Abstract
Background
Exposure to group A Streptococcus (GAS) has been shown to induce maturation of dendritic cells (DC).
Methods
To identify bacterial determinants that modulate DC activation in response to GAS infection, we analyzed the induction of maturation in human monocyte-derived DC following exposure to GAS clinical isolates.
Results
Unexpectedly, only six of 24 GAS strains tested induced surface expression of MHC class II and co-stimulatory molecules CD80 and CD83 to levels consistent with DC maturation. Rather, the majority of the strains did not promote DC maturation and many triggered DC apoptosis. GAS strains that failed to induce DC maturation were those that produced abundant hyaluronic acid (HA) capsular polysaccharide and/or large amounts of the cytotoxin streptolysin O (SLO). Using isogenic mutants deficient in HA and/or SLO, we determined that GAS inhibits DC maturation through two distinct mechanisms: 1) inhibition of bacterial binding and/or phagocytosis by the HA capsule, and 2) SLO-mediated induction of DC apoptosis by intracellular GAS.
Conclusions
These results demonstrate that GAS virulence factors modulate maturation and survival of human DC, effects that are likely to have a critical impact on activation of innate and adaptive immune responses to this important human pathogen.
Keywords: Streptococcus pyogenes, streptolysin O, capsular polysaccharide, dendritic cells, apoptosis, phagocytosis
INTRODUCTION
Streptococcus pyogenes or group A Streptococcus (GAS) is a major cause of bacterial infection in school age children and a less frequent but significant source of acute illness in all age groups. In addition to streptococcal pharyngitis (strep throat), GAS causes a spectrum of infections involving the skin and soft tissues that require antibiotic treatment and that may progress to life-threatening invasive disease [1, 2]. Equally important from a public health perspective is the unique capacity of GAS pharyngeal infection to trigger acute rheumatic fever, a postinfectious inflammatory syndrome responsible for substantial ongoing morbidity in many resource-limited countries [3]. The variety of clinical manifestations associated with GAS infections shows the versatility of this pathogen, which can finely modulate the expression of its virulence determinants in order to overcome the innate barrier constituted by epithelial cells and resident professional phagocytes to successfully infect the human host.
Among professional phagocytes, dendritic cells (DC) are particularly abundant at body surfaces, including pharynx and skin, the most common sites of GAS infection. Upon recognition of pathogen-derived molecules, DC undergo a process of activation or “maturation” that results in the expression of proinflammatory cytokines and chemokines that will orchestrate the innate immune response. DC maturation is also characterized by the up-regulation of cell-surface levels of MHC and co-stimulatory molecules that boost DC immunogenicity or ability to prime T cell responses, thus establishing a bridge between innate and adaptive immunity. Interaction of GAS with human and murine DC in vitro has been shown to induce DC maturation with particularly high production of Th1-polarizing cytokines [4–6]. GAS-induced mature DC can stimulate allogeneic CD4+ T cells to produce IFNγ [5], and can also elicit allogeneic antigen-specific cytotoxicity [7]. Moreover, a recent study has shown that DC contribute to protection against GAS dissemination in a mouse model of skin infection [4]. While these studies provide evidence that GAS can induce DC maturation, the streptococcal factors that may be involved in GAS recognition and in modulation of DC function remain to be identified.
In the present study we report the unexpected finding that only a minority of GAS clinical isolates induce maturation of human monocyte-derived DC in vitro. We demonstrate that GAS strains differ in their ability to induce DC maturation, and we identify some of the virulence factors responsible for this variability. The hyaluronic acid (HA) capsular polysaccharide and the secreted cytotoxin streptolysin O (SLO) inhibit GAS-induced DC maturation through independent mechanisms. Our results suggest that production of these virulence factors may contribute to pathogenesis by preventing early recognition of GAS by DC, resulting in prolonged GAS persistence in the naïve host, and impaired development of acquired immunity.
MATERIALS AND METHODS
GAS strains and growth conditions
Twenty-four GAS clinical isolates used in this study were provided by the Microbiology Laboratory at Children’s Hospital Boston or were from the authors’ strain collection. Production of SLO and of cell-associated HA capsule were quantified as described previously [8, 9]. GAS strain 950771 is a moderately encapsulated M3 clinical isolate from a child with necrotizing fasciitis and sepsis [10]. Isogenic mutants deficient in synthesis of HA and/or SLO were previously derived in the background of strain 950771 [11–13].
GAS strains were grown overnight at 37° C on blood-agar plates, and used to inoculate Todd Hewitt broth supplemented with 0.5% yeast extract. Bacterial cultures were grown to mid-exponential phase, collected, washed extensively, and diluted with cell culture medium prior to inoculation of DC.
Generation of monocyte-derived DC and cell culture conditions
Human peripheral blood mononuclear cells (PBMC) were isolated from freshly collected leukocyte-enriched blood obtained from healthy donors (Children’s Hospital Boston Blood Donor Center) by a density gradient centrifugation over Histopaque-1077 (Sigma-Aldrich). Monocytes were purified by negative selection after magnetic labeling of non-monocyte PBMC using a cocktail of biotin-conjugated antibodies against CD3, CD7, CD16, CD19, CD56, CD123 and Glycophorin A, and Anti-Biotin Microbeads (Miltenyi Biotec). Monocytes were cultured over 5–6 days in RPMI 1640 with 2 mM L-glutamine, 50 μM β-mercaptoethanol, and 10% heat-inactivated fetal bovine serum (FBS; Invitrogen) supplemented with GM-CSF and IL-4 (R&D Systems) each at final concentration of 20 ng/ml. These culture conditions result in a highly pure CD1a+ CD14− CD83− population consistent with immature DC.
Exposure of DC to GAS for in vitro experiments
Immature DC were exposed to a suspension of live GAS in cell culture medium at a GAS to DC ratio ranging from 0.1 to 10 bacteria per DC. Two hours later, DC were extensively washed to remove unbound bacteria and were replated in fresh culture medium containing 20 μg/ml penicillin G and 200 μg/ml gentamicin (Sigma-Aldrich) for the desired chase period. In experiments to block bacteria-cell contact, DC were cultured in 24-well plates and bacteria were added to the upper chamber of a Transwell insert that separated the bacteria from the DC by a membrane with 0.4 μm pores (Corning).
In some experiments, DC were exposed to GAS in the presence of 50 μg/ml of polymyxin B (Sigma-Aldrich) to exclude the possibility of LPS contamination.
Cells incubated with 10 ng/ml LPS from E. coli or 1 μg/ml actinomycin D (Sigma-Aldrich) were used as positive controls for induction of maturation or apoptosis, respectively.
Flow cytometric analysis of DC
DC were exposed to GAS strains for two hours as described above. Twenty hours later, DC were harvested and stained with Abs to human CD83, HLA-DR, CD1a, CD80, CD40, and HLA-DR conjugated to FITC, PE, or PerCP (BD Pharmingen). Stained cells were analyzed in a MoFlo high speed flow cytometer. Median fluorescence intensity values were corrected for nonspecific background staining assessed using isotype control Abs.
Phagocytic capacity of DC
Flow cytometric analysis. GAS-exposed DC were collected at different time points, fixed in 2% paraformaldehyde, and permeabilized prior to staining with an Alexa 488-conjugated IgG fraction of rabbit antiserum to GAS group A carbohydrate (Immucell). The percentage of DC with GAS-labeling was determined by flow cytometry, gated so as to exclude free bacteria. Unstimulated cells were used as controls to determine nonspecific fluorescence.
Confocal microscopy analysis. DC exposed to GAS were collected, washed, and plated on poly-L-lysine (Sigma-Aldrich) coated microscope slides. After 30 min attachment, cells were fixed, permeabilized, and stained with an Alexa 660-conjugated rabbit IgG anti-GAS, an Alexa 488-conjugated goat IgG against LAMP-1 (Santacruz) and phalloidin conjugated to Alexa 568 (Molecular Probes) to label the actin cytoskeleton.
Antibiotic exclusion assay. GAS-exposed DC were collected 2 h post-infection, washed extensively, and incubated with 20 μg/ml penicillin G and 200 μg/ml gentamicin (Sigma-Aldrich) for 1 h to kill extracellular bacteria. Viable intracellular bacteria were recovered after lysis of DC with sterile water and quantified by plating serial dilutions on blood agar plates.
Determination of apoptosis by flow cytometry
At different time points after exposure to GAS, DC were harvested, fixed, and stained with propidium iodide in hypotonic buffer, as described [14]. The percentage of cells containing apoptotic nuclei (i.e., hypodiploid) was measured by flow cytometry.
Determination of cellular caspase activation
A fluorescent inhibitor of caspases (FLICA) was used to detect caspase activation in GAS-exposed DC, following the manufacturer’s instructions (Molecular Probes Invitrogen). Briefly, cells were harvested 2 h or 24 h after exposure to GAS and gently resuspended in medium containing the poly-caspase substrate, FAM-VAD-FMK (FLICA). The percentage of cells stained with FLICA was determined by flow cytometry.
Statistical analysis
Data were expressed as arithmetic mean ± SEM of the individual values. Differences between groups were evaluated by the ANOVA test. A P value of ≤0.05 was considered significant.
RESULTS
GAS clinical isolates differ in their ability to induce DC maturation
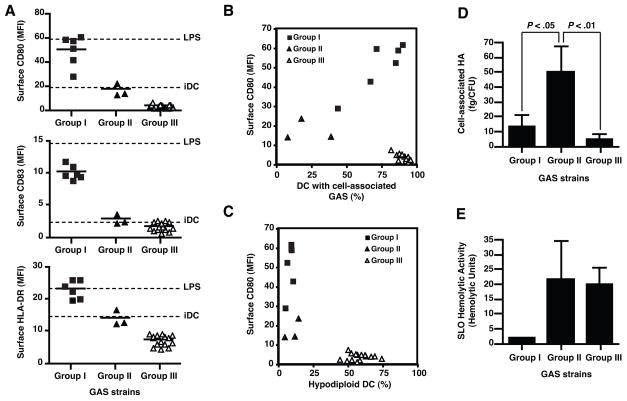
Intracellular signaling triggered upon recognition of microbial factors by members of the Toll-like receptor family stimulates DC maturation [15]. As an initial assessment of the ability of GAS to induce DC maturation, we measured the expression of CD80, CD83, and MHC II on human monocyte-derived DC following exposure of the cells to each of 24 GAS strains representing diverse phenotypic characteristics and clinical syndromes. DC were exposed to live GAS strains at a bacteria to DC ratio of 1 to mimic early infection, although similar results were obtained in preliminary experiments using higher doses of bacteria. After 2 h of bacteria-DC coculture, extracellular bacteria were killed by the addition of antibiotics, and 20 h later DC were stained and analyzed by flow cytometry. We identified three groups of GAS strains based on their capacity to stimulate DC maturation (Fig. 1A and Table 1). Strains that induced levels of expression of MHC II and co-stimulatory molecules similar to those induced by LPS were designated as group I. Group II isolates were those that induced little or no change in the levels of CD80, CD83, and MHC II expression, while group III isolates produced a slight decrease compared to untreated DC. Maturation of GAS-exposed DC was not due to LPS-contamination, since preincubation of the cells with 10 μg/ml of the LPS-inhibitor polymyxin B had no effect on GAS-induced DC maturation, but effectively blocked LPS-induced maturation.
Figure 1.
GAS strains vary in their capacity to induce DC maturation or apoptosis. Human monocyte-derived DC were exposed to live GAS for two h at a GAS to DC ratio of 1:1. Twenty h later, surface expression of CD80, CD83, and HLA-DR was quantified by flow cytometry (A). Values for control untreated DC, and LPS-stimulated DC are shown as dashed lines. Following staining of surface CD80, DC were fixed, permeabilized, and stained with a GAS-specific antiserum to assess binding and internalization of GAS (B), or with propidium iodide to determine percentage of hypodiploid nuclei (apoptotic cells) (C). Each symbol represents the mean of results for three independent donors. 10,000 cells were examined in each measurement. GAS clinical isolates were classified into three groups according to DC responses to each strain: group I, mature DC (filled squares); group II, immature DC (filled triangles); group III, immature apoptotic DC (open triangles). To further characterize the phenotype of GAS strains, bacterial cell-associated HA (D) and SLO-hemolytic activity (E) of the GAS strains were determined. Bars represent mean values±SD of at least three independent experiments for GAS clinical isolates included in each group.
Table 1.
Properties of GAS clinical isolates and effects of GAS exposure on DC phenotype.
| iDC | Group I isolates | Group II isolates | Group III isolates | |
|---|---|---|---|---|
| GAS Phenotype | ||||
| No. of isolates | 6 | 3 | 15 | |
| HA (fg/CFU) | 13.6 ± 7.6 | 50.2 ± 17.2 | 4.9 ± 3.0 | |
| SLO (HU) | 1.8 ± 0.3 | 21.6 ± 13.2 | 20.2 ± 5.5 | |
| DC Phenotype | ||||
| CD83 (MFI) | 2.3 ± 1.1 | 10.3 ± 0.4 | 2.9 ± 0.4 | 1.8 ± 0.2 |
| HLA-DR (MFI) | 14.4 ± 0.7 | 23.5 ± 1.1 | 14.3 ± 1.4 | 7.5 ± 0.4 |
| CD80 (MFI) | 18.6 ± 0.3 | 50.7 ± 5.2 | 17.1 ± 3.1 | 3.7 ± 0.4 |
| Hypodiploid DC (%) | 5.9 ± 1.1 | 8.3 ± 0.8 | 10.5 ± 3.0 | 58.4 ± 2.0 |
| DC with associated GAS (%) | 74.0 ± 6.5 | 21.8 ± 9.1 | 90.6 ± 1.0 | |
Note.- iDC = Untreated DC; MFI = Median Fluorescence Intensity; HU = Hemolytic Units. Values reported correspond to group means±SD.
Because bacterial binding and uptake enhances recognition of bacterial molecules by DC pattern recognition receptors, we next analyzed the phagocytic activity of DC for each of the GAS isolates. After 20 h of exposure to GAS, fixed and permeabilized DC were stained with an antiserum to GAS group A carbohydrate (Fig. 1B), thus revealing DC with attached and/or internalized GAS. For isolates in groups I and II, we observed a correlation between GAS association with DC and expression of DC maturation markers, suggesting that physical association of GAS with DC was necessary to stimulate maturation. In contrast, group III strains associated with DC, but failed to induce DC maturation.
Flow cytometry of DC after staining with propidium iodide revealed hypodiploid nuclear staining consistent with apoptosis in DC exposed to group III GAS strains (Fig. 1C). More than 50% of DC exposed to group III GAS strains exhibited hypodiploid nuclei vs <15% for DC exposed to either group I or II GAS strains or untreated DC. Together, these results suggest that GAS may block DC maturation by resisting attachment and/or phagocytic uptake or by inducing DC apoptosis.
GAS isolates that induce DC maturation are low producers of virulence factors
We next characterized the expression of GAS products with anti-phagocytic and/or pro-apoptotic properties (Fig. 1D–E). The hyaluronic acid (HA) capsule of GAS plays a central role in resistance to phagocytosis by macrophages and neutrophils, and to internalization by keratinocytes [16]. As shown in Fig. 1D, group II GAS strains, which associated relatively inefficiently with human DC, expressed significantly higher amounts of cell-associated HA (50.2 ± 17.2 fg HA/CFU) than did group I (13.6 ± 7.6 fg HA/CFU) or group III (4.9 ± 3.0 fg HA/CFU) strains, suggesting that the capsule might impede phagocytosis of GAS by human DC.
Among the secreted products of GAS, streptolysin O (SLO) can induce apoptosis in keratinocytes [17]. We measured the activity of SLO in culture supernatants from each GAS isolate (Fig. 1E). While SLO hemolytic activity from GAS strains that induced DC maturation (group I) was almost undetectable (1.8 ± 0.3 HU), strains that did not induce maturation (group II) or that triggered apoptosis (group III) were highly hemolytic (>20 HU), suggesting that SLO may contribute to inhibition of DC maturation and/or induction of DC apoptosis.
HA capsule and SLO expression inhibit DC maturation induced by live GAS
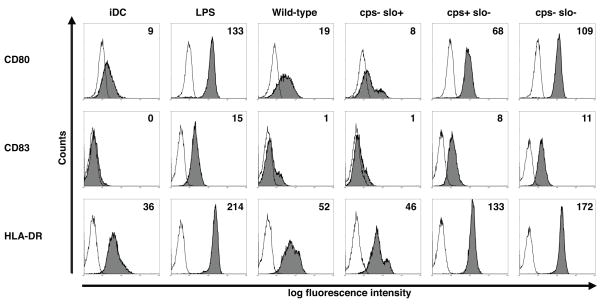
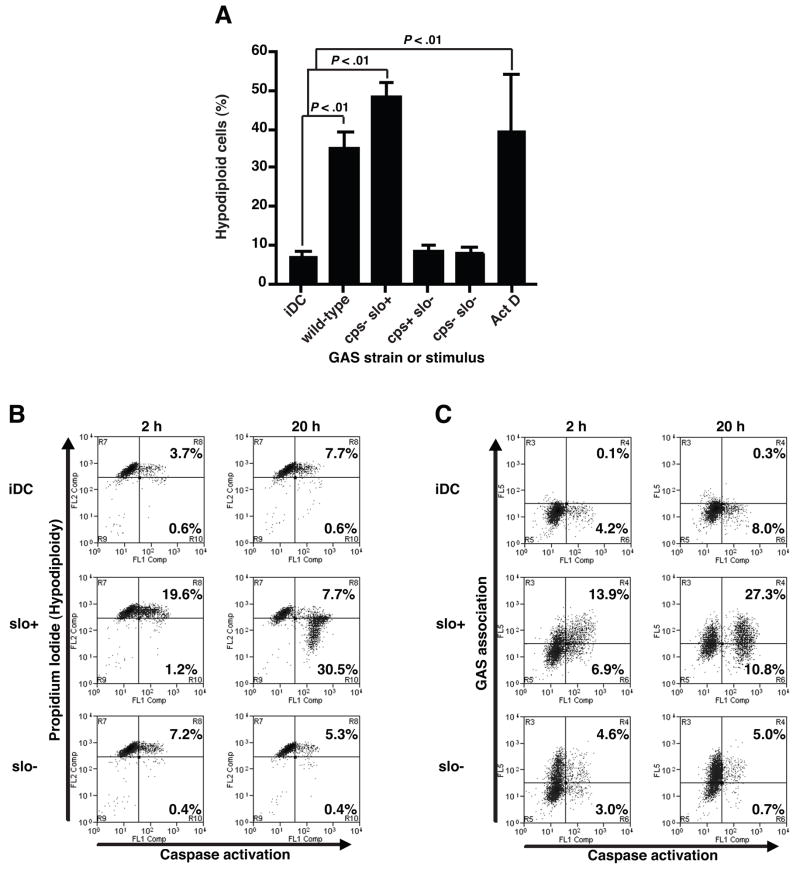
To test directly the effects of capsule and SLO on inhibition of DC maturation, we exposed human DC to isogenic GAS mutants deficient in synthesis of HA, SLO, or both under the experimental conditions described above. Exposure of DC to the live wild-type parent strain 950771 did not significantly increase the expression of MHC II and co-stimulatory molecules over untreated DC (Fig. 2), consistent with the relatively high-level production of both HA (41 fg HA/CFU) and SLO (26 HU) by this strain. Similarly, an acapsular mutant strain (cps−slo+) failed to induce DC maturation. In contrast, DC expression of CD80, CD83, and MHC II was significantly enhanced after exposure to a SLO-deficient mutant (cps+slo−) (Fig. 2). We observed a further increase in the levels of expression of co-stimulatory and MHC II molecules upon exposure of DC to a double mutant strain (cps−slo−). Therefore, consistent with the pattern of low expression of both virulence factors in GAS clinical isolates that triggered DC maturation (Fig. 1D–E), results with isogenic mutants confirmed that SLO and HA have important and independent roles in the inhibition of DC maturation by GAS.
Figure 2.
Expression of MHC II and co-stimulatory molecules by human DC after exposure to GAS. Using the same experimental protocol as in Figure 1, DC were exposed to live wild-type GAS strain 950771 (wild-type), or isogenic mutant strains (bacteria to DC ratio of 1:1): HA-deficient (cps−slo+), SLO-deficient (cps+slo−), or double knockout (cps−slo−). Histograms show the expression of the indicated markers (filled histogram) or staining with appropriate isotype control mAb (open histogram). Values in histograms depict the median channel fluorescence intensity (MFI) for CD80, CD83 and HLA-DR after subtracting the value for the isotype control. The data shown are representative of 10 independent donors.
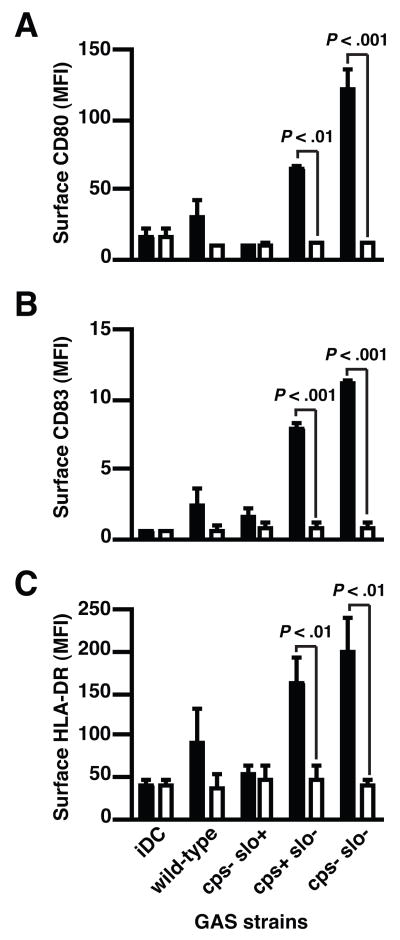
Induction of DC maturation requires physical contact with GAS
To confirm the correlation between bacterial association and DC maturation, we analyzed maturation induction when DC were physically separated from live GAS strains by a membrane with a pore size smaller than GAS cells (Fig. 3). In the absence of bacterial contact, SLO-deficient isogenic mutant strains were no longer able to induce the up-regulation of co-stimulatory and MHC II molecule expression, a result consistent with a requirement for physical contact of GAS for induction of DC maturation rather than stimulation of maturation solely by secreted GAS products.
Figure 3.
Physical contact is required for GAS-mediated DC maturation. Flow cytometric analysis of CD80 (A), CD83 (B) and MHC II (C) expression by DC directly exposed to live GAS wild-type strain and isogenic mutant strains (closed bars) or when bacterial contact was blocked with Transwell inserts (open bars). Means±SD of three independent donors are shown. (P<0.01 and P<0.001 for comparison with direct exposure).
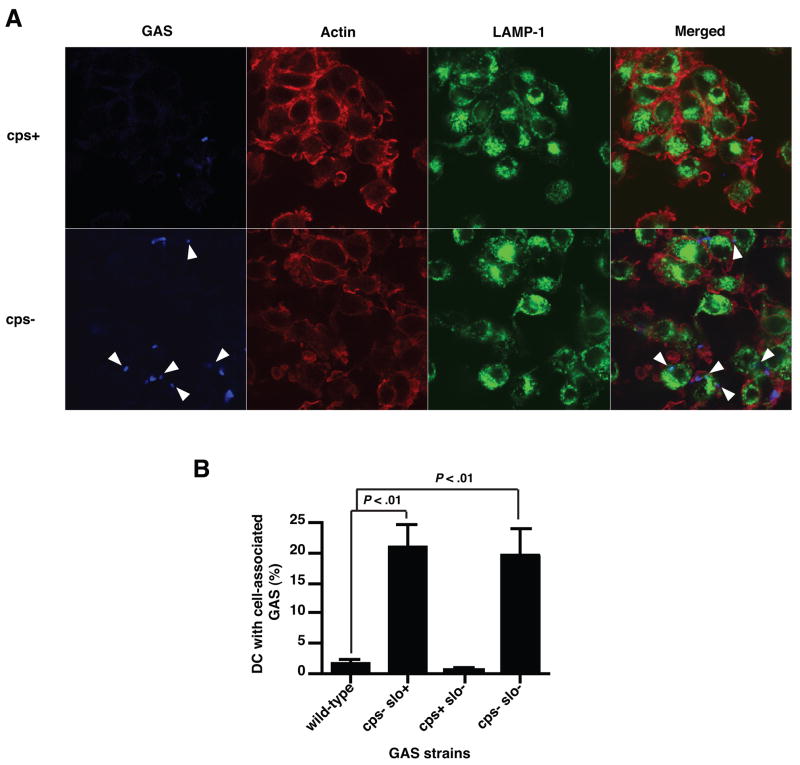
Hyaluronic acid capsule interferes with phagocytosis of GAS by DC
To investigate directly the effect of the HA capsule on the binding and internalization of GAS by DC, we examined the association of DC with live encapsulated wild-type or isogenic acapsular mutant GAS strains by confocal microscopy (Fig. 4A). By 2 h after inoculation, increased numbers of acapsular GAS were found in association with DC compared to encapsulated wild-type GAS. Triple-label experiments in which the actin cytoskeleton and lysosomes were visualized with phalloidin and anti-LAMP-1 antibody, respectively, demonstrated that encapsulated GAS appeared predominantly at the periphery of the DC, while acapsular GAS were located intracellularly and in close association with lysosomes (Fig. 4A, arrowheads). Therefore, the HA capsule not only reduced binding of GAS to DC but may also impede bacterial internalization. In fact, 20-fold more intracellular GAS organisms were recovered from cultures of cell lysates of DC exposed to the acapsular mutant than from DC exposed to the wild-type GAS strain (data not shown). Flow cytometry analysis showed that approximately 20% of the DC exposed to acapsular strains had associated bacteria after 2 hours versus <2% of the DC exposed to encapsulated strains (Fig. 4B). These results indicate that the HA capsule inhibition of DC-bacterial association by the HA capsule is responsible for the failure of DC maturation in response to heavily encapsulated strains.
Figure 4.
The HA capsule prevents binding an internalization of GAS by DC. A, Confocal microscopy analysis of GAS phagocytosis. Human DC pulsed with live GAS for 2 h were allowed to attach to poly-L-lysine coated cover slips, fixed, and permeabilized prior to staining with GAS-specific antiserum (blue). The lysosomal antigen LAMP-1 (green) and the actin cytoskeleton (red) were also visualized. Arrowheads show intracellular GAS. B, FACS analysis of GAS phagocytosis by DC. GAS-pulsed human DC were harvested 2 h post-inoculation, stained with GAS-specific antiserum, and analyzed by flow cytometry. Percentage of DC positive for GAS staining was compared to untreated DC. Means±SD of results from four independent donors are shown.
SLO induces caspase activation and apoptosis of human DC
Expression of SLO prevented DC maturation in response to acapsular GAS despite efficient bacterial phagocytosis (Fig. 4B). Because SLO has been shown previously to induce apoptosis in epithelial cells [17, 18], we assayed induction of apoptosis in DC exposed to GAS. We determined the percentage of DC with nuclear DNA fragmentation using flow cytometry after propidium iodide staining of the cells (Fig. 5A). A significant increase in the percentage of DC that exhibited hypodiploid nuclei was observed 20 h after exposure to live SLO-producing GAS: >35% of DC exposed to wild-type or acapsular GAS vs <9% for untreated DC or DC exposed to SLO-deficient GAS (P<0.01). A 5-fold increase in the percentage of DC containing active caspases was observed immediately after a 2 h exposure to live wild-type SLO-producing GAS compared to untreated DC or DC exposed to SLO-deficient GAS (Fig. 5B). After 20 h, caspase activation was significantly enhanced in DC exposed to SLO-producing GAS, and DC containing active caspases also exhibited hypodiploid nuclei consistent with apoptosis. Dual labeling experiments demonstrated that the apoptotic DC were those directly associated with GAS (Fig. 5C), suggesting that bacterial contact is required for SLO-mediated caspase activation and induction of DC apoptosis.
Figure 5.
SLO-mediated DC apoptosis involves caspase activation and GAS association. Percentages of hypodiploid cells were determined by propidium iodide staining in DC exposed to live wild-type or mutant GAS strains for 2 h followed by a 20 h chase-period (A). Means±SD of at least three independent donors are shown. P<0.01 for comparison with untreated DC (iDC). DC exposed to wild-type (slo+) or SLO-deficient (slo−) GAS strains for 2 h or 20 h were evaluated by 2-color plot of propidium iodide staining (B) or GAS-association (C), each vs a fluorescent marker of activated caspases (FLICA). Percentages of FLICA+ DC that displayed hypodiploid nuclei (B) or were also labeled with GAS-antiserum (C) were determined. Quadrant gates of one representative experiment of three are shown.
Bacterial internalization enhances GAS-induced DC maturation and is required for SLO-dependent DC apoptosis
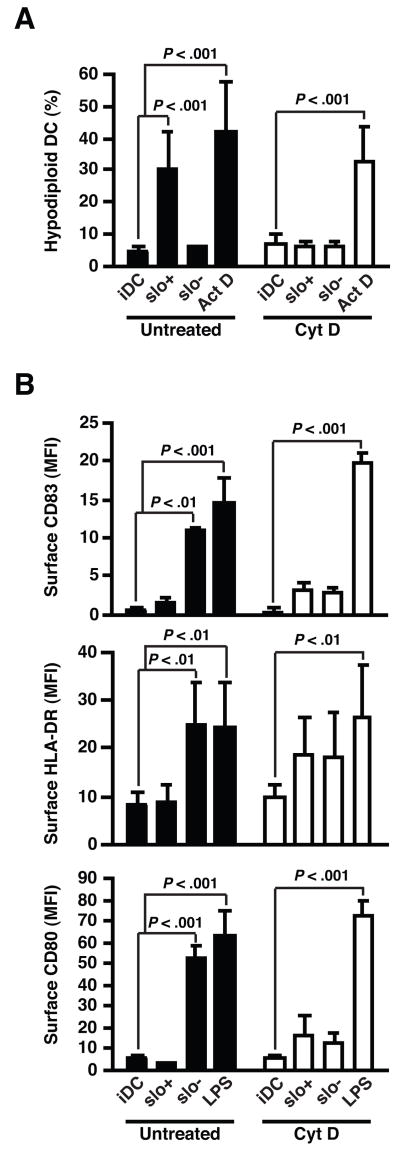
To further examine the relationship between bacterial adherence or internalization and SLO-induced DC apoptosis, we used cytochalasin D to inhibit bacterial internalization by blocking F-actin polymerization (Fig. 6). Since cytochalasin D also reduces GAS adherence to DC, we exposed DC pretreated with cytochalasin D to five times more bacteria to produced similar levels of bacterial association as assessed by flow cytometry, while effectively blocking GAS internalization (data not shown). Treatment with cytochalasin D completely abolished SLO-induced DC apoptosis (Fig. 6A), but not the induction of apoptosis by an unrelated stimulus, actinomycin D. These results suggest strongly that bacterial internalization by DC is required for induction of SLO-dependent apoptosis.
Figure 6.
Bacterial internalization is essential for SLO-mediated DC apoptosis, and contributes to GAS-induced DC maturation. A, Percentages of hypodiploid cells in DC exposed to live wild-type (slo+) or SLO-deficient (slo−) GAS for 2 h at bacteria:DC ratio of 1, followed by 20 h incubation. Prior to infection, DC were treated with 5 μg/ml cytochalasin D (open bars) or untreated (closed bars). Cytochalasin D treatment did not prevent induction of apoptosis by an unrelated stimulus, actinomycin D (ActD). B, Up-regulation of CD83, CD80, and HLA-DR in DC exposed to GAS as described above. LPS-stimulation was used to analyze effect of cytochalasin D on an unrelated maturation stimulus. Means±SD of results from at least three independent experiments are shown.
We also examined the role of GAS internalization in bacterial recognition and induction of phenotypic DC maturation (Fig. 6B). Inhibition of bacterial internalization modestly enhanced the up-regulation of CD83, CD80, and HLA-DR in DC exposed to SLO+ GAS, but it severely reduced the expression of these maturation markers in response to SLO− GAS, while it had no effect on the ability of LPS to induce DC maturation. Together, these results suggest that intracellular detection of GAS contributes to maturation signaling but that SLO production by internalized GAS can interfere with effective induction of DC maturation.
DISCUSSION
Previous studies have demonstrated efficient induction of maturation following exposure of DC to live or penicillin-killed whole GAS [4–6, 19]. However, our study reveals for the first time that GAS strains differ greatly in their ability to induce DC maturation. Remarkably, 18 out of 24 GAS clinical isolates completely failed to enhance the expression of MHC II and co-stimulatory molecules in immature DC, despite the highly conserved nature of GAS constituents involved in TLR-mediated immune recognition [20, 21]. Failure to stimulate DC maturation was correlated with poor attachment and/or phagocytosis of the bacteria, or with induction of DC apoptosis. Significantly, GAS strains that were unable to induce DC maturation produced large amounts of HA capsule and/or SLO, two major virulence factors involved in resistance to phagocytosis and induction of cytotoxicity, respectively [16, 17]. Using isogenic mutant strains deficient in HA capsule and/or SLO, we confirmed that both factors independently inhibit DC maturation in response to GAS.
The inhibitory effect of HA capsule on DC maturation appears to be due to reduced binding and phagocytosis of highly encapsulated GAS strains by DC. Paradoxically, HA oligomers derived from extracellular matrix are recognized by both TLR2 and TLR4, and can act as endogenous ligands for the induction of DC maturation [22, 23]. We did not observe DC maturation in response to GAS culture supernatants although it is possible that stimulatory HA oligomers are generated from the HA capsule by the action of host-derived hyaluronidase(s) during human infection. Moreover, GAS internalization was essential to induce fully mature DC, in agreement with a previous report showing that intracellular processing of GAS enhances TLR signaling and DC activation [24]. By preventing GAS attachment and internalization, the HA capsule may also prevent recognition of GAS ligands by TLRs within the phagosome, and the cytosolic sensing of translocated GAS products [21, 25].
Analysis of isogenic mutant strains revealed that SLO production inhibited DC maturation in response to GAS and triggered DC apoptosis. Several microbes share the ability to induce apoptotic cell death and to block phenotypic maturation of infected DC [26–29]. Our results suggest a cause-and-effect relationship between DC apoptosis and SLO -mediated inhibition of DC maturation, although we cannot exclude the possibility that SLO actively blocks maturation independently of its effect on DC viability. A previous study showed that SLO secreted by extracellular GAS induces keratinocyte apoptosis by augmenting calcium influx through transmembrane pores [18]. Significantly, SLO also prevented internalization of GAS into lysosomes in keratinocytes [13]. In contrast to these observations in epithelial cells, we found that GAS internalized by DC were co-localized with lysosomes, a result that suggests the pro-apoptotic effect of SLO in DC is mediated by SLO poration of the phagolysosome rather than by injury to the cell membrane by SLO secreted from extracellular bacteria. In addition to its cytolytic activity, the ability of SLO to activate TLR4 signaling could also be involved in apoptosis induction, as demonstrated in macrophages for other cholesterol-dependent cytolysins [30–32].
Our study demonstrates that GAS has evolved two independent strategies to prevent bacterial recognition and maturation of DC: 1) inhibition of GAS internalization by the HA capsule, thus reducing intracellular sensing of streptococcal ligands, and 2) induction of DC apoptosis by intracellular SLO-producing GAS. These results, together with the fact that the majority of GAS clinical isolates failed to upregulate costimulatory and MHC II molecules in DC, suggest that blocking DC maturation may enhance GAS virulence. DC have been shown to contribute to GAS clearance in a murine model of skin infection [4]. GAS-induced maturation does not enhance uptake and killing of internalized GAS [33]; however, inhibition of DC maturation may contribute to GAS persistence by preventing IL-12 production at the infection site [4], and may compromise the subsequent development of acquired immunity against this important human pathogen.
Acknowledgments
We thank Emelia DeForce, Nicholas Omniewski, and Emily Babendreier for expert technical assistance.
Footnotes
Potential conflicts of interest: G.C. and M.R.W., no conflicts.
Financial support: This work was supported in part by grants AI29952 and AI070926 and by contract AI30040, all from the National Institutes of Health.
Presented in part: American Society for Microbiology 106th General Meeting, 21–25 May 2006, Orlando, Fl (abstract E-057), and 9th International Conference on Dendritic Cells, 16–20 September 2006, Edinburgh, Scotland (abstract P4.014)
References
- 1.Bisno AL, Brito MO, Collins CM. Molecular basis of group A streptococcal virulence. Lancet Infect Dis. 2003;3:191–200. doi: 10.1016/s1473-3099(03)00576-0. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham MW. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev. 2000;13:470–511. doi: 10.1128/cmr.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carapetis JR, McDonald M, Wilson NJ. Acute rheumatic fever. Lancet. 2005;366:155–68. doi: 10.1016/S0140-6736(05)66874-2. [DOI] [PubMed] [Google Scholar]
- 4.Loof TG, Rohde M, Chhatwal GS, Jung S, Medina E. The Contribution of Dendritic Cells to Host Defenses against Streptococcus pyogenes. J Infect Dis. 2007;196:1794–803. doi: 10.1086/523647. [DOI] [PubMed] [Google Scholar]
- 5.Veckman V, Julkunen I. Streptococcus pyogenes activates human plasmacytoid and myeloid dendritic cells. J Leukoc Biol. 2008;83:296–304. doi: 10.1189/jlb.0707457. [DOI] [PubMed] [Google Scholar]
- 6.Veckman V, Miettinen M, Pirhonen J, Siren J, Matikainen S, Julkunen I. Streptococcus pyogenes and Lactobacillus rhamnosus differentially induce maturation and production of Th1-type cytokines and chemokines in human monocyte-derived dendritic cells. J Leukoc Biol. 2004;75:764–71. doi: 10.1189/jlb.1003461. [DOI] [PubMed] [Google Scholar]
- 7.Nakahara S, Tsunoda T, Baba T, Asabe S, Tahara H. Dendritic cells stimulated with a bacterial product, OK-432, efficiently induce cytotoxic T lymphocytes specific to tumor rejection peptide. Cancer Res. 2003;63:4112–8. [PubMed] [Google Scholar]
- 8.Ruiz N, Wang B, Pentland A, Caparon M. Streptolysin O and adherence synergistically modulate proinflammatory responses of keratinocytes to group A streptococci. Mol Microbiol. 1998;27:337–46. doi: 10.1046/j.1365-2958.1998.00681.x. [DOI] [PubMed] [Google Scholar]
- 9.Gryllos I, Grifantini R, Colaprico A, et al. Mg(2+) signalling defines the group A streptococcal CsrRS (CovRS) regulon. Mol Microbiol. 2007;65:671–83. doi: 10.1111/j.1365-2958.2007.05818.x. [DOI] [PubMed] [Google Scholar]
- 10.Schrager HM, Rheinwald JG, Wessels MR. Hyaluronic acid capsule and the role of streptococcal entry into keratinocytes in invasive skin infection. J Clin Invest. 1996;98:1954–8. doi: 10.1172/JCI118998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashbaugh CD, Warren HB, Carey VJ, Wessels MR. Molecular analysis of the role of the group A streptococcal cysteine protease, hyaluronic acid capsule, and M protein in a murine model of human invasive soft-tissue infection. J Clin Invest. 1998;102:550–60. doi: 10.1172/JCI3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sierig G, Cywes C, Wessels MR, Ashbaugh CD. Cytotoxic effects of streptolysin O and streptolysin S enhance the virulence of poorly encapsulated group A streptococci. Infect Immun. 2003;71:446–55. doi: 10.1128/IAI.71.1.446-455.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hakansson A, Bentley CC, Shakhnovic EA, Wessels MR. Cytolysin-dependent evasion of lysosomal killing. Proc Natl Acad Sci U S A. 2005;102:5192–7. doi: 10.1073/pnas.0408721102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–9. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 15.Rescigno M, Martino M, Sutherland CL, Gold MR, Ricciardi-Castagnoli P. Dendritic cell survival and maturation are regulated by different signaling pathways. J Exp Med. 1998;188:2175–80. doi: 10.1084/jem.188.11.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dale JB, Washburn RG, Marques MB, Wessels MR. Hyaluronate capsule and surface M protein in resistance to opsonization of group A streptococci. Infect Immun. 1996;64:1495–501. doi: 10.1128/iai.64.5.1495-1501.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bricker AL, Cywes C, Ashbaugh CD, Wessels MR. NAD+-glycohydrolase acts as an intracellular toxin to enhance the extracellular survival of group A streptococci. Mol Microbiol. 2002;44:257–69. doi: 10.1046/j.1365-2958.2002.02876.x. [DOI] [PubMed] [Google Scholar]
- 18.Cywes Bentley C, Hakansson A, Christianson J, Wessels MR. Extracellular group A Streptococcus induces keratinocyte apoptosis by dysregulating calcium signalling. Cell Microbiol. 2005;7:945–55. doi: 10.1111/j.1462-5822.2005.00525.x. [DOI] [PubMed] [Google Scholar]
- 19.Kuroki H, Morisaki T, Matsumoto K, et al. Streptococcal preparation OK-432: a new maturation factor of monocyte-derived dendritic cells for clinical use. Cancer Immunol Immunother. 2003;52:561–8. doi: 10.1007/s00262-003-0394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okamoto M, Oshikawa T, Ohe G, et al. Severe impairment of anti-cancer effect of lipoteichoic acid-related molecule isolated from a penicillin-killed Streptococcus pyogenes in toll-like receptor 4-deficient mice. Int Immunopharmacol. 2001;1:1789–95. doi: 10.1016/s1567-5769(01)00103-5. [DOI] [PubMed] [Google Scholar]
- 21.Oshikawa T, Okamoto M, Tano T, et al. Antitumor effect of OK-432-derived DNA: one of the active constituents of OK-432, a streptococcal immunotherapeutic agent. J Immunother (1997) 2006;29:143–50. doi: 10.1097/01.cji.0000189028.18288.6f. [DOI] [PubMed] [Google Scholar]
- 22.Jiang D, Liang J, Fan J, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–9. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 23.Termeer C, Benedix F, Sleeman J, et al. Oligosaccharides of hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okamoto M, Oshikawa T, Tano T, et al. Mechanism of anticancer host response induced by OK-432, a streptococcal preparation, mediated by phagocytosis and Toll-like receptor 4 signaling. J Immunother (1997) 2006;29:78–86. doi: 10.1097/01.cji.0000192106.32206.30. [DOI] [PubMed] [Google Scholar]
- 25.Ratner AJ, Aguilar JL, Shchepetov M, Lysenko ES, Weiser JN. Nod1 mediates cytoplasmic sensing of combinations of extracellular bacteria. Cell Microbiol. 2007;9:1343–51. doi: 10.1111/j.1462-5822.2006.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colino J, Snapper CM. Two distinct mechanisms for induction of dendritic cell apoptosis in response to intact Streptococcus pneumoniae. J Immunol. 2003;171:2354–65. doi: 10.4049/jimmunol.171.5.2354. [DOI] [PubMed] [Google Scholar]
- 27.Erfurth SE, Grobner S, Kramer U, et al. Yersinia enterocolitica induces apoptosis and inhibits surface molecule expression and cytokine production in murine dendritic cells. Infect Immun. 2004;72:7045–54. doi: 10.1128/IAI.72.12.7045-7054.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bosnjak L, Miranda-Saksena M, Koelle DM, Boadle RA, Jones CA, Cunningham AL. Herpes simplex virus infection of human dendritic cells induces apoptosis and allows cross-presentation via uninfected dendritic cells. J Immunol. 2005;174:2220–7. doi: 10.4049/jimmunol.174.4.2220. [DOI] [PubMed] [Google Scholar]
- 29.Arimilli S, Johnson JB, Alexander-Miller MA, Parks GD. TLR-4 and -6 agonists reverse apoptosis and promote maturation of simian virus 5-infected human dendritic cells through NFkB-dependent pathways. Virology. 2007;365:144–56. doi: 10.1016/j.virol.2007.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park JM, Greten FR, Li ZW, Karin M. Macrophage apoptosis by anthrax lethal factor through p38 MAP kinase inhibition. Science. 2002;297:2048–51. doi: 10.1126/science.1073163. [DOI] [PubMed] [Google Scholar]
- 31.Park JM, Ng VH, Maeda S, Rest RF, Karin M. Anthrolysin O and other gram-positive cytolysins are toll-like receptor 4 agonists. J Exp Med. 2004;200:1647–55. doi: 10.1084/jem.20041215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Srivastava A, Henneke P, Visintin A, et al. The apoptotic response to pneumolysin is Toll-like receptor 4 dependent and protects against pneumococcal disease. Infect Immun. 2005;73:6479–87. doi: 10.1128/IAI.73.10.6479-6487.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loof TG, Goldmann O, Medina E. Immune recognition of Streptococcus pyogenes by dendritic cells. Infect Immun. 2008;76:2785–92. doi: 10.1128/IAI.01680-07. [DOI] [PMC free article] [PubMed] [Google Scholar]