Abstract
Background
Controversy exists over the optimal surgical treatment of well-differentiated thyroid cancer. Conservative surgical management reduces the risk of complications and maintains an overall survival rate equivalent to the more extensive approach.
Methods
We conducted a retrospective review of all patients with well-differentiated thyroid cancer greater than 1 cm (180 patients) who underwent surgery between 1982 and 2002 by a single general surgeon at our institution. The prevailing philosophy was to be as conservative as possible, and the predominant resection was lobectomy and isthmusectomy on the affected side.
Results
In total, 90% of patients were in a definable low-risk group: 75% had conservative surgery with 4 recurrences and no mortality, 25% had extensive surgery with 3 recurrences and no mortality. The other 10% were in a definable high-risk group: 90% had extensive surgery with 9 recurrences and 4 deaths. Overall, there were 22 sites of recurrence in 16 patients. There was no recurrence in the residual thyroid tissue, with a median follow-up of 10 years. Three recurrences occurred in the resected thyroid bed; each of these patients had undergone extensive surgery. Twelve recurrences were in lymph nodes; 67% of these patients had extensive surgery. All except 1 of 7 distant metastases occurred in the high-risk group, despite the patient having undergone extensive local surgery. Recurrence did not affect survival in the low-risk group. The extensive surgery group had a 3.4% incidence of recurrent laryngeal nerve injury and a 1.1% incidence of permanent hypocalcemia, with none in the conservative surgery group.
Conclusion
Conservative surgery for low-risk patients with well-differentiated thyroid cancer appears to be sufficient and avoids complications without significantly increased risk for local, regional or distant recurrence.
Abstract
Contexte
Le traitement chirurgical optimal d’un cancer de la thyroïde bien différencié soulève la controverse. Le traitement chirurgical conservateur réduit le risque de complications et maintient un taux de survie global équivalant à celui de l’approche plus étendue.
Méthodes
Nous avons effectué une étude rétrospective portant sur tous les patients atteints d’un cancer de la thyroïde bien différencié de plus de 1 cm (180 patients) qui ont subi, entre 1982 et 2002, une intervention chirurgicale pratiquée par le même chirurgien généraliste à notre établissement. La philosophie dominante consistait à être aussi conservateur que possible et la lobectomie et l’isthmectomie du côté atteint constituaient la principale résection.
Résultats
Au total, 90 % des patients se trouvaient dans un groupe à faible risque définissable : 75 % ont subi une intervention chirurgicale conservatrice à la suite de laquelle il y a eu quatre récurrences et aucune mortalité, 25 % ont subi une intervention chirurgicale étendue à la suite de laquelle il y a eu trois récurrences et aucune mortalité. L’autre tranche de 10 % constituait un groupe à risque élevé définissable : 90 % des patients de ce groupe ont subi une intervention chirurgicale étendue à la suite de laquelle il y a eu 9 récurrences et 4 décès. Dans l’ensemble, le cancer est réapparu à 22 endroits chez 16 patients. Il n’y a pas eu de récurrence dans le reste de tissu thyroïdien à un suivi médian de 10 ans. Il y a eu 3 récurrences dans le lit thyroïdien réséqué : chacun des patients en cause avait subi une intervention chirurgicale étendue. Les 7 métastases éloignées sauf une ont fait leur apparition chez les patients du groupe à risque élevé même si le patient avait subi une intervention chirurgicale locale étendue. La récurrence n’a pas eu d’effet sur la survie des patients du groupe à faible risque. Les patients du groupe ayant subi une intervention chirurgicale étendue présentaient une incidence de 3,4 % de répétition du traumatisme du nerf laryngien et une incidence de 1,1 % d’hypocalcémie permanente, ce qui ne s’est pas produit chez les patients ayant subi une intervention chirurgicale conservatrice.
Conclusion
L’intervention chirurgicale conservatrice chez les patients à faible risque qui ont un cancer de la thyroïde bien différencié semble suffire et évite les complications sans accroître considérablement le risque de récurrence locale, régionale ou éloignée.
Controversy exists over the optimal surgical treatment of well-differentiated thyroid cancer (WDTC). The spectrum of treatment varies from total thyroidectomy with or without modified radical neck dissection to hemithyroidectomy alone if the opposite lobe is grossly normal.
“Total” resection can potentially reduce local recurrence rates. It facilitates the monitoring of serum thyroglobulin levels during follow-up, makes it easier for radioiodine ablation if needed and eliminates possible future anaplastic transformation. However, this surgery is associated with an increased risk of complications, including permanent hypoparathyroidism and recurrent laryngeal nerve (RLN) palsy.1–3 It does not improve patient survival4–7 compared with conservative resection, and cases of anaplastic transformations are exceedingly rare.
Several centres8–11 have developed risk assessment systems in an attempt to preoperatively identify patients whose cancer can be treated with the least extensive resection, with the lowest risk of surgical complication but with adequate excision of a generally indolent malignant tumour.
Our aim was to review our experience with WDTC over a 20-year period. A very conservative approach was used in treating and following these patients, who were operated on by a single surgeon at a tertiary hospital. We support the use of this approach by comparing our results to existing literature with regard to complications, recurrence, disease-free survival and mortality.
Methods
A nonbiased investigator (M.H.) independently performed a retrospective study of all cases with a pathologic diagnosis of WDTC operated on by a single surgeon (M.W.) between 1982 and the end of 2002. In all, 199 patients were identified. We excluded patients with micropapillary tumours (measuring < 1 cm) and those lost to follow-up for more than 2 years, which left 180 patients for whom long-term data were available for analysis.
We reviewed hospital and office charts, McGill Cancer Centre database and death certificates for patient demographics, gross tumour characteristics, extent of the disease and histology. The use of thyroid suppression treatment, radioactive iodine ablation (RAI) or external beam radiation was documented. Operative reports were examined for the extent of thyroid and lymph node resection, intraoperative identification of RLN and parathyroid glands, and completeness of the intended excision.
The prevailing philosophy for surgery throughout this period was to be as conservative as possible. The predominant resection was lobectomy and isthmusectomy on the affected side if digital palpation of the opposite lobe was normal, and selective lymph node removal if it was grossly indicated. All patients received a biannual neck examination by the principle surgeon, often in conjunction with treating endocrinologists, monitoring of thyroglobulin levels and maintenance of complete thyroid suppression with the use of exogenous thyroxine. Ultrasonography of the neck or radioiodine scanning was done only when clinically indicated.
We assigned surgical resections to 1 of 4 categories: lobectomy and isthmusectomy (hemithyroidectomy); extended hemithyroidectomy, which included resection of the medial aspect or lower pole of the opposite lobe; near-total thyroidectomy if only a remnant of thyroid was left behind to protect the RLN or parathyroid; and total thyroidectomy.
Lobectomy was always accompanied by isthmusectomy. Most of the patients were in this operative category. An extended hemithyroidectomy was done if the lesion was located more medially within the thyroid lobe or when major but less dominant nodularity was felt at the lower lobe on the opposite side. Both of these operations are intended to preserve as much healthy thyroid tissue as possible and are classified as conservative resection. The latter 2 operations are intended to remove all possible thyroid tissue and are classified as extensive. We defined multifocal disease as the presence of more than one focus of disease 1 cm apart in the resected segment.
We classified lymph node dissection into 4 categories: none (if no lymph nodes were intentionally resected); selective (if lymph nodes identified intraoperatively as enlarged or suspicious were locally excised); central (if lymph nodes around the trachea [level 6] were dissected); and lateral (if a formal dissection of the lateral compartment [level 3, 4 and 5] was undertaken).
Retrospectively, we staged all patients pathologically using the TNM (tumour, nodes, metastasis) system,12 and we stratified patients clinically into a low- or high-risk group using the AMES (age, distant metastases, extent and size)8 and MACIS (metastases, age, completeness of resection, invasion, size)9 scoring systems (Box 1, Box 2).
Box 1.
AMES clinical risk stratification
Low-risk group
All patients younger than age 41 (for men) or 51 (for women) without distant metastasis
All patients older than above with disease confined to the thyroid (papillary carcinoma), or lack of extensive invasion of the capsule of the tumour (follicular carcinoma), and a primary tumour less than 5 cm in diameter, and without distant metastases
High-risk group
All other patients
AMES = age, metastasis, extent of disease, size.
Reproduced with permission from Elsevier.8 Copyright Elsevier 1988.
Box 2.
MACIS clinical risk scoring system
Score is calculated as follows
3.1 if < 40 years or 0.08 × age if 40+ years
0.3 × tumour size (cm)
1 if incomplete resection
1 if locally invasive
3 if distant metastasis
| Low-risk |
High-risk |
||
|---|---|---|---|
| Stage 1 | score < 6 | Stage 3 | score 7–8 |
| Stage 2 | score 6–7 | Stage 4 | score > 8 |
MACIS = metastasis, age, completeness of resection, invasion, size.
Reproduced with permission from Elsevier.9 Copyright Elsevier 1993.
Pathological data were based on the original reports without an independent review. We included the follicular variant of papillary thyroid cancer as a subtype of papillary cancer, and we recognized and accounted for Hürthle cell and insular subtypes separately.13–16 The presence of micro-or macroinvasion of vessels and/or the capsule was required for the diagnosis of follicular thyroid cancer, and we took the degree of invasion into consideration when we performed the statistical analysis.17,18
We defined 4 categories of recurrent disease: recurrence within the bed of the resected thyroid (TB), recurrence within the unresected (residual) thyroid tissue, lymph node recurrence and distant metastasis.
Statistical analysis
We managed the data using Microsoft Excel and SPSS 15. We calculated the disease-free and overall survival rates at 5, 10 and 20 years using Kaplan–Meier survival plots.
For disease-free survival, we performed univariate survival analyses according to the Kaplan–Meier method, with the log-rank test used for assessment of statistical significance for preoperative variables (sex, age, AMES, presence or absence of metastasis at diagnosis), intraoperative variables (extent of thyroid surgery, extent of nodal dissection and MACIS) and postoperative variables (TNM stage, vascular and/or lymphatic invasion, presence of underlying Hashimoto thyroiditis, tumour multifocality, papillary versus follicular pathologic subtypes and the presence of positive resection margins).
We performed multivariate analysis of the variables using the Cox proportional hazards model. A p value of less than 0.05 was considered significant.
Results
Demographics
Of the 180 included patients, 49 (27.2%) were men and 131 (72.8%) were women. The duration of follow-up was between 4 and 25 years, with a mean of 10 years.
Seven patients (3.9%) presented with distant metastases at the time of the initial diagnosis. Papillary thyroid cancer was present in 150 patients (83.3%), of whom 44 (29.3%) had a follicular variant. Follicular thyroid cancer was found in 24 patients (13.3%). The remaining 6 patients (3.3%) had other subtypes. The right lobe of the thyroid was affected twice as often as the left lobe.
Risk stratification AMES
According to the AMES risk stratification system, which we applied retrospectively, 161 patients (89.4%) were classified as low risk, and 19 patients (10.6%) were classified as high risk. Of those at low risk, 8 patients (5.0%) had a recurrence. Seven patients had lymph node recurrences alone and 1 had recurrence in the TB together with distant metastasis. Of those with lymph node recurrences, 3 had undergone extensive thyroid surgery, and 4 had conservative surgery. All subsequently underwent local nodal excision and were alive and disease free at the last follow-up. The patient with TB and distant metastasis recurrence was in the extensive surgery group. He received RAI and was alive and free of active disease at the last follow-up. There were no deaths in this group.
Of the 19 patients in the high-risk category, there were 8 patients (42.1%) with recurrences. All had undergone extensive surgery. Two had distant metastasis alone: 1 patient died and the other was treated successfully with RAI. Two had recurrences in TB together with a lymph node, and both died from extensive local disease. One had TB and distant metastasis and died. Three patients had recurrence in their lymph node together with distant metastasis and were treated with RAI. Five of the 19 patients (26.3%) in this group died of disease: 4 had recurrences and 1 had never become disease free.
Risk stratification MACIS
According to the MACIS risk scoring system, 144 patients (80.0%) were stage 1, and 9 patients (5.0%) were stage 2. These 85% of patients were considered low risk. Twenty-seven patients (15.0%) were in the high-risk group: 8 were stage 3 and 19 stage 4. All 5 deaths due to disease occurred in the high-risk group.
Pathology
Pathological staging
In the TNM staging system, stages I and II are considered favourable disease with a proven high survival rate that is equivalent to low-risk AMES or MACIS scores. Stages III and above are considered advanced disease with a lower survival rate, equivalent to the clinical high-risk groups.19,20
In total, 135 patients (75.0%) were in pathologically low TNM stages. There were 6 recurrences (4.4%) and no deaths among these patients. Forty-five patients (25.0%) were in high TNM stages with 10 recurrences (22.2%) and 5 deaths (11.1%), 4 within 1 year of surgery.
Histology
There was evidence of underlying lymphocytic thyroiditis (Hashimoto thyroiditis) in 44 (24.4%) patients. There was pathologic evidence of multifocal disease in 57 (31.7%) patients. Fifty-one (28.3) patients had evidence of vascular and/or lymphatic invasion (Table 1). All patients who died had either vascular and/or lymphatic invasion.
Table 1.
Clinical and pathological characteristics of patients with well-differentiated thyroid cancer
| Characteristic | No. (%) of patients, n = 180 |
|---|---|
| AMES score | |
| Low | 161 (89. 5) |
| High | 19 (10.5) |
| MACIS score | |
| Low | 153 (85.0) |
| High | 27 (15.0) |
| TNM stage | |
| Low | 135 (75.0) |
| Stage I | 106 (58.9) |
| Stage II | 29 (16.1) |
| High | 45 (25.0) |
| Stage III | 26 (14.5) |
| Stage IV | 19 (10.5) |
| Multifocality | |
| Absent | 123 (68.3) |
| Present | 57 (31.7) |
| Invasion | |
| Absent | 129 (71.6) |
| Present | 51 (38.3) |
| Vascular | 21 (11.7) |
| Lymphatic | 21 (11.7) |
| Both | 9 (5.0) |
| Hashimoto thyroiditis | |
| Absent | 136 (75.6) |
| Present | 44 (24.4) |
AMES = age, metastasis, extent of disease, size; MACIS = metastasis, age, completeness of resection, invasion, size; TNM = tumour, nodes, metastasis.
Surgery
Ninety-seven (53.9%) had lobectomy and isthmusectomy, and 29 (16.1%) had an extended hemithyroidectomy, giving a total of 126 (70.0%) patients who had conservative surgery. Twenty-nine (16.1%) patients underwent near-total thyroidectomy and 25 (13.9%) had a total thyroidectomy, resulting in 54 patients (30.0%) who had extensive surgery.
Of the 126 patients who had conservative surgery, 4 (3.2%) patients had a recurrence, all of which were in lymph nodes alone. Two of these patients were among the 107 patients with low TNM (1.9%), whereas 2 were among the 19 patients with high TNM (10.5%). All 4 patients required reoperation to resect the local lymph node recurrences. All patients in this group were alive and disease free at last follow-up. No patients had recurrence within the residual unresected thyroid gland (RT).
Of the 54 patients who underwent extensive surgery, 12 (22.2%) patients had recurrences in 19 sites. Within this group, 28 patients (51.9%) were subsequently found to have a pathologically low TNM stage. There were 6 sites of recurrences in 4 patients in this group. Two patients had lymph node recurrences treated with excision. One patient had TB and distant metastasis, and 1 patient had lymph node and distant metastasis; both of these patients received RAI. There were no deaths in this low TNM stage group.
Twenty-six patients (48.1%) in this extensive surgery group were classified as high TNM stage. Eight patients (30.7%) had 13 sites of recurrences. One patient had a lymph node metastasis treated by excision. Two patients had distant metastases, 1 of whom died. Two patients had recurrences in both the TB and lymph nodes, and both died of extensive local disease. One patient had recurrences in both the TB and distant metastasis and died. Two patients had recurrences in the lymph node and distant metastasis. All those with recurrences who were still alive at last follow-up had received RAI.
As with the AMES and MACIS scoring systems, most recurrences and all deaths from disease occurred in the high-risk group, despite the extensive surgery performed at the outset.
Lymph node management
Seventy-five (41.7%) patients had no lymph node dissection, and 73 (40.6%) had only selective local lymph node excision, giving a total of 148 (82.2%) who had conservative nodal management. Twenty-two (12.2%) patients had dissection of the central compartment and 10 (5.6%) had dissection of one lateral compartment (modified neck dissection), giving a total of 32 patients (17.9%) who had extensive nodal management.
Nodal management and AMES
Of the 161 patients in the AMES low-risk group, 138 patients (85.7%) had conservative surgical nodal management. Six of these patients (4.3%) had recurrences; all were in lymph nodes. Twenty-three (14.3%) patients had extensive nodal management. Two of these patients (8.7%) had recurrences: 1 in the lymph node and 1 with TB and distant metastasis.
Of the 19 patients in the AMES high-risk group, 10 (52.6%) had conservative nodal management. Four of these patients (40.0%) had recurrences: 3 were in the lymph node together with distant metastasis and 1 was in the lymph node and TB. Nine patients (47.4%) had extensive nodal management. Four of these patients (44.4%) had recurrences: 2 with distant metastasis, 1 with TB and lymph node and 1 with TB and distant metastasis.
Nodal management and TNM
Of the 148 patients who had conservative nodal management, 118 (79.7%) were subsequently found to be at low TNM stages. Five of these patients (4.2%) had recurrences at 6 sites: 5 were in lymph nodes and 1 was a distant metastasis. There were no deaths. Thirty patients (20.3%) were found to be at high TNM stages. Five of these patients (16.7%) had recurrences in 8 sites (1 TB, 5 lymph node and 2 distant metastases), with 1 death.
Of the 32 patients who underwent extensive nodal management, 15 (46.9%) patients were at high TNM stages. Despite extensive nodal management, 5 of these patients (33.3%) had recurrences at 7 sites (2 TB, 2 lymph node and 3 distant metastases), with 4 deaths. Seventeen patients (53.1%) were at low TNM stages. One patient had recurrence in 2 sites (TB and distant metastasis); there were no deaths.
Other treatment modalities
Fifty-one (28.3%) patients received RAI, 3 (1.7%) patients received external beam radiation, and 9 (5.0%) patients received both therapies. These additional treatments where given to high-risk patients who presented with extensive local disease or distant metastasis.
Complications
There were no local wound complications (hematoma or infection).
Postoperative RLN paresis occurred in 6 (3.3%) patients whose disease was diagnosed by clinical symptoms and confirmed by laryngoscopy. Routine pre- or postoperative laryngoscopy was not preformed unless clinically indicated or reoperation was required. These patients were followed up to document recovery. Five patients of the 6 patients were in the extensive surgery group: 3 had permanent RLN (1 case occurred secondary to reoperation for recurrence) and 2 cases were temporary. One of the 6 patients was in the conservative group and had temporary RLN. There were no cases of bilateral nerve paresis.
All patients had routine measurement of corrected calcium levels in the recovery room and on the morning after the operation. Twelve patients (6.7%) had hypocalcaemia that required treatment either because of classic symptoms or in response to low and falling corrected serum calcium levels. Two (1.1%) had permanently low calcium levels and required lifelong supplementation; both of these patients had total thyroidectomy. The remainder had transiently low calcium levels: 6 of these patients were in the extensive surgery group and 4 were in the conservative surgery group.
Disease-free survival
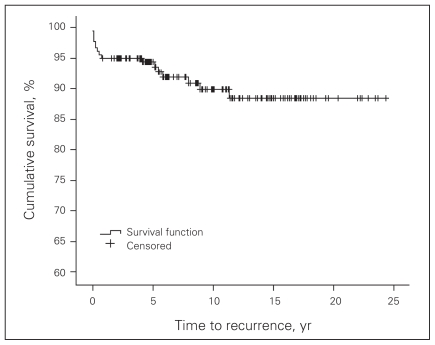
Over a follow-up period of 4–25 years (mean 10 yr), 16 patients (8.9%) had clinical recurrence and 1 (0.6%) was never declared disease free. Seven patients (3.9%) had reoperation; all were for lymph node recurrence. Disease-free survival was 94.5% at 5 years, 90% at 10 years and 88% at 20 years (Fig. 1).
Fig. 1.
Disease-free survival Kaplan–Meier curve for patients with well-differentiated thyroid cancer.
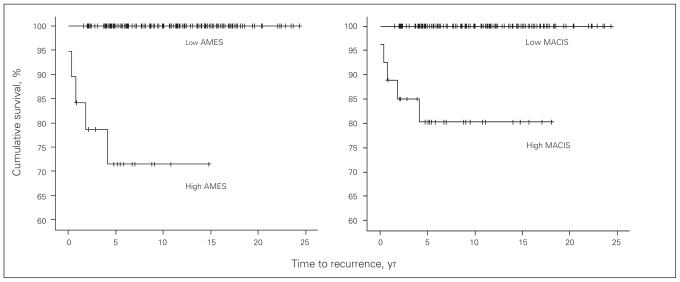
Univariate analysis of disease-free survival showed that the folloving variables were significant: AMES, metastasis at presentation, extent of thyroid and nodal surgery, MACIS, TNM, vascular invasion and positive margins (Table 2, Fig. 2).
Table 2.
Univariate analysis of 20-year disease-free survival
| Variable | Survival, % | p value |
|---|---|---|
| Preoperative | ||
| Sex | 0.14 | |
| Male | 82 | |
| Female | 91 | |
| Age, yr | 0.24 | |
| ≤ 45 | 91.5 | |
| > 45 | 85 | |
| AMES score | < 0.001 | |
| Low | 93.4 | |
| High | 39.3 | |
| Metastasis at presentation | < 0.001 | |
| Absent | 90.8 | |
| Present | 32.3 | |
| Intraoperative | ||
| Extent of thyroid surgery | < 0.001 | |
| Conservative | 96 | |
| Extensive | 71 | |
| Extent of nodal surgery | < 0.001 | |
| Conservative | 93.8 | |
| Extensive | 67.7 | |
| MACIS score | < 0.001 | |
| Low | 94.8 | |
| High | 51.6 | |
| Postoperative | ||
| TNM stage | < 0.001 | |
| Low | 94.2 | |
| High | 71.3 | |
| Invasion | 0.08 | |
| Absent | 92.7 | 0.052 |
| Vascular | 84 | 0.008 |
| Lymphatic | 81.7 | 0.27 |
| Both | 62.4 | 0.30 |
| Hashimoto thyroiditis | 0.08 | |
| Absent | 85.9 | |
| Present | 97 | |
| Multifocality | 0.24 | |
| Absent | 90.6 | |
| Present | 83.6 | |
| Pathologic subtype | 0.68 | |
| Papillary | 86.9 | |
| Follicular | 94.7 | |
| Margins | ||
| Negative | 90.6 | 0.015 |
| Positive | 68.9 | |
AMES = age, metastasis, extent of disease, size; MACIS = metastasis, age, completeness of resection, invasion, size; TNM = tumour, nodes, metastasis.
Fig. 2.
Disease-free survival of patients with high or low AMES (left) and MACIS (right) scores. Note: AMES = age, metastasis, extent of disease, size; MACIS = metastasis, age, completeness of resection, invasion, size.
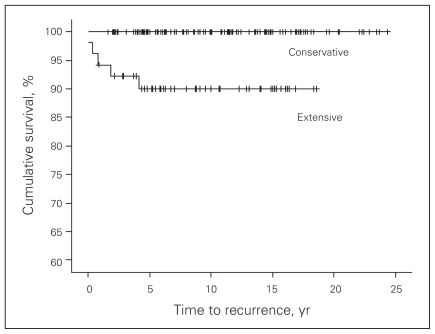
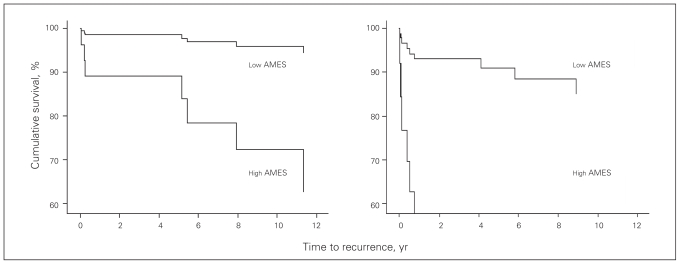
When the extent of thyroid (Fig. 3) or nodal surgery was considered (Fig. 4), more extensive was associated with worse outcomes.
Fig. 3.
Disease-free survival and extent of thyroid surgery.
Fig. 4.
Relation between disease-free survival and high and low AMES scores for conservative surgery (left) and extensive surgery (right). Note: AMES = age, metastasis, extent of disease, size.
Multivariate analyses
We found that AMES and the presence of metastasis at presentation were the only preoperative variables significant for recurrence in the univariate analysis. We performed Cox regression analysis, which found only AMES to be significant (p < 0.001).
We individually tested AMES, MACIS and TNM scores against all other significant variables. We found that AMES and MACIS were significant (p = 0.023, p = 0.02, respectively); TNM stage was not significant (Appendix 1, 2 and 3, available at www.cma.ca/cjs).
Mortality
There were 5 deaths (2.8%) from the disease; 4 of these were within the first year of diagnosis. Four female patients died. Three were below the age of 45 years. All had a high-risk AMES score, and 3 had distant metastases at presentation. All of these patients received extensive thyroid surgery, and 3 had extensive nodal surgery (papillary cancer). All received RAI. Postoperatively, they all were classified as MACIS stage 4 (high risk), and 1 had a positive posterior margin.
One 38-year-old male patient died. He had a high-risk AMES score and metastasis at presentation. He underwent extensive thyroid and nodal surgery for papillary cancer combined with RAI. Postoperatively, he was classified as MACIS stage 4, TNM stage II and had negative margins
Discussion
Risk groups, recurrence and mortality
The adequacy of conservative surgical resection for micro-carcinoma (subcentimetric) has been confirmed by multiple studies.6,21–23 Unilateral lobectomy plus isthmusectomy is sufficient treatment despite the multifocality of papillary carcinoma and the knowledge that microscopic carcinoma will often be present in the opposite lobe on autopsy studies. We therefore excluded these patients from our study. In contrast to their previous study in 1992,24 a recent review from the Mayo clinic involving 900 patients with microcarcinoma treated from 1945 to 2004 confirmed that locoregional recurrence after unilateral lobectomy did not differ significantly from those seen after bilateral resections for lesions smaller than 1 cm. The data for larger lesions are almost identical.25–28
Total thyroidectomy is practised by most experienced thyroid surgeons and is recommended by the American Association of Endocrine Surgery.29 Hay and colleagues30,31 found significantly elevated rates of local or regional recurrences in patients who underwent unilateral resection when compared with patients who underwent bilateral resection and total thyroidectomy. These findings have been supported by others.4,27,32 However, there is evidence to suggest that the extent of thyroidectomy can be tailored to the individual based on clinical stratification systems. Identification of such risk groups come from studies at numerous thyroid cancer centres, including The European Organization for Research and Treatment of Cancer Thyroid Cancer Cooperative Group,33 Sugitani and colleagues from Japan,34 Mayo Clinic,9 Lahey Clinic,8,35 University of Chicago,10 National Thyroid Cancer Treatment Cooperative Study,11 University of Alabama and M.D. Anderson,32 and Memorial Sloan–Kettering Cancer Center.36,37
Despite the differences between various classification systems, most patients (85%–90%) are at very low risk for both recurrence and death regardless of the extent of local treatment. In addition, a large number of patients with benign disease such as adenoma are needlessly subjected to total thyroidectomy because frozen sections are inadequate to reliably distinguish between follicular adenoma and well-differentiated follicular carcinoma, a distinction of questionable clinical relevance in most instances. It is hard to justify an extensive aggressive surgical approach for all patients.
Our study population is similar to that at most centres. There was a marked (73%) female predominance, 75% of patients had papillary thyroid cancer, and 75%–90% of patients were at low risk for recurrence (when examined retrospectively using the various staging systems).
More than 75% of our AMES low-risk patients underwent conservative surgical management, with a resulting recurrence rate of 3%. The remaining 23% had extensive surgery based on “clinical judgment and intraoperative findings.” These patients had a recurrence rate of 11% despite the more extensive surgery, which is undoubtedly a reflection of selection bias; however, the recurrence sites in all except 1 patient were in lymph nodes. This higher recurrence rate with extensive surgery may also reflect the small sample size in this subgroup of patients, and could also indicate weaknesses in each individual staging system.17,18,38,39 There were no clinical recurrences at last follow up in the unresected opposite thyroid lobe. Our recurrence rate is consistent with most reported series with radical surgery.40 The indolent biology of this cancer, especially in the clinically defined low-risk group, explains the rarity of clinically significant metastasis and the absence of death even with local recurrence.28
In contrast, 17 of the 19 patients retrospectively classified as high risk with the AMES system underwent extensive surgical procedures based on clinical judgment at the time. Despite this, 47% had recurrent disease in 14 sites. Recurrence in this group was also associated with high mortality, again not affected by aggressive surgical resection.
Clinical judgment led to conservative surgery in 70% of our patients. Of these, 85% were at pathologically low TNM stages. Recurrence in this group occurred in only 2 patients (1.8%), compared with a recurrence of 10.5% in the 19 patients with high TNM stages. All recurrences in both groups were in lymph nodes.
Clinical judgment led to extensive surgery in 30% of patients, of whom half (52%) were at a low TNM stage with a recurrence rate of 14%. This is explained by the fact that this category contained some patients with locally advanced thyroid cancer or cancers with distant metastasis who were younger than age 45, which led to their classification as low TNM stage. This group of patients is actually in the high-risk AMES group, which is more concordant with their high recurrence rate; however, there were no deaths. This is consistent with our regression analyses, which found AMES to correlate better than TNM staging with disease-free survival. The remaining 48% of those who underwent extensive surgery had a high TNM stage. Despite extensive surgery, the recurrence rate was 30.7%.
More than half of all recurrences in our patients were in lymph nodes (Table 3), which raises the question of the adequacy of nodal removal and its influence on subsequent outcome and survival. Eighty-six percent of low-risk AMES patients had conservative nodal management, with a 4.3% recurrence rate. All recurrences were in lymph nodes and were easily treated with local excision without complications. Similarly, conservative nodal surgery was performed in 80% of patients with a low TNM stage, with a 4.2% recurrence rate. Five of the 6 sites of recurrence were nodal.
Table 3.
Correlation of recurrences, AMES risk stratification and extent of gland surgery
| Recurrence site | Extent of gland surgery; no. of patients | |
|---|---|---|
| Extensive | Conservative | |
| Residual thyroid tissue, n = 0 | ||
| Low-risk AMES score | — | — |
| High-risk AMES score | — | — |
| Resected thyroid tissue, n = 3 | ||
| Low-risk AMES score | 1 | 0 |
| High-risk AMES score | 2 | 0 |
| Lymph nodes, n = 12 | ||
| Low-risk AMES score | 3 | 4 |
| High-risk AMES score | 5 | 0 |
| Distant metastases, n = 7 | ||
| Low-risk AMES score | 1 | 0 |
| High-risk AMES score | 6 | 0 |
AMES = age, distant metastases, extent and size.
In contrast, about half of the high-risk AMES patients had extensive nodal surgery, with a 44.4% recurrence rate despite the extensiveness of their nodal surgeries. Similarly, 47% of all patients who underwent extensive nodal management had a high TNM stage; this group of patients had a recurrence rate of 33.3% despite this aggressive nodal management. In all instances, extensive nodal management reduced the recurrence in lymph nodes but did not eliminate it.
In our multivariate analysis, nodal management was not a significant factor for recurrence. Conservative nodal management resulted in a higher number of lymph node recurrences when compared with the extensive approach. The higher lymph node recurrence in low TNM patients was associated with no mortality. High TNM stage patients had high recurrence and mortality despite the extensive surgery to the gland and/or nodes, and the reduction in lymph node recurrence was not associated with better disease-free survival in this group.
In our univariate analysis, age, different risk stratification systems and the presence of metastasis at presentation were significant predictors of recurrence. Only AMES and MACIS were significant in the multivariate analysis. However, AMES stratification is clinically more valuable because it is available to the clinician for decision-making in the preoperative period. However, MACIS can be a valuable guide to plan further treatment such as RAI.
We found that extensive gland surgery and extensive nodal surgery were associated with a significant increase in recurrence and poorer outcomes (Table 2). This is an expression of the selection bias in treating patients with more advanced or aggressive disease and was not significant in the multivariate analysis. In total, there were 22 sites of recurrence in 16 patients. Extensive gland surgery was performed in all except 4 lymph node recurrences.
The presence of positive margins was a significant variable for recurrence in the univariate analysis (Table 2). However, this was only true for patients who had advanced-stage disease. Other variables such as site, sex, age, the presence of Hashimoto thyroiditis, the presence of follicular variant of papillary and histological subtype did not show any significant relation to disease-free survival. The need for reoperation for nodal recurrence did not change mortality.
Although our follow-up period ranged from 4 to 25 years, our mean follow-up was only 10 years. Disease recurrence continues to occur during the lifetime of the individual, as with all cancers. However, as seen from our study and most others, most recurrences are seen within the first 5 years of initial presentation.5,41,42 The possibility of a missed subclinical recurrence exists in our patients based on our conservative follow-up protocol. However, these recurrences are of a questionable clinical significance, as recently reviewed by Hay and colleagues.28
Shaha and coworkers37,43 reviewed their experience with low-risk WDTC over a long period. They found no difference in local or regional recurrence rates or in long-term survival for patients who underwent partial lobectomy, total lobectomy or total thyroidectomy. Cady and colleagues42,44 found similar results when they reviewed their data. Haigh and colleagues7 reviewed 5432 patients and found similar survival rates regardless of whether patients underwent total or partial thyroidectomy for the same risk group. Yildirim45 found no survival advantage of extensive operations in patients classified as having favourable disease in his own model for predicting outcomes. Gemsenjager and colleagues3,32 also showed no survival advantage during a 25-year follow-up period for lymph node dissection or extensive thyroid resection in low-risk patients. Similar results have been reported for follicular cancer when evaluated seperately.19,46
The failure to improve survival with the use of RAI as reported by numerous investigators28,45 eliminates another argument in support of total thyroidectomy (i.e., to facilitate ablation).
Surgical complications
The procedure of thyroidectomy had prohibitive morbidity and mortality in the decades before Kocher, who received a Nobel Prize in 1909 for his contribution to the understanding of thyroid physiology and thyroid surgery. Complications were principally related to infection and intraoperative hemorrhage, and mortality was about 40%.47
Today, the frequency of surgical complications is comparatively small in the hands of an experienced thyroid surgeon. Nonetheless, bilateral lobectomy and total thyroidectomy have a substantial risk (1%–3%) of RLN paralysis1 and loss of blood supply to the parathyroid gland.1, 48–58 Whereas the latter is often transient, it carries a 20%–30% frequency, even in contemporary literature, with permanent hypocalcaemia reported in as many as 8% of patients.48,49,51,52 Moreover, it is unknown whether these results are generalizable to the average general surgeon if total thyroidectomy is advocated for all patients. We had no patients with permanent hypoparathyroidism or permanent RLN in the conservative surgery group or in the conservative nodal surgery group.
Wound infection (< 1%) and hematoma (1%–2%)56,58,59 are rare. Meticulous dissection and hemostasis resulted in no local wound complications in any of our patients.
Conclusion
Our results from this retrospective review are similar to most reported series in terms of risk stratification, disease-free survival and overall survival, despite our conservative surgical approach, and with very low complication rate. Recurrence in unresected thyroid glands was negligible even in cases of multifocal disease.
It appears that conservative surgery for low-risk patients with WDTC is adequate and avoids complications without a significantly increased risk for local, regional or distant recurrence. Lymph node recurrence is easily managed by local excision. Whereas high-risk patients require an aggressive surgical approach, it is not without high local recurrence and relatively high mortality.
Biological factors and initial risk stratification are important determinants of outcome and not the extent of surgery required.
Footnotes
Preliminary results were presented as an oral presentation at the Canadian Surgery Forum in Montréal, Que., Sept. 8–11, 2009. Abstract published in Can J Surg 2005;48(Suppl):29.
Competing interests: None declared.
Contributors: Dr. Wexler designed the study, and Dr. Hassanain acquired the data. Both authors analyzed the data, wrote and reviewed the article and approved its publication.
References
- 1.Jatzko GR, Lisborg PH, Muller MG, et al. Recurrent nerve palsy after thyroid operations — principal nerve identification and a literature review. Surgery. 1994;115:139–44. [PubMed] [Google Scholar]
- 2.Moulton-Barrett R, Crumley R, Jalilie S, et al. Complications of thyroid surgery. Int Surg. 1997;82:63–6. [PubMed] [Google Scholar]
- 3.Filho JG, Kowalski LP. Postoperative complications of thyroidectomy for differentiated thyroid carcinoma. Am J Otolaryngol. 2004;25:225–30. doi: 10.1016/j.amjoto.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Mazzaferri EL. An overview of the management of papillary and follicular thyroid carcinoma. Thyroid. 1999;9:421–7. doi: 10.1089/thy.1999.9.421. [DOI] [PubMed] [Google Scholar]
- 5.Shaha AR, Shah JP, Loree TR. Low-risk differentiated thyroid cancer: the need for selective treatment. Ann Surg Oncol. 1997;4:328–33. doi: 10.1007/BF02303583. [DOI] [PubMed] [Google Scholar]
- 6.Shaha AR. Thyroid cancer: extent of thyroidectomy. Cancer Control. 2000;7:240–5. doi: 10.1177/107327480000700303. [DOI] [PubMed] [Google Scholar]
- 7.Haigh PI, Urbach DR, Rotstein LE. Extent of thyroidectomy is not a major determinant of survival in low- or high-risk papillary thyroid cancer. Ann Surg Oncol. 2005;12:81–9. doi: 10.1007/s10434-004-1165-1. [DOI] [PubMed] [Google Scholar]
- 8.Cady B, Rossi R. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery. 1988;104:947–53. [PubMed] [Google Scholar]
- 9.Hay ID, Bergstralh EJ, Goellner JR, et al. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993;114:1050–7. discussion 7–8. [PubMed] [Google Scholar]
- 10.DeGroot LJ, Kaplan EL, McCormick M, et al. Natural history, treatment, and course of papillary thyroid carcinoma. J Clin Endocrinol Metab. 1990;71:414–24. doi: 10.1210/jcem-71-2-414. [DOI] [PubMed] [Google Scholar]
- 11.Sherman SI, Brierley JD, Sperling M, et al. Prospective multicenter study of thyroid carcinoma treatment: initial analysis of staging and outcome. National Thyroid Cancer Treatment Cooperative Study Registry Group. Cancer. 1998;83:1012–21. doi: 10.1002/(sici)1097-0142(19980901)83:5<1012::aid-cncr28>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 12.Greene FL, Page DL, Fleming ID, et al., editors. American Joint Committee on Cancer. AJCC cancer staging handbook: from the AJCC cancer staging manual. 6th ed. New York (NY): Springer; 2002. [Google Scholar]
- 13.Liu J, Singh B, Tallini G, et al. Follicular variant of papillary thyroid carcinoma: a clinicopathologic study of a problematic entity. Cancer. 2006;107:1255–64. doi: 10.1002/cncr.22138. [DOI] [PubMed] [Google Scholar]
- 14.Pellegriti G, Giuffrida D, Scollo C, et al. Long-term outcome of patients with insular carcinoma of the thyroid: the insular histotype is an independent predictor of poor prognosis. Cancer. 2002;95:2076–85. doi: 10.1002/cncr.10947. [DOI] [PubMed] [Google Scholar]
- 15.Mills SC, Haq M, Smellie WJ, Harmer C. Hürthle cell carcinoma of the thyroid: retrospective review of 62 patients treated at the Royal Marsden Hospital between 1946 and 2003. Eur J Surg Oncol. 2009;35:230–4. doi: 10.1016/j.ejso.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Stojadinovic A, Hoos A, Ghossein RA, et al. Hurthle cell carcinoma: a 60-year experience. Ann Surg Oncol. 2002;9:197–203. doi: 10.1007/BF02557374. [DOI] [PubMed] [Google Scholar]
- 17.Furlan JC, Bedard YC, Rosen IB. Clinicopathologic significance of histologic vascular invasion in papillary and follicular thyroid carcinomas. J Am Coll Surg. 2004;198:341–8. doi: 10.1016/j.jamcollsurg.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 18.D’Avanzo A, Treseler P, Ituarte PH, et al. Follicular thyroid carcinoma: histology and prognosis. Cancer. 2004;100:1123–9. doi: 10.1002/cncr.20081. [DOI] [PubMed] [Google Scholar]
- 19.Lin JD, Chao TC, Chen ST, et al. Operative strategy for follicular thyroid cancer in risk groups stratified by pTNM staging. Surg Oncol. 2007;16:107–13. doi: 10.1016/j.suronc.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Lo CY, Chan WF, Lam KY, et al. Follicular thyroid carcinoma: the role of histology and staging systems in predicting survival. Ann Surg. 2005;242:708–15. doi: 10.1097/01.sla.0000186421.30982.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wein RO, Weber RS. Contemporary management of differentiated thyroid carcinoma. Otolaryngol Clin North Am. 2005;38:161–78. doi: 10.1016/j.otc.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Haas SN. Management of papillary microcarcinoma of the thyroid. S D Med. 2006;59:425–7. [PubMed] [Google Scholar]
- 23.Topliss D. Thyroid incidentaloma: the ignorant in pursuit of the impalpable. Clin Endocrinol (Oxf) 2004;60:18–20. doi: 10.1111/j.1365-2265.2004.01956.x. [DOI] [PubMed] [Google Scholar]
- 24.Hay ID, Grant CS, van Heerden JA, et al. Papillary thyroid micro-carcinoma: a study of 535 cases observed in a 50-year period. Surgery. 1992;112:1139–46. discussion 46–7. [PubMed] [Google Scholar]
- 25.Loh KC, Greenspan FS, Gee L, et al. Pathological tumor-node-metastasis (pTNM) staging for papillary and follicular thyroid carcinomas: a retrospective analysis of 700 patients. J Clin Endocrinol Metab. 1997;82:3553–62. doi: 10.1210/jcem.82.11.4373. [DOI] [PubMed] [Google Scholar]
- 26.Tsang RW, Brierley JD, Simpson WJ, et al. The effects of surgery, radioiodine, and external radiation therapy on the clinical outcome of patients with differentiated thyroid carcinoma. Cancer. 1998;82:375–88. [PubMed] [Google Scholar]
- 27.Bilimoria KY, Bentrem DJ, Ko CY, et al. Extent of surgery affects survival for papillary thyroid cancer. Ann Surg. 2007;246:375–81. doi: 10.1097/SLA.0b013e31814697d9. discussion 81–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hay ID, Hutchinson ME, Gonzalez-Losada T, et al. Papillary thyroid microcarcinoma: a study of 900 cases observed in a 60-year period. Surgery. 2008;144:980–7. doi: 10.1016/j.surg.2008.08.035. discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 29.Cobin RH, Gharib H, Bergman DA, et al. AACE/AAES medical/surgical guidelines for clinical practice: management of thyroid carcinoma. American Association of Clinical Endocrinologists. American College of Endocrinology. Endocr Pract. 2001;7:202–20. [PubMed] [Google Scholar]
- 30.Hay ID, Grant CS, Bergstralh EJ, et al. Unilateral total lobectomy: Is it sufficient surgical treatment for patients with AMES low-risk papillary thyroid carcinoma. Surgery. 1998;124:958–64. discussion 64–6. [PubMed] [Google Scholar]
- 31.Hay ID, Bergstralh EJ, Grant CS, et al. Impact of primary surgery on outcome in 300 patients with pathologic tumor-node-metastasis stage III papillary thyroid carcinoma treated at one institution from 1940 through 1989. Surgery. 1999;126:1173–81. doi: 10.1067/msy.2099.101435. discussion 81–2. [DOI] [PubMed] [Google Scholar]
- 32.Beenken S, Roye D, Weiss H, et al. Extent of surgery for intermediate-risk well-differentiated thyroid cancer. Am J Surg. 2000;179:51–6. doi: 10.1016/s0002-9610(99)00254-8. [DOI] [PubMed] [Google Scholar]
- 33.Byar DP, Green SB, Dor P, et al. A prognostic index for thyroid carcinoma. A study of the E.O.R.T.C. Thyroid Cancer Cooperative Group. Eur J Cancer. 1979;15:1033–41. doi: 10.1016/0014-2964(79)90291-3. [DOI] [PubMed] [Google Scholar]
- 34.Sugitani I, Kasai N, Fujimoto Y, et al. A novel classification system for patients with PTC: addition of the new variables of large (3 cm or greater) nodal metastases and reclassification during the follow-up period. Surgery. 2004;135:139–48. doi: 10.1016/s0039-6060(03)00384-2. [DOI] [PubMed] [Google Scholar]
- 35.Hundahl SA, Fleming ID, Fremgen AM, et al. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995. Cancer. 1998;83:2638–48. doi: 10.1002/(sici)1097-0142(19981215)83:12<2638::aid-cncr31>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 36.Shah JP, Loree TR, Dharker D, et al. Prognostic factors in differentiated carcinoma of the thyroid gland. Am J Surg. 1992;164:658–61. doi: 10.1016/s0002-9610(05)80729-9. [DOI] [PubMed] [Google Scholar]
- 37.Shaha AR, Loree TR, Shah JP. Intermediate-risk group for differentiated carcinoma of thyroid. Surgery. 1994;116:1036–40. discussion 40–1. [PubMed] [Google Scholar]
- 38.Jukkola A, Bloigu R, Ebeling T, et al. Prognostic factors in differentiated thyroid carcinomas and their implications for current staging classifications. Endocr Relat Cancer. 2004;11:571–9. doi: 10.1677/erc.1.00826. [DOI] [PubMed] [Google Scholar]
- 39.Lang BH, Lo CY, Chan WF, et al. Prognostic factors in papillary and follicular thyroid carcinoma: their implications for cancer staging. Ann Surg Oncol. 2007;14:730–8. doi: 10.1245/s10434-006-9207-5. [DOI] [PubMed] [Google Scholar]
- 40.Hay ID, Thompson GB, Grant CS, et al. Papillary thyroid carcinoma managed at the Mayo Clinic during six decades (1940–1999): temporal trends in initial therapy and long-term outcome in 2444 consecutively treated patients. World J Surg. 2002;26:879–85. doi: 10.1007/s00268-002-6612-1. [DOI] [PubMed] [Google Scholar]
- 41.Schlumberger M. Papillary and follicular thyroid carcinoma. N Engl J Med. 1998;338:297–306. doi: 10.1056/NEJM199801293380506. [DOI] [PubMed] [Google Scholar]
- 42.Cady B. Papillary carcinoma of the thyroid gland: treatment based on risk group definition. Surg Oncol Clin N Am. 1998;7:633–44. [PubMed] [Google Scholar]
- 43.Shaha AR. Implications of prognostic factors and risk groups in the management of differentiated thyroid cancer. Laryngoscope. 2004;114:393–402. doi: 10.1097/00005537-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 44.Sanders LE, Cady B. Differentiated thyroid cancer: reexamination of risk groups and outcome of treatment. Arch Surg. 1998;133:419–25. doi: 10.1001/archsurg.133.4.419. [DOI] [PubMed] [Google Scholar]
- 45.Yildirim E. A model for predicting outcomes in patients with differentiated thyroid cancer and model performance in comparison with other classification systems. J Am Coll Surg. 2005;200:378–92. doi: 10.1016/j.jamcollsurg.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 46.Thompson LD, Wieneke JA, Paal E, et al. A clinicopathologic study of minimally invasive follicular carcinoma of the thyroid gland with a review of the English literature. Cancer. 2001;91:505–24. doi: 10.1002/1097-0142(20010201)91:3<505::aid-cncr1029>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 47.Giddings AE. The history of thyroidectomy. J R Soc Med. 1998;91(Suppl 33):3–6. doi: 10.1177/014107689809133s02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shindo ML, Sinha UK, Rice DH. Safety of thyroidectomy in residency: a review of 186 consecutive cases. Laryngoscope. 1995;105:1173–5. doi: 10.1288/00005537-199511000-00006. [DOI] [PubMed] [Google Scholar]
- 49.Herranz-Gonzalez J, Gavilan J, Matinez-Vidal J, et al. Complications following thyroid surgery. Arch Otolaryngol Head Neck Surg. 1991;117:516–8. doi: 10.1001/archotol.1991.01870170062014. [DOI] [PubMed] [Google Scholar]
- 50.Tovi F, Noyek AM, Chapnik JS, et al. Safety of total thyroidectomy: review of 100 consecutive cases. Laryngoscope. 1989;99:1233–7. doi: 10.1288/00005537-198912000-00005. [DOI] [PubMed] [Google Scholar]
- 51.de Roy van Zuidewijn DB, Songun I, Kievit J, et al. Complications of thyroid surgery. Ann Surg Oncol. 1995;2:56–60. doi: 10.1007/BF02303703. [DOI] [PubMed] [Google Scholar]
- 52.Scanlon EF, Kellogg JE, Winchester DP, et al. The morbidity of total thyroidectomy. Arch Surg. 1981;116:568–71. doi: 10.1001/archsurg.1981.01380170050009. [DOI] [PubMed] [Google Scholar]
- 53.Prim MP, de Diego JI, Hardisson D, et al. Factors related to nerve injury and hypocalcemia in thyroid gland surgery. Otolaryngol Head Neck Surg. 2001;124:111–4. doi: 10.1067/mhn.2001.112305. [DOI] [PubMed] [Google Scholar]
- 54.Steinmuller T, Klupp J, Wenking S, et al. Complications associated with different surgical approaches to differentiated thyroid carcinoma. Langenbecks Arch Surg. 1999;384:50–3. doi: 10.1007/s004230050173. [DOI] [PubMed] [Google Scholar]
- 55.Wingert DJ, Friesen SR, Iliopoulos JI, et al. Post-thyroidectomy hypocalcemia. Incidence and risk factors. Am J Surg. 1986;152:606–10. doi: 10.1016/0002-9610(86)90435-6. [DOI] [PubMed] [Google Scholar]
- 56.Bergamaschi R, Becouarn G, Ronceray J, et al. Morbidity of thyroid surgery. Am J Surg. 1998;176:71–5. doi: 10.1016/s0002-9610(98)00099-3. [DOI] [PubMed] [Google Scholar]
- 57.Bhattacharyya N, Fried MP. Assessment of the morbidity and complications of total thyroidectomy. Arch Otolaryngol Head Neck Surg. 2002;128:389–92. doi: 10.1001/archotol.128.4.389. [DOI] [PubMed] [Google Scholar]
- 58.Moulton-Barrett R, Crumley R, Jalilie S, et al. Complications of thyroid surgery. Int Surg. 1997;82:63–6. [PubMed] [Google Scholar]
- 59.Shaha A, Jaffe BM. Complications of thyroid surgery performed by residents. Surgery. 1988;104:1109–14. [PubMed] [Google Scholar]