Abstract
Telomere length (TL), a measure of replicative senescence, decreases with aging, but the factors involved are incompletely understood. To determine if age-associated reductions in TL are related to habitual endurance exercise and maximal aerobic exercise capacity (maximal oxygen consumption, VO2max), we studied groups of young (18 – 32 years; n = 15, 7m) and older (55 – 72 years; n = 15, 9m) sedentary and young (n = 10, 7m) and older endurance exercise-trained (n = 17, 11m) healthy adults. Leukocyte TL (LTL) was shorter in the older (7059 ± 141 bp) vs. young (8407 ± 218) sedentary adults (P < 0.01). LTL of the older endurance-trained adults (7992 ± 169 bp) was ~900 bp greater than their sedentary peers (P < 0.01) and was not significantly different (P=0.12) from young exercise-trained adults (8579 ± 413). LTL was positively related to VO2max due to a significant association in older adults (r = 0.44, P < 0.01). Stepwise multiple regression analysis revealed that VO2max independently explained ~60% of the variance in LTL. Our results indicate that LTL is preserved in healthy older adults who perform vigorous aerobic exercise and is positively related to maximal aerobic exercise capacity. This may represent a novel molecular mechanism underlying the "anti-aging" effects of maintaining high aerobic fitness.
Telomere length (TL) is a measure of replicative senescence (Aubert & Lansdorp, 2008) and a proposed marker of biological aging (Bekaert et al., 2007). TL decreases progressively with aging (Gilley et al., 2008) and is associated with numerous pathologies (Brouilette et al., 2007; Demissie et al., 2006; Fitzpatrick et al., 2007) and all-cause mortality (Cawthon et al., 2003; Farzaneh-Far et al., 2008). Moreover, cellular senescence induced by telomere shortening is a potential mechanism underlying reductions in physiological function with aging (Muller, 2009; Fuster & Andrés, 2006).
At any age, TL is determined by the combination of the TL at birth and the rate of age-related decline (Aubert & Lansdorp, 2008). Despite its biological and biomedical importance, the factors that influence the age-associated decrease in TL are incompletely understood, particularly with regard to factors that may act to preserve TL. In this context, habitual aerobic exercise and high aerobic exercise capacity generally are associated with better maintenance of cellular function with aging compared with a sedentary lifestyle. However, associations between TL and habitual physical activity are inconsistent (Cherkas et al., 2008; Woo et al., 2008; Ludlow et al., 2008, Shin et al., 2008) and there is no information on TL and objective measures of aerobic capacity.
Accordingly, we tested the hypothesis that TL is at least partially preserved with aging in humans who perform regular vigorous aerobic exercise, and is positively related to maximal aerobic exercise capacity. We studied 57 men and women: 15 young (18 – 32 years, 7 male) and 15 older (55 – 72 years) sedentary (9 male), and 10 young (7 male) and 17 older habitually exercising (11 male). Subjects were non-obese, non-smokers and healthy as assessed by medical history, physical examination, blood chemistries and exercise ECG (older subjects only). Sedentary subjects performed no regular aerobic exercise (< 2 days/week, < 30 min/day), whereas exercising subjects had performed regular vigorous aerobic exercise (≥ 5 days/week, > 45 min/day) for ≥5 years.
Leukocyte TL (LTL), the most commonly used human telomere sample and a proposed marker of systemic biological aging (Bekaert et al., 2007; Aviv, 2002; Cherkas et al., 2008), was measured in genomic DNA extracted from isolated leukocytes using a modification of the Southern blot analysis described previously (Middleman et al., 2006). Maximal oxygen consumption (VO2max) was assessed during treadmill exercise and used as a measure of maximal aerobic exercise capacity as described earlier (DeSouza et al., 2000). Subject characteristics were assessed using standard techniques described elsewhere (Pierce et al., 2008). All measurements were performed after a 12-h fast and 24-h abstention from alcohol and physical activity. Group differences were determined by analysis of variance. Bivariate Pearson correlation analyses were used to assess relations of interest. Stepwise linear regression analysis was performed to identify independent predictors of LTL.
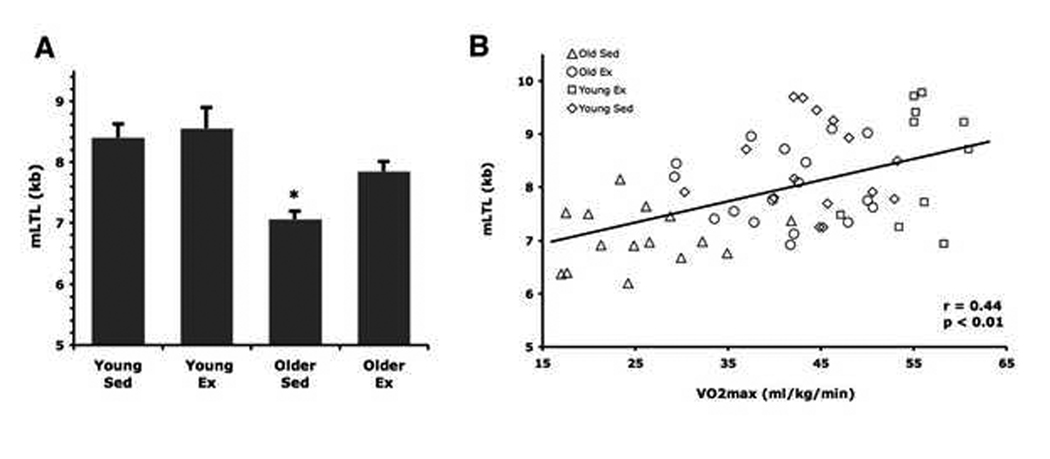
Several subject characteristics differed among the groups in the expected directions (Table 1). Diet composition and nutrient intake, adjusted for total calories, were similar among the groups (data not shown). LTL was shorter in the older (7059 ± 141 bp) vs. young (8407 ± 218) sedentary adults (P < 0.01, Figure 1). LTL of the older endurance-trained adults (7992 ± 169 bp) was ~900 bp greater than their sedentary peers (P < 0.01) and was not significantly different (P = 0.12) from young exercise-trained adults (8579 ± 413).
Table 1.
Subject Characteristics
| Young Sedentary | Young Exercising | Older Sedentary | Older Exercising | |
|---|---|---|---|---|
| n | 15 | 10 | 15 | 17 |
| Sex | 7M,8F | 7M, 3F | 9M,6F | 11M,6F |
| Age | 23 ± 1 | 21 ± 1 | 65 ± 1* | 62 ± 2# |
| VO2max, ml/kg/min | 43.7 ± 2.0 | 55.8 ± 1.4* | 25.9 ± 1.8* | 40.5 ± 1.7#† |
| Leisure time physical activity, (MET hrs/wk) | 32 ± 6 | 137 ± 22* | 12 ± 3* | 75 ± 11#† |
| Body mass index, kg/m2 | 22.1 ± 0.7 | 22.3 ± 0.4 | 25.5 ± 1* | 23.2 ± 0.9 |
| % Body Fat | 24.6 ± 2.6 | 13.4 ± 2.5* | 33.7 ± 2.7* | 21.4 ± 2.0#† |
| Waist:hip ratio | 0.76 ± 0.02 | 0.71 ± 0.03 | 0.87 ± 0.03* | 0.85 ± 0.02# |
| Systolic blood pressure, mm Hg | 104 ± 9 | 102 ± 10 | 125 ± 4* | 120 ± 4# |
| Diastolic blood pressure, mm Hg | 57 ± 2 | 54 ± 5 | 75 ± 2* | 75 ± 3# |
| Total cholesterol, mg/dL | 150 ± 11 | 144 ± 19 | 186 ± 7* | 190 ± 11#† |
| LDL cholesterol, mg/dL | 82 ± 9 | 88 ± 11 | 110 ± 7* | 109 ± 10 |
| HDL cholesterol, mg/dL | 53 ± 3 | 50 ± 4 | 56 ± 4 | 63 ± 3# |
| Triglycerides, mg/dL | 76 ± 7 | 77 ± 7 | 99 ± 11* | 110 ± 15# |
| Glucose, mg/dL | 84 ± 3 | 87 ± 3 | 88 ± 1 | 89 ± 2 |
| C-reactive Protein, mg/L | 0.5 ± 0.2 | 0.6 ± 0.2 | 1.3 ± 0.5 | 1.2 ± 0.4 |
Values are means ± SEs. MET, metabolic equivalent; LDL, low-density lipoprotein; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance;
P < 0.01 vs. Young Sedentary;
P < 0.01 Older Exercising vs. Younger Exercising;
P < 0.01 Older Exercising vs. Older Sedentary.
Figure 1.
(A) Compared with sedentary (Sed) and exercising (Ex) young adults, mean LTL was shorter in sedentary, but not exercising older adults (*P < 0.01 vs. young groups and older exercising group). (B) Mean LTL is positively related to maximal aerobic exercise capacity.
LTL was positively related to VO2max in the overall sample (r = 0.44, P < 0.01, Figure 1) due to a positive correlation among the older adults (r = 0.44, P < 0.01), as there was no relation among young adults (r = −0.02, P = 0.92). Stepwise multiple regression analysis revealed that in the overall sample VO2max was the only variable that entered and independently explained 62% of the variance in LTL (R2 = 0.62, b = 0.51, P < 0.01), whereas in the older subjects alone VO2max explained 60% of the variance (R2 = 0.60, b = 0.077, P < 0.01) with age explaining an additional 28% (R2 = 0.88, b = −0.051, P < 0.01). The relation between LTL and VO2max in the overall and older groups was not affected by other subject characteristics. LTL did not correlate with leisure-time physical activity in any group (r = 0.15 – 0.28, P = 0.11 – 0.52). LTL was not influenced by the sex of the subjects (P = 0.21).
The results of the present study provide evidence that LTL is related to regular vigorous aerobic exercise and maximal aerobic exercise capacity with aging in healthy humans. LTL is not influenced by aerobic exercise status among young subjects, presumably because TL is intact (i.e., already normal) in sedentary healthy young adults. However, as LTL shortens with aging it appears that maintenance of aerobic fitness, produced by chronic strenuous exercise and reflected by higher VO2max, acts to preserve LTL.
The exact nature of these relations and the underlying mechanisms are not clear. Risk factors for cardiovascular disease may influence LTL (Chen et al., 2009; Fitzpatrick et al., 2007), but did not explain the relations with VO2max in the present study. Habitual aerobic exercise modulates VO2max, but as much as ~50% of the latter is determined primarily by genetic factors (Bouchard et al., 1998 & 1999). Shorter-term exercise training has no effect on LTL in humans or mice (Shin et al., 2008; Werner et al., 2008), but does modulate telomere-regulating proteins (Werner et al., 2008). This may suggest that longer-term or more strenuous exercise is required to influence LTL. It also is possible that habitual exercise behavior, higher VO2max and longer LTL all are part of the same phenotype expressed in some older adults. Although previous findings are inconsistent (Cherkas et al., 2008; Ludlow et al., 2008; Woo et al., 2008), the fact that leisure-time physical activity was unrelated to LTL in our study suggests a more direct association with maximal aerobic exercise capacity.
In conclusion, LTL is preserved with age in endurance exercise-trained humans and is related to maximal aerobic exercise capacity among healthy older adults. This may represent a novel molecular mechanism linking vigorous habitual exercise/aerobic fitness to reduced cellular senescence and preserved physiological function with aging.
Acknowledgments
We thank John Anderson for his efforts in DNA isolations and Arthur Zaug for his assistance with Southern blot procedures.
Sources of funding
Supported by National Institutes of Health awards AG013038, AG006537, AG022241, AG015897, AG031141, AG000279 and RR00051.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aubert G, Lansdorp PM. Telomeres and Aging. Physiol. Rev. 2008;88:557–579. doi: 10.1152/physrev.00026.2007. [DOI] [PubMed] [Google Scholar]
- Aviv A. Chronology versus biology: telomeres, essential hypertension, and vascular aging. Hypertension. 2002;40:229–232. doi: 10.1161/01.hyp.0000027280.91984.1b. [DOI] [PubMed] [Google Scholar]
- Bekaert S, De Meyer T, Rietzschel ER, De Buyzere ML, De Bacquer D, Langlois M, Segers P, Cooman L, Van Damme P, Cassiman Pa, Van Criekinge W, Verdonck P, De Backer GG, Gillebert TC, Van Oostveldt P. Telomere length and cardiovascular risk factors in a middle-aged population free of overt cardiovascular disease. Aging Cell. 2007;6:639–647. doi: 10.1111/j.1474-9726.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- Bouchard C, Daw EW, Rice T, Pérusse L, Gagnon J, Province MA, Leon AS, Rao DC, Skinner JS, Wilmore JH. Familial resemblance for VO2max in the sedentary state: the HERITAGE Family Study. Med. Sci. Sports Exerc. 1998;30:252–258. doi: 10.1097/00005768-199802000-00013. [DOI] [PubMed] [Google Scholar]
- Bouchard C, Daw EW, Rice T, Pérusse L, Gagnon J, Province MA, Leon AS, Rao DC, Skinner JS, Wilmore JH. Familial aggregation of VO2max response to exercise training: results from the HERITAGE Family Study. J. Appl. Physiol. 1999;87:1003–1008. doi: 10.1152/jappl.1999.87.3.1003. [DOI] [PubMed] [Google Scholar]
- Brouilette SW, Moore JS, McMahon AD, Thompson JR, Ford I, Shepherd J, Packard CJ, Samani NJ. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet. 2007;13:107–114. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- Chen W, Gardner JP, Kimura M, Brimacombe M, Cao X, Srinivasan SR, Berenson GS, Aviv A. Leukocyte telomere length is associated with HDL cholesterol levels: The Bogalusa heart study. Atherosclerosis. 2009;205:620–625. doi: 10.1016/j.atherosclerosis.2009.01.021. [DOI] [PubMed] [Google Scholar]
- Cherkas LF, Hunkin JL, Kato BS, Richards JB, Gardner JP, Surdulescu GL, Kimura M, Lu X, Spector TD, Aviv A. The association between physical activity in leisure time and leukocyte telomere length. Arch. Intern. Med. 2008;168:154–158. doi: 10.1001/archinternmed.2007.39. [DOI] [PubMed] [Google Scholar]
- Demissie S, Levy D, Benjamin EJ, Cupples LA, Gardner JP, Herbert A, Kimura M, Larson MG, Meigs JB, Keaney JF, Aviv A. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell. 2006;5:325–330. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- Farzaneh-Far R, Cawthon RM, Na B, Browner WS, Schiller NB, Whooley MA. Prognostic value of leukocyte telomere length in patients with stable coronary artery disease: data from the Heart and Soul Study. Arterioscler. Thromb. Vasc. Biol. 2008;28:1379–1384. doi: 10.1161/ATVBAHA.108.167049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick AL, Kronmal RA, Gardner JP, Psaty BM, Jenny NS, Tracy RP, Walston J, Kimura M, Aviv A. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am. J. Epidemiol. 2007;165:14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- Fuster JJ, Andrés V. Telomere biology and cardiovascular disease. Circ. Res. 2006;99:1167–1180. doi: 10.1161/01.RES.0000251281.00845.18. [DOI] [PubMed] [Google Scholar]
- Gilley D, Herbert BS, Huda N, Tanaka H, Reed T. Factors impacting human telomere homeostasis and age-related disease. Mech. Ageing. Dev. 2008;129:27–34. doi: 10.1016/j.mad.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Ludlow AT, Zimmerman JB, Witkowski S, Hearn JW, Hatfield BD, Roth SM. Relationship between physical activity level, telomere length, and telomerase activity. Med. Sci. Sports. Exerc. 2008;40:1764–1771. doi: 10.1249/MSS.0b013e31817c92aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleman EJ, Choi J, Venteicher AS, Cheung P, Artandi SE. Regulation of cellular immortalization and steady-state levels of the telomerase reverse transcriptase through its carboxy-terminal domain. Mol. Cell. Biol. 2006;26:2146–2159. doi: 10.1128/MCB.26.6.2146-2159.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M. Cellular senescence: molecular mechanisms, in vivo significance, and redox considerations. Antioxid. Redox. Signal. 2009;11:59–98. doi: 10.1089/ars.2008.2104. [DOI] [PubMed] [Google Scholar]
- Pierce GL, Beske SD, Lawson BR, Southall KL, Benay FJ, Donato AJ, Seals DR. Weight loss alone improves conduit and resistance artery endothelial function in young and older overweight/obese adults. Hypertension. 2008;52:72–79. doi: 10.1161/HYPERTENSIONAHA.108.111427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin YA, Lee JH, Song W, Jun TW. Exercise training improves the antioxidant enzyme activity with no changes of telomere length. Mech. Ageing. Dev. 2008;129:254–260. doi: 10.1016/j.mad.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Werner C, Hanhoun M, Widmann T, Kazakov A, Semenov A, Pöss J, Bauersachs J, Thum T, Pfreundschuh M, Müller P, Haendeler J, Böhm M, Laufs U. Effects of physical exercise on myocardial telomere-regulating proteins, survival pathways, and apoptosis. J. Am. Coll. Cardiol. 2008;52:470–482. doi: 10.1016/j.jacc.2008.04.034. [DOI] [PubMed] [Google Scholar]
- Woo J, Tang N, Leung J. No association between physical activity and telomere length in an elderly Chinese population 65 years and older. Arch. Intern. Med. 2008;168:2163–2164. doi: 10.1001/archinte.168.19.2163. [DOI] [PubMed] [Google Scholar]