Abstract
The ρ1 GABA receptor is inhibited by a number of neuroactive steroids. A previous study (J Pharmacol Exp Ther 323:236–247, 2007) focusing on the electrophysiological effects of inhibitory steroids on the ρ1 receptor found that steroid inhibitors could be divided into three major groups based on how mutations to residues in the M2 transmembrane domain modified inhibition. It was proposed that the steroids act through distinct mechanisms. We selected representatives of the three groups (pregnanolone, tetrahydrodeoxycorticosterone, pregnanolone sulfate, allopregnanolone sulfate, and β-estradiol) and probed how these steroids, as well as the nonsteroidal inhibitor picrotoxinin, modify GABA-elicited fluorescence changes from the Alexa 546 C5 maleimide fluorophore attached to residues in the extracellular region of the receptor. The fluorophore responds with changes in quantum yield to changes in the environment, allowing it to probe for structural changes taking place during channel activation or modulation. The results indicate that the modulators have specific effects on fluorescence changes suggesting that distinct conformational changes accompany inhibition. The findings are consistent with the steroids acting as allosteric inhibitors of the ρ1 GABA receptor and support the hypothesis that divergent mechanisms underlie the action of inhibitory steroids on the ρ1 GABA receptor.
The ionotropic GABA receptor belongs to the cysteine loop family of transmitter-gated ion channels. The ρ1 GABA receptors have especially high expression in the retina, in which they modulate the visual signal as it passes from the photoreceptors to the ganglion cells. Lower levels of the receptor are found in other parts of the nervous system, as well as in the gastrointestinal tract and testis. Drugs acting on ρ1 GABA receptors are considered useful in the treatment of visual, sleep, and cognitive disorders (Johnston et al., 2003).
Here, we have examined the mechanism of action of several endogenous neurosteroids on the human ρ1 GABA receptor using simultaneous measurements of electrophysiological activity and site-specific fluorescence. This approach was first described for voltage-gated potassium channels (Mannuzzu et al., 1996) and has recently been used to study conformational changes in a number of transmitter-gated ion channels (Chang and Weiss, 2002; Dahan et al., 2004; Muroi et al., 2006, 2009; Pless et al., 2007; Khatri et al., 2009; Zhang et al., 2009). The goal is to label a residue in the region of interest with an environmentally sensitive fluorescent reporter. These fluorescent reporters respond to changes in the environment with changes in quantum yield: if the environment around the fluorophore changes (e.g., from hydrophilic to hydrophobic), the intensity of the fluorescence signal changes. This method can be applied to probe for structural changes taking place during channel activation or modulation.
A previous electrophysiological study (Li et al., 2007) found that steroid inhibitors of the human ρ1 receptor could be divided into three major groups based on how mutations to residues in the M2 transmembrane domain modified inhibition. It was shown that the P294S mutation (P2′S mutation in the M2) had little effect on channel inhibition by charged (sulfated or carboxylated) steroids or β-estradiol but essentially eliminated inhibition by the 5β-reduced steroids 3α5βP and 3α5βPOH. When the receptors contained the threonine-to-phenylalanine mutation to the 298 site (6′ residue in the M2), channel inhibition by β-estradiol, but not the charged or uncharged 5β-reduced steroids, was greatly reduced. It was proposed that differential sensitivity to mutations results from distinct mechanisms of action of the steroids. We have selected representatives of the three groups (3α5βP, 3α5βPOH, 3α5βPS, 3α5αPS, and β-estradiol) and probed how these steroids modify fluorescence signals from the Alexa 546 C5 maleimide fluorophore attached to various regions of the receptor. In addition, we examined modulation of ΔF by the plant-derived nonsteroidal inhibitor picrotoxinin. The results indicate that the drugs have specific and distinct effects on fluorescence changes, thus bolstering the hypothesis of divergent mechanisms of action of inhibitory drugs.
Materials and Methods
The experiments were conducted on wild-type (GenBank accession no. M62400) and mutant (K217C, Y241C, L166C, S66C, L166C + T298F, and S66C + T298F; numbered according to the first residue in the predicted mature subunit) human ρ1 GABA receptors expressed in Xenopus laevis oocytes. The cDNAs for the receptor subunits were subcloned into the pGEMHE expression vector in the T7 orientation. The cDNA was linearized by NheI (New England Biolabs, Ipswich, MA) digestion, and the RNA was produced using mMessage mMachine (Ambion, Austin, TX). The oocytes were injected with 7 to 14 ng of cRNA in a volume of 20 to 60 nl and incubated at 16°C for 3 to 4 days before labeling and recording.
Labeling with Alexa Fluor 546 C5 maleimide (A5m; Invitrogen, Carlsbad, CA) was carried out using incubation for 30 to 45 min at room temperature in the dark with 20 μM A5m dissolved in OR2 (92.5 mM NaCl, 2.5 mM KCl, 1 mM MgCl2, and 10 mM HEPES) at pH 7.2. The oocytes were washed in the bath solution (OR2, pH 7.5) before transferring to the recording chamber.
We used a custom-made recording chamber. The chamber consists of two compartments, separated by a 0.8-mm aperture on which the oocyte is placed. The oocyte was impaled in the top compartment and the agonist (and modulators) was applied in the lower compartment. An inverted microscope (Diaphot TMD; Nikon, Tokyo, Japan) fitted with a Nikon 20×, long working distance, 0.4 numerical aperture objective lens was used to image the fluorescence signal from the lower chamber. The microscope holds dichroic (565DCLP) and emission (D605/55m) filters (Chroma Technology Corp., Brattleboro, VT) for fluorescence detection. Because of a relatively tight seal between the oocyte and the aperture, there is little leakage between the two compartments, and the fluorescence and current signals were measured from the same population of receptors.
The fluorescence measurement system was purchased from Photon Technology International (Birmingham, NJ). The system consists of a DeltaRAM monochromator, from which the light (546 nm) is passed into the microscope via a liquid light guide, a photomultiplier tube (Hamamatsu Photonics, Bridgewater, NJ) mounted on the side port of the microscope, BryteBox acquisition hardware, and FeliX32 software for control of excitation (Photon Technology International). A 75-W Xenon short-arc lamp (Ushio Inc., Tokyo, Japan) served as a light source.
Standard two-electrode voltage clamp was used to record the currents. Both voltage and current electrodes were patch-clamp electrodes filled with 3 M KCl and had resistances of 0.5 to 1.5 MΩ. The oocytes were clamped at −60 mV. The chamber was perfused continuously at approximately 5 ml/min. Bath solution was perfused between all test applications. Solutions were switched by hand using Teflon rotary valves (Cobert Associates, St. Louis, MO) or via pClamp (Molecular Devices, Sunnyvale, CA) using a VC-8T valve controller (Warner Instruments, Hamden, CT). Solutions were applied from glass reservoirs via metal or Teflon tubing to reduce adsorption.
A previous study (Li et al., 2007) found that the effect of inhibitory steroids on receptor function was reduced at higher concentrations of GABA. As a result, we conducted our experiments in the presence of GABA that produced a submaximal response (approximately 35–70% of maximal response). The protocol was to expose an oocyte to one or more 20- to 40-s GABA applications, separated by washouts in bath solution for 1 to 3 min. The oocyte was then exposed to GABA plus modulator followed by washout and an additional application of GABA alone to ascertain the lack of irreversible effects or rundown. In some experiments, the modulator alone was applied.
The current responses were amplified with an Axoclamp 900A amplifier (Molecular Devices), digitized with a Digidata 1320 series digitizer (Molecular Devices) at a 100-Hz sampling rate and stored using pClamp. Current and fluorescent transients were analyzed with Clampfit. The baseline was corrected for bleaching when needed. The fluorescence data are presented as the percentage change in baseline fluorescence, with positive values indicating an increase in fluorescence. Statistical analyses were carried out using paired t test (Excel; Microsoft Corp., Redmond, WA).
Modeling of the ρ1 subunits was done using the DeepView/Swiss-Pdb viewer freeware version 4.01 (available at http://spdbv.vital-it.ch/). Two copies of the human ρ1 protein sequence from residues Ile60 to Arg258 were aligned against the human GABA-A receptor β3 subunit sequence. The α-carbon atoms of the aligned residues were then threaded on the Protein Data Bank structure of the β3 subunit (Bracamontes and Steinbach, 2008) to obtain two three-dimensional adjacent copies of the subunit. The pentameric receptor model showing the position of the Ser66 residue is from the Protein Data Bank file of the acetylcholine binding protein, marking the homologous residue in the acetylcholine binding protein. The presentation of the structural models was done using the three-dimensional molecule viewer of the Vector NTI Advance version 11 software (Invitrogen).
The steroids 3α5βP, 3α5βPOH, 3α5βPS, 3α5αPS, and β-estradiol were purchased from Sigma-Aldrich (St. Louis, MO) or Steraloids (Newport, RI). Stock solutions (10–20 mM) of the steroids were made in dimethyl sulfoxide, and dilutions to test concentrations were made on the day of the experiment. Picrotoxinin was purchased from Sigma-Aldrich, and solubilized in OR2 at 1 mM concentration with the final dilution made on the day of the experiment.
Results
Sites Selected for Labeling.
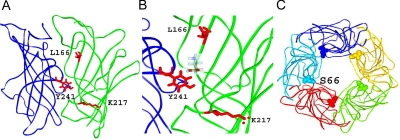
We selected four sites for labeling with the fluorescent reporter A5m: Tyr241, Lys217, Leu166, and Ser66 (Fig. 1). Each of the residues was individually mutated to cysteine, the mutant receptor was labeled with A5m, and the ability of GABA, steroid inhibitors, or picrotoxinin to affect fluorescence signals was examined.
Fig. 1.
Structures of the GABA receptor. A, structural model of the extracellular domain of the human ρ1 GABA receptor. The model was constructed by homology with the acetylcholine-binding protein. The model shows two neighboring subunits (blue and green) containing a single transmitter binding pocket. B, a higher-resolution image of the transmitter binding pocket. Three of the four residues used for labeling with A5m are shown with molecular structures (Tyr241 of the “+” side of the interface, and Lys217 and Leu166 of the “−” side of the interface are shown in red). A GABA molecule is placed in the putative transmitter binding pocket. C, a top view of the receptor showing the Ser66 residue located near the subunit-subunit interface in each of the five subunits of the GABA receptor.
The tyrosine residue at position 241 in loop C is crucial to ρ1 receptor activation. The Y241C mutation shifts the GABA concentration-effect curve by more than 1000-fold to higher concentrations (Amin and Weiss, 1994; Chang and Weiss, 2002). A previous study showed that the Y241C mutant receptor labeled with A5m responds to applications of GABA with an increase in fluorescence intensity. Enhanced fluorescence intensity is consistent with a movement of the A5m fluorophore into an environment with a lower dielectric constant (Chang and Weiss, 2002).
The Lys217 residue belongs to loop F, contributing to the transmitter binding site. A recent dynamics characteristics analysis of the related nicotinic acetylcholine receptor found that loop F undergoes structural rearrangement upon exposure to agonist; however, the movements, although possibly important to regulating affinity to the transmitter, are not part of global channel motions that lead to the opening of the gate (Szarecka et al., 2007). A recent study on site-specific fluorescence of loop F residues found no clear correlation between the ability of a ligand to elicit currents and fluorescence changes accompanying channel activation and concluded that loop F is not along the pathway for channel opening (Khatri et al., 2009). In the ρ1 receptor, the K217C mutation has a relatively small effect on the GABA concentration-effect relationship (Khatri et al., 2009). Previous work has shown that the K217C receptor labeled with A5m responds to applications of GABA with a decrease in fluorescence intensity (Khatri et al., 2009; Zhang et al., 2009).
The Leu166 residue (loop E) is located at the top of the transmitter binding site where it may help stabilize the ligand through hydrophobic contacts. In the ρ1 receptor, a substitution to cysteine in this position shifts the GABA concentration-effect curve by approximately 10-fold to higher concentrations. Exposure to GABA results in an increase in fluorescence intensity from receptors labeled with A5m at the Leu166 position (Chang and Weiss, 2002).
The location of the residue Ser66 is distant from the transmitter binding pocket but is within the subunit-subunit interface. A mutation to cysteine in this position slightly shifts the GABA concentration-effect curve to higher concentrations. The labeled S66C mutant receptor responds to applications of GABA with a reduction in fluorescence intensity (Chang and Weiss, 2002).
Sulfated Steroids but Not β-Estradiol or Unsulfated 5β-Reduced Steroids Affect Fluorescence Change from Labeled K217C.
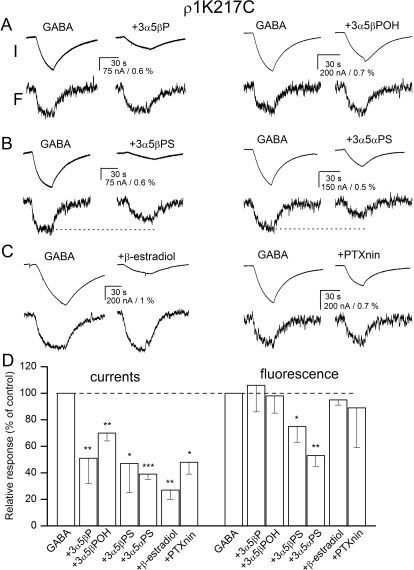
We labeled the human ρ1 receptors expressed in X. laevis oocytes with Alexa 546 fluorophore at position Lys217, and examined the effects of several inhibitory steroids and picrotoxinin on GABA-elicited currents and changes in fluorescence intensity.
Both the electrophysiological and fluorescence responses were found to be dependent on the concentration of GABA used to activate the receptor. The application of 20 μM GABA (a saturating concentration) elicited an average peak current of 1017 ± 623 nA (mean ± S.D.; n = 8 cells). This was accompanied by a ΔF of −4.4 ± 2.7% (the application of GABA led to a decrease in fluorescence intensity, indicating that channel gating results in the fluorophore being exposed to a more polar environment). Exposure of the same cells to 1 μM GABA resulted in a peak current of 432 ± 451 nA and a ΔF of −2.4 ± 1.3%. The calculated fractional current and fluorescence responses at 1 μM GABA were 42 and 55%, respectively, of that observed in the presence of 20 μM GABA, indicating that GABA sensitivity of the current response and ΔF are similar.
The coapplication of inhibitory steroids (at 20 μM) or picrotoxinin (at 10 μM) with 1 μM GABA significantly reduced the electrophysiological response. The greatest effect was observed for β-estradiol that reduced the peak current to 27% of control (paired t test, p < 0.01). 3α5βPOH was the least effective, reducing the current response to 70% of control (p < 0.01). In parallel with the inhibitory effect on the electrophysiological response, the application of two of the steroids had a significant effect on ΔF. Exposure to 3α5βPS reduced the ΔF to 75% (p < 0.05) of that observed in the presence of GABA alone. When 3α5αPS was coapplied with GABA, the ΔF was reduced to 53% (p < 0.01) of control. Sample recordings are shown in Fig. 2, A to C. The summary of the data are given in Fig. 2D and Supplemental Table 1.
Fig. 2.
Current and fluorescence responses from the ρ1 receptor labeled with A5m at the K217C site. Current and fluorescence responses from receptors activated by 1 μM GABA in the absence and presence of 20 μM 3α5βP or 3α5βPOH (A), 3α5βPS or 3α5αPS (B), or β-estradiol or 10 μM picrotoxinin (PTXnin) (C). The top traces show currents (I), and the bottom traces show the concomitant changes in fluorescence (F). The modulators are sorted according to their proposed classification into groups. All drugs inhibit the electrophysiological response, but only 3α5βPS and 3α5αPS reduce the change in fluorescence (shown with dotted lines). D, the summary of effects on electrophysiology and fluorescence responses. ∗, p < 0.05; ∗∗, p < 0.01; ∗∗∗, p < 0.001.
To gain insight into the concentration-effect relationship for the actions of steroids, we compared the effects of 2, 20, and 50 μM 3α5βPS on receptors activated by 1 μM GABA. To reduce errors caused by cell-to-cell variability, these experiments were conducted on the same set of three oocytes. The application of 2 μM 3α5βPS was without effect on receptor function and ΔF. The peak current was 91 ± 6% of control (p > 0.1), and the ΔF was 125 ± 28% of control (p > 0.25). Exposure to 20 or 50 μM steroid had significant effects on both current and fluorescence responses. In the presence of 20 μM 3α5βPS, the peak current was reduced to 35 ± 7% of control (p < 0.01) and ΔF to 65 ± 10% of control (p < 0.05). When 50 μM 3α5βPS was coapplied with GABA, the peak current was reduced to 18 ± 9% of control (p < 0.01), and ΔF was reduced to 32 ± 9% of control (p < 0.01). The data indicate that an increase in the concentration of the inhibitory steroid affects both the electrophysiological and fluorescence responses.
A previous study demonstrated that the ability of 3α5βPS to inhibit the current response is reduced at higher GABA concentrations (Li et al., 2007). To determine whether reduced electrophysiological inhibition is accompanied by reduced modulation of ΔF, we tested the effect of 3α5βPS on receptors activated by 20 μM GABA (a saturating concentration). In four cells, coapplication of 20 μM 3α5βPS with GABA had essentially no effect on the current response (106 ± 25% of control, p > 0.65). The ΔF was slightly reduced (87 ± 11% of control), but the effect was not significant (p > 0.1). We infer from the data that the fluorescence response reflects conformational changes associated with channel activation. This experiment also demonstrates the need to use low, submaximal concentrations of GABA to examine the effects of the inhibitors on GABA-induced conformational changes.
β-Estradiol but Not Sulfated or 5β-Reduced Unsulfated Steroids Affect Fluorescence Change from Labeled L166C.
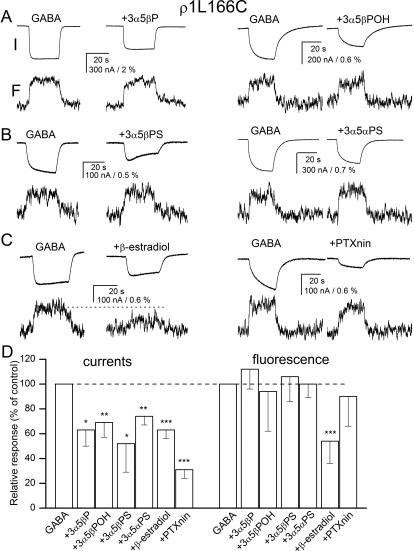
We next examined the effects of the steroids and picrotoxinin on current and fluorescence changes from receptors labeled at the L166C site. The labeled receptors were activated by 60 μM GABA. This concentration elicited 39 ± 13% of the maximal response (n = 14 cells). Coapplication of 20 μM steroid inhibitors or 10 μM picrotoxinin reduced the peak current to 31 to 74% of control. The biggest effect was seen in the presence of picrotoxinin, the smallest in the presence of 3α5αPS. The inhibitors selectively affected changes in fluorescence. No significant effects in ΔF were observed in the presence of 3α5βP, 3α5βPOH, 3α5βPS, 3α5αPS, or picrotoxinin. The coapplication of 20 μM β-estradiol with GABA reduced the change in fluorescence to 54% of control (p < 0.01). Figure 3, A to C, gives sample current and fluorescence recordings. A full summary of the data are given in Fig. 3D and Supplemental Table 1.
Fig. 3.
Current and fluorescence responses from the ρ1 receptor labeled with A5m at the L166C site. Current and fluorescence responses from receptors activated by 60 μM GABA in the absence and presence of 20 μM 3α5βP or 3α5βPOH (A), 3α5βPS or 3α5αPS (B), or β-estradiol or 10 μM picrotoxinin (PTXnin) (C). The top traces show currents (I), and the bottom traces show the concomitant changes in fluorescence (F). The modulators are sorted according to their proposed classification into groups. All drugs inhibit the electrophysiological response, but only β-estradiol reduces the change in fluorescence (shown with dotted line). D, the summary of effects on electrophysiology and fluorescence responses. ∗, p < 0.05; ∗∗, p < 0.01; ∗∗∗, p < 0.001.
We conducted limited concentration-effect relationship studies for β-estradiol-mediated reduction in ΔF. In six cells labeled at the L166C site, exposure to 2, 20, or 50 μM β-estradiol reduced the fluorescence change to 91 ± 21% (p > 0.3), 55 ± 20% (p < 0.01), or 7 ± 18% (p < 0.001) of control. The corresponding peak current amplitudes were reduced to 92 ± 2% (p < 0.001), 62 ± 7% (p < 0.01), or 56 ± 6% (p < 0.01) of control. The data suggest that β-estradiol has a stronger effect on ΔF than the peak current. The application of 20 μM β-estradiol in the absence of GABA did not elicit an electrophysiological response or a ΔF (data not shown).
We also tested the effect of β-estradiol on labeled L166C receptors activated by 1 mM GABA (a saturating concentration). Coapplication of 20 μM β-estradiol with GABA slightly reduced the peak current response (87 ± 4%, n = 4 cells, p < 0.001) but was without effect on ΔF (96 ± 12%, p > 0.4).
Sulfated Steroids, β-Estradiol, and Picrotoxinin Reduce Fluorescence Change from Labeled S66C.
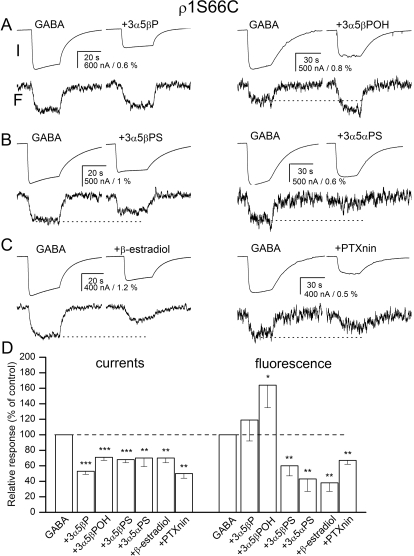
To probe for the structural changes at the subunit-subunit interface, we labeled the S66C mutant receptor with A5m and probed for changes in fluorescence intensity after the application of GABA in the absence and presence of inhibitory steroids and picrotoxinin. The labeled S66C receptor was activated by 5 μM GABA, a concentration eliciting 72 ± 7% of maximal response (n = 12 cells).
The application of each of the inhibitors studied significantly reduced the electrophysiological response to GABA. The peak current in the presence of the inhibitors ranged from 50 to 71% of the control response. The drugs selectively affected ΔF. The GABA-elicited change in fluorescence was significantly reduced in the presence of the sulfated steroids 3α5βPS and 3α5αPS, β-estradiol, or picrotoxinin. The relative ΔF in the presence of these drugs ranged from 38 (β-estradiol) to 67% (picrotoxinin) of control. In contrast, the application of 3α5βPOH enhanced the GABA-elicited fluorescence change. The effect (164% of control) was statistically significant (p < 0.05). The ΔF in the presence of 3α5βP demonstrated a slight increase (119% of control), but the effect did not reach statistical significance. Sample current and fluorescence traces are shown in Fig. 4, A to C. The data are summarized in Fig. 4D and Supplemental Table 1.
Fig. 4.
Current and fluorescence responses from the ρ1 receptor labeled with A5m at the S66C site. Current and fluorescence responses from receptors activated by 5 μM GABA in the absence and presence of 20 μM 3α5βP or 3α5βPOH (A), 3α5βPS or 3α5αPS (B), or β-estradiol or 10 μM picrotoxinin (PTXnin) (C). The top traces show currents (I), and the bottom traces show the concomitant changes in fluorescence (F). The modulators are sorted according to their proposed classification into groups. All drugs inhibit the electrophysiological response. The application of 3α5βPOH enhances whereas the application of 3α5βPS, 3α5αPS, β-estradiol or PTXnin reduce the change in fluorescence (shown with dotted lines). D, the summary of effects on electrophysiology and fluorescence responses. ∗, p < 0.05; ∗∗, p < 0.01; ∗∗∗, p < 0.001.
We also examined the actions of two compounds, 3α5βPS and β-estradiol, on currents and fluorescence elicited by saturating GABA. The data indicate that the presence of 20 μM 3α5βPS or β-estradiol had no effect on the peak current from receptors activated by 100 μM. The peak current was 107 ± 15% of control (n = 4 cells, p > 0.4) or 97 ± 4% of control (n = 5 cells, p > 0.25) in the presence of 3α5βPS or β-estradiol, respectively. The application of 3α5βPS had no effect on ΔF (89 ± 14% of control, p > 0.2) in the presence of 100 μM GABA. In contrast, 20 μM β-estradiol reduced the fluorescence change to 64 ± 15% of control (p < 0.01). Thus, β-estradiol could exert an effect on ΔF without a concomitant effect on receptor function.
Finally, we probed the effects of the inhibitory drugs in the absence of GABA. The application of 20 μM 3α5βPOH, 3α5βPS, 3α5αPS, β-estradiol, or 10 μM picrotoxinin did not elicit a functional response from the receptors and was without effect on the fluorescence response (data not shown).
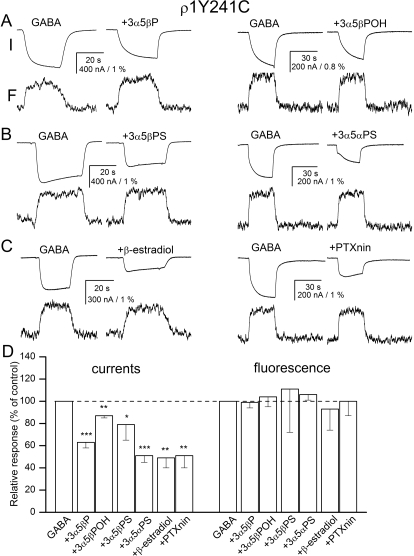
Fluorescence Change from Labeled Y241C Is Not Affected by the Inhibitory Steroids or Picrotoxinin.
We also tested the effects of the five inhibitory steroids and picrotoxinin on the electrophysiological and fluorescence responses from a receptor labeled with A5m at the Y241C site. The Y241C mutation has a dramatic effect on the receptor activation properties, rightward-shifting the activation concentration-effect curve so that no clear saturation is observed at GABA concentrations up to 100 mM (Chang and Weiss, 2002). In the present study, we used 20 mM GABA to activate the receptors.
Coapplication of the inhibitors with GABA in each case significantly reduced the electrophysiological response. The peak currents in the presence of the inhibitors ranged from 49 to 87% of control. β-Estradiol had the strongest effect, whereas 3α5βPOH was the least effective at producing inhibition. It is interesting that none of the drugs at the concentrations used in these experiments affected GABA-elicited ΔF. Figure 5, A to C, shows sample recordings in the presence of representative steroids. The data are summarized in Fig. 5D and Supplemental Table 1.
Fig. 5.
Current and fluorescence responses from the ρ1 receptor labeled with A5m at the Y241C site. Current and fluorescence responses from receptors activated by 5 μM GABA in the absence and presence of 20 μM 3α5βP or 3α5βPOH (A), 3α5βPS or 3α5αPS (B), or β-estradiol or 10 μM picrotoxinin (PTXnin) (C). The top traces show currents (I) and the bottom traces show the concomitant changes in fluorescence (F). The modulators are sorted according to their proposed classification into groups. All drugs inhibit the electrophysiological response. None of the drugs affects the change in fluorescence. D, the summary of effects on electrophysiology and fluorescence responses. ∗, p < 0.05; ∗∗, p < 0.01; ∗∗∗, p < 0.001.
The T298F Mutation Reduces the Ability of β-Estradiol to Affect Currents and Fluorescence.
We tested the effect of the ρ1T298F mutation on the ability of the steroids to inhibit the current response and the concomitant change in fluorescence. The motivation from these experiments comes from previous work demonstrating that the threonine to phenylalanine mutation of the 6′ residue in the second transmembrane domain (ρ1T298F) removes block by β-estradiol (Li et al., 2007). We hypothesized that if the ρ1Thr298 residue acts as a transduction or gating element for steroid-elicited block, then the mutation will reduce or abolish the ability of β-estradiol to inhibit the electrophysiological response but may be without effect on ΔF generated at the L166C site. In contrast, if the ρ1T298F mutation prevents the binding of steroid to the receptor, steroid effects on both current and fluorescence responses will be affected to a similar degree.
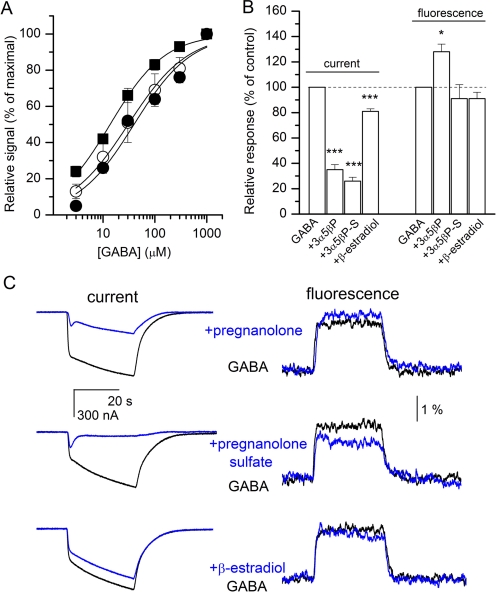
We started by examining the GABA concentration-response properties of mutant receptors. Oocytes expressing the ρ1L166C + T298F receptors were exposed to 3 to 1000 μM GABA. The normalized current responses from seven cells were averaged and fitted to the Hill equation. We estimate that the midpoint of the concentration-response curve is at 30 ± 4 μM, and the Hill slope is 0.8 ± 0.1 (Fig. 6A).
Fig. 6.
Current and fluorescence responses from the ρ1T298F receptor labeled with A5m at the L166C site. A, GABA concentration-effect relationships for currents (○, unlabeled ρ1L166C + T298F mutant receptors; ●, receptors labeled with A5m) and fluorescence changes (■, receptors labeled with A5m). The curves were fitted to the Hill equation. The best-fit parameters for currents from unlabeled receptors are EC50 = 30 ± 4 μM, nH = 0.8 ± 0.1. The best-fit parameters for currents from receptors labeled with A5m are EC50 = 40 ± 9 μM, nH = 0.8 ± 0.1. The best-fit parameters for fluorescence from receptors labeled with A5m are EC50 = 14 ± 1 μM, nH = 0.8 ± 0.1. B, summary of steroid effects on electrophysiology and fluorescence responses. 3α5βP, pregnanolone; 3α5βP-S, pregnanolone sulfate. ∗, p < 0.05; ∗∗, p < 0.01; ∗∗∗, p < 0.001. C, sample current and fluorescence traces from the ρ1L166C + T298F receptors. The traces show responses to 30 μM GABA in the absence and presence of 20 μM pregnanolone (top), pregnanolone sulfate (middle), or β-estradiol (bottom). The data indicate that the T298F mutation reduces the ability of β-estradiol to inhibit the current and fluorescence responses (compare with Fig. 3C).
We also examined the GABA concentration-response properties of receptors labeled with A5m. From six cells, we estimate that the EC50 of the concentration-response curve for currents is 40 ± 9 μM and the Hill slope is 0.8 ± 0.1. For simultaneously recorded fluorescence responses, the EC50 was 14 ± 1 μM and the Hill slope was 0.8 ± 0.1. The concentration-response curves are shown in Fig. 6A.
Next, we tested the ability of steroids to inhibit the electrophysiological and fluorescence responses from ρ1L166C + T298F receptors. Coapplication of 20 μM β-estradiol with 30 μM GABA slightly reduced the peak current (81 ± 4% of control, n = 5 cells, p < 0.001). In the same cells, the magnitude of ΔF remained unchanged (91 ± 11%, p > 0.12). For comparison, in cells expressing L166C receptors, β-estradiol reduced the peak current to 63%, whereas ΔF was reduced to 54% of control (Fig. 3, C and D). Thus, the T298F mutation reduces the ability of β-estradiol to modulate both current and fluorescence responses. Sample current and fluorescence traces are shown in Fig. 6.
We also tested the ability of 3α5βP and 3α5βPS to modulate the ρ1L166C + T298F receptor. Coapplication of 20 μM 3α5βP with GABA reduced the peak current to 35 ± 8% of control (n = 5 cells, p < 0.001), whereas the ΔF was slightly increased (128 ± 14% of control, p < 0.05). The presence of 20 μM 3α5βPS reduced the peak current response to 26 ± 7% (n = 5 cells, p < 0.001) of that observed in the presence of 30 μM GABA but was without effect on ΔF (91 ± 24% of control, p > 0.4). Thus, the T298F mutation does not preclude inhibition of the electrophysiological response by 3α5βP and 3α5βPS.
Discussion
The ρ1 GABA receptor constitutes a dominant inhibitory force in the retina but is expressed, albeit at lower levels, throughout the nervous system. Drugs acting on the ρ1 receptor can be useful in the treatment of a variety of visual, sleep, and cognitive disorders. A recent study demonstrated that cis- and trans-(3-aminocyclopentanyl)methylphosphinic acid, selective antagonists of ρ receptors, can prevent the development of myopia (Chebib et al., 2009). The same study indicated that after intraperitoneal injection, the drugs enhanced learning and memory in rats in the Morris water maze task. Furthermore, receptors containing the ρ subunit have been implicated in apoptosis of hippocampal neurons (Yang et al., 2003) and regulation of hormone release in the pituitary gland (Boue-Grabot et al., 2000), and it has been proposed that antagonism of the ρ receptor underlies 4,5,6,7-tetrahydroisoxazolo[4,5-c]pyridin-3-ol-induced analgesia in rats (Zorn and Enna, 1987; Johnston et al., 2003).
In this study, we focused on the structural correlates of ρ1 GABA receptor inhibition by selected neurosteroids and the nonsteroidal inhibitor picrotoxinin. An inhibitor is operationally defined as a compound that diminishes the response to an agonist. The potential mechanisms of action for the inhibitor are many. A drug may act as a simple pore blocker. Alternatively, the inhibitor may compete with the agonist for the transmitter binding site (competitive inhibition) or prevent the binding of the transmitter or channel opening through interaction with a separate site (allosteric inhibition). The goal of the study was to provide structural insights into the mechanisms of receptor inhibition by selected inhibitors of the ρ1 receptor.
For that, we used an approach of simultaneous electrophysiological and fluorescence recordings. The GABA-elicited fluorescence signal, probably resulting from conformational changes associated with channel activation, was generated by the A5m fluorophore attached to one of four selected residues in the extracellular region of the receptor. To probe for conformational changes after channel inhibition, we recorded the effects of six inhibitory agents on fluorescence intensity.
It should be noted that the approach has limitations. A change in fluorescence signal contains no information about the extent or the physical direction of the conformational change. Fluorescence intensity is dependent on the polarity of the environment surrounding the fluorophore, and it is conceivable that two ligands could induce different conformational changes yet show similar fluorescence signals if the microenvironment surrounding the fluorophore is similar. Furthermore, a movement of the residue to which the fluorophore is attached relative to its environment and a movement of neighboring residues around a static fluorophore could produce identical changes in fluorescence. Nonetheless, the approach is highly valuable in correlating the development of conformational changes with the electrophysiological response. The approach can be especially useful in comparing the structural effects of related drugs or conformational changes at homologous sites in different subunits.
A previous electrophysiological study (Li et al., 2007) comparing ρ1 GABA receptor inhibition by a large number of steroid analogs distinguished three apparent mechanisms for producing inhibition. The steroids used in the present study (3α5βP, 3α5βPOH, 3α5βPS, 3α5αPS, and β-estradiol) are representatives of groups demonstrating different inhibition mechanisms. In addition, we used the nonsteroidal inhibitor picrotoxinin that had been proposed to share a common mechanism with β-estradiol (Li et al., 2007).
The present findings demonstrate that the inhibitors differentially affect fluorescence generated by A5m attached to different residues in the receptor. We found that the sulfated steroids 3α5βPS and 3α5αPS, but not unsulfated 5β-reduced steroids and β-estradiol, or the nonsteroidal inhibitor picrotoxinin, diminish the ΔF produced by GABA at the K217C position. When the L166C residue was labeled, only β-estradiol reduced ΔF. At the S66C position, exposure to the unsulfated 5β-reduced steroids and to β-estradiol or picrotoxinin reduced ΔF. Exposure to 3α5βP was without effect on ΔF, whereas the application of 3α5βPOH enhanced the fluorescence change produced by GABA. None of the drugs tested influenced ΔF at position Y241C. All compounds tested were shown to inhibit the electrophysiological response from the labeled receptors.
We have attempted to interpret the results in a framework in which an effect on the fluorescence change results from the ability of the antagonist to interfere with the underlying conformational change. The findings are not consistent with the steroids or picrotoxinin acting as classic open-channel blockers. An open pore-blocking mechanism may be expected to be without effect on ΔF from sites in the extracellular domain. Previous work has shown that the application of drugs capable of acting as competitive inhibitors can elicit ΔF at sites near the transmitter binding site (Chang and Weiss, 2002; Muroi et al., 2006; Khatri et al., 2009; Zhang et al., 2009). Thus, the lack of effect of the compounds on ΔF when applied in the absence of GABA suggests that inhibitory steroids do not act as competitive antagonists of the ρ1 receptor. We propose that the compounds act through an allosteric mechanism.
Furthermore, we infer that the steroids act through different molecular mechanisms. A common mechanism is strictly defined as one in which compounds bind to the same docking site and cause identical conformational changes, resulting in modification of the same transition rate. We have drawn this conclusion based on the finding that the steroids differentially affect fluorescence changes produced by labeling the residues in the extracellular domain.
A previous study examining the electrophysiological effects of inhibitory steroids found that the steroids could be divided into three major groups based on how mutations to residues in the M2 transmembrane domain modified inhibition (Li et al., 2007). Thus, the unsulfated 5β-reduced steroids formed one group, sulfated and carboxylated steroids formed another, and the estradiols shared some features with the nonsteroidal agent picrotoxinin. In the present study, we found that such classification is generally maintained when the effects of the inhibitors on ΔF are examined. The one notable exception was that β-estradiol but not picrotoxinin modulated ΔF produced at the L166C site, suggesting that the actions of picrotoxinin and β-estradiol on the ρ1 GABA receptor are not identical.
The T298F mutation (T6′F in the second transmembrane domain) strongly reduces channel inhibition by β-estradiol (Li et al., 2007). We examined whether the mutation has any effect on the ability of the steroid to affect ΔF generated at the L166C site. We hypothesized that if the Thr298 residue acts as a blocking element, then the T298F mutation will abolish inhibition of the current response but may still allow inhibition of ΔF generated in the extracellular domain. On the other hand, if the T298F mutation acts by preventing the binding of the steroid to the receptor, both processes (current and fluorescence) will be modulated. The data indicate that the presence of the T298F mutation similarly affected β-estradiol-mediated inhibition of the electrophysiological response and changes in fluorescence. Based on this finding we propose that the T298F mutation interferes with the ability of β-estradiol to bind to the receptor.
At present, the molecular mechanisms that underlie the effects of the steroids on fluorescence are not clear. However, the finding that steroids can have specific and distinct effects on ΔF indicates that different conformational changes accompany inhibition, bolstering the hypothesis of divergent mechanisms of action of inhibitory steroids. Future studies will undoubtedly refine the map of the GABA receptor-channel to illustrate in greater detail the conformational changes taking place in the presence of modulators.
In summary, we have examined the effects of inhibitory neurosteroids and the plant-derived inhibitor picrotoxinin on the structural rearrangements taking place during channel activation. The data indicate that the drugs have specific and unique effects on how they modify fluorescence, suggesting distinct underlying inhibitory mechanisms.
Supplementary Material
Acknowledgments
We thank Chuck Zorumski and Steve Mennerick for providing Xenopus laevis oocytes and Amanda Taylor for technical help with oocyte harvest.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant NS35291]; National Institutes of Health National Institute of General Medical Sciences [Grant GM47969]; and National Institutes of Health National Institute of Environmental Health Sciences [Grant ES16350].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.109.062885.
- 3α5βP
- pregnanolone
- 3α5βPS
- pregnanolone sulfate
- 3α5αPS
- allopregnanolone sulfate
- 3α5βPOH
- tetrahydrodeoxycorticosterone
- ΔF
- fluorescence change
- A5m
- Alexa Fluor 546 C5 maleimide.
References
- Amin J, Weiss DS. (1994) Homomeric ρ1 GABA channels: activation properties and domains. Receptors Channels 2:227–236 [PubMed] [Google Scholar]
- Boue-Grabot E, Taupignon A, Tramu G, Garret M. (2000) Molecular and electrophysiological evidence for a GABAC receptor in thyrotropin-secreting cells. Endocrinology 141:1627–1632 [DOI] [PubMed] [Google Scholar]
- Bracamontes JR, Steinbach JH. (2008) Multiple modes for conferring surface expression of homomeric β1 GABAA receptors. J Biol Chem 283:26128–26136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Weiss DS. (2002) Site-specific fluorescence reveals distinct structural changes with GABA receptor activation and antagonism. Nat Neurosci 5:1163–1168 [DOI] [PubMed] [Google Scholar]
- Chebib M, Hinton T, Schmid KL, Brinkworth D, Qian H, Matos S, Kim HL, Abdel-Halim H, Kumar RJ, Johnston GA, et al. (2009) Novel, potent, and selective GABAC antagonists inhibit myopia development and facilitate learning and memory. J Pharmacol Exp Ther 328:448–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan DS, Dibas MI, Petersson EJ, Auyeung VC, Chanda B, Bezanilla F, Dougherty DA, Lester HA. (2004) A fluorophore attached to nicotinic acetylcholine receptor βM2 detects productive binding of agonist to the αδ site. Proc Natl Acad Sci USA 101:10195–10200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston GA, Chebib M, Hanrahan JR, Mewett KN. (2003) GABAC receptors as drug targets. Curr Drug Targets CNS Neurol Disord 2:260–268 [DOI] [PubMed] [Google Scholar]
- Khatri A, Sedelnikova A, Weiss DS. (2009) Structural rearrangements in loop F of the GABA receptor signal ligand binding, not channel activation. Biophys J 96:45–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Jin X, Covey DF, Steinbach JH. (2007) Neuroactive steroids and human recombinant ρ1 GABAC receptors. J Pharmacol Exp Ther 323:236–247 [DOI] [PubMed] [Google Scholar]
- Mannuzzu LM, Moronne MM, Isacoff EY. (1996) Direct physical measure of conformational rearrangement underlying potassium channel gating. Science 271:213–216 [DOI] [PubMed] [Google Scholar]
- Muroi Y, Czajkowski C, Jackson MB. (2006) Local and global ligand-induced changes in the structure of the GABAA receptor. Biochemistry 45:7013–7022 [DOI] [PubMed] [Google Scholar]
- Muroi Y, Theusch CM, Czajkowski C, Jackson MB. (2009) Distinct structural changes in the GABAA receptor elicited by pentobarbital and GABA. Biophys J 96:499–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pless SA, Dibas MI, Lester HA, Lynch JW. (2007) Conformational variability of the glycine receptor M2 domain in response to activation by different agonists. J Biol Chem 282:36057–36067 [DOI] [PubMed] [Google Scholar]
- Szarecka A, Xu Y, Tang P. (2007) Dynamics of heteropentameric nicotinic acetylcholine receptor: implications of the gating mechanism. Proteins 68:948–960 [DOI] [PubMed] [Google Scholar]
- Yang L, Omori K, Omori K, Otani H, Suzukawa J, Inagaki C. (2003) GABAC receptor agonist suppressed ammonia-induced apoptosis in cultured rat hippocampal neurons by restoring phosphorylated BAD level. J Neurochem 87:791–800 [DOI] [PubMed] [Google Scholar]
- Zhang J, Xue F, Chang Y. (2009) Agonist- and antagonist-induced conformational changes of loop F and their contributions to the ρ1 GABA receptor function. J Physiol 587:139–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn SH, Enna SJ. (1987) The GABA agonist THIP, attenuates antinociception in the mouse by modifying central cholinergic transmission. Neuropharmacology 26:433–437 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.