Summary of recent advances
In mammals, auditory perception is initially mediated through sensory cells located in a rigorously patterned mosaic of unique cell types located within the coiled cochlea. Identification of the factors that direct multipotent progenitor cells to develop as each of these specialized cell types has the potential to enhance our understanding of the development of the auditory system and to identify potential targets for regenerative therapies. Recent results have identified specific signaling molecules and pathways, including Notch, Hedgehog, Sox2 and Fgfs, that guide progenitor cells to develop first as a sensory precursor, referred to as a prosensory cell, and subsequently as one of the specialized cell types within the sensory mosaic.
Introduction
In mammals, the snail-like cochlea located in the ventral region of the inner ear serves as the primary auditory sensory organ. The structure of the cochlear duct represents a remarkable achievement in developmental patterning and regulation. While the cochlea can extend to lengths greater than 60 mm in particularly large animals, the width of the sensory epithelium rarely exceeds 100 μm (1). Moreover, the sensory epithelium is comprised of mechanosensory hair cells and associated non-sensory supporting cells that are arrayed in a rigorous mosaic of regular rows that extends along the length of the cochlear duct (Fig. 1). The factors that regulate the formation of this structure from a population of otic progenitor cells remain largely unknown; however recent results have provided valuable insights regarding the signaling pathways and cellular interactions that are required for cochlear development.
Figure 1.
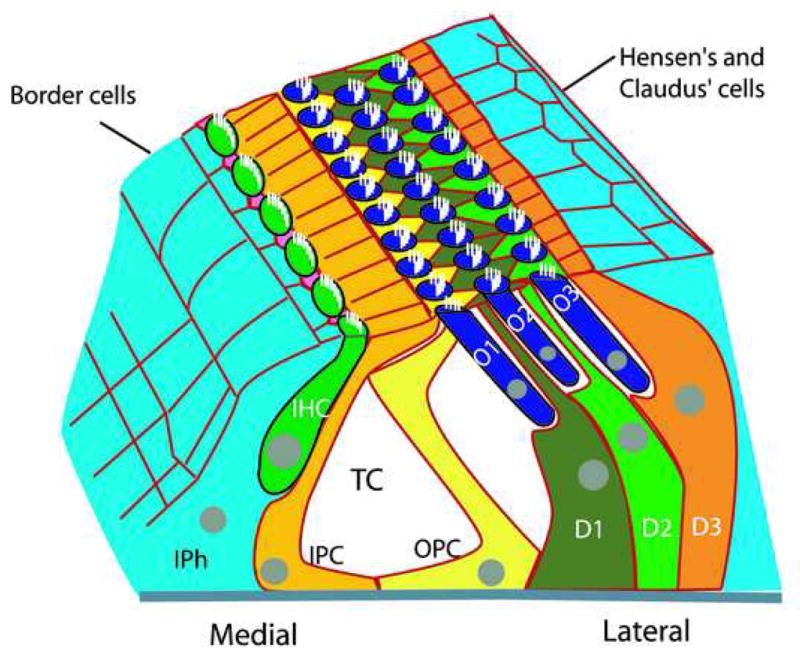
Three-Dimensional depiction of the mammalian auditory sensory epithelium (the organ of Corti). The sensory epithelium extends along the full length of the cochlear spiral and as a result has a medial-to-lateral axis (relative to the spiral) as noted. The epithelium is asymmetrically patterned with a single inner hair cell (IHC, green) and inner phalangeal cells (IPh, pink) on the medial boundary followed by inner and outer pillar cells (IPC, gold and OPC, yellow)), and three rows of outer hair cells (O1–O3, blue) and Deiters’ cells (D1–D3, green and orange). The single rows of IPCs and OPCs form walls of the tunnel of Corti (TC). Border cells (light blue) are located medial to inner hair cells and Hensen’s and Claudius’ cells are located lateral to outer hair cells.
Specification of prosensory cells
Virtually all of the cell types within the membranous labyrinth of the inner ear are derived from multipotent epithelial progenitor cells initially located in the otocyst (Fig. 1). Otocyst-derived cells develop into three major lineages, prosensory (cells that will develop as either hair cells or associated supporting cells), proneural (cells that will develop as auditory or vestibular neurons), and nonsensory (all other otocyst derived cells) with cells within each lineage developing in topologically and temporally defined domains of the otocyst (2–6). Cells within the prosensory lineage are thought to possess a unique ability to develop as hair cells or supporting cells, however, recent studies (to be discussed below) have suggested that specification as a prosensory cell may not be absolutely required for hair cell or supporting cell formation.
The precise timing of the specification of prosensory cells remains unclear, however expression of Jagged1, Lfng and Bmp4, all of which mark prosensory patches to some extent, can be detected in discrete patches of the otocyst by E10 in the mouse, suggesting at least some level of prosensory identity at that time and identifying several factors as candidates for induction of prosensory fate (7). Deletion of either Lfng or Bmp4 does not lead to loss of hair cells or supporting cells (8,9), however, reduced function or complete deletion of Jagged1 results in the reduction or absence of most of the prosensory cells within the ear (10–13). These results demonstrate an early role for Notch signaling via Jagged1 in prosensory specification, a conclusion that is supported by the observation that inhibition of Notch activity by the gamma-secretase inhibitor, DAPT, in vitro leads to loss of prosensory marker expression (14–16). Moreover, ectopic expression of a constitutively active form of Notch1 (Notch1 intracellular domain, (NICD)) leads to the expression of prosensory markers in embryonic mammalian cochlea (16) and to the induction of ectopic sensory patches in developing chick inner ear (17). Together, these results indicate a role for Jagged1-dependent Notch activation in specification of prosensory identity and subsequent formation of sensory patches. These results also demonstrate dual roles for Notch signaling in inner ear development; an initial role in induction of prosensory patches followed by a second, well established, role in the regulation of lateral inhibition between hair cells and supporting cells.
Another molecule that has recently been demonstrated to play a role in prosensory specification is the high-mobility-group transcription factor, Sox2. At E10, Sox2 is broadly expressed in both the prosensory and proneural regions of the otocyst (18, Puligilla et al., unpublished). However, Sox2 expression subsequently becomes refined to roughly overlap with Jagged1 in putative prosensory domains. A previous study by Kiernan et al (2005) demonstrated that mutations in an otocyst-specific promoter of Sox2 (Sox2Lcc and Sox2Ysb) in mice leads to failure of prosensory domain formation and a complete (Sox2Lcc) or nearly complete (Sox2Ysb) absence of both mechanosensory hair cells and support cells, a result that is consistent with a role for Sox2 in prosensory specification. Expression of Sox2 is down-regulated, although some expression persists, in Jagged1-deficient cochleae suggesting that Sox2 acts downstream of Jagged1 (13), a conclusion that is supported by the demonstration of induction of Sox2 expression in response to ectopic expression of NICD (16). Together, these results suggest that early Jagged1-mediated activation of one or more of the Notch receptors acts to induce prosensory identity through induction of Sox2.
An additional study has examined a possible role for Eyes absent homolog 1 (Eya1), a transcriptional co-activator, in prosensory specification. In humans and mice, deletion of EYA1/Eya1 leads to various anomalies including profound defects in inner ear development (20). A recent study demonstrated that Eya1 initially co-localizes with Sox2 in the ventral wall of the otocyst, the region that gives rise to prosensory lineage. As development continues Eya1 and Sox2 become restricted to partially over-lapping expression domains, with Eya1 ultimately becoming restricted to hair cells while Sox2 expression becomes restricted to supporting cells (16,19,20). Deletion of Eya1 leads to a complete absence of sensory formation and expression of the prosensory markers, Jagged1, Bmp4, and Lfng, suggesting a failure of prosensory specification in the absence of Eya1. However, while Sox2 expression is reduced in the absence of Eya1, it is not completely absent (19), suggesting that Sox2 may act in a parallel pathway with Eya1 to regulate prosensory specification.
Finally, the hedgehog signaling pathway has recently been implicated as a negative regulator of prosensory fate, but may only be active in the developing cochlea. Gli3 is a zinc-finger transcription factor that mediates hedgehog signaling. Mice with a targeted-truncating mutation in Gli3 that mimics the mutations found in individuals with Pallister-Hall syndrome have shortened cochleae that contain an expanded sensory epithelium and ectopic sensory patches in non-sensory regions of the cochlea (21). The truncating mutation leads to the formation of a repressor form of Gli3 that acts to partially inhibit the hedgehog pathway, suggesting that hedgehog acts to inhibit sensory formation within the cochlea. In vitro studies confirmed an antagonistic role for sonic hedgehog in sensory formation and simultaneous modulation of Notch signaling demonstrated that hedgehog acts upstream of Jagged1-Notch interactions (21).
Overall, these recent results have provided exciting new data regarding the specification of prosensory domains within the otocyst. Considering that many inner ear pathologies often result in the loss of both hair cells and supporting cells, the identification of factors that specify progenitor cells with the ability to develop as either cell type has the potential to provide valuable insights regarding both congenital and acquired hearing deficits.
Specification of hair cells
Once a prosensory domain is specified, individual cells within the domain are thought to make a subsequent choice to develop as either a hair cell or a supporting cell. Previous morphological studies, as well as Notch pathway deletions, have demonstrated that a hair cell is the primary fate choice within this population (12,13,22). Moreover, a large body of data has demonstrated that the basic helix-loop-helix transcription factor Atoh1 (formerly Math1) is both necessary and sufficient to induce a hair cell fate (23–26). However, the factors that regulate Atoh1 expression within the inner ear remain poorly understood. Atoh1 expression is dependent on the presence of a prosensory cell population, and as a result, is lost in prosensory mutants; however a direct role for any of the known prosensory genes in the onset of Atoh1 expression has not been demonstrated. In fact, a rather intriguing relationship has recently been described between Sox2 and Atoh1 (16). While ectopic expression of Sox2 in non-sensory regions of the cochlea is sufficient to induce expression of the homeodomain transcription factor Prox1, a downstream marker of a subset of prosensory cells, expression of Atoh1 or activation of the Atoh1 promoter was never observed in Sox2-transfected cells. In fact, forced expression of Sox2 actually acted to inhibit prosensory cells from developing into hair cells. These results, along with the observation that following a period of initial overlap, expression of Atoh1 and Sox2 becomes segregated to hair cells and supporting cells respectively, led to the suggestion that Sox2 and Atoh1 might mutually antagonize one another. This hypothesis was supported by the demonstration that Sox2 is sufficient to directly antagonize the ability of Atoh1 to induce a hair cell fate and that conversely, Atoh1 expression is sufficient to down-regulate Sox2 in P19 embryonal carcinoma cell lines. Moreover, low levels of Sox2 (hypomorphic Sox2EGFP/LP) leads to precocious differentiation and overproduction of hair cells, presumably as a result of a reduction in the antagonistic effects of Sox2 on Atoh1. Finally, overexpression of the Sox2 target gene, Prox1 also inhibits Atoh1 activity. These results demonstrate that although expression of Sox2 is initially required for the establishment of prosensory identity, continued expression of Sox2 essentially acts to inhibit hair cell formation, suggesting that subsequent down-regulation of Sox2 is required for normal sensory development (16).
The Fibroblast growth factor (Fgf) signaling pathway has been shown to be crucial for inner ear development in most vertebrates (27–31). In addition to essential roles in early otic induction and morphogenesis (28, 32, 33), Fgf receptor1 (Fgfr1) is required for the formation of both hair cells and supporting cells within the cochlea (34). Analysis of cochleae from Fgfr1 hypomorphs or animals with a conditional otocyst deletion of Fgfr1 indicates sparse mis-patterned sensory patches containing only inner hair cells. While the prosensory domain was reported to still be present in these mutants, a dose dependent decrease in Atoh1 was observed, suggesting that Fgfr1 acts downstream of prosensory specification. The ligand for Fgfr1 in the cochlea has not been determined, but recent results demonstrated that inhibition of Fgf20 causes a severe reduction in hair cells and support cells and a loss of Atoh1 expression (35), a phenotype that is consistent with results from Fgfr1 mutants. These results suggest that Fgf20 is a likely ligand for Fgfr1 and that ligand-dependent activation of Fgfr1 is a necessary step for sensory formation, however the specific target genes that are regulated by Fgfr1 remain to be determined.
Specification of supporting cells
While considerable progress has been made in the identification of factors that specify a hair cell fate, similar insights regarding supporting cell fates are lacking. Hair cells are known to induce supporting cells, but, in general, the specific signaling molecules that mediate this process have not been identified. One exception is the specification of inner pillar cells, a unique cell type only found adjacent to inner hair cells in the mammalian cochlea. Prior to morphological differentiation, progenitor cells that will develop as pillar cells, along with adjacent progenitors that will develop as outer hair cells and Deiters’ cells, begin to express Fgfr3. At the same time, developing inner hair cells become positive for Fgf8, suggesting a potential inductive interaction. Consistent with this hypothesis, deletion of Fgfr3 or a tissue-specific deletion of Fgf8 leads to a defect in pillar cell formation (36–38). Further analysis of Fgfr3 mutants indicated that inner pillar cells are missing in these mutant cochleae and that the progenitors have undergone a cell fate switch to develop as additional outer hair cells (37). Moreover, the outer hair cell phenotype in Fgfr3−/− cochleae is rescued by inhibition of Bmp4 suggesting that reciprocal signaling interactions between Fgfr3 and Bmp4 defines the number of cells that develop into either pillar cells or outer hair cells (37).
Cochlear patterning
One of the most striking aspects of the cochlear sensory epithelium is the inherent asymmetry in cellular patterning. As illustrated in Fig. 1, a single row of inner hair cells and two rows of pillar cells are located on the medial side while the lateral side contains three rows of outer hair cells. The factors that specify this pattern are unknown, with the exception that disruption of Fgf signaling leads to small patches of loosely organized hair cells. However this is more likely the result of a defect in cell specification rather than patterning. Historically, studies on other asymmetric structures, such as the vertebrate limb bud, have gained insights from the identification of factors that lead to mirror image duplications of these patterns (39–43). Therefore, the recent demonstration of mirror-image duplications of the cochlear sensory epithelium in mice with a spontaneous mutation in Sobp1 (also called Jxc1) is particularly intriguing (44). Sobp is a vertebrate homolog of the Drosophila sine oculis-binding protein encoding a nuclear zinc-finger protein that is mutated in Jackson Circler mice. Cochleae from animals with homozygous mutations in Sobp contain ectopic, vestibular-like hair cells, supernumerary hair cells within the sensory epithelium and, mirror-image duplications of the sensory epithelium, including inner hair cells, pillar cells, and the tunnel of Corti (44). These results suggest that Sobp regulates cell fate and gross patterning of the organ of Corti. However, it remains to be seen whether Sobp acts as a transcriptional activator to regulate these processes. Sobp is broadly expressed within the cochlear duct, providing limited clues as to its specific role in cellular patterning and fate. In addition, Sobp mutant cochleae are shorter than controls suggesting that some of the patterning defects could be a result of gross morphological defects rather than a specific role in cell patterning. However, the presence of mirror image duplications of the sensory epithelium is, to date, unique to Sobp mutants, and provides the first clue to the factors that determine asymmetric patterning within the cochlear duct.
Conclusions
The mammalian cochlear sensory epithelium is a remarkable example of developmental patterning. Multiple unique cell types are specified from a small proportion of multipotent otocyst progenitor cells and then arranged into a highly rigorous cellular mosaic. While our understanding of the factors that direct cells initially into the prosensory lineage and subsequently to develop as specialized types of hair cells or supporting cells remains limited, recent results have identified at least some of the pathways that regulate each of these decisions. Extracellular signaling pathways, such as Notch and Hedgehog, have positive and negative effects respectively on prosensory specification that are mediated through intracellular factors such as Sox2 and Eya1. Once formed, prosensory cells develop as either hair cells or supporting cells as a result of cross-regulation between factors that either promote hair cell fate, in particular Atoh1, and factors such as Sox2 and Prox1 that act to prevent hair cell formation through antagonism of Atoh1 (Fig. 3). In a final step, specialized supporting cell types are specified, most probably through specific inductive interactions, which largely remain to be determined. Future research will hopefully be able to build upon these results to identify factors that specify individual hair cell and supporting cell types as well as the factors that regulate asymmetric cellular patterning.
Figure 3.
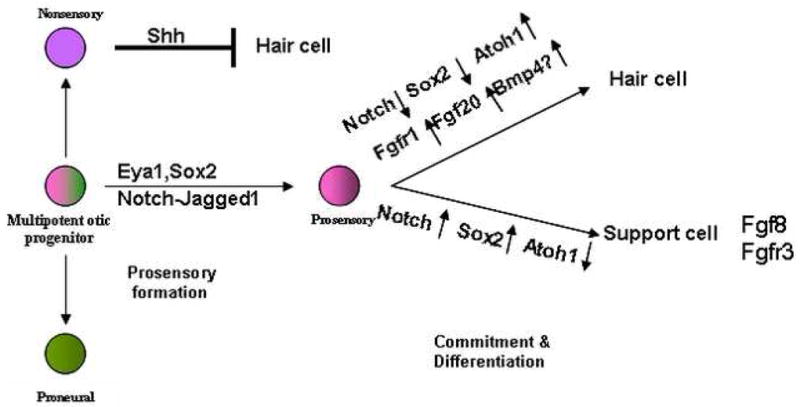
Schematic of specification of different cell types from progenitor cells within the mouse otocyst and the signaling factors that play a role in prosensory specification and subsequent specification of hair cell and support cell fates. See text for details.
Figure 2.
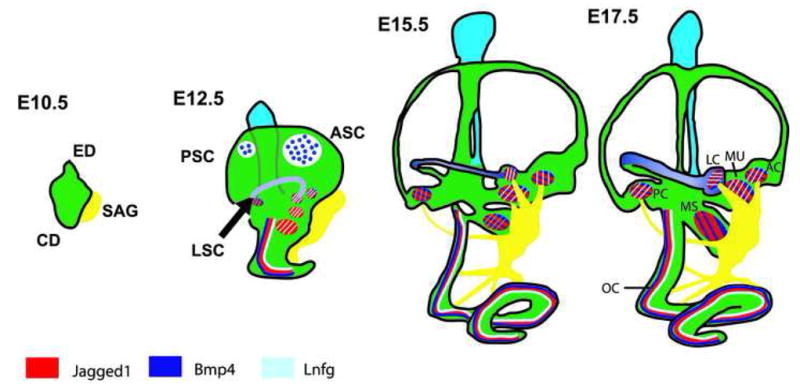
Development of the inner ear. The inner ear develops from the otic placode which initially invaginates to form otocyst around E9.5. By E10.5, dorsal and ventral protrusions are evident. These will subsequently develop into the endolymphatic (ED) and cochlear (CD) ducts. In addition, at around the same time, neuroblasts (yellow) that will coalesce to form the statoacoustic ganglion (SAG) delaminate from the ventral region of the otocyst. By E12.5, the developing cochlear duct starts to form a spiral and anterior (ASC), posterior (PSC) and lateral semicircular canals (LAC) can be identified. The speckled regions represent the areas of resorption in the central region of the outgrowths to form the mature canal phenotype (45). By this stage the 6 different sensory patches in the sensory organs of the inner ear (3 cristae associated with the semicircular canals; maculae of utricle and saccule; and the organ of Corti) can be identified based on gene expression. All patches are initially positive for Jag1 (red) and Lfng (blue). Bmp4 (purple) is also present in the developing cristae, but is absent from the maculae of utricle and saccule. In the cochlea Bmp4 is expressed in a domain located just lateral to the developing sensory epithelium. Between E15.5 and E17.5 all the inner ear structures continue to grow and by E17.5 the cochlea reaches its mature length of 1.75 turns. Expression of Jag1, Lfng and Bmp4 persists in the cristae and by E15.5 Bmp4 is also expressed in the maculae of utricle and saccule. However, expression of Bmp4 never overlaps with Jag1 and Lfng in the cochlea and instead, Bmp4 remains expressed in a lateral domain. The regions that are positive for Jag1, Bmp4 and Lnfg are in red with blue and purple stripes. PC, posterior crista; LC, lateral crista; AC, anterior crista; MS, macula saccule; MU, macula utricle.
Acknowledgments
The authors’ research is supported by the Intramural Program of the National Institute on Deafness and Other Communication Disorders at National Institutes of Health. The authors wish to apologize to any of the colleagues whose work was unavoidably excluded from this review due to space constraints.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parks SE, Ketten DR, O’Malley JT, Arruda J. Anatomical predictions of hearing in the North Atlantic right whale. Anat Rec (Hoboken) 2007;290:734–744. doi: 10.1002/ar.20527. [DOI] [PubMed] [Google Scholar]
- 2.Sher AE. The embryonic and postnatal development of the inner ear of the mouse. Acta Otolaryngol Suppl. 1971;285:1–77. [PubMed] [Google Scholar]
- 3.Carney PR, Silver J. Studies on cell migration and axon guidance in the developing distal auditory system on the mouse. J Comp Neurol. 1983;215:359–369. doi: 10.1002/cne.902150402. [DOI] [PubMed] [Google Scholar]
- 4.Torres M, Giraldez F. The development of the vertebrate inner ear. Mech Dev. 1998;71:5–21. doi: 10.1016/s0925-4773(97)00155-x. [DOI] [PubMed] [Google Scholar]
- 5.Brown ST, Martin K, Groves AK. Molecular basis of inner ear induction. Curr Top Dev Biol. 2003;57:115–119. doi: 10.1016/s0070-2153(03)57004-1. [DOI] [PubMed] [Google Scholar]
- 6.Barald KF, Kelley MW. From placode to polarization: new tunes in inner ear development. Development. 2004;131:4119–4130. doi: 10.1242/dev.01339. [DOI] [PubMed] [Google Scholar]
- 7.Morsli H, Choo D, Ryan A, Johnson R, Wu DK. Development of the mouse inner ear and origin of its sensory organs. J Neurosci. 1998;18:3327–3335. doi: 10.1523/JNEUROSCI.18-09-03327.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang N, Martin GV, Kelley MW, Gridley T. A mutation in the Lunatic fringe gene suppresses the effects of a Jagged2 mutation on inner hair cell development in the cochlea. Curr Biol. 2000;10:659–662. doi: 10.1016/s0960-9822(00)00522-4. [DOI] [PubMed] [Google Scholar]
- 9.Chang W, Lin Z, Kulessa H, Hebert J, Hogan BL, Wu DK. Bmp4 is essential for the formation of the vestibular apparatus that detects angular head movements. PLoS Genet. 2008;11:e1000050. doi: 10.1371/journal.pgen.1000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsai H, Hardisty RE, Rhodes C, Kiernan AE, Roby P, Tymowska-Lalanne Z, Mburu P, Rastan S, Hunter AJ, Brown SD, Steel KP. The mouse slalom mutant demonstrates a role for Jagged1 in neuroepithelial patterning in the organ of Corti. Hum Mol Genet. 2001;10:507–512. doi: 10.1093/hmg/10.5.507. [DOI] [PubMed] [Google Scholar]
- 11.Kiernan AE, Ahituv N, Fuchs H, Balling R, Avraham KB, Steel KP, Hrabe de Angelis M. The Notch ligand Jagged1 is required for inner ear sensory development. Proc Natl Acad Sci U S A. 2001;98:3873–3878. doi: 10.1073/pnas.071496998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brooker R, Hozumi K, Lewis J. Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development. 2006;133:1277–1286. doi: 10.1242/dev.02284. [DOI] [PubMed] [Google Scholar]
- 13**.Kiernan AE, Xu J, Gridley T. The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genet. 2006;2:e4. doi: 10.1371/journal.pgen.0020004. This work uses a tissue-specific deletion of Jagged1 to demonstrate that Jagged1 signaling is required upstream of Sox2 to mediate formation of prosensory patches within the inner ear. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daudet N, Ariza-McNaughton L, Lewis J. Notch signaling is needed to maintain, but not to initiate, the formation of prosensory patches in chick inner ear. Development. 2007;134:2369–2378. doi: 10.1242/dev.001842. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi T, Kokubo H, Hartman BH, Ray CA, Reh TA, Bermingham-McDonogh O. Hesr1 and Hesr2 may act as early effectors of Notch signaling in the developing cochlea. Dev Biol. 2008;316:87–99. doi: 10.1016/j.ydbio.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16*.Dabdoub A, Puligilla C, Jones JM, Fritzsch B, Cheah KS, Pevny LH, Kelley MW. Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proc Natl Acad Sci U S A. 2008;105:18396–18401. doi: 10.1073/pnas.0808175105. This paper demonstrated the existence of mutual antagonism between Sox2 and Atoh1 and suggested that the interplay between these molecules plays a key role in mediating the number of cells that develop as hair cells versus supporting cells. The results also suggest that while Sox2 is required for prosensory specification, subsequent down-regulation of Sox2 is required for hair cell development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daudet N, Lewis J. Two contrasting roles for Notch activity in chick inner ear development: specification of prosensory patches and lateral inhibition of hair-cell differentiation. Development. 2005;132:541–551. doi: 10.1242/dev.01589. [DOI] [PubMed] [Google Scholar]
- 18.Kiernan AE, Pelling AL, Leung KKH, Tang ASP, Bell DM, Tease C, Lovell-Badge R, Steel KP, Cheah KSE. Sox2 is required for sensory organ development in the mammalian inner ear. Nature. 2005;434:1031–1035. doi: 10.1038/nature03487. [DOI] [PubMed] [Google Scholar]
- 19.Zou D, Erickson C, Kim E, Jin D, Fritzsch B, Xu P. Eya1 gene dosage critically affects the development of sensory epithelia in the mammalian inner ear. Hum Mol Genet. 2008;17:3340–3356. doi: 10.1093/hmg/ddn229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hume CR, Bratt DL, Oesterle EC. Expression of LHX3 and SOX2 during mouse inner ear development. Gene Expr Patterns. 2007;7:798–807. doi: 10.1016/j.modgep.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21*.Driver EC, Pryor SP, Hill P, Turner J, Ruther U, Biesecker LG, Griffith AJ, Kelley MW. Hedgehog signaling regulates sensory cell formation and auditory function in mice and humans. J Neurosci. 2008;28:7350–7358. doi: 10.1523/JNEUROSCI.0312-08.2008. This manuscript demonstrated auditory deficits in patients with Pallister-Hall Syndrome that were correlated with cochlear defects in a mouse model of Pallister-Hall. A direct inhibitory effect of sonic hedgehog on prosensory formation was also demonstrated in vitro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiernan AE, Cordes R, Kopan R, Gossler A, Gridley T. The Notch ligands DLL1 and JAG2 act synergistically to regulate hair cell development in the mammalian inner ear. Development. 2005;132:4353–4362. doi: 10.1242/dev.02002. [DOI] [PubMed] [Google Scholar]
- 23.Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal inner ears. Nat Neurosci. 2000;3:580–586. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- 24.Kawamoto K, Ishimoto S, Minoda R, Brough DE, Raphael Y. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neurosci. 2003;23:4395–4400. doi: 10.1523/JNEUROSCI.23-11-04395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woods C, Montcouquiol M. Kelley MW Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nat Neurosci. 2004;7:1310–1318. doi: 10.1038/nn1349. [DOI] [PubMed] [Google Scholar]
- 26*.Gubbels SP, Woessner DW, Mitchell JC, Ricci AJ, Brigande JV. Functional auditory hair cells produced in the mammalian cochlea by in utero gene transfer. Nature. 2008;455:537–541. doi: 10.1038/nature07265. This work utilized a newly developed technique to transfer genes into otocyst cells in embryonic mice in vivo. Hair cells generated through transfection of Atoh1 were shown to be functional and to have mechanosensitive properties that are comparable with endogenous hair cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wright TJ, Ladher R, McWhirter J, Murre C, Schoenwolf GC, Mansour GL. Mouse FGF15 is the ortholog of human and chick FGF19, but is not uniquely required for otic induction. Dev Biol. 2004;269:264–275. doi: 10.1016/j.ydbio.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Wright TJ, Mansour SL. Fgf3 and Fgf10 are required for mouse otic placode induction. Development. 2003;130:3379–3390. doi: 10.1242/dev.00555. [DOI] [PubMed] [Google Scholar]
- 29.Ladher RK, Wright TJ, Moon AM, Mansour SL, Schoenwolf GC. FGF8 initiates inner ear induction in chick and mouse. In: Ladher, et al., editors. Genes Dev. Vol. 19. 2005. pp. 603–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leger S, Brand M. Fgf8 and Fgf3 are required for zebrafish ear placode induction, maintenance and inner ear patterning. Mech Dev. 2002;119:91–108. doi: 10.1016/s0925-4773(02)00343-x. [DOI] [PubMed] [Google Scholar]
- 31.Maroon H, Walshe J, Mahmood R, Kiefer P, Dickson C, Mason I. Fgf3 and Fgf8 are required together for formation of the otic placode and vesicle. Development. 2002;129:2099–2108. doi: 10.1242/dev.129.9.2099. [DOI] [PubMed] [Google Scholar]
- 32.Groves AK, Bronner-Fraser M. Competence, specification and commitment in otic placode induction. Development. 2000;127:3489–3499. doi: 10.1242/dev.127.16.3489. [DOI] [PubMed] [Google Scholar]
- 33.Solomon KS, Kwak SJ, Fritz A. Genetic interactions underlying otic placode induction and formation. Dev Dyn. 2004;230:419–433. doi: 10.1002/dvdy.20067. [DOI] [PubMed] [Google Scholar]
- 34.Pirvola U, Ylikoski J, Trokovic R, Hebert JM, McConnell SK, Partanen J. FGFR1 is required for the development of the auditory sensory epithelium. Neuron. 2002;35:671–680. doi: 10.1016/s0896-6273(02)00824-3. [DOI] [PubMed] [Google Scholar]
- 35.Hayashi T, Ray CA, Bermingham-McDonogh O. Fgf20 is required for sensory epithelial specification in the developing cochlea. J Neursci. 2008;28:5991–5999. doi: 10.1523/JNEUROSCI.1690-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayashi T, Cunningham D, Bermingham-McDonogh O. Loss of Fgfr3 leads to excess hair cell development in the mouse organ of Corti. Dev Dyn. 2007;236:525–533. doi: 10.1002/dvdy.21026. [DOI] [PubMed] [Google Scholar]
- 37.Puligilla C, Feng F, Ishikawa K, Bertuzzi S, Dabdoub A, Griffith AJ, Fritzsch B, Kelley MW. Disruption of fibroblast growth factor receptor 3 signaling results in defects in cellular differentiation, neuronal pattering, and hearing impairment. Dev Dyn. 2007;236:1905–1917. doi: 10.1002/dvdy.21192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacques BE, Montcouquiol ME, Layman EM, Lewandoski M, Kelley MW. Fgf8 induces pillar cell fate and regulates cellular patterning in the mammalian cochlea. Development. 2007;134:3021–3029. doi: 10.1242/dev.02874. [DOI] [PubMed] [Google Scholar]
- 39.Duprez DM, Kostakopoulou K, Francis-West PH, Tickle C, Brickell PM. Activation of Fgf-4 and HoxD gene expression by BMP-2 expressing cells in the developing chick limb. Development. 1996;122:1821–1828. doi: 10.1242/dev.122.6.1821. [DOI] [PubMed] [Google Scholar]
- 40.Niederreither K, Ward SJ, Dolle P, Chambon P. Morphological and molecular characterization of retinoic acid-induced limb duplications in mice. Dev Biol. 1996;176:185–98. doi: 10.1006/dbio.1996.0126. [DOI] [PubMed] [Google Scholar]
- 41.Duprez D, Lapointe F, Edom-Vovard F, Kostakopoulou K, Robson L. Sonic hedgehog (SHH) specifies muscle pattern at tissue and cellular chick level, in the chick limb bud. Mech Dev. 1999;82:151–163. doi: 10.1016/s0925-4773(99)00040-4. [DOI] [PubMed] [Google Scholar]
- 42.Wada N, Kawakami Y, Nohno T. Sonic hedgehog signaling during digit pattern duplication after application of recombinant protein and expressing cells. Dev Growth Differ. 1999;41:567–574. doi: 10.1046/j.1440-169x.1999.00452.x. [DOI] [PubMed] [Google Scholar]
- 43.McFadden DG, McAnally J, Richardson JA, Charite J, Olson EN. Misexpression of dHAND induces ectopic digits in the developing limb bud in the absence of direct DNA binding. Development. 2002;129:3077–3088. doi: 10.1242/dev.129.13.3077. [DOI] [PubMed] [Google Scholar]
- 44*.Chen Z, Montcouquiol M, Calderon R, Jenkins NA, Copeland NG, Kelley MW, Noben-Trauth K. Jxc1/Sobp, encoding a nuclear zinc finger protein, is critical for cochlear growth, cell fate, and patterning of the organ of Corti. J Neurosci. 2008;28:6633–6641. doi: 10.1523/JNEUROSCI.1280-08.2008. This work identifies a point mutation in Sobp/Jcx1 as the defect in the Jackson Circler mouse line. The cochleae of these animals are shown to have numerous defects including an overall shortening, patterning defects including mirror image duplications of the organ of Corti and aberrant planar cell polarity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin P, Swanson GJ. Descriptive and experimental analysis of the epithelial remodellings that control semicircular canal formation in the developing mouse inner ear. Dev Biol. 1993;159:549–558. doi: 10.1006/dbio.1993.1263. [DOI] [PubMed] [Google Scholar]
- 46.Morsli H, Choo D, Ryan A, Johnson R, Wu DK. Development of the mouse inner ear and origin of its sensory organs. J Neurosci. 1998;18:3327–3335. doi: 10.1523/JNEUROSCI.18-09-03327.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]


