Abstract
Models of selective attention predict that focused attention to spatially contiguous stimuli may result in enhanced activity in areas of cortex specialized for processing task-relevant and task-irrelevant information. We examined this hypothesis by localizing color-sensitive areas (CSA) and word and letter sensitive areas of cortex and then examining modulation of these regions during performance of a modified version of the Stroop task in which target and distractors are spatially coincident. We report that only the incongruent condition with the highest cognitive demand showed increased activity in CSA relative to other conditions, indicating an attentional enhancement in target processing areas. We also found an enhancement of activity in one region sensitive to word/letter processing during the most cognitively demanding incongruent condition indicating greater processing of the distractor dimension. Correlations with performance revealed that top-down modulation during the task was critical for effective filtering of irrelevant information in conflict conditions. These results support predictions made by models of selective attention and suggest an important mechanism of top-down attentional control in spatially contiguous stimuli.
Keywords: Attentional control, Top-down modulation, Stroop task, Color-sensitive, Visual word form area
1. Introduction
In a complex visual scene, attentional selection allows relevant items to be processed while irrelevant items are ignored. Top-down selection processes are thought to bias competition in favor of task-relevant information and against task-irrelevant information. In support of this, neuroimaging research has reported that directed attention effectively modulates activity in sensorimotor brain regions. For example, using positron emission tomography (PET), Corbetta et al. [10] reported that directing attention to certain features of a visual stimulus, such as color, shape, or speed, increased activity in distinct areas of visual cortex that were specialized for processing the particular feature. Directing attention to a particular location also increases activity in extrastriate regions of monkeys [59] and humans [20,35,38] and anticipation of a stimulus before any visual stimulation occurs can also alter activity levels in task-relevant extrastriate regions that process color [7] and motion [7,22,36]. Therefore, attention can modulate activity in striate and extrastriate regions of cortex for a variety of task-relevant stimulus dimensions.
Both enhancement and suppressive processes in striate and extrastriate cortices have been purported to be essential components in a push–pull mechanism of attentional selection [49,52]. For example, attentional enhancement of activity in cortical regions that process an attended stimulus location or other feature (e.g. [10,46,48]) has been related to improved behavioral performance and better task-related selection processes [60]. In addition, attention can reduce, inhibitor suppress, visual cortex activity associated with processing to-be-ignored stimuli or task-irrelevant distractors [48,57].
The efficacy of attentional selection to filter task-irrelevant information and bias task-relevant information is dependent on the cognitive demands of the task [32,31,42,47]. Specifically, the cognitive demands can moderate the influence that task-irrelevant distractors have on performance. For example, behavioral studies have shown that under conditions of greater cognitive load, interference from distractors is increased, suggesting that (1) the ability for selective attention to ignore irrelevant distractors is dependent on the cognitive demands of the task and (2) distractor information is processed to a greater degree under conditions of high cognitive load [31,32]. Consistent with behavioral results, De Fockert et al. [12] demonstrated that regions of the visual cortex that were related to processing distractor information exhibited increased activity under high memory loads, supporting the claim that distractor information is more extensively processed under conditions of higher cognitive demand. Therefore, both behavioral and neuroimaging results argue that the efficacy of attentional selection in filtering task-irrelevant material is dependent upon cognitive load and that the processing of distractors increases in cognitively demanding conditions.
The processing of distractor information, however, may require spatial separation between the targets and distractors [33,40]. Lavie [33] suggested that when the target and distractor form part of the same stimulus, increased attention to the target might result in a concomitant increase in attention towards the distractor. This argument predicts that for spatially contiguous or coincident stimuli, cortical processors specialized for processing the task-relevant and the task-irrelevant features should show increases in activity under attentionally demanding conditions.
The Stroop task is an example of an attentionally demanding task in which target and distractor information are not just spatially contiguous, but spatially coincident. In this task, participants are instructed to respond to the ink color of a printed word while ignoring the meaning of the printed word. Therefore, both target (i.e. responding to the ink color) and distractor information (i.e. the printed word) are spatially coincident and cognitive demands are higher in conditions in which the target and distractor information are incongruent with each other (e.g. when the word RED is printed in blue ink).
Models of selective attention and top-down control [33], predict that in the Stroop task, regions of visual cortex that are sensitive to processing color (target) information should be selectively enhanced as attentional demands are increased. Increased activity in target-sensitive areas of cortex would be representative of increased top-down bias to the task-relevant attribute. Greater top-down bias would then lead to better task performance. Therefore, the magnitude of activity in color-sensitive areas of striate and extrastriate cortex should be positively correlated with performance (negatively correlated with reaction times) when attentional demands are highest, that is when the printed word and ink color conflict.
Models of selective attention and top-down control also predict that areas of striate and extrastriate cortex sensitive to processing word (distractor) information should be selectively enhanced under conditions of high cognitive demand since the distractor information is more extensively processed in spatially coincident stimuli. Therefore, the magnitude of activity in distractor processing regions of cortex, such as those that are involved in lexico-semantic processing and grapho-phonological processing, can be considered an index of the degree of distractor processing. Greater distractor processing should result in greater interference during the most demanding incongruent Stroop task condition and less so in less demanding conditions, such as the congruent condition. Therefore, the magnitude of activity in some of the word-sensitive distractor regions that are involved in letter and symbol recognition, semantic processing, and grapho-phonological conversion, should be negatively correlated with performance (positively correlated with reaction times) in the incongruent Stroop condition.
We tested these hypotheses by localizing areas of the visual cortex that were sensitive to the presentation of passively viewed word/letter and color stimuli. Previous studies have successfully utilized a similar passive-viewing localizer approach to assess attentional modulation of face stimuli [14,15], color-checkerboards [29], word-finding processes [64], testing localization of theory-of-mind [45], and testing areas responsive to number symbols and numerosities [51] and objects and houses [39].
In the present study we employed a modified version of the Stroop task to examine the way sin which cognitive demand, engendered through conflict, in spatially coincident stimuli modulates activity in target-sensitive and distractor-sensitive regions of cortex. We examined this on individually assigned regions-of-interest (ROIs), which allowed us to define regions sensitive to the manipulation on an individual-by-individual basis. We also computed correlations between these regions and Stroop task performance to test the predictions of models of selective attention and top-down control, as described above.
2. Materials and methods
2.1. Participants
Fourteen participants (9 male; 5 female) between the ages of 18 and 29 (mean age = 23.46) signed an informed consent approved by the University of Illinois and met or surpassed all criteria for participating in an MRI experiment including no previous head trauma, no claustrophobia, and no metallic implants. Participants were paid $ 15 dollars an hour for volunteering.
2.2. Procedure
Visual stimuli were presented with MRI-safe fiber optic goggles (Resonance Technologies Inc.) and manual responses to Stroop stimuli were obtained by the use of an MRI-safe five-button pad. Three buttons were used during the task with each button corresponding to a different ink color (see Stroop task). Participants were first run in the Stroop task followed by the localizer tasks and structural scans.
2.3. MRI parameters and preprocessing
A 3T Siemens Allegra head-only MRI scanner was used for structural and functional MRI measures. For the fMRI protocol, we employed a fast echo-planar imaging (EPI) sequence with Blood Oxygenation Level Dependent (BOLD) contrast and collected a total of 150 T2* weighted volumes per participant (TR = 1.5; TE = 26; flip angle = 60) for the checkerboard tasks and a total of 220 T2* weighted volumes per participant (TR = 1.5; TE = 26, flip angle = 60) for the word tasks. For the Stroop task we collected a total of 380 T2* weighted volumes per participant (TR = 1.5; TE = 26; flip angle = 60). Twenty-eight slices (4 mm thickness with a 0% gap) were collected in an ascending and sequential fashion parallel to the anterior and posterior commissures for all three tasks.
In addition, a high-resolution T1-weighted MPRAGE (1.3 mm × 1.3 mm × 1.3 mm) anatomical image was collected for each participant. The anatomical images were skull-stripped using a brain extraction technique [61] and subsequently used for registration purposes.
The functional MRI data for each participant was preprocessed using FSL version 3.2 [62]. For the localizer tasks, the images were slice-time corrected, motion-corrected using MCFLIRT [24], temporally filtered with a Gaussian high pass cut-off of 120 s and a low pass cut-off of 9.32 s, and spatially smoothed with a 5 mm full-width half-max 3D Gaussian kernel. The data from the Stroop task were also slice-time corrected and motion-corrected using the same protocols as those for the localizer tasks. The Stroop task data was temporally smoothed with a high-pass cut-off at 80 s and a low-pass cut-off of 1.5 s, and spatially smoothed with an 8 mm full-width half-max 3D Gaussian kernel.
2.4. Localization of CSA and VWFA
In order to localize CSA we presented flashing black and white checkerboards and flashing color checkerboards at a rate of 8 Hz. Each condition was presented in two separate 30 s blocks that alternated with 30 s blocks of a crosshair baseline. Participants were instructed to keep their eyes open and attend to the screen. The luminance of the black and white checkerboards (22.40 cd/m2) was slightly higher than the luminance for color checkerboards (10.93 cd/m2) while the Michelson contrast ratio was equivalent (color, .978; black and white, .979). A slightly higher luminance of the black and white checkerboard display compared with the color-checkerboard display should be a conservative estimate of the differences in which the brain shows greater activation for color when compared to black and white images.
After preprocessing, the data were convolved with a Gamma function to model the hemodynamic response to each block for each condition. This first-level analysis resulted in voxel-wise parameter estimate maps for the entire brain for each condition and for the direct comparison between color checkerboards and black and white checkerboards. These maps were then registered into standard space (MNI space) and forwarded to a higher-level group analysis where a fixed-effects analysis was performed to find areas across participants that were sensitive to color. A fixed-effects analysis was chosen at this stage because we were only interested in the CSA specific to the participants in this sample since our ROI analysis would be performed on an individual-by-individual basis. The result from the color versus black and white checkerboard comparison was thresholded at a voxel-wise z-score of 2.33 (p<.01) and a cluster-wise threshold of p<.05. The resulting statistical map was then converted into a binary map and used as a mask to find peaks for each participant. Therefore, the peaks that we report for each participant are located within the mask from the group analysis. The statistical peak from each participant within this mask was found for each person and voxels contiguous with the peak within 5 mm (10 mm diameter) were then used as an ROI for each participant. The mean of these voxels were then extracted for the color checkerboard condition versus baseline, the black and white checkerboard condition versus baseline, as well as each condition in the word localizer task (see below) versus baseline. This was done to (a) ensure that the peak was more sensitive to color checkerboards than black and white checkerboards, words, pseudo-words, and letter strings, and thus an area we could validly call ‘CSA’, and (b) to use as ROIs in the Stroop task to examine top-down modulatory effects of conflict.
In addition to these empirically derived CSA ROIs, we extracted the activity for each condition from V4, a classically defined region sensitive to processing color [69]. Specifically, we used Tal2MNI software (http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach) to convert Talairach coordinates for V4 to MNI space as reported by Zeki et al. [69]. The left hemisphere coordinates for V4 (−26, −68, −8) were converted to −26, −69, −13 and the right hemisphere coordinates for V4 (20, −66, −4) were converted to 20, −68, −8 (see Table 1). Others have also reported similar coordinates for V4 [3,27,41]. A 10 mm diameter sphere around each of the points was used as a pre-defined ROI, and the mean activity in these regions was extracted for each condition and compared using a repeated-measures ANOVA with planned comparisons.
Table 1.
Regions from previous studies (Cohen and Dehaene [9]; Vigneau et al. [67]; Zeki et al. [69]) and a meta-analysis (Jobard et al. [26]) showing V4 localization for color-processing and areas specialized for word/letter processing
| Region | X | Y | Z | Study |
|---|---|---|---|---|
| Left hemisphere V4 | −26 | −69 | −13 | Zeki et al. [69] |
| Right hemisphere V4 | 20 | −68 | −8 | Zeki et al. [69] |
| Fusiform gyrus (VWFA) | −43 | −54 | −12 | Cohen and Dehaene [9] |
| Inferior occipital gyrus | −42 | −78 | −10 | Jobard et al. [26]; Vigneau et al. [67] |
| Posterior middle temporal gyrus | −50 | −44 | −10 | Jobard et al. [26]; Vigneau et al. [67] |
These areas were used as predefined ROIs in this study by creating a sphere around each of these points with a radius of 5 mm.
In order to localize word and letter sensitive areas of cortex we presented 30 s blocks of words that were matched for frequency [30] and length with the color words and neutral words used during the Stroop task. In addition, we presented 30 s blocks of pronounceable pseudo-words and letter strings that were also matched for length with the Stroop words. Similar to the checkerboards, each condition was presented in two separate 30 s blocks that alternated with 30 s blocks of a crosshair baseline. Participants were instructed to keep their eyes open and attend to the screen. All letter strings, pseudo-words, and words were presented in white font on a black background.
Similar to the analyses for the CSA, the blocks of trials were convolved with a gamma function. Planned comparisons between words and both pseudo-words and letter strings resulted in parameter estimate maps for each individual participant. These maps were then registered into standard space (MNI space) and forwarded to a higher-level group analysis.
Because these main comparisons did not result in any regions that were specific to printed words, we performed a few other analyses to try and isolate word/letter sensitive areas of cortex. Therefore, we first used coordinates from previously published manuscripts (see Table 1) that have reported localization of a visual word form area (VWFA) as well as other regions associated with lexico-semantic processing from a recent meta-analysis [26,67]. This resulted in three areas in the posterior left hemisphere that were subsequently used as ROIs with a 5 mm radius. The mean percent signal change for each Stroop condition from each ROI was extracted for each individual and evaluated for modulatory effects. Second, we examined areas that were commonly active for words, pseudo-words, and letter strings compared to baseline, but were not active for either color or black and white checkerboards. These areas represent regions involved in processing the symbolic representation of letters. We then used the clusters from this comparison as ROIs and extracted the peaks for each participant. Similar to the checkerboard analysis, we extracted the values from these ROIs for each participant and took an average of the immediately contiguous 124 voxels around the peak (10 mm diameter). The mean of these voxels were then extracted for each condition versus baseline as well as each condition in the checkerboard localizer task versus baseline. This was done to (a) ensure that the peak was more sensitive to words, pseudo-words, and letter strings than either color checkerboards or black and white checkerboards, and (b) to use as ROIs in the Stroop task to examine modulatory effects of conflict on regions processing task-irrelevant information. These analyses resulted in five separate regions for word area analysis (3 predefined ROIs; 2 empirically defined ROIs).
2.5. Stroop task
We used incongruent, neutral, and congruent stimuli in an event-related design. Congruent stimuli were words that matched the ink color (e.g. RED in red ink). Neutral stimuli were words that were matched for frequency and word length with the color words, but that were not associated with color (e.g. SHIP in red ink). Incongruent-eligible stimuli were stimuli in which the printed word matched one of the potential responses (e.g. RED in blue ink if ‘red’ is one of the potential responses). In congruent-ineligible stimuli were stimuli in which the printed word did not match the set of potential responses (e.g. PURPLE in blue ink if ‘purple’ is not one of the potential responses). We had two ink color sets that were counter balanced across participants. For one of the ink color sets the eligible colors were red, orange, and purple while the ineligible words were blue, green, and yellow. The other color set had eligible responses of blue, green, and yellow while the ineligible words were red, orange, and purple. Each condition consisted of 36 trials. For the incongruent-eligible condition, each ink color was paired with both response eligible color words 12 times during a session (six times per word). However, for the incongruent-ineligible trials, each ink color was paired with one of three ineligible words 12 times (four times per word). Each stimulus was displayed for 1 s with a 1.5 s response window and a 3 s stimulus-onset asynchrony (SOA). A crosshair (+) was presented on the screen during all interstimulus intervals. We employed an event-related stimulus design with a 40% jitter, such that the timing between trials varied, in order to optimize the stimulus sequence and timing. Stimulus sequence and jitter for each participant was generated using OptSeq2 (http://surfer.nmr.mgh.harvard.edu/optseq/). Participants were instructed to respond as quickly and accurately as possible during the task.
Whole-head data from this task was analyzed by using a double-gamma convolution with each condition as a separate explanatory variable. The errors and motion parameters were modeled separately and used as covariates of no interest in the model. All comparisons between conditions were performed on an individual basis and then forwarded to a higher-level group analysis where a mixed effect ANOVA was performed on all participants. In order to examine the modulatory effects of conflict on enhancement or suppression of activity, we extracted the average percent signal change in each of the ROIs for all participants for each of the Stroop conditions (congruent, neutral, ineligible, eligible). These values were then subjected to statistical analysis using SPSS (11.01 for Mac).
2.6. Data analysis
Analysis of the functional MRI data was carried out using FEAT (FMRI Expert Analysis Tool) Version 5.1, part of FSL (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl). Higher-level analyses were carried out using FLAME [62]. All results from the final whole-head analysis resulted in Z statistic images that were thresholded using clusters determined by Z > 3.1 and a (corrected) cluster significance threshold of p<0.01.
For the Stroop task, the ROIs from the localizer scans (see above) were applied to the individual parameter estimate maps in the participant’s original space (before registration into standard space). The mean parameter estimates from each ROI for each condition and for each participant was extracted and subsequently used in an ANOVA in SPSS version 11.02. Correlations were carried out in SPSS between subject’s response times for each condition of the Stroop task and percent signal change in each of the ROIs.
3. Results
3.1. Response times
The response times were analyzed using a repeated-measures ANOVA with condition as a within-subjects factor (see Table 2). We found a significant main effect of condition (F(3,39) = 8.83; p<.001). Planned comparisons revealed a marginal difference between the congruent and neutral conditions (p<.06), supporting previous claims for as light facilitation effect of congruent word and ink color information. We also found that the response times for the neutral condition were significantly faster than the incongruent-eligible condition (p<.003) and the incongruent-ineligible condition (p<.05). Furthermore, the response times for the incongruent-eligible condition were reliably slower than the response times for the incongruent-ineligible condition (p<.032), replicating previous findings that the incongruent-eligible condition differs in its level of conflict from that of the incongruent-ineligible condition [34].
Table 2.
Response times and error rates for all conditions
| Congruent | Neutral | Incongruent-ineligible | Incongruent-eligible | |
|---|---|---|---|---|
| Response times (ms) | 630.98 (59.6) | 656.05 (60.0) | 670.82 (72.2) | 684.04 (68.6) |
| %Error | 10.1 (9.1) | 9.3 (8.2) | 16.2 (13.7) | 19.0 (13.7) |
Standard deviations are represented in parentheses
3.2. Accuracy
The accuracy rates were also analyzed by a repeated-measures ANOVA with condition as a within-subjects factor (see Table 2). We found a main effect of condition (F(3,39) = 9.48; p<.001). Planned comparisons revealed that the error rates between congruent and neutral conditions were not different (p<.32). However, the incongruent-eligible (p<.001)and incongruent-ineligible(p<.007) trials had significantly higher error rates than the neutral condition. The error rates for the incongruent-eligible and incongruent-ineligible condition were not significantly different (p<.20).
3.3. Color-sensitive areas
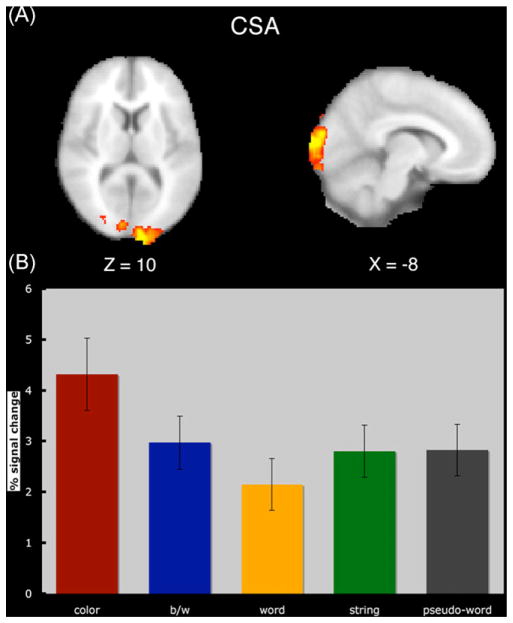
The results from the group analysis comparing color-checkerboards with black and white checkerboards revealed a large cluster of activation in visual cortex (Fig. 1). This cluster was used as an inclusive mask, and the peaks from each subject were extracted for each of the localizer conditions (see Section 2). In a repeated-measures ANOVA with localizer (color checkerboards, black and white checkerboards, words, letter strings, pseudo-words) as a within-subjects variable, we found that the overall effect of condition was significant (F (4,52) = 4.154; p<.005). Importantly, in a series of planned comparisons we found that the CSA had significantly greater activity when processing color than when processing black and white checkerboards (p<.026), words (p<.015), letter strings (p<.049), or pseudowords (p<.016) (see Fig. 1). There were no differences in this ROI between black and white checkerboards, words, letter strings, or pseudo-words. This result indicates that we successfully localized color-sensitive regions of cortex.
Fig. 1.
A comparison of color checkerboards versus black and white checkerboards across all participants resulted in a cluster of activity in visual cortex (A). This cluster meets a voxel-wise threshold of Z = 2.33 and a cluster-wise threshold of p<.01. (B) Shows that the activity in this region is reliably more responsive to color checkerboards than to either black and white checkerboards, words, letter strings, or pseudo-words. It should be noted that these areas are not specialized for processing only color since all other conditions also show significant activity in this region above baseline. Therefore, this area is only more sensitive to color than to other stimuli – thus, we call it a color-sensitive area (CSA). The images are displayed in neurological convention (right on right).
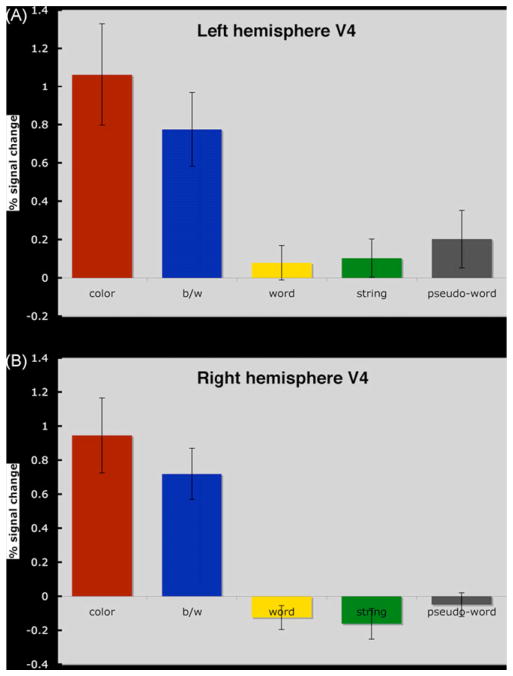
We also created predefined ROIs in the left and right V4 region based on previous research on color-processing in the visual cortex [69] (see Fig. 2). For the left hemisphere, in a repeated-measures ANOVA with localizer (color checkerboards, black and white checkerboards, words, letter strings, pseudo-words) as a within-subjects variable, we found that the overall effect of condition was significant (F (4,52) = 14.28; p<.001). In a series of planned comparisons we found that activation to color checkerboards was significantly greater than activity for black and white checkerboards (p<.05), words (p<.001), letter strings (p<.001), and pseudo-words(p<.001). For the right hemisphere, in a repeated-measures ANOVA with localizer (color checkerboards, black and white checkerboards, words, letter strings, pseudo-words) as a within-subjects variable, we found that the overall effect of condition was significant (F(4,52) = 37.88; p<.001). In a series of planned comparisons we found that there was a trend for activation to color checkerboards to be greater than activity for black and white checkerboards (p<.09). In addition, activity to color checkerboards was significantly greater than activity to words (p<.001), letter strings (p<.001), and pseudo-words (p<.001).
Fig. 2.
(A) Mean activity and standard errors in the left hemisphere V4 region for all localizer conditions. Color checkerboards elicited more activity in this region compared to black and white checkerboards (p<.05) and more activity than the word and letter conditions (all p<.001). (B) Mean activity and standard errors in the right hemisphere V4 region for all localizer conditions. There was a trend for color checkerboards to elicit more activity in this region compared to black and white checkerboards (p<.09). Color checkerboards significantly differed from word and letter conditions (all p<.001).
3.4. Word-sensitive areas: empirically defined
The results from the group analysis that compared word activity with either letter strings or pseudo-words failed to show any clusters of activity at our threshold. However, many studies that have reported VWFA have reported greater activation for both letters and words compared to non-word stimuli such as checkerboards, pictures, textures, or faces [8,18,19,25,53–56,58] and even current arguments for extrastriate specialization for reading have focused on specialization for letters rather than entire words [9]. Since our localizer conditions all consisted of letters, we examined if there were any areas sensitive to processing letters relative to either color or black and white checkerboards. Therefore, we first found the statistical conjunction of word activation, letter string activation, and activation for pseudo-words. This resulted in two brain regions sensitive to the presence of letters: left superior parietal lobule near a location that others have reported associated with word encoding [28], and a left middle temporal region located more anterior to the commonly reported VWFA [9,63], but overlapping with the middle temporal region reported in studies examining the lexico-semantic route of word reading [26,67]. We then found the peak for each participant within these clusters and extracted the percent signal change from this defined ROI for all five localizer tasks for each participant. We found that for the left parietal lobule the overall effect of condition from the repeated-measures ANOVA model was significant (F(4,52) = 4.328; p<.004). Planned comparisons revealed that the activity for letter strings was significantly greater than the activity for color (p<.043) and black and white checkerboards (p<.013) and the activity for pseudo-words was significantly greater than the activity for color (p<.011) and black and white checkerboards (p<.005). However, there were no differences between words and color (p<.723) or black and white checkerboards (p<.415) (see Fig. 3). In the left temporal region we found that the overall ANOVA from the repeated-measures analysis was also significant (F(4,52) = 3.98; p<.007). Planned comparisons revealed that activity in this region marginally differed between words and color checkerboards (p<.06) and significantly differed from black and white checkerboards (p<.027). Letter strings significantly differed from color checkerboards (p<.043) and black and white checkerboards (p<.037). Finally, pseudo-words also significantly differed from color checkerboards (p<.021) and black and white checkerboards (p<.048). Therefore, although we failed to find any areas specific to processing words, we successfully found areas specialized in processing letters compared to processing either color or black and white checkerboard displays. This result replicates prior research reporting areas in the occipito-parietal regions involved in processing words as well as middle temporal engagement during lexico-semantic processing [9,67].
Fig. 3.
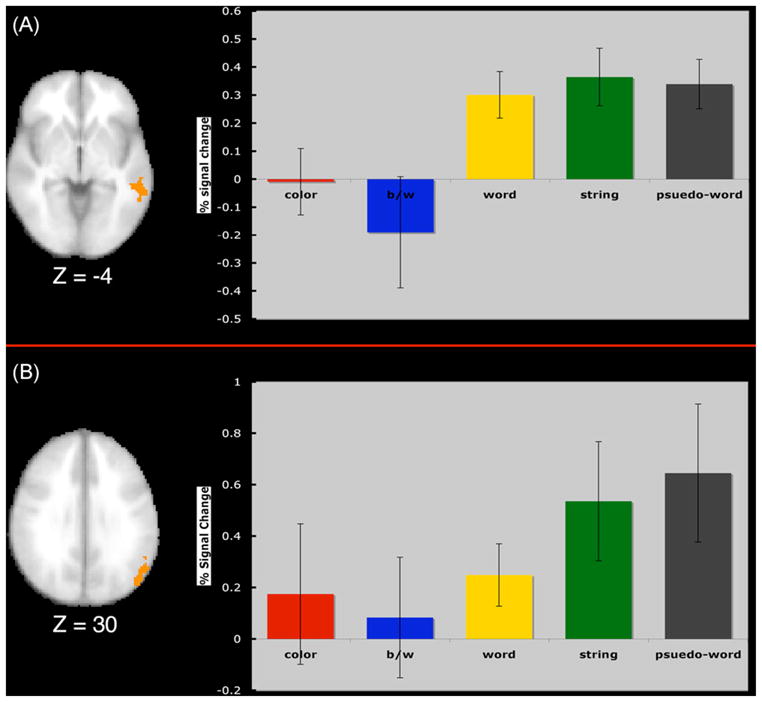
(A) Displays the left middle temporal region mask resulting from the conjunction between blocks of words, letter-strings, and pseudo-words. The graph shows that this region was significantly more active for the presentation of letters than color or black and white checkerboards. (B) Displays the left parietal region mask resulting from the conjunction of activity for words, letter-strings, and pseudo-words. The graph shows that this region was significantly more active for the presentation of letter strings and non-words than color or black and white checkerboards.
3.5. Stroop effects
In all Stroop task comparisons, we found a similar pattern of activity that others have reported for the incongruent relative to neutral conditions including left and right dorsolateral prefrontal cortex, ventrolateral prefrontal cortex, anterior cingulate cortex, left and right parietal cortices, and extrastriate cortex [1,2,15,16,37,42–44,50]. Furthermore, we replicated the pattern of eligibility effects that others have reported previously [34,42,44]. These results suggest that the right ventral prefrontal regions are involved in response conflict, while bilateral dorsolateral prefrontal regions are involved in maintenance of an attentional set in working memory. Since others have spent considerable time describing and explaining effects of the Stroop task on the attentional network, in this paper we focus on the modulation of CSA and word areas instead of an in depth discussion of the Stroop results.
3.6. Modulatory effects of the Stroop task on CSA
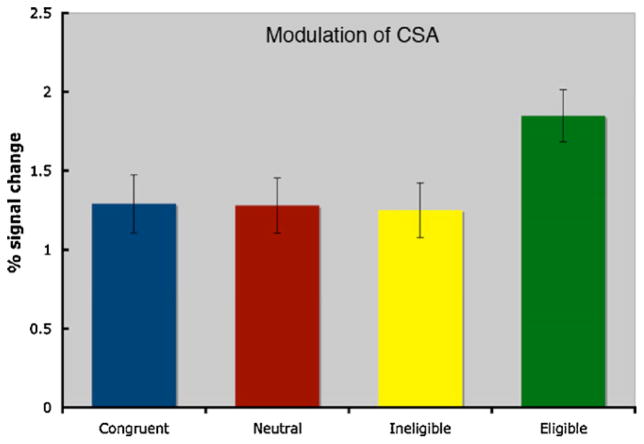
To address the question of attentional modulation on target (color) areas of extrastriate cortex, we analyzed the CSA regions described above during each condition of the Stroop task. To do this we conducted a repeated-measures ANOVA with condition (neutral, congruent, incongruent-ineligible, incongruent-eligible) as a within-subjects factor. We found that the main effect of condition was significant (F(3,39) = 4.892; p<.006). Consistent with predictions made by models of selective attention and top-down control, planned comparisons revealed that in the CSA, the activity associated with the incongruent-eligible condition was significantly greater than the activity for the congruent condition (p<.015), the neutral condition (p<.02), and the incongruent-ineligible condition (p<.001) suggesting that activity in the CSA was significantly enhanced under the most cognitively demanding condition, that is, the incongruent-eligible condition relative to the other three conditions (see Fig. 4).
Fig. 4.
Percent signal change in the CSA as a function of condition. The activity associated with the incongruent-eligible condition was significantly greater than the other three conditions.
Interestingly, the incongruent-ineligible condition failed to show any significant enhancement of activity in the CSA relative to the neutral (p<.855) or congruent conditions (p<.787) suggesting that it is not simply the presence of conflict that results in enhanced activity in CSA, but specifically the greater cognitive demands manifested within the incongruent-eligible condition.
In the predefined V4 regions of visual cortex, we found a non-significant effect of condition using a repeated-measures ANOVA with condition (neutral, congruent, incongruent-ineligible, incongruent-eligible) as a within-subjects factor for the left hemisphere (F(3,39) = .421; p<.739) and the right hemisphere (F(3,39) = 2.03; p<.126). None of the planned comparisons reached significance at p<.05 for the left hemisphere region. For the right hemisphere the incongruent-ineligible condition had significantly greater activity than the congruent condition (p<.03) and there was also a trend for the incongruent-eligible condition to have greater activity than the congruent condition (p<.07). Therefore, we failed to find any task-related modulation of activity in the predefined V4 region of the left extrastriate cortex, but at least a trend for the incongruent conditions of the Stroop task to show task-related modulation in the right hemisphere.
It is possible that the modulated activity in CSA regions were being driven by a general increase in activity throughout visual cortex for the incongruent-eligible condition. To test this we extracted the mean signal from a 10 mm diameter ROI from primary visual cortex (x = −12, y = −96, z = −12) from each of the four Stroop conditions. If the modulation of activity during the incongruent-eligible condition was due to a non-specific and a general increase of activity throughout visual cortex, then this region should also demonstrate a significant increase in activity during the incongruent-eligible condition relative to the other conditions. However, if the modulation during the incongruent-eligible condition was specific to color-sensitive regions, then there should not be modulation of activity in the non-specific ROI. We failed to find any main effect of condition (F(3,39) = 1.062; p<.376)in this region. Furthermore, all of the planned comparisons between the conditions were not significant. This result suggests that there is not a general increase in visual cortex activity in response to the most demanding condition, but rather a specific increase in category-specific regions that process the target information.
3.7. Modulatory effects of the Stroop task on word/letter areas
To address the question of attentional modulation on distractor (word/letter) areas that were empirically derived by the localizer conditions, we conducted a repeated-measures ANOVA with condition (neutral, congruent, incongruent-ineligible, incongruent-eligible) as a within-subjects factor for each region separately. First, we found that for the left temporal area (F(3,39) = .15; p<.929) and the left parietal area (F(3,39) = .994; p<.406) the overall effect of condition was not significant. Further-more, planned comparisons revealed that in both the temporal and parietal cortex, none of the conditions were reliably different from one another. This effect suggests that in our empirically derived ROIs, there was neither enhancement nor suppression of distractor related areas of cortex during any condition of the Stroop task.
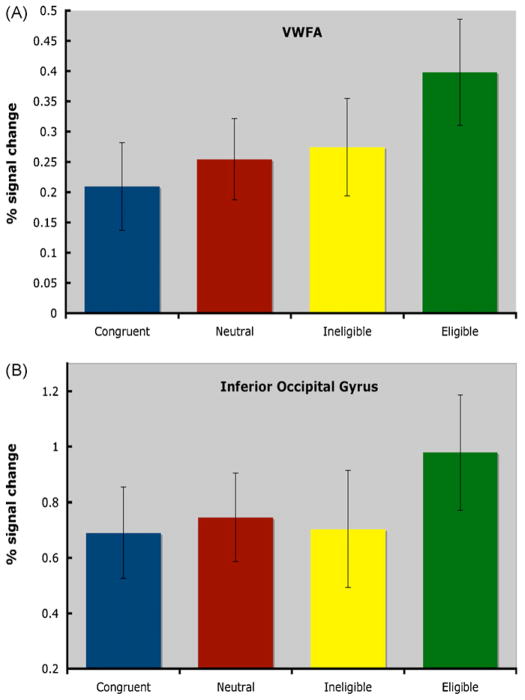
We also analyzed the predefined ROIs based on previous studies using a repeated-measures ANOVA with condition (neutral, congruent, incongruent-ineligible, incongruent-eligible) as a within-subjects factor for each region separately. We found that in the VWFA (coordinates: −43, −54, −12) there was a non-significant trend for a main effect of condition (F(3,39) = 1.99; p<.13; see Fig. 5). Planned comparisons in this region revealed that there was greater activity in this region for the incongruent-eligible condition than the neutral condition (p<.03) and significantly greater activity for the incongruent-eligible condition than the congruent condition (p<.016). No other effects were significant in this region. Furthermore, in the inferior occipital gyrus ROI (coordinates: −42, −78, −10), we found a non-significant trend for condition (F(3,39) = 2.12; p<.11). Planned comparisons revealed that there was a trend for activity for the incongruent-eligible condition to be greater than the activity for the neutral condition (p<.07). There were no significant effects for any of the other predefined ROIs. These results argue that greater attentional demands in the incongruent-eligible conditions of the Stroop task tend to be related to increased activity in word/letter sensitive regions of cortex. However, this modulation was dependent on the region examined – only one out of the five regions examined showed significant task-related modulation during the Stroop task (VWFA), with an additional region trending towards significance (inferior occipital gyrus).
Fig. 5.
Percent signal change in the predefined ROIs (A) VWFA, and (B) inferior occipital gyrus. We found that the incongruent-eligible condition had significantly higher levels of activity compared to the neutral condition.
3.8. Correlations between CSA activity and response times
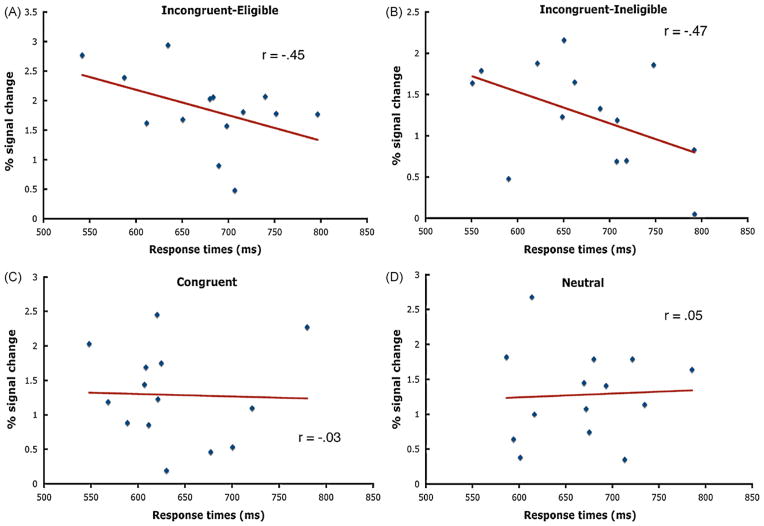
We predicted that enhancement of activity in CSA regions would be associated with enhanced performance manifested by reduced reaction times during the Stroop task. To this end, we performed a series of one-tailed correlations that examined whether the level of activity in the CSA during each condition of the Stroop task and for each subject was correlated with response times. As predicted, we found that faster response times for the in congruent-eligible condition were associated with higher activity levels in the CSA (r = −.45; p<.05) (Fig. 6). In addition, the incongruent-ineligible condition also showed a significant negative correlation between response times and activity in the CSA (r = −.47; p<.04) despite the lack of any significant modulation of activity in the CSA for this condition (Fig. 6). The response times for the neutral (r = .05; p<.433) and the congruent (r = −.03; p<.456) conditions were not correlated with activity in the CSA. These results argue that activity in color-sensitive regions of cortex only impacts performance when interference and attentional demands are highest and that an up-regulation of this region during the Stroop task was associated with enhanced task performance (Table 3).
Fig. 6.
Scatterplots of the mean response times (ms) for the incongruent-eligible, incongruent-ineligible, neutral, and congruent conditions as a function of percent signal change in the empirically derived CSA. Each point corresponds to an individual participant. The Incongruent-Eligible and Incongruent-Ineligible correlations were significant at p<.05.
Table 3.
We predicted both the incongruent conditions to show modulation of activity relative to either the congruent or neutral condition
| Condition | Observed modulation | Correlations with response times | |
|---|---|---|---|
| CSA | Incongruent-ineligible | – | −.47 |
| Incongruent-eligible | √ | −.45 | |
| Left V4 | Incongruent-ineligible | – | −.60 |
| Incongruent-eligible | – | −.81 | |
| Right V4 | Incongruent-ineligible | √ | n.s. |
| Incongruent-ineligible | * | n.s. | |
| VWFA | Incongruent-ineligible | – | n.s. |
| Incongruent-eligible | √ | −.46 | |
| IOG | Incongruent-ineligible | – | −.54 |
| Incongruent-eligible | * | −.56 | |
| Left anterior MTG | Incongruent-ineligible | – | n.s. |
| Incongruent-eligible | – | .42 | |
| Left posterior MTG | Incongruent-ineligible | – | n.s. |
| Incongruent-eligible | – | n.s. | |
| Left parietal | Incongruent-ineligible | – | n.s |
| Incongruent-eligible | – | .87 |
In the second column we display the regions and conditions that were consistent with our predictions ((*) p<.10; (√) p<.05). The third column displays the correlations (Pearson correlation coefficients) with response times for each of the incongruent conditions.
In the predefined V4 region in the left hemisphere we failed to find any task-related modulation of activity for any Stroop task condition. In a similar vein, the correlations with activity in this region and behavioral performance were not limited to the conflict conditions. Specifically, correlations between performance and activity revealed that, similar to the empirically defined ROIs described above, there were significant negative correlations between reaction times and percent signal change for all conditions but neutral. Specifically, we found that faster response times were associated with higher activity levels in left V4 for the incongruent-eligible condition (r = −.81; p<.001), the incongruent-ineligible condition (r = −.60; p<.001), the congruent condition (r = −.50; p<.03) and a trend for the neutral condition (r = −.42; p<.06). For the right hemisphere V4 region, the incongruent-ineligible condition showed a trend for greater activity than the other Stroop task conditions. However, unlike the left hemisphere V4 region none of the correlations between reaction time and activity were significant (incongruent-eligible condition (r = −.18; p<.265), the incongruent-ineligible condition (r = −.39; p<.08), the congruent condition (r = .26; p<.18) and neutral condition (r = .12; p<.33)).
To ensure that these correlations between activity in CSA regions and behavioral performance were not being driven by a general increase in activity throughout visual cortex for the better performers, we correlated the activity in a region of visual cortex that was unresponsive to our color manipulation with performance on the incongruent conditions. We extracted the mean signal from a 10 mm diameter ROI from primary visual cortex (x = −12, y = −96, z = −12). If enhanced performance on the Stroop task was associated with a non-specific and a general increase of activity in visual cortex, then this region should also be correlated with performance, however, if enhanced performance was specific to color-sensitive areas, then there should not be a correlation between performance and activity in the non-specific ROI. Indeed, we failed to find a significant correlation between activity in this ROI and performance on the incongruent-eligible condition (r = −.04) or performance on the incongruent-ineligible condition (r = −.07) suggesting that performance-related correlations were limited to category-specific regions of cortex and not due to a general, and non-specific increase of activity throughout visual cortex.
3.9. Correlations between word areas and response times
Similar to the CSA regions, we examined whether behavioral performance for each condition was correlated with the level of activity in the temporal and parietal regions defined empirically as well as the level of activity in each of the predefined ROIs. Top-down models of selective attention predict positive correlations between activity and response times in distractor regions of cortex. That is, performance should be worse with greater processing of the distractor information.
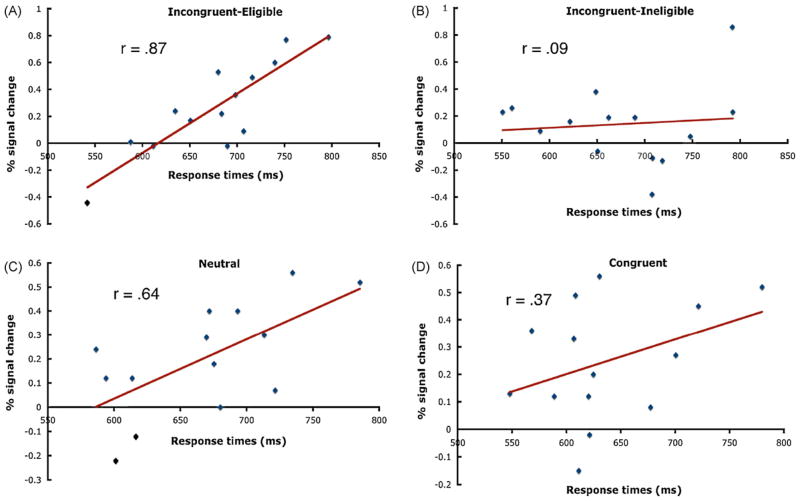
First, despite the lack of modulation during the Stroop task, we found that response times for the incongruent-eligible (r = .87; p<.001), congruent (r = .37; p<.09), and neutral (r = .64; p<.007) conditions were positively correlated, at least marginally, with the level of activity in the left parietal lobule such that faster response times, or better performance, were associated with less activity in this region (Fig. 7). The correlation between the response times for the incongruent-ineligible condition and activity in the left parietal region was not significant (r = .09; p<.37). Therefore, the magnitude of activity in this region does not seem to be dependent on the cognitive demands or the degree of conflict in the task, but rather a general mechanism associated with performing all task conditions.
Fig. 7.
Scatterplots and correlation coefficients between the response times for the (A) incongruent-eligible, (B) incongruent-ineligible, (C) neutral, and (D) congruent conditions and activity levels within the left parietal lobule ROI. Each point corresponds to an individual participant. All correlations were significant (one-tailed) at p<.05 except for the incongruent-ineligible condition.
For the left middle temporal lobe region we found that the response times for the incongruent-eligible condition (r = .42; p<.06) were marginally correlated with the level of activity, but neither the incongruent-ineligible (r = −.14; p<.31), congruent (r = −.12; p<.34), nor neutral (r = −.02; p<.47) response times were significantly correlated with activity in this region. The trend for a positive correlation specific to the incongruent-eligible condition suggests that like the parietal region, faster response times were marginally associated with less activity. However, unlike the parietal region, this correlation was specific for the most demanding condition, that is, the condition with the greatest degree of conflict.
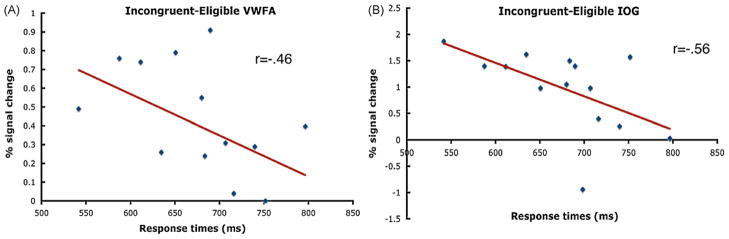
Second, for the predefined ROIs, we failed to find evidence that the activity in these regions was positively correlated with reaction times. Instead, we found the opposite pattern of correlations. Specifically, the VWFA ROI showed a negative correlation between activity for the incongruent-eligible condition and response times (r = −.46; p<.05) such that faster response times were associated with greater levels of activity (Fig. 8). In addition, for the inferior occipital gyrus (coordinates: −42, −78, −10) we found a negative correlation between activity for the incongruent-eligible condition and response times (r = −.56; p<.01), as well as for the incongruent-ineligible condition and response times (r = −.54; p<.02). Interestingly, the direction of these effects is opposite to that which we report for the middle temporal region and parietal region, and are therefore inconsistent with predictions made from models of top-down control (Table 3). However, the tendency for these regions to show negative correlations with the conditions showing the greatest degree of conflict is partially consistent with push–pull mechanisms of top-down control. This is discussed in more detail below.
Fig. 8.
Scatterplots and correlation coefficients between the response times for the (A) incongruent-eligible condition in the VWFA, and (B) incongruent-eligible condition for the inferior occipital gyrus. Each point corresponds to an individual participant. Both correlations were significant (one-tailed) at p<.05.
4. Discussion
In this study, we examined whether cognitive demands engendered through conflict in the Stroop task would modulate activity in brain regions sensitive to processing target and distractor information. Consistent with theories of selective attention and top-down control [33] we found that activity in color-sensitive regions of cortex was up-regulated during conditions of high cognitive demand and behavioral conflict and that the magnitude of this up-regulation was positively correlated with performance (negatively correlated with response times). In addition, we found limited evidence for the hypothesis that there should be enhanced activity in distractor regions of cortex under highly demanding conditions. In only one of the five word/letter regions examined did we find significant enhancement of activity during the task, and this activity was positively, rather than negatively, correlated with performance. This effect might suggest that top-down control operations in this type of selective attention task are more associated with enhancement processes in target-related visual cortical areas rather than increased or decreased modulation of distractor regions. However, the correlations with performance in distractor related processing regions suggests that the magnitude of activity in these regions is still related to task performance, despite the lack of task-related modulation.
Interestingly, although we failed to find task-related modulation in many of the word/letter areas, we found that some of these regions were correlated with performance. Specifically, activity in the left parietal region was negatively correlated with performance, whereas activity in the VWFA and inferior occipital cortex was positively correlated with performance. These effects suggest that (1) Stroop task performance is related to the degree of activity in word/letter areas, despite a non-significant degree of modulation in many of these regions during the task, and (2) greater activity in some word/letter sensitive regions and less activity in other word/letter sensitive regions is related to enhanced task performance. These effects indicate that the capabilities of selective attention to influence category-specific regions of visual cortex are directly related with task performance and that the direction of the relationship is not monotonic.
The magnitude that attention during the Stroop task modulates, or correlates with, activity levels in word/letter areas of the visual cortex might vary as a function of the role that these regions play in word/letter recognition or processing. For example, the left middle temporal gyrus and the left superior parietal gyrus reported in this study have been linked to lexico-semantic processing and grapho-phonological processing, respectively (e.g. [26]). Neither of these regions showed task-related modulation of activity levels, but activity in the left parietal region was positively correlated with performance in nearly all of the Stroop task conditions suggesting that grapho-phonological processing plays a critical role in Stroop task performance, but not necessarily with resolving Stroop conflict. It is likely that the attentional set during the Stroop task (“Attend to the color and ignore the word”) is applied to all conditions regardless of the presence of conflict, and the level of activity in the left parietal region is representative of control operations that reduce grapho-phonological conversion of the distractor (word), resulting in an improvement in performance. In contrast, the VWFA and inferior occipital gyrus regions defined a priori based on prior investigations of word/letter string localization are responsive to a variety of symbols and letter strings [26]. Both the VWFA and inferior occipital regions were negatively correlated with Stroop task performance. It is possible that semantic similarity between the color-word and the word itself results in a spreading of activation in early visual regions that process word/letter information, a hypothesis consistent with one connectionist model of Stroop task performance [21].
The different directions of the correlations between performance and activity and their locus within the extrastriate cortices suggests that a push–pull mechanism of attentional selection (enhancement and suppression mechanisms) might not only be dependent on the cognitive demands and semantic similarity between distractors and targets, but may also be dependent on the complexity of the receptive fields and processors of the region being moderated. The correlations and results discussed in this manuscript lend some support to this hypothesis. However, an alternative hypothesis is that better performers employ a different strategy during the Stroop task then their poorer performing counterparts. A difference in the type of top-down control mechanism may account for some of the differences in the direction of the correlations across the regions examined.
Although retinotopic mapping procedures would have been a more accurate method of demarcating regions of the visual cortex, we were able to successfully localize color-sensitive areas of cortex using our localizer method. These color-sensitive areas were distributed throughout primary and extrastriate cortices replicating other findings that regions involved with processing color extend from V1 through V4 [70]. As discussed above, we demonstrated an increase in target (color) related activity during the most demanding condition of the Stroop task, thereby supporting predictions made by a number of models of attentional control [11,15,21,33,34,44,65,66] as well as arguments that top-down attention enhances target processing under high cognitive loads [32]. In addition, better task performance for the incongruent conditions was related to greater activity in CSA. These correlations suggest that the efficacy of top-down modulation during the Stroop task has a direct effect on the performance capabilities to selectively attend to the task-relevant color dimension.
Interestingly, although response times were fastest for the congruent condition and reliably increased with attentional demands(congruent RT < neutral RT < incongruent-ineligible RT < incongruent-eligible RT), modulation of CSA was found only for the most demanding condition. This apparent discrepancy between the behavioral and neuroimaging results suggests that there is not a one-to-one relationship between cognitive demands, as measured behaviorally, and attentional modulation of target-related fMRI activity. This may highlight a limitation of models of top-down attentional control when target and distractor information are spatially contiguous or coincident [32].
The results described in this study are inline with previous work showing that posterior regions of the brain are up-regulated during an object-Stroop task [2]. Other studies of attention have also reported modulation of activity in ventral visual cortex [10,20,48]. Previous Stroop studies have suggested that color areas of cortex would be up-regulated during the incongruent condition while word and letter related areas would be down-regulated [44]. We failed to find evidence that distractor-related regions were down-regulated during the Stroop task. However, consistent with connectionist models of the Stroop task as well as models of perceptual and cognitive load [12,33], both task-relevant and task-irrelevant areas of cortex were up-regulated during the task.
One important caveat of this study is that empirically we failed to find any area of cortex specific to processing words, even at a lower threshold (p<.05). Our comparisons revealed that there were no differences between words, letter strings, and pseudo-words, and only by performing a conjunction analysis between the word, letter-string, and pseudo-word conditions and comparing the activation within the inclusive conjunction mask to the activity from color checkerboards and black and white checkerboards did we reveal areas that were sensitive to processing letters. Although difficult to explain a null result, our result is consistent with a large literature reporting the presence of word/letter related activity in extrastriate and occipito-temporal regions when contrasting letter strings or words with non-word stimuli such as textures, symbols, checkerboards, or a rest period [4–6,13,17–19,23,56,68]. In addition, the locality of our results are also consistent with other studies reporting an area of the left middle temporal region as involved in lexico-semantic processing and occipito-parietal engagement during memory for words [28] and grapho-phonological processing [26,67]. It should also be noted that our word/letter localizers were passively viewed and it is unknown whether the participants engaged in any deep processing of the words and letters. This also could have contributed to the data reported here. In short, our results complement the literature on localization of VWFA and demonstrate that areas of cortex sensitive to processing words and letters could only be found by contrasts with checkerboard displays.
In sum and consistent with our hypotheses, we report that selective attention enhances activity in cortical processors that are sensitive to target information (color), and also enhances activity in some distractor (word) sensitive areas of cortex. Importantly, these results provide partial support for models of attentional control that argue that attention towards distractors may be enhanced when target and distractor dimensions are spatially contiguous or coincident [33]. Therefore, we conclude that selective attention, when task-relevant and task-irrelevant information are spatially contiguous, can function by enhancing the processing associated with the task-relevant target and some of the regions associated with distractor processing. Both positive and negative correlations with performance argue that these top-down processes play a critical role in performance on selective attention tasks.
Acknowledgments
We would like to thank Nancy Dodge, Holly Tracy, Maritza Alvarado, and Michelle Voss for their help in data collection and to Diane Beck for her helpful comments on previous versions of the manuscript. This research was supported by grants R37 AG25667 and RO1 AG25032 from the National Institute on Aging.
References
- 1.Banich MT, Milham MP, Atchley RA, Cohen NJ, Webb A, Wszalek T, et al. Prefrontal regions play a predominant role in imposing an attentional ‘set’: evidence from fMRI. Cogn Brain Res. 2000;10:1–9. doi: 10.1016/s0926-6410(00)00015-x. [DOI] [PubMed] [Google Scholar]
- 2.Banich MT, Milham MP, Jacobson BL, Webb A, Wszalek T, Cohen NJ, et al. Attentional selection and the processing of task-irrelevant information: insights from fMRI examinations of the Stroop task. Prog Brain Res. 2001;134:459–70. doi: 10.1016/s0079-6123(01)34030-x. [DOI] [PubMed] [Google Scholar]
- 3.Beauchamp MS, Haxby JV, Jennings JE, DeYoe EA. An fMRI, version of the Farnsworth-Munsell 100-Hue test reveals multiple color-sensitive areas in human ventral occipitotemporal cortex. Cereb Cortex. 1999;9:257–63. doi: 10.1093/cercor/9.3.257. [DOI] [PubMed] [Google Scholar]
- 4.Beauregard M, Chertkow H, Bub D, Murtha S, Dixon R, Evans A. The neural substrates for concrete, abstract, and emotional word lexica: a positron emission tomography study. J Cogn Neurosci. 1997;9:441–61. doi: 10.1162/jocn.1997.9.4.441. [DOI] [PubMed] [Google Scholar]
- 5.Binder JR, McKiernan KA, Parsons ME, Westbury CF, Pssing ET, Kaufman JN, et al. Neural correlates of lexical access during visual word recognition. J Cogn Neurosci. 2003;15:372–93. doi: 10.1162/089892903321593108. [DOI] [PubMed] [Google Scholar]
- 6.Brem S, Bucher K, Halder P, Summers P, Dietrich T, Martin E, et al. Evidence for developmental changes in the visual word processing network beyond adolescence. NeuroImage. 2006;29(3):822–37. doi: 10.1016/j.neuroimage.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 7.Chawla D, Rees G, Friston KJ. The physiological basis of attentional modulation in extrastriate visual areas. Nat Neurosci. 1999;2(7):671–6. doi: 10.1038/10230. [DOI] [PubMed] [Google Scholar]
- 8.Cohen L, Lehiricy S, Chochon F, Lemer C, Rivaud S, Dehaene S. Language-specific tuning of visual cortex? Functional properties of the visual word form area. Brain. 2002;125(Pt 5):1054–69. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- 9.Cohen L, Dehaene S. Specialization within the ventral stream: the case for the visual word form area. NeuroImage. 2004;22(1):466–76. doi: 10.1016/j.neuroimage.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 10.Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Peterson SE. Selective and divided attention during visual discrimination of shape, color, and speed: functional anatomy by positron emission tomography. J Neurosci. 1991;11(8):2383–402. doi: 10.1523/JNEUROSCI.11-08-02383.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–15. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 12.De Fockert JW, Rees G, Frith CD, Lavie N. The role of working memory in visual selective attention. Science. 2001;291(5509):1803–6. doi: 10.1126/science.1056496. [DOI] [PubMed] [Google Scholar]
- 13.Dehaene S, Le Clec’ HG, Poline JB, Le Bihan D, Cohen L. The visual word form area: a prelexical representation of visual words in the fusiform gyrus. NeuroReport. 2002;13(3):321–5. doi: 10.1097/00001756-200203040-00015. [DOI] [PubMed] [Google Scholar]
- 14.Druzgal TJ, D’Esposito M. Dissecting contributions of prefrontal cortex and fusiform face area to face working memory. J Cogn Neurosci. 2003;15(6):771–84. doi: 10.1162/089892903322370708. [DOI] [PubMed] [Google Scholar]
- 15.Egner T, Hirsch J. The neural correlates and functional integration of cognitive control in a Stroop task. NeuroImage. 2005;24:539–47. doi: 10.1016/j.neuroimage.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Erickson KI, Milham MP, Colcombe SJ, Kramer AF, Banich MT, Webb A, et al. Behavioral conflict, anterior cingulate cortex, and experiment duration: implications of diverging data. Hum Brain Mapp. 2004;21(2):98–107. doi: 10.1002/hbm.10158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiez JA, Balota DA, Raichle ME, Peterson SE. Effects of lexicality, frequency, and spelling-to-sounds consistency on the functional anatomy of reading. Neuron. 1999;24(1):205–18. doi: 10.1016/s0896-6273(00)80833-8. [DOI] [PubMed] [Google Scholar]
- 18.Gauthier I, Tarr MJ, Moylan J, Skudlarski P, Gore JC, Anderson AW. The fusiform “face area” is part of a network that processes faces at the individual level. J Cogn Neurosci. 2000;12:495–504. doi: 10.1162/089892900562165. [DOI] [PubMed] [Google Scholar]
- 19.Hasson U, Levy I, Behrmann M, Hendler T, Malach R. Eccentricity bias as an organizing principle for human high-order object areas. Neuron. 2002;34:479–90. doi: 10.1016/s0896-6273(02)00662-1. [DOI] [PubMed] [Google Scholar]
- 20.Heinze HJ, Mangun GR, Burchert W, Hinrichs H, Scholz M, Munte TF, et al. Combined spatial and temporal imaging of brain activity during visual selective attention in humans. Nature. 1994;372(6506):543–6. doi: 10.1038/372543a0. [DOI] [PubMed] [Google Scholar]
- 21.Herd SA, Banich MT, O’Reilly RC. Neural mechanisms of cognitive control: an integrative model of Stroop task performance and fMRI data. J Cogn Neurosci. 2006;18(1):22–32. doi: 10.1162/089892906775250012. [DOI] [PubMed] [Google Scholar]
- 22.Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci. 2000;3(3):284–91. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- 23.James KH, James TW, Jobard G, Wong AC, Gauthier I. Letter processing in the visual system: different activation patterns for single letters and strings. Cogn Affect Behav Neurosci. 2005;5(4):452–66. doi: 10.3758/cabn.5.4.452. [DOI] [PubMed] [Google Scholar]
- 24.Jenkinson M, Bannister PR, Brady JM, Smith SM. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17(2):825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 25.Jessen F, Erb M, Klose U, Lotze M, Grodd W, Heun R. Activation of human language processing regions after the presentation of random letter strings demonstrated with event-related functional magnetic resonance imaging. Neurosci Lett. 1999;270:13–6. doi: 10.1016/s0304-3940(99)00453-x. [DOI] [PubMed] [Google Scholar]
- 26.Jobard G, Crivello F, Tzourio-Mazoyer N. Evaluation of the dual-route theory of reading: a meta-analysis of 35 neuroimaging studies. NeuroImage. 2003;20(2):693–712. doi: 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- 27.Kastner S, DeWeerd P, Desimone R, Ungerleider LC. Mechanisms of directed attention in the human extrastriate cortex as revealed by functional MRI. Science. 1998;282:108–11. doi: 10.1126/science.282.5386.108. [DOI] [PubMed] [Google Scholar]
- 28.Kelley WM, Miezen FM, McDermott KB, Buckner RL, Raichle ME, Cohen NJ, et al. Hemispheric specialization in human dorsal cortex and medial temporal lobe for verbal and nonverbal memory encoding. Neuron. 1998;20:927–36. doi: 10.1016/s0896-6273(00)80474-2. [DOI] [PubMed] [Google Scholar]
- 29.Kraft A, Schira MM, Hagendorf H, Schmidt S, Olma M, Brandt SA. fMRI localizer technique: efficient acquisition and functional properties of single retinotopic positions in the human visual cortex. NeuroImage. 2005;28(2):453–63. doi: 10.1016/j.neuroimage.2005.05.050. [DOI] [PubMed] [Google Scholar]
- 30.Kucera, Francis WN. Computational analysis of present-day American English. Providence: Brown University Press; 1967. [Google Scholar]
- 31.Lavie N. Perceptual load as a necessary condition for selective attention. J Exp Psychol: Hum Percept Perform. 1995;21:451–68. doi: 10.1037//0096-1523.21.3.451. [DOI] [PubMed] [Google Scholar]
- 32.Lavie N, Hirst A, De Fockert JW, Viding E. Load theory of selective attention and cognitive control. J Exp Psychol: Gen. 2004;133(3):339–54. doi: 10.1037/0096-3445.133.3.339. [DOI] [PubMed] [Google Scholar]
- 33.Lavie N. Distracted and confused?: Selective attention under load. Trends Cogn Sci. 2005;9(2):75–82. doi: 10.1016/j.tics.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Liu X, Banich MT, Jacobson BL, Tanabe JL. Functional dissociation of attentional selection within PFC: response and non-response related aspects of attentional selection as ascertained by fMRI. Cereb Cortex. 2006;16(6):827–34. doi: 10.1093/cercor/bhj026. [DOI] [PubMed] [Google Scholar]
- 35.Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J Neurophysiol. 1997;77(1):24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- 36.Luks TL, Simpson GV. Preparatory deployment of attention to motion activates higher-order motion-processing brain regions. NeuroImage. 2004;22:1515–22. doi: 10.1016/j.neuroimage.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 37.MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–8. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 38.Martinez A, Anllo-Vento L, Sereno MI, Frank LR, Buxton RB, Dubowitz DJ, et al. Involvement of striate and extrastriate visual cortical areas in spatial attention. Nat Neurosci. 1999;2(4):364–8. doi: 10.1038/7274. [DOI] [PubMed] [Google Scholar]
- 39.Maurer D, O’Craven KM, Le Grand R, Mondloch CJ, Springer MV, Lewis TL, et al. Neural correlates of processing facial identity based on features versus their spacing. Neuropsychologia. 2007;45(7):1438–51. doi: 10.1016/j.neuropsychologia.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 40.McCarley JS, Mounts JRW, Kramer AF. Age-related differences in localized attentional interference. Psychol Aging. 2004;19(1):203–10. doi: 10.1037/0882-7974.19.1.203. [DOI] [PubMed] [Google Scholar]
- 41.McKeefry D, Zeki S. The position and topography of the human colour centre as revealed by functional magnetic resonance imaging. Brain. 1997;120:2229–42. doi: 10.1093/brain/120.12.2229. [DOI] [PubMed] [Google Scholar]
- 42.Milham MP, Banich MT, Webb A, Barad V, Cohen NJ, Wszalek T, et al. The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Cogn Brain Res. 2001;12:467–73. doi: 10.1016/s0926-6410(01)00076-3. [DOI] [PubMed] [Google Scholar]
- 43.Milham MP, Erickson KI, Banich MT, Kramer AF, Webb A, Wszalek T, et al. Attentional control in the aging brain: insights from an fMRI study of the Stroop task. Brain Cogn. 2002;49:467–73. doi: 10.1006/brcg.2001.1501. [DOI] [PubMed] [Google Scholar]
- 44.Milham MP, Banich MT, Barad V. Competition for priority in processing increases prefrontal cortex’s involvement in top-down control: an event-related fMRI study of the Stroop task. Cogn Brain Res. 2003;17:212–22. doi: 10.1016/s0926-6410(03)00108-3. [DOI] [PubMed] [Google Scholar]
- 45.Mitchell JP. Activity in right temporo-parietal junction is not selective for theory-of-mind. Cereb Cortex. 2008;18(2):262–71. doi: 10.1093/cercor/bhm051. [DOI] [PubMed] [Google Scholar]
- 46.Moran J, Desimone R. Selective attention gates visual processing in the extrastriate cortex. Science. 1985;229(4715):782–4. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- 47.Nobre AC, Allison T, McCarthy G. Modulation of human extrastriate visual processing by selective attention to colours and words. Brain. 1998;121:1357–68. doi: 10.1093/brain/121.7.1357. [DOI] [PubMed] [Google Scholar]
- 48.O’Conner DH, Fukui MM, Pinsk MA, Kastner S. Attention modulates responses in the human lateral geniculate nucleus. Nat Neurosci. 2002;5(11):1203–9. doi: 10.1038/nn957. [DOI] [PubMed] [Google Scholar]
- 49.Pessoa L, Kastner S, Ungerleider LG. Neuroimaging studies of attention: from modulation of sensory processing to top-down control. J Neurosci. 2003;23(10):3990–8. doi: 10.1523/JNEUROSCI.23-10-03990.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peterson BS, Kane MJ, Alexander GM, Lacadie C, Skudlarski P, Leung HC, et al. An event-related functional MRI study comparing interference effects in the Simon and Stroop tasks. Cogn Brain Res. 2002;13:427–40. doi: 10.1016/s0926-6410(02)00054-x. [DOI] [PubMed] [Google Scholar]
- 51.Piazza M, Pinel P, Le Bihan D, Dehaene S. A magnitude code common to numerosities and number symbols in human intraparietal cortex. Neuron. 2007;53(2):293–305. doi: 10.1016/j.neuron.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 52.Pinsk MA, Doniger GM, Kastner S. Push-pull mechanism of selective attention in human extrastriate cortex. J Neurophysiol. 2004;92:622–9. doi: 10.1152/jn.00974.2003. [DOI] [PubMed] [Google Scholar]
- 53.Price CJ, Moore CJ, Humphreys GW, Wise RJ. Segregating semantic from phonological processes during reading. J Cogn Neurosci. 1997;9:727–33. doi: 10.1162/jocn.1997.9.6.727. [DOI] [PubMed] [Google Scholar]
- 54.Price CJ. The functional anatomy of word comprehension and production. Trends Cogn Sci. 1998;2:281–8. doi: 10.1016/s1364-6613(98)01201-7. [DOI] [PubMed] [Google Scholar]
- 55.Price CJ, Devlin JT. The myth of the visual word form area. NeuroImage. 2003;19(3):473–81. doi: 10.1016/s1053-8119(03)00084-3. [DOI] [PubMed] [Google Scholar]
- 56.Puce A, Allison T, Asgari M, Gore JC, McCarthy G. Differential sensitivity of human visual cortex to faces, letterstrings, and textures: a functional magnetic resonance imaging study. J Neurosci. 1996;16:5205–15. doi: 10.1523/JNEUROSCI.16-16-05205.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rees G, Frith CD, Lavie N. Modulating irrelevant motion perception by varying attentional load in an unrelated task. Science. 1997;278:1616–9. doi: 10.1126/science.278.5343.1616. [DOI] [PubMed] [Google Scholar]
- 58.Rees G, Russell C, Frith CD, Driver J. Inattentional blindness amnesia for fixated but ignored words. Science. 1999;286:2504–7. doi: 10.1126/science.286.5449.2504. [DOI] [PubMed] [Google Scholar]
- 59.Reynolds JH, Chelazzi L. Attentional modulation of visual processing. Annl Rev Neurosci. 2004;27:611–47. doi: 10.1146/annurev.neuro.26.041002.131039. [DOI] [PubMed] [Google Scholar]
- 60.Rose M, Schmid C, Winzen A, Sommer T, Buchel C. The functional and temporal characteristics of top-down modulation in visual selection. Cereb Cortex. 2004;15:1290–8. doi: 10.1093/cercor/bhi012. [DOI] [PubMed] [Google Scholar]
- 61.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(S1):208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 63.Tagamets MA, Novick JM, Chalmers ML, Friedman RB. A parametric approach to orthographic processing in the brain: an fMRI study. J Cogn Neurosci. 2000;12:281–97. doi: 10.1162/089892900562101. [DOI] [PubMed] [Google Scholar]
- 64.Tan LH, Chan AH, Kay P, Khong PL, Yip LK, Luke KK. Language affects patterns of brain activation associated with perceptual decision. Proc Natl Acad Sci USA. 2008;105(10):4004–9. doi: 10.1073/pnas.0800055105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS. Anterior cingulate cortex, conflict monitoring, and levels of processing. NeuroImage. 2001;14:1302–8. doi: 10.1006/nimg.2001.0923. [DOI] [PubMed] [Google Scholar]
- 66.Van Veen V, Holroyd CB, Cohen JD, Stenger VA, Carter CS. Errors without conflict: implications for performance monitoring theories of anterior cingulate cortex. Brain Cogn. 2004;56:267–76. doi: 10.1016/j.bandc.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 67.Vigneau M, Jobard G, Mazoyer B, Tzourio-Mazoyer N. Word and non-word reading: what role for the visual word form area? NeuroImage. 2005;27(3):694–705. doi: 10.1016/j.neuroimage.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 68.Wagner AD, Poldrack RA, Eldridge LL, Desmond JE, Glover GH, Gabrieli JD. Material-specific lateralization of prefrontal activation during episodic encoding and retrieval. NeuroReport. 1998;9(16):3711–7. doi: 10.1097/00001756-199811160-00026. [DOI] [PubMed] [Google Scholar]
- 69.Zeki S, Watson JDG, Lueck CJ, Friston KJ, Kennard C, Frackowiak RSJ. A direct demonstration of functional specialization in human visual cortex. J Neurosci. 1991;11:641–9. doi: 10.1523/JNEUROSCI.11-03-00641.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zeki S, Marini L. Three cortical stages of colour processing in the human brain. Brain. 1998;121:1669–85. doi: 10.1093/brain/121.9.1669. [DOI] [PubMed] [Google Scholar]