Abstract
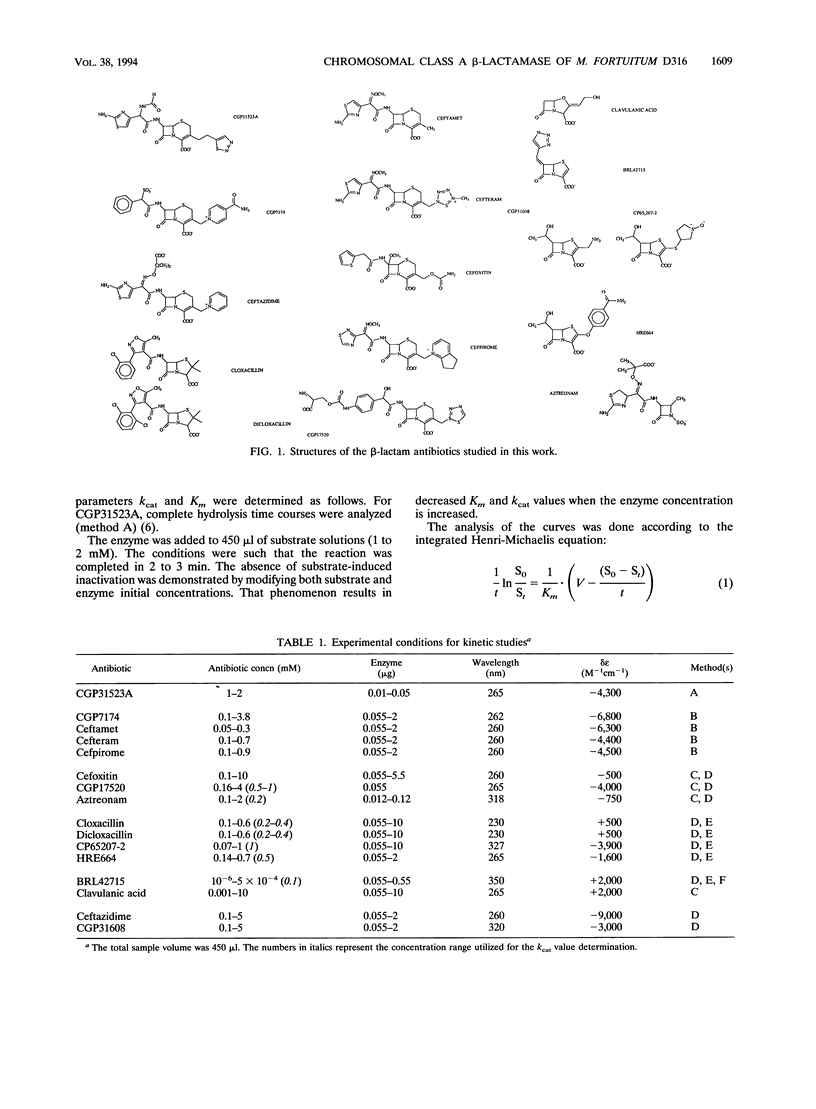
Sixteen different compounds usually considered beta-lactamase stable or representing potential beta-lactam inhibitors and inactivators were tested against the beta-lactamase produced by Mycobacterium fortuitum. The compounds exhibiting the most interesting properties were BRL42715, which was by far the best inactivator, and CGP31608 and ceftazidime, which were not recognized by the enzyme. These compounds thus exhibited adequate properties for fighting mycobacterial infections. Although cloxacillin, dicloxacillin, cefoxitin, and CP65207-2 exhibited poor inhibitory efficiency against the enzyme, they were also rather poor substrates and might be considered potential antimycobacterial agents. By contrast, CGP31523A and ceftamet were good substrates.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amicosante G., Franceschini N., Segatore B., Oratore A., Fattorini L., Orefici G., Van Beeumen J., Frere J. M. Characterization of a beta-lactamase produced in Mycobacterium fortuitum D316. Biochem J. 1990 Nov 1;271(3):729–734. doi: 10.1042/bj2710729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnas R. L., Fisher J., Knowles J. R. Chemical studies on the inactivation of Escherichia coli RTEM beta-lactamase by clavulanic acid. Biochemistry. 1978 May 30;17(11):2185–2189. doi: 10.1021/bi00604a025. [DOI] [PubMed] [Google Scholar]
- Cullmann W., Stieglitz M. Antibacterial activity of HRE 664, a new parenteral penem. Antimicrob Agents Chemother. 1988 Jul;32(7):1090–1093. doi: 10.1128/aac.32.7.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meester F., Joris B., Reckinger G., Bellefroid-Bourguignon C., Frère J. M., Waley S. G. Automated analysis of enzyme inactivation phenomena. Application to beta-lactamases and DD-peptidases. Biochem Pharmacol. 1987 Jul 15;36(14):2393–2403. doi: 10.1016/0006-2952(87)90609-5. [DOI] [PubMed] [Google Scholar]
- Elks J. Structural formulae and nomenclature of the cephalosporin antibiotics. Drugs. 1987;34 (Suppl 2):240–246. doi: 10.2165/00003495-198700342-00017. [DOI] [PubMed] [Google Scholar]
- Fattorini L., Orefici G., Jin S. H., Scardaci G., Amicosante G., Franceschini N., Chopra I. Resistance to beta-lactams in Mycobacterium fortuitum. Antimicrob Agents Chemother. 1992 May;36(5):1068–1072. doi: 10.1128/aac.36.5.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattorini L., Scardaci G., Jin S. H., Amicosante G., Franceschini N., Oratore A., Orefici G. Beta-lactamase of Mycobacterium fortuitum: kinetics of production and relationship with resistance to beta-lactam antibiotics. Antimicrob Agents Chemother. 1991 Sep;35(9):1760–1764. doi: 10.1128/aac.35.9.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J., Charnas R. L., Knowles J. R. Kinetic studies on the inactivation of Escherichia coli RTEM beta-lactamase by clavulanic acid. Biochemistry. 1978 May 30;17(11):2180–2184. doi: 10.1021/bi00604a024. [DOI] [PubMed] [Google Scholar]
- Frère J. M., Dormans C., Lenzini V. M., Duyckaerts C. Interaction of clavulanate with the beta-lactamases of Streptomyces albus G and Actinomadura R39. Biochem J. 1982 Dec 1;207(3):429–436. doi: 10.1042/bj2070429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gootz T., Retsema J., Girard A., Hamanaka E., Anderson M., Sokolowski S. In vitro activity of CP-65,207, a new penem antimicrobial agent, in comparison with those of other agents. Antimicrob Agents Chemother. 1989 Aug;33(8):1160–1166. doi: 10.1128/aac.33.8.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarlier V., Gutmann L., Nikaido H. Interplay of cell wall barrier and beta-lactamase activity determines high resistance to beta-lactam antibiotics in Mycobacterium chelonae. Antimicrob Agents Chemother. 1991 Sep;35(9):1937–1939. doi: 10.1128/aac.35.9.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matagne A., Misselyn-Bauduin A. M., Joris B., Erpicum T., Granier B., Frère J. M. The diversity of the catalytic properties of class A beta-lactamases. Biochem J. 1990 Jan 1;265(1):131–146. doi: 10.1042/bj2650131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waley S. G. Kinetics of suicide substrates. Biochem J. 1980 Mar 1;185(3):771–773. doi: 10.1042/bj1850771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Andrews J. M., Piddock L. J. In vitro activity of CGP 31608, a new penem. Antimicrob Agents Chemother. 1987 Feb;31(2):267–273. doi: 10.1128/aac.31.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Steingrube V. A., Wallace R. J., Jr beta-Lactamase inhibitors and the inducibility of the beta-lactamase of Mycobacterium tuberculosis. Am Rev Respir Dis. 1992 Mar;145(3):657–660. doi: 10.1164/ajrccm/145.3.657. [DOI] [PubMed] [Google Scholar]



