Abstract
Objectives
To characterize the expression of fibroblast growth factor binding protein (FGF-BP) messenger RNA (mRNA) in head and neck squamous cell carcinoma (HNSCC) and to study the association of FGF-BP with vascularity.
Design
The expression of FGF-BP mRNA in HNSCC was studied in 35 primary and 8 metastatic HNSCC specimens and 7 control tissues using in situ hybridization and reverse transcriptase–polymerase chain reaction (RT-PCR). Microvessels in tumor specimens were identified with endothelial cell markers (von Willebrand factor [vWF] and CD34-specific antibodies). Correlates between FGF-BP and microvessel counts were evaluated statistically.
Setting
University of Minnesota Hospitals and Clinics.
Patients
Forty-two surgically treated patients with HNSCC.
Interventions
The patients were routinely treated in the study hospitals and clinics.
Main Outcome Measures
The expression of FGF-BP and angiogenesis in tumors were evaluated.
Results
In situ hybridization and RT-PCR demonstrated that FGF-BP mRNA transcripts were expressed in 34 of 35 primary HNSCC specimens and 5 of 8 metastatic tumor specimens but not in adjacent control tissues. The microvessel counts in HNSCC specimens were closely related to the expression level of FGF-BP (P<.001).
Conclusion
The expression of FGF-BP is statistically linked to the angiogenesis of HNSCC, suggesting that FGF-BP participates in the angiogenesis of HNSCC.
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common tumor in humans1; it is a solid tumor and heavily dependent on angiogenesis. 2 One of the angiogenic mechanisms for tumors relies on the recruitment and utilization of locally stored fibroblast growth factors (FGFs).3 It is known that FGFs are involved in angiogenesis4 and neoplasia,5 and FGF binding protein (FGF-BP) has been detected in several HNSCC cell lines and tissues.3 However, whether FGF-BP is extensively expressed in HNSCC tissues and plays a role in the angiogenesis of HNSCC remains to be elucidated.
A secreted protein, FGF-BP binds to acidic and basic FGFs in a noncovalent reversible manner.6,7 It was originally isolated from human epidermoid carcinoma cells.8 The expression of FGF-BP is downregulated in normal adult tissues but upregulated in skin cancer.9 Overexpression or, conversely, reduced expression of FGF-BP has indicated a role in the angiogenesis of tumors.4 Expression of the mouse FGF-BP gene was found to be prominent in the skin and intestine during the prenatal phase but is downregulated in adult mice.9 The expression of FGF-BP was reported to facilitate a non-tumorigenic-to-tumorigenic switch in a human cell line (SW-13).3 Expression of FGF-BP in these cells recruits basic FGF from the extracellular storage and allows it to reach the FGF receptor.4,10
Nuclear factor κB (NF-κB) is highly active in the angiogenesis of tumors. It is known that NF-κB binds to the promoter of interleukin 8 (IL-8), vascular endothelial growth factor (VEGF), and cyclooxygenase 2 (COX-2) and thus increases the transcription of these angiogenic factors.11
We hypothesized for this study that FGF-BP is related to the angiogenesis of HNSCC and used cellular methods to investigate FGF-BP expression in HNSCC specimens. The correlates between FGF-BP and NF-κB as well as FGF-BP and microvessel counts were evaluated by statistical analysis.
METHODS
Thirty-five primary HNSCC specimens, 8 metastatic tumor specimens, and 7 histologically adjacent control tissues were procured from 42 patients treated surgically at the University of Minnesota Hospitals and Clinics (UMHC). All specimens and clinical data in this study were procured, handled, and maintained according to the protocols approved by the institutional review board at the University of Minnesota. Of the patients with primary HNSCC, 28 were men, and 7 were women; mean (SD) age was 63.8 (12.6) years (age range, 36–93 years ). Eight tumors were located in the larynx, 4 in the pharynx, 11 in the oral cavity, 3 in the nasal cavity, 4 on the face, and 5 in the neck. Histologically, there were 4 well-differentiated SCCs, 20 moderately differentiated SCCs, and 11 poorly differentiated SCCs.
REVERSE TRANSCRIPTASE–POLYMERASE CHAIN REACTION
Head and neck SCC specimens (approximately 0.5–1.0 g of tissue per specimen) were obtained from surgical resections. In the operating room, the tissues were immediately frozen in liquid nitrogen and homogenized in a solution of 5M guanidine isothiocyanate, 0.5% sarcosyl, 25mM sodium citrate (pH 7.0), and 0.1M 2-mercaptoethanol. Total cellular RNA was isolated by extraction with guanidium thiocyanate-acidic-phenol-chloroform, as previously described.12 The primer sequences used to amplify FGF-BP complementary DNA (cDNA) were designed based on the cDNA sequence published in GenBank. Primer 1, sense mutations, included 98 to 120 base pairs (bp); primer 2, antisense, 777 to 799 bp; primer 3, sense, 209–232 bp; and primer 4, antisense, 546–568 bp.
Total RNA was reverse transcribed in a volume of 50 µL using GeneAmp RNA PCR Core Kit (Roche Molecular Systems Inc, Branchburg, New Jersey) according to the manufacturer’s instructions. Polymerase chain reaction was performed as previously described13 using primer pair combinations 1 + 2, 1 + 4, 3 + 2, and 3 + 4. The RT-PCR reaction generated 4 fragments, and 1 of the fragments was 702 bp long.
SUBCLONING
Polymerase chain reaction products underwent electrophoresis in 1% agarose gel. They were then dissected out, purified using a PCR product clean kit (Promega Corporation, Madison, Wisconsin), cut with EcoRI endonuclease (Promega Corporation), and cloned into the multiple cloning site of a plasmid (pBluescript II vector; Stratagene, La Jolla, California). The plasmid was amplified with Escherichia coli, extracted using a MAXprep Kit (Promega), sequenced, and compared with the original sequence using BLAST software, version 2.0.6 (National Center for Biotechnology Information; http://www.ncbi.nlm.nih.gov). The 702-bp fragment in the pBluescript II was used as a template for making sense and antisense probes for in situ hybridization.
IN SITU HYBRIDIZATION
In situ hybridization was performed as previously described.14 Briefly, FGF-BP cDNA from the plasmid was reverse transcribed into mRNA transcripts with DNA-dependent RNA polymerase using T7/T3 promoters. Both sense and antisense riboprobes with sulfur 35 (35S) radiolabels were prepared using the MAXIscript In vitro Transcription Kit (Applied Biosystems/Ambion, Austin, Texas). The antisense riboprobe was used for hybridization and the sense riboprobe used as a control. Paraffin-embedded specimens were cut and deparaffinized with xylene and graded ethanol solutions. Slides were pretreated with digitonin, digested with proteinase K, acetylated with acetic anhydride, dehydrated through graded ethanol solutions, and hybridized with 105 counts per minute per microgram of 35S riboprobes. Slides were stringently washed, digested with ribonucleases A and T1, dehydrated with graded ethanol solutions, coated with film emulsion (Eastman Kodak, Rochester, New York), exposed for 24 hours, counterstained with hematoxylin-eosin, and documented microscopically.
IMMUNOHISTOCHEMICAL ANALYSIS AND MICROVESSEL QUANTITATION
To identify microvessels in tumor specimens, immunohistochemical analysis was performed by using specific antibodies against von Willebrand factor (vWF), 1:25 dilution Dako North America Inc, Carpinteria, California) and/or CD34, 1:50 dilution (Zymed Laboratories Inc, Carlsbad, California) routinely on the 35 primary HNSCC specimens. Nonspecific IgG was used for a control. Slides were examined under a microscope at low power (original magnification × 40) for identification of the region rich in blood vessels. Five highly vascularized areas within the tumor mass for each slide were taken for microvessel counting. In each of these specimens, 5 areas found to be positive for vWF and/or CD34 were counted under a microscope with original magnification × 100 added, and averaged. To clearly see the microvessel details, photographs were taken at original magnifications ×100, ×200, and ×400. Large vessels with thick muscular walls or with lumens greater than 50 µm were excluded from the count. Results were expressed as the average number of microvessels identified within 10 consecutive high-power fields, as previously described.15 For cultured cell immunohistochemical analysis, we used specific antibodies against NF-κB subunit p65 from Abcam (Cambridge, Massachusetts), 1:100 dilution (ab7970), and a secondary antibody fluorescein isothiocyanate conjugated to green.
STATISTICAL ANALYSIS
We used the χ2 test to evaluate correlates between NF-κB expression intensities and FGF-BP levels. The Spearman rank correlation was used to evaluate correlates between FGF-BP expression levels and microvessel counts. The method was chosen because of the nondeterminant associations (positive or negative) between FGF-BP and microvessel counts. Significance was set at P<.05.
RESULTS
EXPRESSION OF FGF-BP IN HNSCC
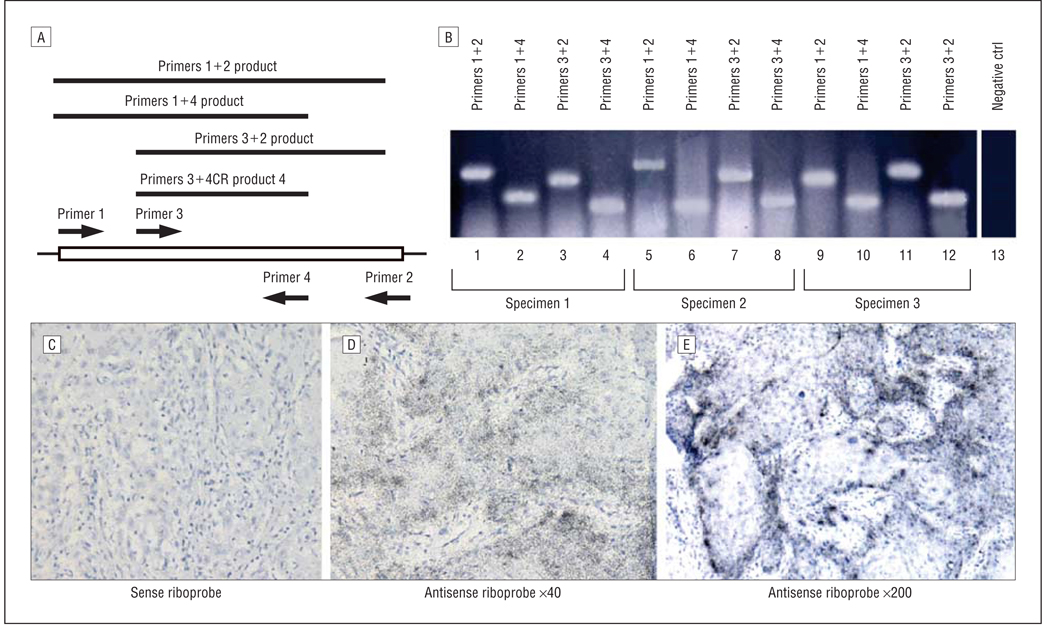
Four overlapping cDNA fragments encoding SCC FGF-BP were isolated by RT-PCR using the FGF-BP primer combinations. The amplified RT-PCR products from HNSCC specimens using 4 sets of primer pairs are schematically presented in Figure 1A. Using these primer pairs, the PCR products amplified from 3 individual HNSCC specimens were obtained (Figure 1B, lanes 1–12). The RT-PCR product was not observed when the mRNA sample was omitted (Figure 1B, lane 13). The PCR products in Figure 1B were sequenced and matched to the sequence of FGF-BP originally from epidermoid carcinoma by a nucleotide BLAST-searching tool on the National Institutes of Health Web site (http://www.ncbi.nlm.nih.gov/). To localize the expression site of FGF-BP in HNSCC specimens, in situ hybridization on all the tissue specimens (HNSSC and control tissues) was performed. The representative results for FGF-BP in situ hybridization are presented in Figure 1C–E: the sense riboprobe for FGF-BP, Figure 1C, and antisense riboprobe for FGF-BP (with 2 different magnifications, Figure 1D and E). Note that FGF-BP is frequently expressed at the edge of tumor lumps (Figure 1E).
Figure 1.
Expression of fibroblast growth factor binding protein (FGF-BP). Expression of FGF-BP messenger RNA (mRNA) transcripts in head and neck squamous cell carcinoma (HNSCC) specimens. A, Reverse transcriptase–polymerase chain reaction (RT-PCR) was performed using primer pairs indicated in (not scaled for the schematic bars, box, and arrows). B, Individual RT-PCR products from 3 HNSCC specimens (lanes 1–4, the products from specimen 1; lanes 5–8, the products from specimen 2; and lanes 9–12, the products from specimen 3); lane 13 represents the PCR negative control (ctrl). C–E, By in situ hybridization, the FGF-BP mRNA transcript signals were not detected when the control sense riboprobe was used (C) (original magnification ×40), whereas the signals were detected when the antisense riboprobe was used (D, original magnification ×40; E, original magnification ×200). The FGF-BP mRNA transcript signals are located at the edge of tumor lumps.
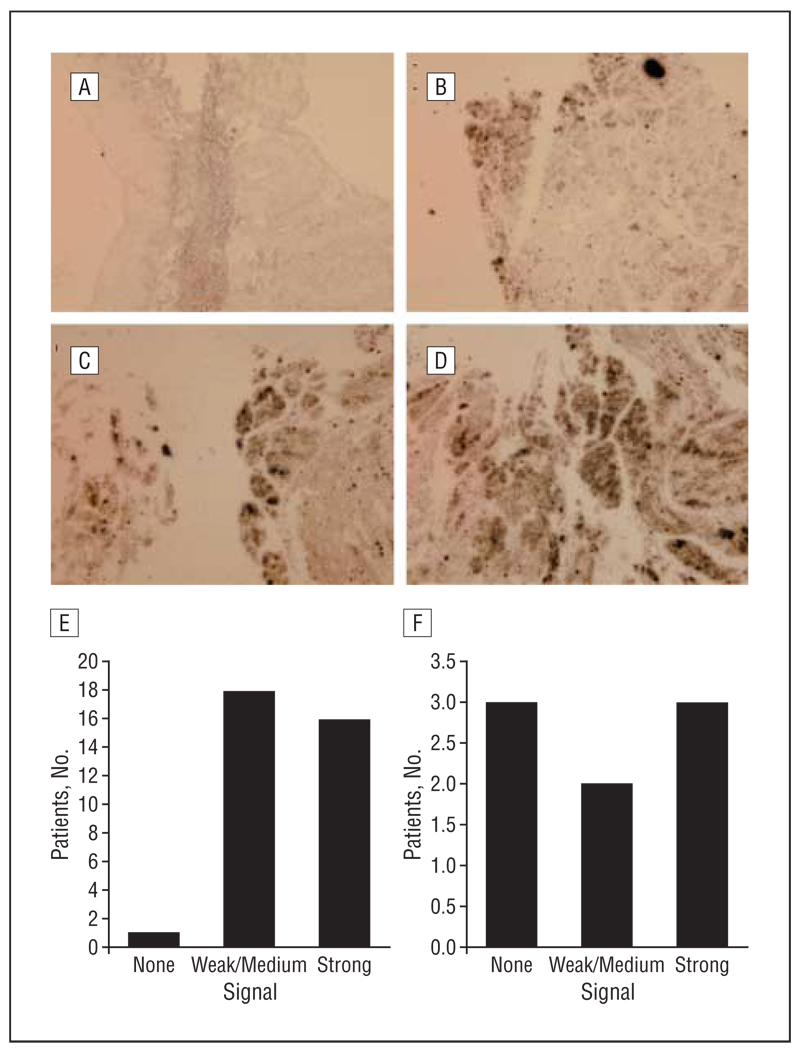
In the in situ hybridization experiment, there were no detectable FGF-BP signals in the normal adult larynx (Figure 2A), tongue, and lymph node. Of the 35 primary HNSCC specimens, no signals were found in 1; weak to medium signals were found in 18 (Figure 2B and C); and strong signals were found in 16 (Figure 2D). The profile of FGF-BP mRNA transcripts in 35 primary HNSCC tumors by in situ hybridization is summarized in Figure 2E. In the metastatic cases, the expression of FGF-BP mRNA transcripts was examined in 8 lymph nodes and soft tissues containing metastatic HNSCC cells. Strong signals were found in 3 of the 8; weak to medium signals in 2; and no signals in 3 (Figure 2F). In FGF-BP highly expressed specimens, there was apparent NF-κB expression. No signals were detected when a sense riboprobe for FGF-BP was used for hybridization with HNSCC sections.
Figure 2.
The expression profiles of fibroblast growth factor binding protein (FGF-BP). The expression profiles of FGF-BP messenger RNA (mRNA) transcripts in primary and metastatic head and neck squamous cell carcinoma (HNSCC) specimens. By in situ hybridization, sulfur 32 (32S)–labeled FGF-BP riboprobes were used to detect the mRNA transcripts in 35 primary and 8 metastatic tumors. A, No mRNA signals (black dots) were detected in normal adjacent larynx mucosa. B–D, In the primary HNSCC specimens, weak (B), moderate (C), and strong (D) signals for FGF-BP were detected. E, Among the 35 primary tumors studied, 34 were positive for FGF-BP expression. F, Among the 8 metastatic tumors studied, 5 were positive for FGF-BP expression.
ASSOCIATION OF FGF-BP EXPRESSION WITH NF-κB IN HNSCC
To study the correlation between FGF-BP and NF-κB expression, we evaluated NF-κB expression in 35 primary HNSCC specimens. Among 16 FGF-BP highly expressed specimens and 18 FGF-BP weakly to moderately expressed specimens, NF-κB expression was evaluated by immunohistochemical analysis. Two independent observers blindly evaluated the expression of FGF-BP and NF-κB. It was found that there was an association between FGF-BP expression and NF-κB levels (Table 1). Statistical analysis using the χ2 test showed P<.05 (Minitab software, version 15; Minitab Inc, State College, Pennsylvania).
Table 1.
Correlates Between FGF-BP Expression and NF-κB Level in HNSCC Specimens
Abbreviations: FGF-BP, fibroblast growth factor binding protein; HNSCC, head and neck squamous cell carcinoma; NF-κB, nuclear factor κB.
Low FGF-BP level indicates weak to medium signals; high level indicates strong signals.
χ2 Test (using Minitab software, version 15 [Minitab Inc, State College, Pennsylvania]).
CORRELATION BETWEEN MICROVESSEL COUNTS AND FGF-BP EXPRESSION
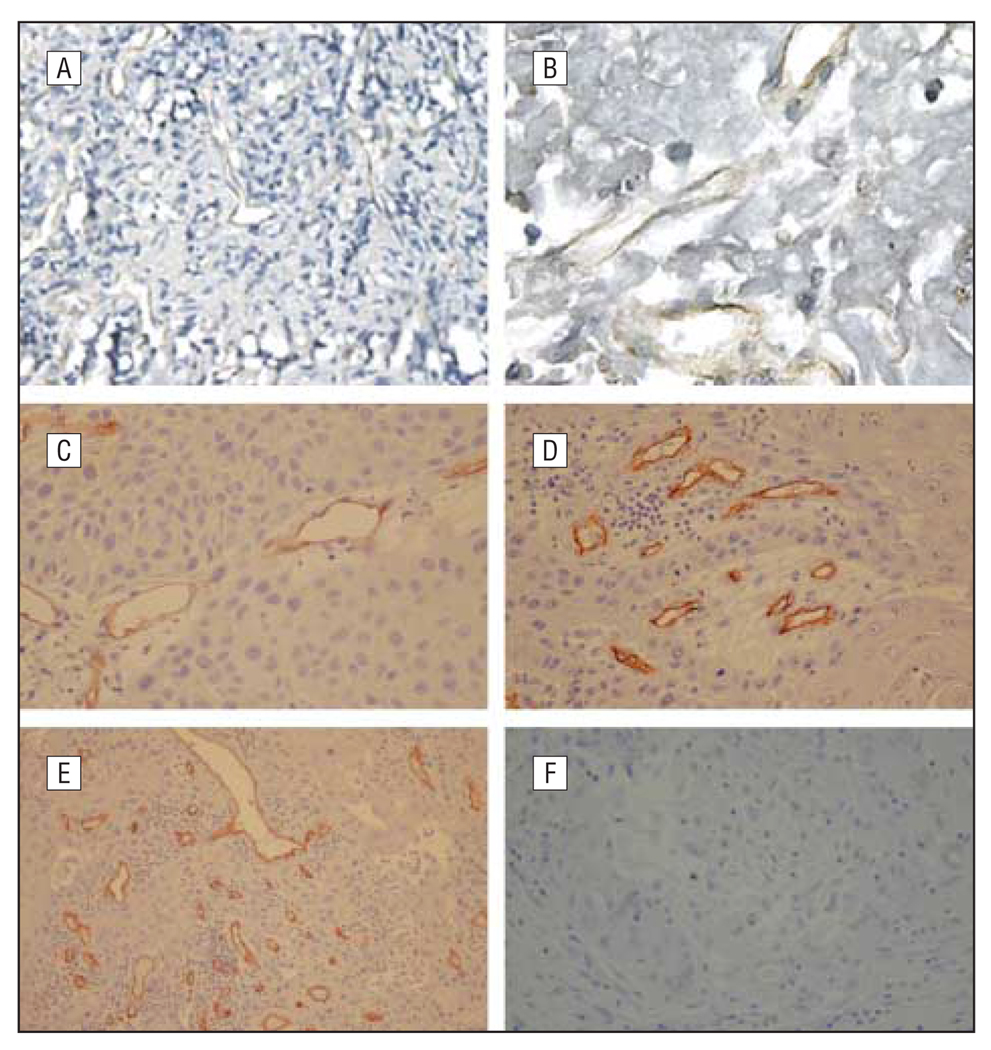
No specific staining was observed in the immunohistochemical controls when anti-vWF and anti-CD34 antibodies were replaced by a nonspecific IgG (sc-2709; Santa Cruz Biotechnology Inc, Santa Cruz, California). Frequently, the highest density of microvessels was observed at the invasive edge of tumors where the expression of NF-κB was also high (data not shown). Microvessels were identified from HNSCC specimens by immunohistochemical stain on the tissue sections with antiendothelial cell marker antibodies, namely anti-vWF and anti-CD34 antibodies (Figure3). Microvessel counts in 35 HNSCC specimens ranged from 26 to 134 with a mean (SD) of 57 (24) for vWF and CD34 staining when viewed under a microscope at a low-power field (original magnification ×100). There was no statistically significant association between vessel counts and age, sex, or cancer stage (data not shown). However, the vessel counts were significantly higher in patients with metastatic tumors (lymphatic node metastasis or protrusions into the neighborhood) than in those without metastatic tumors (P<.05).
Figure 3.
Identification of microvessels. Identification of microvessels in head and neck squamous cell carcinoma (HNSCC) specimens with endothelial cell markers. Specific von Willebrand factor (vWF) and CD34-specific antibodies were used for staining HNSCC specimens. Microvessels were observed in the HNSCC specimens at various densities. A and B, Anti-vWF antibody weakly stained the microvessel endothelial cells (original magnifications ×100 [A] and ×400 [B]). C–E, Anti-CD34 clearly stained the endothelial cells in the HNSCC specimens (original magnifications ×200 [C], ×200 [D], and ×100 [E]). F, Nonspecific IgG did not stain (original magnification ×100).
There was a close correlation between the microvessel density and FGF-BP expression in primary HNSCC tumors (P<.001 using the Spearman test). In tumors with microvessel counts of more than 60 per low-power field, 84.4% of samples were highly positive for FGF-BP. The mean (SD) microvessel counts in highly FGF-BP–positive HNSCC specimens (61 [24]) were significantly higher (P<.001) than in weakly to moderately FGF-BP–positive tumors (40 [13]). Correlates between microvessel counts and FGF-BP expression levels are summarized in Table 2.
Table 2.
Correlates Between FGF-BP Levels and Microvessel Counts in HNSCC Specimens
| FGF-BP Level | ||||
|---|---|---|---|---|
| Characteristic | Lowa | High | Correlation Coefficient | P Valueb |
| Microvessel count, mean (SD), No. | 40 (13) | 61 (24) | r = 0.332 | <.001 |
Abbreviations: FGF-BP, fibroblast growth factor binding protein; HNSCC, head and neck squamous cell carcinoma.
Low FGF-BP level indicates weak to medium signals; high level indicates strong signals.
Spearman rank correlation test.
COMMENT
In this study we found that FGF-BP is extensively expressed in primary HNSCC tumors and some metastatic cells but not in normal adult mucosal tissues. This is consistent with the findings of Czubayko et al3 that FGF-BP was expressed in 9 of 11 HNSCC specimens. Fibroblast growth factor binding protein cDNA was isolated from HNSCC specimens with its sequence matched to that in the A431 human epidermoid carcinoma cells.8
Angiogenesis is a critical cellular process for cancer growth and subsequent metastasis.16 Some studies have shown that in histologic sections of primary tumors, microvessel counts reflect angiogenesis and are associated with tumor growth and metastasis.15,17–19 Dray et al20 demonstrated a strong correlation between high microvessel counts and recurrent or metastatic disease in HNSCC. We found that 5 of 8 metastatic tumors were positive for FGF-BP, and 3 of 8 were negative. In the future, it will be important to study larger numbers of metastatic tumors to be able to definitively comment on the contribution of FGF-BP or other angiogenic growth factors to the critical level of microvessel density reliably associated with HNSCC metastases. It may be that other microenvironment interactions between tumor and stromal cells exclusive of FGF-BP contribute to this process or that structurally altered FGF-BP may exist in some tumors. Since the metastatic tumor numbers were small in this study (only 8 specimens), it is too early to state whether FGF-BP is associated with HNSCC metastasis. Some of the metastatic tumors (3 of 8 specimens) were actually negative for FGF-BP expression when they spread to the surrounding lymphatic nodes, whereas only 1 primary tumor specimen showed no signals. This suggests the FGF-BP expression may be related to the unique microenvironments of tumor cells: interactions between stromal cells and tumor cells drive the production of FGF-BP. There is accumulating evidence that stromal cells activated by tumor cells certainly affect the behavior of tumor cells.
High concentrations of acidic FGF and basic FGF have been found in tumor tissues of different origins, including HNSCC.21,22 However, their role in the angiogenesis is not clearly understood. Fibroblast growth factors stimulate the proliferation of keratinocytes and a number of other cell types including fibroblasts and endothelial cells in vitro.23 The association of FGF-BP with increased microvessel counts in HNSCC specimens and the increased activity of NF-κB in HNSCC cells caused by basic FGF together suggest that FGF-BP plays a role in the angiogenesis of HNSCC.
Although the detailed molecular mechanisms for FGF-BP to stimulate the angiogenesis of HNSCC are unclear, our present study indicates that FGF-BP is involved in the angiogenesis of HNSCC, and inhibitors of FGF-BP may be potentially important for the suppression of tumor angiogenesis.
In conclusion, the results of present study demonstrate that FGF-BP is present in HNSCC. The mean microvessel count in highly FGF-BP–positive HNSCC was significantly higher than in weakly to moderately FGF-BP–positive tumors. This suggests that FGF-BP is linked to the angiogenic activities of HNSCC. If this is proven, blockage of FGF-BP expression might inhibit the growth and progression of HNSCC.
Acknowledgments
Funding/Support: This study was supported in part by the National Institutes of Health grants R03CA107989 and R01DC008165 (Dr Lin) and the Minnesota Medical Foundation and the Lions 5-M foundation (Drs Juhn and Lin).
Footnotes
Author Contributions: Dr Lin had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Acquisition of data: Li, Wang, and Lin. Analysis and interpretation of data: Juhn, Ondrey, and Lin. Drafting of the manuscript: Li, Wang, Ondrey, and Lin. Critical revision of the manuscript for important intellectual content: Juhn and Ondrey. Statistical analysis: Li, Wang, and Ondrey. Obtained funding: Juhn and Lin. Administrative, technical, and material support: Lin. Study supervision: Juhn, Ondrey, and Lin.
Financial Disclosure: None reported.
Additional Contributions: The late George L. Adams, MD, provided the clinical head and neck cancer specimens; Sherry Fulton, MD, provided technical assistance in tissue sections; and Brian Hunter, MD, provided editorial assistance in the preparation of the manuscript.
REFERENCES
- 1.Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics 1996. CA Cancer J Clin. 1996;46(1):5–27. doi: 10.3322/canjclin.46.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1(1):27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 3.Czubayko F, Smith RV, Chung HC, Wellstein A. Tumor growth and angiogenesis induced by a secreted binding protein for fibroblast growth factors. J Biol Chem. 1994;269(45):28243–28248. [PubMed] [Google Scholar]
- 4.Czubayko F, Liaudet-Coopman ED, Aigner A, Tuveson AT, Berchem GJ, Wellstein A. A secreted FGF-binding protein can serve as the angiogenic switch in human cancer. Nat Med. 1997;3(10):1137–1140. doi: 10.1038/nm1097-1137. [DOI] [PubMed] [Google Scholar]
- 5.Berking C, Takemoto R, Satyamoorthy K, Elenitsas R, Herlyn M. Basic fibroblast growth factor and ultraviolet B transform melanocytes in human skin. AmJ Pathol. 2001;158(3):943–953. doi: 10.1016/S0002-9440(10)64041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu DQ, Kan MK, Sato GH, Okamoto T, Sato JD. Characterization and molecular cloning of a putative binding protein for heparin-binding growth factors. J Biol Chem. 1991;266(25):16778–16785. [PubMed] [Google Scholar]
- 7.Gospodarowicz D, Neufeld G, Schweigerer L. Fibroblast growth factor. Mol Cell Endocrinol. 1986;46(3):187–204. doi: 10.1016/0303-7207(86)90001-8. [DOI] [PubMed] [Google Scholar]
- 8.Klagsbrun M, Moses MA. Molecular angiogenesis. Chem Biol. 1999;6(8):R217–R224. doi: 10.1016/S1074-5521(99)80081-7. [DOI] [PubMed] [Google Scholar]
- 9.Kurtz A, Wang HL, Darwiche N, Harris V, Wellstein A. Expression of a binding protein for FGF is associated with epithelial development and skin carcinogenesis. Oncogene. 1997;14(22):2671–2681. doi: 10.1038/sj.onc.1201117. [DOI] [PubMed] [Google Scholar]
- 10.Rak J, Kerbel RS. bFGF and tumor angiogenesis–back in the limelight? Nat Med. 1997;3(10):1083–1084. doi: 10.1038/nm1097-1083. [DOI] [PubMed] [Google Scholar]
- 11.Cherukuri DP, Goulet AC, Inoue H, Nelson MA. Selenomethionine regulates cyclooxygenase-2 (COX-2) expression through nuclear factor-kappa B (NF-kappaB) in colon cancer cells. Cancer Biol Ther. 2005;4(2):175–180. doi: 10.4161/cbt.4.2.1438. [DOI] [PubMed] [Google Scholar]
- 12.Lin J, Ozeki M, Javel E, et al. Identification of gene expression profiles in rat ears with cDNA microarrays. Hear Res. 2003;175(1–2):2–13. doi: 10.1016/s0378-5955(02)00704-9. [DOI] [PubMed] [Google Scholar]
- 13.Lin J, Tsuboi Y, Pan W, Giebink GS, Adams GL, Kim Y. Analysis by cDNA microarrays of altered gene expression in middle ears of rats following pneumococcal infection. Int J Pediatr Otorhinolaryngol. 2002;65(3):203–211. doi: 10.1016/s0165-5876(02)00130-1. [DOI] [PubMed] [Google Scholar]
- 14.Lin J, Tsuboi Y, Rimell F, et al. Expression of mucins in mucoid otitis media. J Assoc Res Otolaryngol. 2003;4(3):384–393. doi: 10.1007/s10162-002-3023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanigawa N, Matsumura M, Amaya H, et al. Tumor vascularity correlates with the prognosis of patients with esophageal squamous cell carcinoma. Cancer. 1997;79(2):220–225. [PubMed] [Google Scholar]
- 16.Nakopoulou L, Lekkas N, Lazaris AC, et al. An immunohistochemical analysis of angiogenesis in invasive breast cancer with correlations to clinicopathologic predictors. Anticancer Res. 1999;19(5C):4547–4553. [PubMed] [Google Scholar]
- 17.Yoshida A, Anand-Apte B, Zetter BR. Differential endothelial migration and proliferation to basic fibroblast growth factor and vascular endothelial growth factor. Growth Factors. 1996;13(1–2):57–64. doi: 10.3109/08977199609034566. [DOI] [PubMed] [Google Scholar]
- 18.Beatrice F, Cammarota R, Giordano C, et al. Angiogenesis: prognostic significance in laryngeal cancer. Anticancer Res. 1998;18(6B):4737–4740. [PubMed] [Google Scholar]
- 19.Inoue K, Ozeki Y, Suganuma T, Sugiura Y, Tanaka S. Vascular endothelial growth factor expression in primary esophageal squamous cell carcinoma: association with angiogenesis and tumor progression. Cancer. 1997;79(2):206–213. doi: 10.1002/(sici)1097-0142(19970115)79:2<206::aid-cncr2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 20.Dray TG, Hardin NJ, Sofferman RA. Angiogenesis as a prognostic marker in early head and neck cancer. Ann Otol Rhinol Laryngol. 1995;104(9 pt 1):724–729. doi: 10.1177/000348949510400911. [DOI] [PubMed] [Google Scholar]
- 21.Dellacono FR, Spiro J, Eisma R, Kreutzer D. Expression of basic fibroblast growth factor and its receptors by head and neck squamous carcinoma tumor and vascular endothelial cells. Am J Surg. 1997;174(5):540–544. doi: 10.1016/s0002-9610(97)00169-4. [DOI] [PubMed] [Google Scholar]
- 22.Petruzzelli GJ, Benefield J, Taitz AD, et al. Heparin-binding growth factor(s) derived from head and neck squamous cell carcinomas induce endothelial cell proliferations. Head Neck. 1997;19(7):576–582. doi: 10.1002/(sici)1097-0347(199710)19:7<576::aid-hed3>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 23.Steenfos HH. Growth factors and wound healing. Scand J Plast Reconstr Surg Hand Surg. 1994;28(2):95–105. doi: 10.3109/02844319409071186. [DOI] [PubMed] [Google Scholar]