Figure 1.
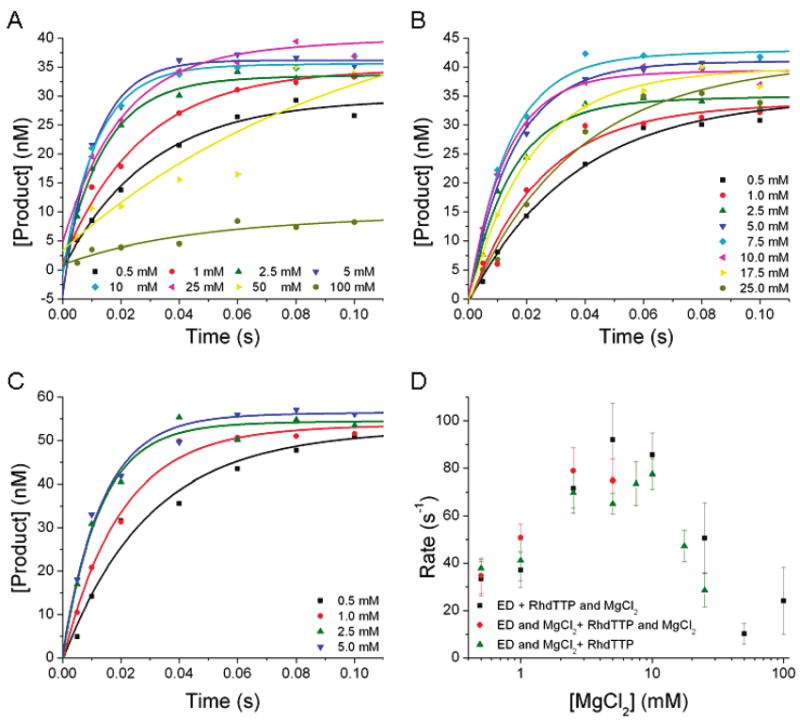
Incorporation of Rh·dTTP opposite dA at varying concentrations of MgCl2. The final reaction conditions for all experiments depicted consisted of 100 nM RB69 pol, 90 nM DNA, 250 μM Rh·dTTP, 50 mM MOPS (pH 7.0), and varying concentrations of MgCl2 from 0.5 to 100 mM. (A) The reaction was initiated by premixing Rh·dTTP and MgCl2 and then rapidly mixing this solution with one containing RB69 pol and DNA. The incorporation rate is 92 ± 15 s−1. (B) The reaction was initiated by premixing RB69 pol, DNA, and MgCl2 and then rapidly mixing this solution with one containing Rh·dTTP. The incorporation rate is 77 ± 6 s−1. (C) The reaction was initiated by premixing RB69 pol, DNA, and MgCl2 and then rapidly mixing this solution with one containing Rh·dTTP and MgCl2. The incorporation rate is 80 ± 10 s−1. (D) The rates of product formation are plotted vs MgCl2 concentration for each of the three experiments depicted in panels A–C. Regardless of when MgCl2 is added, the observed maximum rate of the reaction fits to approximately 80 s−1, at a MgCl2 concentration of approximately 10 mM. Because of the mixing procedure, each solution was prepared at twice the final reported concentration. The product concentrations were plotted vs time and fit to a single-exponential equation.
