responding (Brimijoin et al., 2008), we set out to derive a mutant of CocE that would be more appropriate for cocaine abuse therapy. Here we describe CocE-L169K/G173Q, the most thermostable CocE variant characterized to date (t1/2 at 37°C = 2.9 days). CocE-L169K/G173Q was characterized in both in vitro stability assays and in vivo duration of action studies. We also provide structural evidence as to why this enzyme is the most thermostable variant to date. Initial pharmacokinetic data from this long-acting mutant compared with wt CocE is also presented.
Materials and Methods
Site-Directed Mutagenesis.
Point mutations were introduced into the CocE sequence present in the bacterial expression vector pET-22b (+) using a modified QuikChange (Stratagene, La Jolla, CA) mutagenesis protocol and confirmed by sequencing in both directions over the entire coding region. Wt and CocE mutants were expressed and purified as described previously (Gao et al., 2009; D. Narasimhan and R. K. Sunahara, unpublished observations).
Spectrophotometric Cocaine Assay.
Cocaine hydrolysis was measured spectrophotometrically using a protocol adapted from (Turner et al., 2002). Varying concentrations of cocaine (0.5, 2.5, 5, 12.5, 25, 50, 100, and 150 μM) in phosphate-buffered saline (50 mM Tris, pH 8.0, and 150 mM NaCl) were added to a UV-permeable 96-well plate (100 μl). CocE was added to the 96-well plate to give a final concentration of 10 ng/ml and a final volume of 200 μl. Cocaine hydrolysis was followed by the change in absorbance at 240 nm at 10-s time points for 20 min using a SpectraMax Plus 384 UV plate reader (Molecular Devices, Sunnyvale, CA) using SOFTmax Pro software (Version 3.1.2). The Vmax and Km of the enzyme were determined using Prism (GraphPad Software, San Diego, CA).
In Vitro Measurements of Thermostability.
Mutants were incubated at 37°C in human plasma (obtained from the University of Michigan Hospital blood bank) at a concentration of 60 μg/ml. All samples were prepared immediately before incubation for the indicated times, and cocaine hydrolyzing activity was assayed as described above.
Crystallization and Structure Determination.
CocE L169K/G173Q was purified as described previously (Gao et al., 2009). CocE crystals were grown by hanging drop vapor diffusion in VDX plates on siliconized glass coverslips (Hampton Research, Aliso Viejo, CA). One microliter of CocE at 5 mg/ml was combined with 1 μl of well solution (1.7 M ammonium sulfate, 10 mM Tris-HCl, pH 7.3, and 25 mM NaCl) and incubated at 293 K over 1 ml of well solution. Crystals reached their maximum size within 2 days and were harvested within 1 week of tray setup. Five microliter of cryo-protectant (1.5 M ammonium sulfate, 5 mM Tris-HCl, pH 7.3, 10 mM HEPES, pH 7.5, 2 mM MgCl2, 1 mM EDTA, 825 mM NaCl, and 25% glycerol) were added to the drop during harvest to prevent ammonium sulfate crystal formation. CocE crystals were transferred to a 20-μl drop of cryoprotectant for 1 to 5 min then flash-frozen in liquid nitrogen. X-ray diffraction data were collected at the Advanced Photon Source, Argonne National Laboratory (Argonne, IL) with the use of LS-CAT beamline 21-ID-D calibrated for 1-s exposures at a wavelength of 1.02 Å. Data were integrated and scaled using HKL2000 (Otwinowski and Minor, 1997) and refined against the structure of wt CocE (Larsen et al., 2002). Modeling was performed by alternating rounds of refinement using refmac5 (Collaborative Computational Project Number 4, 1994) and manual density fitting using Coot (Emsley and Cowtan, 2004). Coordinates were validated by MolProbity (Lovell et al., 2003) and deposited into the Protein Data Bank under identification code 3ida.
Intravenous Enzyme Administration.
Mice were placed in small restraint chambers (outer tube diameter, 30 mm; inner tube diameter, 24 mm; Harvard Apparatus Inc., Holliston, MA) that left the tail exposed. The tail was then placed under a heat lamp for 7 s to increase blood flow and bring the tail vein to the surface. The tail was wiped with an alcohol pad and a 30.5 gauge precision glide needle was inserted into one of the lateral veins of the tail. After injection of 0.2 ml, the needle was removed and the bleeding was stanched using sterile gauze and pressure on the injection site.
Behavioral Toxicity.
Cocaine-induced toxicity was evaluated by observing the occurrence of convulsions and/or lethality in male NIH Swiss mice (25–30 g; Harlan Inc., Indianapolis, IN). Mice were housed in groups of nine mice per cage (16 × 28 × 20 cm) and allowed ad libitum access to food and water. Animals were maintained on a 12-h light/dark cycle with lights on at 7:00 AM. Experiments were performed according to guidelines established by the National Institutes of Health in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996). Experimental protocols were approved by the University Committee on the Use and Care of Animals at the University of Michigan.
Mice were given intravenous tail vein injections of CocE (1, 0.3, 0.1, 0.032, and 0.01 mg) or saline 1 min before an intraperitoneal challenge dose of cocaine (100, 180, 320, 560, or 1000 mg/kg). The mice were observed for behavior in a Plexiglas container (16 × 28 × 20 cm) for 45 min or until death. Assessment of the in vivo duration of action used the above protocol except that CocE (1 mg) was given at longer times before treatment to a challenge dose of 180 mg/kg cocaine. Convulsions were defined as loss of righting for more than 5 s. Lethality was defined as a loss of respiration and heart beat.
Serum Collection.
Blood was collected via cheek pouch blood sampling with the use of a mouse bleeding lancet (GoldenRod 4.0-mm animal lancet; MEDIpoint Inc., Mineola, NY). Blood was collected in tubes (Microtainer; BD Biosciences, San Jose, CA) and placed on ice. Bleeding was stopped using a sterile gauze pad applied with direct pressure. The animal was then returned to its home cage and allowed to recover. Samples were spun at 4000 rpm for 5 min to separate the serum fraction. Each mouse gave approximately 50 μl of serum.
Western Blot Analysis.
Plasma samples (20 μg of total plasma protein) were resolved by SDS-polyacrylamide gel electrophoresis on 10% polyacrylamide gels. Protein was transferred to a nitrocellulose membrane and probed with a rabbit anti-CocE polyclonal primary antibody (a kind gift from Dr. Donald Landry at Columbia University) and horseradish peroxidase-linked anti-rabbit secondary antibody (Bio-Rad Laboratories, Hercules, CA). The membranes were stripped and re-probed with rabbit anti-mouse apolipoprotein antibody (Affinity BioReagents, Golden, CO) and the same secondary antibody for loading control. Western blots for CocE using these conditions can detect CocE to approximately 10 ng. Analysis of band densities from scanned films was performed with ImageJ software (http://rsbweb.nih.gov/ij/). Area-under-the-curve analysis was done to determine densities of both CocE and ApoA1 bands. To normalize CocE densities, the fraction of the mean ApoA1 density was calculated for each ApoA1 band and divided from each CocE density.
Cocaine Self-Administration.
Male Sprague-Dawley rats (300–350 g; Harlan Inc., Indianapolis, IN) were housed in groups of three animals per cage with ad libitum access to food and water on a 12-h light/dark cycle (lights on at 7:00 AM). After catheter implantation, rats were housed individually. All experimental protocols were approved by University Committee on the Use and Care of Animals at the University of Michigan.
Rats were implanted with an indwelling femoral vein catheter under ketamine (90 mg/kg) and xylazine (10 mg/kg) anesthesia. The catheter was run under the skin and fixed to a metal plate sewn into the muscle of the back under the skin. This plate could then be attached to an intravenous catheter line present in the self-administration box. After a 5-day recovery period after surgery, rats were initiated into the self-administration procedure. Training and testing occurred at the same time each day at the middle of the light cycle. Rats were placed for 1-h sessions into a standard operant chamber equipped with a “nose-poke” apparatus, in which entry into a hole was recorded with a photo-beam break and counted as one response. Rats were required to respond with a nose poke for an injection of 0.56 mg/kg cocaine (0.1 ml/kg injection volume) on a fixed ratio (FR) 1 schedule (one nose poke delivers one cocaine injection). Once responding stabilized in this phase of training, rats were gradually moved up to an FR 5 and then switched to a dose of 0.1 mg/kg/injection cocaine to maintain the most reliable behavior throughout the session. Once animals responded consistently (≤20% variation in responding and no upward or downward trend) for 3 days, they were given either 1 mg of CocE-L169K/G173Q or saline at the indicated times before the session. After the test day, rats continued the training schedule until responding recovered to baseline. Animals were also tested by substituting saline for cocaine. Immediate CocE pretreatment and saline substitution conditions were compared with cocaine-reinforced responding behavior maintained by 0.1 mg/kg/injection by one-way ANOVA with Bonferroni post-tests. CocE pretreatments at 1 and 2 h before the session were compared with baseline responding by Student's t test.
Drugs.
Cocaine hydrochloride was obtained from the National Institute on Drug Abuse (Bethesda, MD).
Results
Thermostability of Mutants.
Previous studies showed that CocE-L169K alone has a 33-fold longer half-life at 37°C than wt CocE (measured by time to 50% loss of Vmax) (D. Narasimhan and R. K. Sunahara, unpublished observations). However, CocE-L169K had an increased Km that decreased the catalytic efficiency of the enzyme 12-fold. To compensate for this deficiency, L169K was paired with single mutations to test whether the elevated Km could be corrected or the half-life could be extended in a synergistic manner. Mutants were assessed for initial activity using the spectrophotometric cocaine hydrolysis assay and (if initial kinetics were better than or comparable with wt CocE) were subjected to incubation at 37°C for 24 h to test for thermostability (Table 1). The L169K mutant retained 55% of its catalytic efficiency after a 24-h incubation period at 37°C. The combination of L169K and T172R [t1/2 at 37°C CocE-T172R = 46.8 min (D. Narasimhan and R. K. Sunahara, unpublished observations)] produced an enzyme with a lower Vmax and a higher Km than wt (5.6-fold loss in catalytic efficiency). Combination of L169K and N197K (predicted to create novel contacts with the dimer partner of CocE) had kinetics similar to that of wt CocE but retained no measurable activity after 24 h. The addition of other point mutations that we predicted to either increase the stabilization of domain 2 by creating additional buried surface area between domains 1 and 2 (N42V), or create a new hydrogen bond between the subunits of the CocE dimer (A193D), failed to increase thermostability, increase Vmax, or further reduce the Km.
TABLE 1.
Cocaine hydrolysis by CocE variants Vmax and Km, were measured spectrophotometrically immediately after thaw from −80°C and after 24 h incubation at 37°C
Catalytic efficiency was calculated from the Vmax and Km values. CocE variants that lost all activity, or had negligible activity, are reported here as having no Michaelis-Menten kinetics remaining.
| CocE Variant | Vmax | Km | Catalytic Efficiency | 24 h, 37°C |
Catalytic Efficiency Remaining at 24 h | ||
|---|---|---|---|---|---|---|---|
| Vmax | Km | Catalytic Efficiency | |||||
| min−1 | μM | min−1 · μM−1 | min−1 | μM | min−1 · μM−1 | % | |
| Wild-type | 2510 | 7.2 | 348.6 | 270 | 7.3 | 37.0 | 10.8 |
| L169Ka | 3100a | 105.0a | 29.5a | 950b | 59.8b | 15.9 | 55 |
| L169K/T172R | 1510 | 24.2 | 62.4 | N.D. | N.D. | ||
| L169K/N197K | 3120 | 58.3 | 53.5 | N.M. | N.M. | ||
| L169K/G173Q | 6670 | 30.3 | 220.1 | 3890 | 22.9 | 170.0 | 77.5 |
| L169K/G173Q/N42V | 3180 | 56.4 | 56.4 | N.M. | N.M. | ||
| L169K/G173Q/A193D | 2350 | 21.4 | 110.0 | 340 | 30.6 | 11.1 | 10.0 |
N.D., not determined; N.M., no Michaelis-Menten kinetics remaining at 24 h.
D. Narasimhan, M. R. Nance, D. Gao, M. C. Ko, J. Macdonald, P. Tamburi, D. Yoon, D. M. Landry, J. H. Woods, C. G. Zhan, et al., submitted.
D. Narasimhan, unpublished observations.
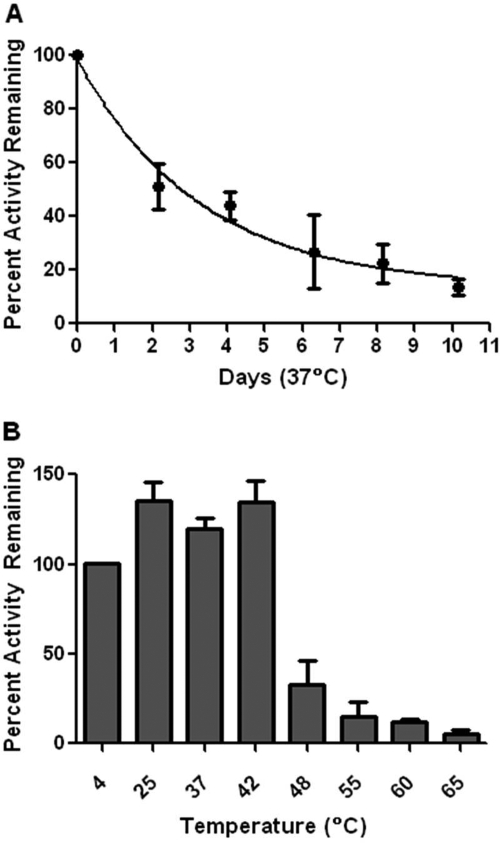
However, combining L169K and G173Q conferred a prolonged half-life at 37°C, and retained nearly 75% activity after 24 h. Although the Km was 4-fold elevated above that of the wt Km (30 compared with 7 μM), it was half that of CocE-L169K. It is noteworthy that CocE-L169K/G173Q exhibited a higher Vmax than any CocE variant characterized to date. The higher Vmax of CocE-L169K/G173Q compensates for its slightly increased Km, such that CocE-L169K/G173Q had a catalytic efficiency of 219.7 min−1 · μM−1, similar to that of wt (349.7 min−1 · μM−1), which represented a 7-fold improvement over CocE-L169K alone (29.6 min−1 · μM−1). The half-life of CocE-L169K/G173Q at 37°C (Fig. 1a) was an impressive 340-fold longer than wt, corresponding to 2.9 days. After a 10-day incubation at 37°C, CocE-L169K/G173Q retained approximately 15% activity.
Fig. 1.
In vitro stability of CocE-L169K/G173Q. A, 10-day time course of CocE activity at 37°C. CocE was incubated at 60 μg/ml for the time indicated. Activity was assessed using the spectrophotometric cocaine hydrolysis assay. Vmax and kcat were determined and the catalytic efficiency was plotted as a percentage of the catalytic efficiency of nonincubated enzyme. A single-phase exponential decay model shows that 50% activity is retained until approximately 2.9 days. B, heat inactivation of CocE-L169K/G173Q. CocE was incubated at 60 μg/ml at the temperatures indicated for 10 min. Incubations were stopped on ice, and Vmax was determined by the spectrophotometric assay. Vmax activity was plotted as a percentage of the unincubated CocE Vmax kept at 4°C. CocE-L169K/G173Q retains over 50% activity up to 42°C. At higher temperatures, the activity rapidly drops, but residual activity is observed up to 65°C.
Temperature of inactivation experiments were conducted by incubating CocE-L169K/G173Q for 10 min at a variety of temperatures (Fig. 1b). The Vmax of CocE-L169K/G173Q decreased as the temperature of incubation increased above 42°C, and the temperature of inactivation was between 42 and 48°C. This is a significant improvement over wt, which has a temperature of inactivation of 37° (D. Narasimhan and R. K. Sunahara, unpublished observations), and closer to that of BchE, which inactivates between 54 and 57°C (Edwards and Brimijoin, 1983).
Crystal Structure of CocE-L169K/G173Q.
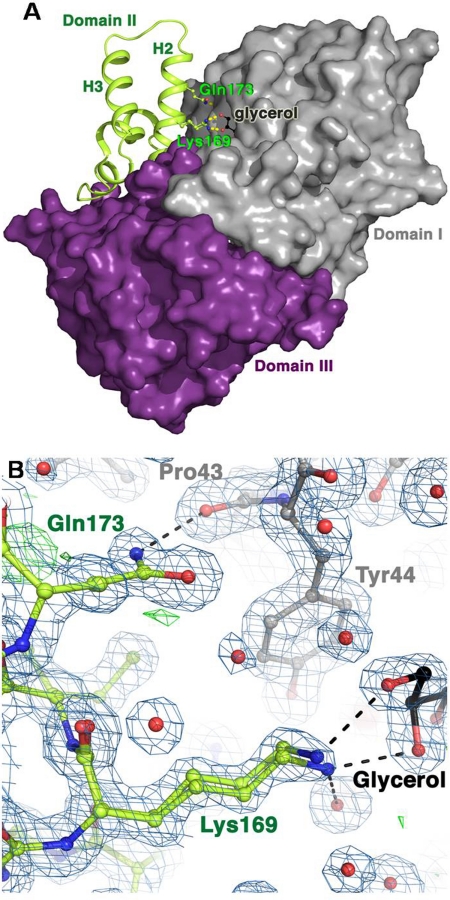
CocE consists of three domains. Domain I has an α/β hydrolase fold and contains the catalytic residues, Domain II consists primarily of a pair of large helices, and domain III has a jelly-roll–like topology. All three domains contribute to the active site. The CocE-L169K/G173Q structure was determined to a resolution of 1.6 Å (Table 2) and can be superimposed onto the four previously determined structures of wt CocE with an RMSD of <0.58 Å for all Cα atoms (Protein Data Bank identification code: 1ju3, 1ju4, 1l7r, 1l7q). The crystal packing of CocE-L169K/G173Q is the same as in previous structures and is consistent with the concept that CocE exists as a homodimer.
TABLE 2.
Crystallographic data and refinement statistics
| X-ray source | APS LS-CAT 21-ID-D |
| Wavelength (Å) | 1.02 |
| Resolution (Å) | 25.0–1.60 |
| Space group | P6522 |
| Cell constants (Å, °) | a = b = 108.3 |
| c = 227.2 | |
| α = β= 90.0° | |
| γ = 120.0° | |
| Unique reflections | 104,076 |
| Average redundancy | 9.5 (8.3) |
| Rsym (%)a | 9.4 (61.3) |
| Completeness (%) | 99.1 (98.3) |
| <I>/<σI> | 22.4 (3.1) |
| Refinement resolution (Å) | 24.6–1.60 |
| Total reflections used | 97,839 |
| Protein atoms | 4,799 |
| Non-protein atoms | 761 |
| RMSD bond lengths (Å) | 0.012 |
| RMSD bond angles (°) | 1.19 |
| Estimated coordinate error (Å) | 0.08 |
| Ramachandran plot statistics | |
| Most favored, disallowed (%) | 96.7, 0.2 |
| Rworkb | 17.7 (22.5) |
| Rfreec | 19.4 (25.5) |
Rsym = ΣhklΣi |I(hkl)i − I(hkl)|/ Σhkl I(hkl)i, where I(hkl) is the mean intensity of i reflections after rejections.
Rwork = Shkl‖Fobs(hkl)| − |Fcalc(hkl)‖/Shkl|Fobs(hkl)|; no I/S cutoff was used during refinement.
Five percent of the truncated data set was excluded from refinement.
The L169K and G173Q mutations are both located in helix 2 of domain II. The mutated side chains are easily distinguished in the electron density maps (Fig. 2b), and each creates new interactions between domains I and II. The Gln173 creates a hydrogen bond to the backbone oxygen of Pro43 in domain I. The Lys169 reaches over a portion of the entrance to the active site to create van der Waals interactions with Tyr44 of domain I. The ζ-nitrogen of Lys169, unlike a leucine side chain, is able to favorably interact with solvent, and in the structure stabilizes a glycerol molecule that was used as a cryoprotectant. It is noteworthy that the loop between helices 2 and 3 of domain II, which exhibits multiple conformations in other CocE structures determined, adopts a single conformation in the CocE-L169K/G173Q structure, suggesting greater stability in this region of the protein. As reported previously, dithiothreitol and CO2 present during the protein purification was observed to form a tetrahedral 1,4-dithio-2,3-butylene carbonate adduct with Ser117 in the active site (D. Narasimhan and R. K. Sunahara, unpublished observations).
Fig. 2.
Crystal structure of CocE-L169K/G173Q. A, domain structure of CocE. Domain I (gray) contains the catalytic residues and domain II (green) is helical, with two pronounced antiparallel helices (H2 and H3). Domain III is shown in purple. The active site is formed at their intersection. Domains I and III are shown as their solvent-excluded surfaces. The L169K and G173Q mutations (ball-and-stick side chains) are found in H2 of domain II, close to the entrance to the active site, partially occupied here by a molecule of glycerol. B, refined model of CocE-L169K/G173Q. 2|Fo| − |Fc| electron density is shown as a blue wire cage contoured at the 1 σ level, with |Fo| − |Fc| density show in green contoured at 3 σ and red at −3 σ. Lys169 is observed in two distinct conformations that interact with a bound molecule of glycerol from the harvesting solution. Carbon atoms are shown as their respective domain colors (see A), oxygens are red, and nitrogens blue.
Duration of Action against Cocaine-Induced Lethality.
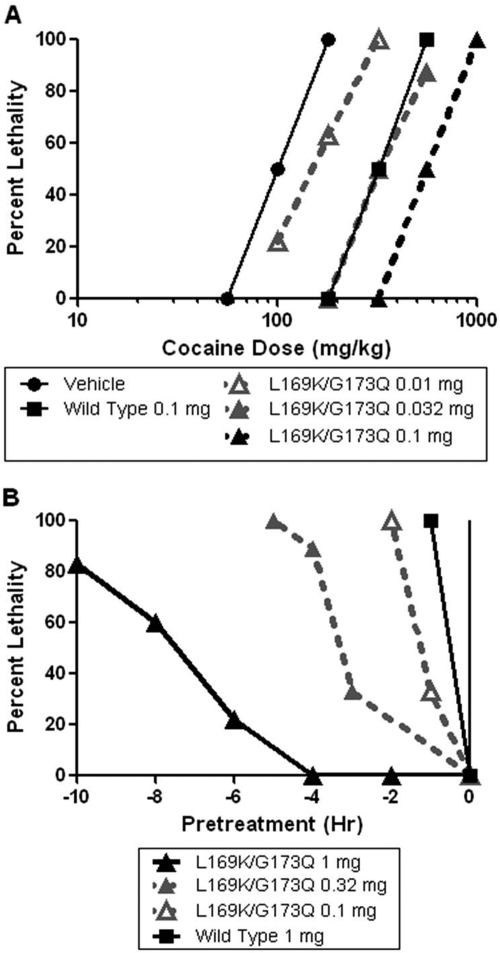
Cocaine administration at a dose of 180 mg/kg into the intraperitoneal cavity of NIH Swiss mice produced lethality in 100% of animals. Pretreatment with CocE-L169K/G173Q produced dose-dependent, rightward shifts in the cocaine dose-response curve (Fig. 3a). CocE-L169K/G173Q exhibited a subtle degree of protection even with a very low dose of 0.01 mg, and increased the LD100 of cocaine to 1000 mg/kg at a dose of 0.1 mg of CocE-L169K/G173Q, 0.25 log unit lower than what is needed for the same effect with wt CocE (0.32 mg). The time-to-death after cocaine injection was also increased by CocE-L169K/G173Q. With saline pretreatment, animals died within 2 to 3 min after administration of 180 mg/kg cocaine. However, with a pretreatment of 0.032 mg of CocE-L169K/G173Q, it took a dose of 560 mg/kg cocaine to produce near 100% lethality and produced a slight increase in the time to death of some animals (animals died within 2–7 min after 560 mg/kg cocaine; data not shown).
Fig. 3.
In vivo potency and duration of action of CocE-L169K/G173Q. A, potency of CocE-L169K/G173Q against cocaine induced lethality. CocE-L169K/G173Q was administered to NIH Swiss mice intravenously 1 min before a cocaine challenge given intraperitoneally. Increasing doses of CocE-L169K/G173Q cause significant rightward shifts in the cocaine dose-response curve. B, CocE-L169K/G173Q pretreatment protects against cocaine lethality more than wt CocE. CocE is administered intravenously at time of pretreatment. A lethal dose of 180 mg/kg is used as a challenge dose at time 0. The percentage of mice experiencing lethality after a 1-h postcocaine period is plotted.
CocE-L169K/G173Q pretreatments of longer duration were able to protect animals from cocaine-induced lethality (Fig. 3b). We have previously demonstrated that wt CocE is rendered ineffective at preventing lethality from a challenge dose of cocaine after a 30-min pretreatment (Ko et al., 2007). CocE-T172R/G173Q (1 mg) protected 50% of animals from a LD100 dose (180 mg/kg) of cocaine up to 4 h after CocE administration (Gao et al., 2009). CocE-L169K/G173Q (1 mg), given 7 to 8 h before administration of 180 mg/kg cocaine, protected 50% of the animals, representing a nearly 16-fold improvement over the wt enzyme.
In Vivo Protection against the Reinforcing Effects of Cocaine.
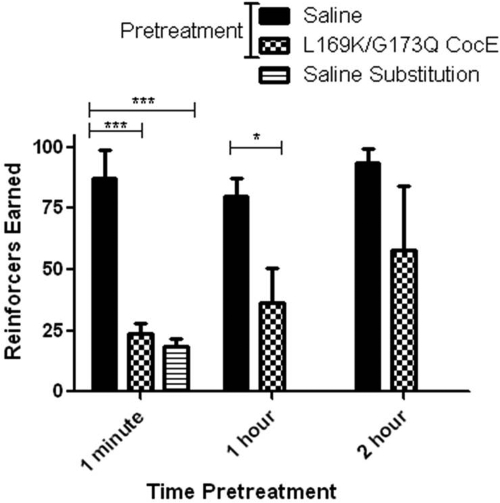
Sprague-Dawley rats were trained to respond for cocaine (0.1 mg/kg injection) on a fixed ratio (FR) 5 schedule. After saline pretreatments, animals responded for 75 to 100 injections of 0.1 mg/kg cocaine per 1-h session. When saline was substituted as the reinforcer, total responses were reduced and clustered at the beginning of the sessions (data not shown). Immediate pretreatment with CocE-L169K/G173Q produced a reduction in the number of reinforcers earned to a level not significantly different from saline-reinforced responding (Fig. 4). Both saline substitution and immediate pretreatment with CocE-L169K/G173Q produced only approximately 25% of the responding of baseline sessions, similar to the behavior seen in the initial session of responding extinction. One-hour pretreatment times also resulted in a reduction in responding, the degree of which was smaller than that with the immediate pretreatment and not statistically significant (three of five animals reduced responding to under 50% of control levels). Two-hour pretreatments with CocE-L169K/G173Q resulted in a cumulative average of responses that was not significantly different from saline pretreatment.
Fig. 4.
CocE-L169K/G173Q protection against cocaine-reinforced operant responding in Sprague-Dawley rats. CocE-L169K/G173Q (1 mg) was given as a pretreatment to cocaine self-administration sessions at the times indicated. Rats in the saline substitution condition received no cocaine from nose-pokes during the session. ***, one-way ANOVA F(2,12) = 27.4; Bonferroni post test, p < 0.001. *, Student's t test p < 0.05.
In Vivo Serum Half-Life.
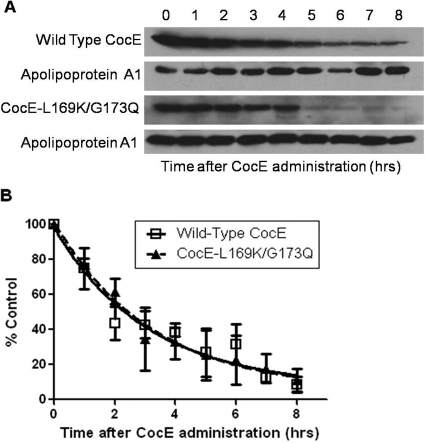
The presence of wt and CocE-L169K/G173Q remaining in the serum of NIH Swiss mice was analyzed using Western blot analysis on serum samples taken at increasing times after CocE administration (Fig. 5, a and b). wt and CocE-L169K/G173Q seem to be eliminated from serum after a similar time course. The elimination time course yields a serum half-life of 2.3 h for CocE-L169K/G173Q and 2.2 h for wild-type CocE (one-phase decay model, r2 = 0.8 for both enzymes tested). Although the in vitro half-lives of these enzymes at 37°C is 340-fold different, the in vivo serum half-life is virtually identical and follows the same pattern of elimination.
Fig. 5.
In vivo CocE plasma half-life. A, representative Western blots of CocE from mouse serum over time. CocE (L169K/G173Q or wt) was administered to mice intravenously via the lateral tail vein. Blood samples were taken at the times indicated by submandibular sampling. Serum was collected and 20 μg of total serum protein was run on a 10% SDS polyacrylamide gel. Blotting was performed with rabbit anti-CocE antibody and rabbit anti-Apolipoprotein A1 antibody. Wild-type and CocE-L169K/G173Q were both tested in three independent groups of animals followed by serum analysis. B, quantification of wt and CocE-L169K/G173Q Western blot densities analyzed with Image J software. All time points are adjusted as a fraction of the apoA1 loading control. Fit to a one-phase exponential decay model, the half-life of CocE-L169K/G173Q was determined to be 2.3 h after administration (wt = 2.2 h). two-way ANOVA, p = 0.92.
Discussion
This study demonstrates the superior stability of CocE-L169K/G173Q in vitro and its extended duration of action in vivo, and it provides the first description of CocE pharmacokinetics in rodents. wt CocE prevents the short-term toxic effects of cocaine (Cooper et al., 2006; Ko et al., 2007; Jutkiewicz et al., 2009; Wood et al., 2010), but its instability at 37°C and its short duration of action in vivo limit its therapeutic potential. However, CocE-L169K/G173Q seems to be the first attractive CocE candidate for treatment of cocaine abuse (should its half-time in serum be extended) because of its long duration of action and ability to block the reinforcing properties of cocaine.
CocE-L169K/G173Q displays a significantly higher Vmax than the wt or T172R/G173Q mutant (Gao et al., 2009). The increase in catalytic turnover is accompanied by a slight increase in the Km of the enzyme. Crystallographic evidence suggests that the elevation in Km is most likely to be due to the extended side chain of Lys169, which may partially occlude access to the active site pocket. This increase in Km, however, does not affect the enzyme's function at the very high cocaine concentrations that cause toxicity in vivo. Our data also suggest that this defect does not seem to hinder the efficacy of CocE-L169K/G173Q at rapidly clearing low concentrations of cocaine as seen in rats in self-administration models. The increased Km does not hinder the ability of CocE-L169K/G173Q to hydrolyze cocaine at a faster rate than CocE-T172R/G173Q, even at the very low cocaine concentrations seen in these studies (0.1 mg/kg injection or approximately 0.49 μM when dispersed in total body water of the rat), due to the much higher Vmax of CocE-L169K/G173Q (predicted by Michaelis-Menten rate equation).
The L169K/G173Q mutations dramatically increased the thermostability of the enzyme from 37°C for wt to 48 to 55°C for CocE-L169K/G173Q. Butyrylcholinesterase has been reported to have a temperature of inactivation of 54 to 57°C (Brimijoin et al., 2008). Furthermore, these two mutations slow aggregation of CocE, as seen by size exclusion chromatography (data not shown). After incubation at 37°C, wt CocE is 100% aggregated within 1 h (D. Narasimhan and R. K. Sunahara, unpublished observations), whereas CocE-L169K/G173Q is only 30 to 40% aggregated after 24 h.
Analysis of the crystal structure of CocE-L169K/G173Q reveals a complex molecular mechanism for its improved thermal stability. Lys169 and Gln173 provide additional anchor points between domain I and the conformationally flexible region of domain II. The newly introduced hydrogen bond between Gln173 (domain II) and Pro43 (domain I), the novel van der Waals interaction between Lys169 (domain II) and Tyr44 (domain I), and more favorable interactions with solvent by the Lys169 side chain are also likely to contribute to the thermostability of CocE-L169K/G173Q.
In vivo studies in mice confirm that CocE-L169K/G173Q displays an increased potency and duration of action over wt. The higher potency of CocE-L169K/G173Q is most likely to be due to the combination of its higher Vmax and increased stability at 37°C, allowing it to quickly degrade high concentrations of cocaine and remain active over a longer period of time. It is reasonable to expect that at concentrations of cocaine resembling a human overdose situation, cocaine would be hydrolyzed more rapidly by CocE-L169K/G173Q than by wt CocE, although less of a difference in turnover rate would be seen at lower cocaine concentration because of the elevated Km of CocE-L169K/G173Q.
The rat self-administration study presented here, which examines the effects of CocE-L169K/G173Q, expands on the work previously conducted by Collins et al. (2009). They demonstrated that CocE-T172R/G173Q did not suppress operant responding reinforced by food or WIN-35065-2 (a nonhydrolyzable cocaine analog) at doses that suppressed cocaine-reinforced responding. These results demonstrate that CocE specifically eliminates the reinforcing properties of cocaine and does not simply suppress operant responding. The Collins study also highlighted a dose-response relationship between CocE-T172R/G173Q and cocaine-reinforced responding. No change in responding was observed with the lowest dose of 0.032 mg of CocE-T172R/G173Q given to rats responding for 0.1 mg/kg injection of cocaine. A dose of 0.1 mg of CocE-T172R/G173Q increased the rate of responding for 0.1 mg/kg injection (as would be expected to surmount a low dose of an “antagonist”). At 0.32 and 1.0 mg, the higher doses of CocE-T172R/G173Q, responding for cocaine decreased by half and to saline levels, respectively. The time course data presented here on CocE-L169K/G173Q (Fig. 5) demonstrate that after a 2-h pretreatment, enough circulating CocE remains to reduce operant responding slightly. It is likely that with longer pretreatment times, operant responding for cocaine would increase because of very low levels of CocE remaining in the serum.
The duration of action of CocE-L169K/G173Q in vitro greatly surpasses wt and the previously reported thermostable mutant CocE-T172R/G173Q. However, its in vivo duration of action (7–8 h) is significantly shorter than the in vitro half-life at 37°C (2.9 days). In measures of protection against cocaine-induced lethality, wt, CocE-L169K, and CocE-T172R/G173Q all lose their protective effects over a time course similar to their respective in vitro half-lives at 37°C (Gao et al., 2009; D. Narasimhan and R. K. Sunahara, unpublished observations). This discrepancy between the CocE-L169K/G173Q t1/2 in vitro and in vivo is also observed in the protection against the reinforcing properties of cocaine measured in the rat self-administration model. Some reduction in responding can be seen after a 1-h pretreatment of CocE-L169K/G173Q; however, this protection is almost eliminated after 2 h. CocE dose-response analyses by Collins et al. (2009) illustrate that plasma levels of CocE-T172R/G173Q need to be sufficiently high or else the effect of CocE is surmountable. This leads us to hypothesize that the discrepancy between the in vitro stability and in vivo protection against lethality and self-administration data of CocE-L169K/G173Q may be due to a reduction in plasma levels of the enzyme.
Indeed, Western blot analysis on serum from mice injected with CocE-L169K/G173Q shows that the enzyme is eliminated from the serum with a t1/2 of 2.3 h. Processes unrelated to thermostability may explain the relatively short pharmacokinetic properties of the enzyme. One possibility is protease degradation, which may remove or destroy the regions of CocE recognized by the polyclonal anti-body and yield results that mimic the disappearance of CocE. Glomerular filtration is unlikely, because CocE is approximately 134 kDa as a dimer (D. Narasimhan and R. K. Sunahara, unpublished observations). However, it is possible that the dimer interface may be disrupted in vivo, and the monomer or smaller proteolysed products are filtered from the blood before forming aggregates (CocE aggregates can be observed in mouse serum with size exclusion chromatography; data not shown).
It is of great interest that both the thermally stable CocE-L169K/G173Q and the wt CocE disappear from the plasma with the same kinetics. Active CocE is a dimer, and a monomeric form has never been isolated in vitro. Because CocE is heat-inactivated, the protein forms aggregates both in vitro and in vivo (determined by size exclusion chromatography; D. Narasimhan and R. K. Sunahara, unpublished observations). Wild-type CocE aggregates in vitro within 1 h at 37°C (D. Narasimhan and R. K. Sunahara, unpublished observations), consistent with its thermal inactivation; however, CocE-L169K/G173Q maintains as a dimer form for at least 120 h (data not shown). The observation that both dimers and aggregates are eliminated with the same half-time illustrates that the state in which the esterase exists does not affect the CocE elimination process in vivo, despite corresponding to the activity of the enzyme.
The long in vitro half-life of CocE-L169K/G173Q at 37°C greatly surpasses any combination of mutations to date. The three-day half-life in vitro is a promising step toward development of a therapy against cocaine addiction. After 10 days at 37°C, this enzyme still retains 15% of its original activity and displays intact Michaelis-Menten kinetics. The concentrations of cocaine typically seen in cocaine abusers [0–1 mg/liter or 0.6–3 μM (Couper and Logan, 2004)] should be cleared in approximately the same time by both the wt and L169K/G173Q enzyme. The extended half-life and improved Michaelis-Menten parameters of CocE-L169K/G173Q suggest that this CocE enzyme represents a strong candidate for a cocaine abuse therapy indication.
Creation of the thermally stable CocE-L169K/G173Q was a necessary advancement toward treatment of cocaine addiction by CocE. This enzyme demonstrates that the instability of wt CocE at 37°C can be surmounted. The improved thermostability of CocE-L169K/G173Q over wt CocE, however, does not affect the in vivo rate of elimination from the serum. The short plasma half-life of CocE-L169K/G173Q presents an obstacle that must be overcome if CocE is to be used as a treatment for cocaine abuse. Strategies that have shown success at increasing circulating protein half-life in vivo, including PEGylation, encapsulation in red blood cells, and sugar modification, are currently under investigation.
Acknowledgments
We thank Davina Barron and Adam Kynaston for excellent technical assistance. Thanks also to Dr. R. D. Davenport for providing the human plasma samples.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants DA021416, DA025100, DA013930]; the National Institutes of Health National Institute of General Medical Sciences [Grant GM007767]; and the National Institutes of Health National Heart, Lung, and Blood Institute [Grants HL086865, HL071818 (both to J.J.G.T.)].
This work was previously presented in part: Brim RL, Nance M, Tesmer J, Sunahara RK, and Woods JH (2009) Mutant bacterial cocaine esterase with a three-day half-life possess characteristics of a cocaine abuse therapy, ASPET Annual Meeting; Apr 18–22, 2009; New Orleans, LA.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.109.060806.
- BchE
- butyrylcholinesterase
- CocE
- cocaine esterase
- wt
- wild-type
- WIN-35065-2
- troparil
- ANOVA
- analysis of variance
- FR
- fixed ratio
- RMSD
- root-mean-square deviation.
References
- Benowitz NL. (1993) Clinical pharmacology and toxicology of cocaine. Pharmacol Toxicol 72:3–12 [DOI] [PubMed] [Google Scholar]
- Billman GE. (1990) Mechanisms responsible for the cardiotoxic effects of cocaine. FASEB J 4:2469–2475 [DOI] [PubMed] [Google Scholar]
- Bresler MM, Rosser SJ, Basran A, Bruce NC. (2000) Gene cloning and nucleotide sequencing and properties of a cocaine esterase from Rhodococcus sp. strain MB1. Appl Environ Microbiol 66:904–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimijoin S, Gao Y, Anker JJ, Gliddon LA, Lafleur D, Shah R, Zhao Q, Singh M, Carroll ME. (2008) A cocaine hydrolase engineered from human butyrylcholinesterase selectively blocks cocaine toxicity and reinstatement of drug seeking in rats. Neuropsychopharmacology 33:2715–2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne SP, Slaughter EA, Couch RA, Rudnic EM, McLean AM. (1998) The influence of plasma butyrylcholinesterase concentration on the in vitro hydrolysis of cocaine in human plasma. Biopharm Drug Dispos 19:309–314 [DOI] [PubMed] [Google Scholar]
- Carroll FI, Howard JL, Howell LL, Fox BS, Kuhar MJ. AAPS J 8:E196–E203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborative Computational Project Number 4 (1994) The CCP4 suite: programs for protein crystallography. Acta Crystallogr D Biol Crystallogr 50:760–763 [DOI] [PubMed] [Google Scholar]
- Collins GT, Brim RL, D. Narasimhan D, Ko MC, Sunahara RK, Zhan CG, Woods JH. (2009) Cocaine esterase prevents cocaine-induced toxicity and the ongoing intravenous self-administration of cocaine in rats. J Pharmacol Exp Ther 331:445–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, D. Narasimhan D, Sunahara RK, Mierzejewski P, Jutkiewicz EM, Larsen NA, Wilson IA, Landry DW, Woods JH. (2006) Rapid and robust protection against cocaine-induced lethality in rats by the bacterial cocaine esterase. Mol Pharmacol 70:1885–1891 [DOI] [PubMed] [Google Scholar]
- Couper FJ, Logan BK. (2004) Drug and Human Performance Fact Sheets, Report No. DOT HS 809 725, National Highway Traffic Safety Administration, Washington DC: Available at http://www.nhtsa.gov/PEOPLE/INJURY/research/job185drugs/drugs_web.pdf [Google Scholar]
- Crumb WJ, Jr, Clarkson CW. (1990) Characterization of cocaine-induced block of cardiac sodium channels. Biophys J 57:589–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels A, Ayala E, Chen W, Coop A, Matsumoto RR. (2006) N-[2-(m-methoxyphenyl)ethyl]-N-ethyl-2-(1-pyrrolidinyl)ethylamine (UMB 116) is a novel antagonist for cocaine-induced effects. Eur J Pharmacol 542:61–68 [DOI] [PubMed] [Google Scholar]
- Duysen EG, Bartels CF, Lockridge O. (2002) Wild-type and A328W mutant human butyrylcholinesterase tetramers expressed in Chinese hamster ovary cells have a 16-hour half-life in the circulation and protect mice from cocaine toxicity. J Pharmacol Exp Ther 302:751–758 [DOI] [PubMed] [Google Scholar]
- Edwards JA, Brimijoin S. (1983) Thermal inactivation of the molecular forms of acetylcholinesterase and butyrylcholinesterase. Biochimica et Biophysica Acta 742:509–516 [DOI] [PubMed] [Google Scholar]
- Emsley P, Cowtan K. (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60:2126–2132 [DOI] [PubMed] [Google Scholar]
- Gao D, D. Narasimhan DL, Macdonald J, Brim R, Ko MC, Landry DW, Woods JH, Sunahara RK, Zhan CG. (2009) Thermostable variants of cocaine esterase for long-time protection against cocaine toxicity. Mol Pharmacol 75:318–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Brimijoin S. (2004) An engineered cocaine hydrolase blunts and reverses cardiovascular responses to cocaine in rats. J Pharmacol Exp Ther 310:1046–1052 [DOI] [PubMed] [Google Scholar]
- Gao Y, LaFleur D, Shah R, Zhao Q, Singh M, Brimijoin S. (2008) An albumin-butyrylcholinesterase for cocaine toxicity and addiction: catalytic and pharmacokinetic properties. Chem Biol Interact 175:83–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick DA. (1997) Enhancing cocaine metabolism with butyrylcholinesterase as a treatment strategy. Drug Alcohol Depend 48:159–165 [DOI] [PubMed] [Google Scholar]
- Hoffman RS, Morasco R, Goldfrank LR. (1996) Administration of purified human plasma cholinesterase protects against cocaine toxicity in mice. J Toxicol Clin Toxicol 34:259–266 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed.Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC [Google Scholar]
- Johanson CE, Fischman MW. (1989) The pharmacology of cocaine related to its abuse. Pharmacol Rev 41:3–52 [PubMed] [Google Scholar]
- Jutkiewicz EM, Baladi MG, Cooper ZD, D. Narasimhan D, Sunahara RK, Woods JH. (2009) A Bacterial Cocaine Esterase Protects Against Cocaine-Induced Epileptogenic Activity and Lethality. Ann Emerg Med 54:409–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Bowen LD, D. Narasimhan D, Berlin AA, Lukacs NW, Sunahara RK, Cooper ZD, Woods JH. (2007) Cocaine esterase: interactions with cocaine and immune responses in mice. J Pharmacol Exp Ther 320:926–933 [DOI] [PubMed] [Google Scholar]
- Larsen NA, Turner JM, Stevens J, Rosser SJ, Basran A, Lerner RA, Bruce NC, Wilson IA. (2002) Crystal structure of a bacterial cocaine esterase. Nat Struct Biol 9:17–21 [DOI] [PubMed] [Google Scholar]
- Lovell SC, Davis IW, Arendall WB, III, de Bakker PIW, Word JM, Prisant MG, Richardson JS, Richardson DC. (2003) Structure validation by C-alpha geometry: phi, psi, and C-beta deviation. Protein Struct Funct Genet 50:437–450 [DOI] [PubMed] [Google Scholar]
- Lynch TJ, Mattes CE, Singh A, Bradley RM, Brady RO, Dretchen KL. (1997) Cocaine detoxification by human plasma butyrylcholinesterase. Toxicol Appl Pharmacol 145:363–371 [DOI] [PubMed] [Google Scholar]
- Mattes CE, Lynch TJ, Singh A, Bradley RM, Kellaris PA, Brady RO, Dretchen KL. (1997) Therapeutic use of butyrylcholinesterase for cocaine intoxication. Toxicol Appl Pharmacol 145:372–380 [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Kimmel HL, Howell LL, Carroll FI. (2009) Effects of the monoamine uptake inhibitors RTI-112 and RTI-113 on cocaine- and food-maintained responding in rhesus monkeys. Pharmacol Biochem Behav 91:333–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of National Drug Control Policy (2004) The Economic Costs of Drug Abuse in the United States, 1992–2002, Publication No. 207303, Executive Office of the President, Washington, DC: Available at http://www.ncjrs.gov/ondcppubs/publications/pdf/economic_costs.pdf [Google Scholar]
- Otwinowski Z, Minor W. (1997) Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol 276:307–326 [DOI] [PubMed] [Google Scholar]
- Pan Y, Gao D, Yang W, Cho H, Yang G, Tai HH, Zhan CG. (2005) Computational redesign of human butyrylcholinesterase for anticocaine medication. Proc Natl Acad Sci USA 102:16656–16661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Baumann MH. (2007) Dual dopamine/serotonin releasers as potential medications for stimulant and alcohol addictions. AAPS J 9:E1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (2007) Results from the 2006 National Survey on Drug Use and Health: National Findings, Office of Applied Studies, NSDUH Series H-32, DHHS Publication no. SMA 07-4293, Rockville, MD [Google Scholar]
- Turner JM, Larsen NA, Basran A, Barbas CF, 3rd, Bruce NC, Wilson IA, Lerner RA. (2002) Biochemical characterization and structural analysis of a highly proficient cocaine esterase. Biochemistry 41:12297–12307 [DOI] [PubMed] [Google Scholar]
- Uhl GR, Hall FS, Sora I. (2002) Cocaine, reward, movement and monoamine transporters. Mol Psychiatry 7:21–26 [DOI] [PubMed] [Google Scholar]
- Wood SK, D. Narasimhan D, Cooper Z, Sunahara RK, Woods JH. (2010) Prevention and reversal by cocaine esterase of cocaine-induced cardiovascular effects in rats. Drug Alcohol Depend 106:219–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Altamirano CV, Bartels CF, Speirs RJ, Cashman JR, Lockridge O. (1999) An improved cocaine hydrolase: the A328Y mutant of human butyrylcholinesterase is 4-fold more efficient. Mol Pharmacol 55:83–91 [DOI] [PubMed] [Google Scholar]
- Zheng F, Yang W, Ko MC, Liu J, Cho H, Gao D, Tong M, Tai HH, Woods JH, Zhan CG. (2008) Most efficient cocaine hydrolase designed by virtual screening of transition states. J Am Chem Soc 130:12148–12155 [DOI] [PMC free article] [PubMed] [Google Scholar]