Abstract
We evaluated the cryosurvival of rat epididymal sperm preserved in raffinose–modified Krebs-Ringer bicarbonate–egg yolk extender supplemented with various energy-yielding substrates (glucose, pyruvate, lactate, and ATP) and assessed the effect on sperm oxygen consumption. The incubation of sperm at 37 °C for 10 min in lactate-free extender decreased sperm motility and oxygen consumption before and after thawing compared with those of sperm in glucose- and pyruvate-free mediums. We then focused on the effect of supplementing the extender with lactate (0, 10.79, 21.58, 32.37, and 43.16 mM) and found that sperm frozen and thawed in extender supplemented with 32.37 mM lactate exhibited the highest motility. When we supplemented extender containing 32.37 mM lactate with ATP (0, 0.92, 1.85, 3.70, and 5.55 mM), sperm frozen and thawed in the extender supplemented with 1.85 mM ATP exhibited considerably higher motility and viability than those of sperm frozen and thawed in ATP-free extender. These results provide the first evidence that supplementation of the raffinose-modified Krebs–Ringer bicarbonate–egg yolk extender with 32.37 mM lactate and 1.85 mM ATP increases of number of motile sperm before freezing and enhances the cryosurvival of rat sperm. These supplements to the extender may enhance sperm cryosurvival by improving the metabolic capacity of sperm before freezing.
Abbreviations: mKRB, modified Krebs–Ringer bicarbonate
We previously showed that freezing rat epididymal sperm in an extender of raffinose dissolved in modified Krebs–Ringer bicarbonate (mKRB) solution containing egg yolk enhances their cryosurvival of sperm as measured by viability and acrosomal integrity; this finding suggested that a mKRB-based freezing extender containing glucose, pyruvate, and lactate can protect sperm against freezing injury.33 A possible reason for this finding is that sperm in this extender retain high metabolic capacity before freezing which, in turn, may enhance the cryosurvival of rat sperm. However, the mechanism by which the mKRB-based extender promotes the metabolic capacity and cryosurvival of rat sperm is unclear.
The process of cryopreservation imposes numerous stresses on not only the physical features of sperm but also the energy production to support motility before and after freezing, and improving energy production in frozen–thawed sperm is important for successful cryopreservation.19 Sugars play various roles in sperm extender solution, including providing an energy substrate for sperm during cooling and acting as a cryoprotectant.1 The beneficial effects of adding glucose to the extender on the viability of frozen–thawed sperm have been reported for various species;2 this nutritional effect may involve the synthesis and provision of ATP through the glycolytic pathway to provide the energy required for sperm motility. Therefore, glucose may play a key role in generating energy in motile sperm and preventing freezing damage.
The addition of an exogenous substrate (such as lactate) improved the sperm motility characteristics of cattle,11 boars,14,21 and rabbits.29 A previous study9 showed that a shuttle involving the redox couple lactate–pyruvate and lactate dehydrogenase isozyme C4 is active in rat and rabbit mitochondria but not in mouse mitochondria. This finding suggests that sperm from various species, including rats, may find lactate a suitable substrate for maintaining the energy production and consumption as well as oxygen consumption. However, which of the major biochemical pathways—glycolysis or oxidative phosphorylation—is involved in supplying energy to mobilize sperm has been a long-lasting debate, because the pathway of energy production is species-specific.8,28,32 Regardless of the identity of the pathway involved in energy supply, the development of an appropriate freezing extender likely would improve sperm motility and survival during cryopreservation through enhancement of the metabolic capacities of sperm. However, no previous studies have reported on the optimal components, especially the energy substrates, of a cryodiluent for the freezing of rat sperm.
Sperm may attain access to eggs by mobilizing metabolic energy production in the form of ATP to drive motility.30 ATP is hydrolyzed by the dynein adenosine triphosphatase, which converts the chemical energy of ATP into mechanical energy used for the movement of sperm.3,15 ATP can have several downstream effects leading to improvement in the motility of sperm by means of an increase in the calcium level.10,17,18,20,25,27 Moreover, the amount of ATP required for metabolic energy is higher in the cytosol than the mitochondria.16 Therefore, supplementation of the freezing extender with exogenous ATP may improve the cryosurvival of rat epididymal sperm.
Here we evaluated the cryosurvival and parameters of mitochondrial activity, including oxygen consumption, of rat sperm diluted in raffinose–mKRB–egg yolk extender supplemented with various energy-yielding substrates, including glucose, pyruvate, lactate, and ATP. In addition, we identified the optimal energy substrates and other components of a cryodiluent for the freezing of rat sperm.
Materials and Methods
Principles of laboratory animal care were followed during this study, and all procedures were conducted in accordance with guidelines of the Ethics Committee for Care and Use of Laboratory Animals for Research of the Graduate School of Agricultural Science (Tohoku University, Japan). Wistar rats were used throughout the experiments. Animals were kept in polycarbonate cages (25 × 40 × 20 cm) under controlled conditions with lights on at 0800 and off at 2000. They were given food and tap water ad libitum.
Preparation of the raffinose–mKRB–egg yolk extender.
The basic extender used in this study was the raffinose–mKRB–egg yolk freezing solution defined previously;33 it comprised 0.1 M raffinose (Sigma, St Louis, MO), 94.6 mM NaCl (Wako Pure Chemical Industries, Osaka, Japan), 4.78 mM KCl (Wako), 1.71 mM CaCl2·2H2O (Wako), 1.19 mM MgSO4·7H2O (Wako), 1.19 mM KH2PO4 (Wako), 25.07 mM NaHCO3 (Wako), 21.58 mM sodium dl-lactate (Sigma), 0.5 mM sodium pyruvate (Wako), 5.56 mM glucose (Wako), 50 μg/mL streptomycin (Sigma), and 75 μg/mL penicillin (Sigma); egg yolk was separated from the albumin, and 20% (v:v) egg yolk was added to the raffinose–mKRB solution. Egg yolk lipids were solubilized by adding 0.04% (w:v) SDS (Wako) to the solution. The solution was centrifuged twice at 7000 × g for 30 min. The pH of the solution was adjusted to 7.3 with HCl and its osmotic pressure to 400 mOsm. The supernatant was aspirated and filtered through a 0.45-μm membrane filter (Sartorius, Goettingen, Germany).
Evaluation of sperm motility parameters.
Sperm motility parameters were assessed by using a sperm motility analysis system (version 1.0, Kashimura, Tokyo, Japan) and a 10-μm deep Makler chamber (Sefi Medical Instruments, Haifa, Israel); the protocol was described previously.6 At least 100 sperm and 5 fields were assessed by the sperm motility analysis system for each treatment group. The following parameters were assessed in this study: motility (%), straight line velocity (μm/s), curvilinear velocity (μm/s), amplitude of lateral head displacement (μm), and beat cross frequency (Hz).
Evaluation of sperm acrosome integrity.
The acrosomal integrity of fresh and frozen–thawed sperm was assessed by staining with FITC-conjugated peanut agglutinin (Wako) according to the procedure described previously.33
Collection of rat epididymal sperm.
Both caudae epididymides were excised from 24 sexually mature male Wistar rats older than 15 wk. The excised epididymis was rinsed and carefully blotted free of blood and adipose tissues. A small part of the caudae epididymides tract was excised with fine scissors. The droplet of sperm that welled up was transferred to a 1.5-mL microfuge tube containing 1 mL of freezing medium at 37 °C. After 5 min, the solution was examined macroscopically to verify that sperm were dispersed adequately.
Cryopreservation and thawing.
Experiment 1a.
In this experiment, we investigated the effect of the substrates glucose, pyruvate, and lactate in raffinose–mKRB–egg yolk freezing extender on the motility characteristics of fresh sperm after collection and frozen–thawed sperm. Sperm from both the caudae epididymides from 3 rats were used in this experiment. Immediately after collection, aliquots of sperm were exposed to the following 5 solutions: raffinose–mKRB–egg yolk extender containing the substrates glucose, pyruvate, and lactate (control); glucose-free extender; pyruvate-free extender; lactate-free extender; and substrate-free extender. The osmotic pressure of these solutions was adjusted to 400 mOsm with sucrose (Wako) and the pH to 7.3 with HCl. Each sperm suspension was incubated at 37 °C for 5 min to allow the sperm to disperse, and the sperm concentration and motility parameters then were evaluated by the sperm motility analysis system. The sperm were processed and frozen by using a modification of a previously published protocol.33 The diluted sperm samples were cooled at 5 °C for 90 min. The sperm samples were further diluted 1:1 with each extender containing 1.5% of a commercial cryoprotectant (Equex STM, Nova Chemical Sales, Scituate, MA) to obtain a sperm concentration of 5 × 106 sperm/mL and then were equilibrated at 5 °C for 30 min before freezing. Afterward, the samples were loaded into standard 0.5-mL straws and the straws were heat-sealed. The straws were placed in liquid nitrogen vapor for 10 min, plunged into liquid nitrogen (–196 °C), and stored for 3 d at this temperature. The straws were thawed rapidly by holding them in water (37 °C) for 10 s. The sperm were transferred to a 1.5-mL microfuge tube and incubated at 37 °C for 5 min, after which motility parameters after thawing were assessed by using the sperm motility analysis system. The acrosome status of frozen–thawed sperm was assessed by staining with FITC-conjugated peanut agglutinin.1
Experiment 1b.
Building on the results of Experiment 1a, this experiment was conducted to analyze the characteristics of fresh and frozen–thawed sperm in the raffinose–mKRB–egg yolk extender containing various concentrations of lactate. Sperm from both caudae epididymides from 3 rats were used. Aliquots of sperm were suspended in 1 mL raffinose–mKRB–egg yolk extender containing 0, 10.79, 21.58, 32.37, or 43.16 mM lactate; all of these solutions had an osmotic pressure of 400 mOsm and a pH of 7.3, except the solution containing 43.16 mM lactate (430 mOsm and pH 7.3). The procedures followed for cryopreservation and evaluation of sperm were the same as described for experiment 1a.
Experiment 1c.
This experiment was designed to compare the cryosurvival of rat sperm frozen in raffinose–mKRB–egg yolk extender solution containing 32.37 mM lactate supplemented with various concentrations of ATP. Both caudae epididymides from 3 male rats were used in this experiment. After collection, sperm was divided into 5 aliquots and suspended in 1 mL of extender solution containing 32.37 mM lactate and 0, 0.92, 1.85, 3.70, or 5.55 mM ATP (400 mOsm and pH 7.3). The freezing protocol and evaluation of sperm were the same as described previously. For the evaluation of sperm viability, frozen–thawed sperm samples were incubated in a water bath at 37 °C for 5 min. For each treatment, 3 samples were evaluated after 1, 2, and 3 h of incubation to determine sperm motility, straight-line velocity, curvilinear velocity, and amplitude of lateral head displacement.
Measurement of oxygen consumption of sperm.
Experiment 2a.
The aim of this experiment was to assess the effect of substrates in the raffinose–mKRB–egg yolk medium on the mitochondrial activity of sperm. Sperm was collected from 5 mature male rats and extended in substrate-free raffinose–mKRB–egg yolk medium at 37 °C. The sample was incubated for 5 min to allow the sperm to disperse and then equal volumes were resuspended in each of the following solutions: raffinose–mKRB–egg yolk medium containing 11.12 mM glucose, 1 mM pyruvate, and 43.16 mM lactate (control); glucose-free control (containing pyruvate and lactate); pyruvate-free control (containing glucose and lactate); lactate-free control (containing glucose and pyruvate); and substrate-free raffinose–mKRB–egg yolk medium. The final concentrations of the various substrates in medium containing sperm were 5.56 mM glucose, 0.5 mM pyruvate, and 21.58 mM lactate. The osmotic pressure of these solutions was adjusted to 400 mOsm with sucrose and the pH to 7.3 with HCl. The oxygen consumption rates of the sperm were measured by using Clark-type oxygen electrodes (Rank Brothers, Cambridge, UK) maintained at 37 °C for 10 min and calibrated with air-saturated water at 37 °C, which was assumed to contain 406 nmol oxygen/mL.26 A sperm sample in a volume of 1 mL was suspended in the reaction chamber by stirring carefully to prevent the addition of any external air. The final concentration of sperm in the incubation chamber was approximately 1 × 107 sperm/mL. Data were acquired by using a commercial software program (LabChart version 5.2, AD Instruments, Castle Hill, Australia). The oxygen consumed by the sperm was calculated as:26
 |
The rate of oxygen consumption by sperm was expressed as nmol/min/1 × 107 sperm.
Experiment 2b.
The effect of various concentrations of lactate in the raffinose–mKRB–egg yolk extender on the oxygen uptake of sperm was analyzed. Sperm were collected from the caudae epididymides of 5 rats and suspended in lactate-free raffinose–mKRB–egg yolk medium and then incubated for 5 min at 37 °C. Equal volumes of raffinose–mKRB–egg yolk medium containing 0, 21.58, 43.16, 64.74, and 86.32 mM lactate were diluted with lactate-free raffinose–mKRB–egg yolk medium, resulting in solutions with final lactate concentrations of 0, 10.79, 21.58, 32.37, and 43.16 mM, respectively. The osmotic pressure and the pH of all these solutions were adjusted to 400 mOsm and 7.3, respectively, except the solution containing 43.16 mM lactate (430 mOsm and pH 7.3). The oxygen consumption of each sperm suspension was determined in relation to air-saturated medium as described for experiment 2b.
Experiment 2c.
The effect of adding ATP to raffinose–mKRB–egg yolk medium containing 32.37 mM lactate on the rate of oxygen consumption of sperm was examined. Sperm from 5 rats were flushed the sperm out by using the medium, and then the suspensions in ATP-free raffinose–mKRB–egg yolk medium were incubated for 5 min at 37 °C. Each treated sample was placed in raffinose–mKRB–egg yolk medium containing 0, 1.84, 3.70, 7.4, or 11.1 mM ATP. Subsequently, equal volumes of extended sperm were added to these solutions, resulting in solutions with final ATP concentrations of 0, 0.92, 1.85, 3.70, and 5.55 mM (400 mOsm and pH 7.3). The oxygen consumption of the sperm was determined as described for experiment 2a.
Statistical analysis.
The data were subjected to ANOVA and the Fisher protected least-significant difference post hoc test (StatView, Abacus Concepts, Berkeley, CA). All data are expressed as mean ± SEM. A P value of less than 0.05 indicated statistical significance.
Results
Effect of various substrates in raffinose–mKRB–egg yolk extender on fresh and frozen–thawed sperm (experiment 1a).
The first experiment in this series was aimed at assessing the effect of various energy-yielding substrates in the raffinose–mKRB–egg yolk extender on the motility characteristics of fresh and frozen–thawed sperm. The motility of sperm added to the medium without the substrates glucose, pyruvate, and lactate was significantly (P < 0.05) lower than that of sperm added to the medium containing all 3 of these substrates (control; Table 1); this result was obtained from both fresh and frozen–thawed sperm. In contrast, the sperm motility and motion parameters did not differ significantly between fresh and frozen–thawed sperm when glucose-free and pyruvate-free solutions were used. The medium that contained glucose, pyruvate, and lactate resulted in the highest motility of frozen–thawed sperm. The percentage of intact acrosomes did not differ significantly among sperm treated with the various extenders for both fresh and frozen–thawed sperm (Table 1).
Table 1.
Effect of the substrates glucose, pyruvate, and lactate in raffinose–mKRB–egg yolk extender on fresh and frozen–thawed sperm
| Sperm characteristics | Control | –Glucose | –Pyruvate | –Lactate | Substrate-free | |
| Fresh sperm | Motility (%) | 78.2 ± 8.0 | 70.8 ± 2.6 | 67.3 ± 8.0 | 54.9 ± 6.1 | 44.8 ± 3.9a |
| VSL (μm/s) | 17.4 ± 1.2 | 16.5 ± 3.3 | 10.9 ± 1.4 | 15.4 ± 1.0 | 11.1 ± 1.9 | |
| VCL (μm/s) | 124.2 ± 3.1 | 115.1 ± 8.6 | 109.8 ± 1.9 | 108.3 ± 2.9 | 102.4 ± 4.1 | |
| ALD (μm) | 6.9 ± 0.1 | 6.5 ± 0.5 | 6.5 ± 0.2 | 6.5 ± 0.1 | 5.8 ± 0.3 | |
| BCF (Hz) | 22.2 ± 0.8 | 23.3 ± 1.8 | 25.4 ± 1.6 | 23.4 ± 1.0 | 29.0 ± 0.6 | |
| Acrosomal integrity (%) | 84.5 ± 2.9 | 76.2 ± 1.9 | 79.6 ± 4.9 | 77.1 ± 2.4 | 81.4 ± 4.4 | |
| Frozen–thawed sperm | Motility (%) | 21.5 ± 1.4 | 23.2 ± 0.9 | 19.8 ± 1.3 | 13.8 ± 2.4a | 13.7 ± 1.5a |
| VSL (μm/s) | 3.9 ± 0.4 | 3.2 ± 0.5 | 3.9 ± 0.4 | 3.9 ± 0.3 | 4.4 ± 0.8 | |
| VCL (μm/s) | 85.4 ± 8.7 | 71.8 ± 4.7 | 89.2 ± 13.2 | 77.8 ± 5.5 | 69.3 ± 7.4 | |
| ALD (μm) | 4.2 ± 0.5 | 3.3 ± 0.2 | 4.1 ± 0.5 | 3.8 ± 0.2 | 3.2 ± 0.5 | |
| BCF (Hz) | 32.2 ± 3.7 | 31.3 ± 1.6 | 36.2 ± 1.8 | 34.1 ± 0.9 | 31.7 ± 2.5 | |
| Acrosomal integrity (%) | 68.1 ± 4.0 | 71.3 ± 5.8 | 70.0 ± 2.0 | 64.1 ± 5.3 | 70.6 ± 2.3 |
ALD, amplitude of lateral head displacement; BCF, beat cross frequency; VCL, curvilinear velocity; VSL, straight-line velocity.
Data are presented as mean ± SEM (n = 3).
Value significantly (P < 0.05) different from control value.
Effect of lactate in raffinose–mKRB–egg yolk extender on fresh and frozen–thawed sperm (experiment 1b).
According to the results of experiment 1a, lactate was the most effective agent for increasing the motility of both fresh and frozen–thawed sperm. We therefore investigated the effect of adding lactate at 0, 10.79, 21.58, 32.37, and 43.16 mM to the raffinose–mKRB–egg yolk medium on the motility of sperm. Sperm diluted in lactate-free extender showed significantly (P < 0.05) lower motility than did sperm diluted in extender containing 21.58 or 32.37 mM lactate (Table 2). The data revealed that sperm frozen in the raffinose–mKRB–egg yolk extender containing 32.37 mM lactate showed significantly (P < 0.05) higher motility after thawing than did sperm frozen in substrate-free extender. The proportion of sperm with intact acrosomes either before or after thawing did not differ significantly among extenders containing 0, 10.79, 21.58, 32.37, or 43.16 mM lactate.
Table 2.
Effect of lactate in raffinose–mKRB–egg yolk extender on fresh and frozen–thawed sperm
| Lactate concentration (mM) |
||||||
| Sperm characteristics | 0 | 10.79 | 21.58 | 32.37 | 43.16 | |
| Fresh sperm | Motility (%) | 47.4 ± 4.9 | 46.3 ± 13.2 | 61.3 ± 2.1 | 67.5 ± 3.8a | 55.1 ± 3.7 |
| VSL (μm/s) | 12.5 ± 1.7 | 8.8 ± 1.0 | 16.5 ± 2.6 | 12.3 ± 1.2 | 9.8 ± 3.0 | |
| VCL (μm/s) | 98.3 ± 6.3 | 86.0 ± 2.7 | 94.6 ± 10.0 | 92.9 ± 3.1 | 95.9 ± 6.5 | |
| ALD (μm) | 5.3 ± 0.4 | 5.3 ± 0.3 | 4.8 ± 0.6 | 4.7 ± 0.3 | 5.1 ± 0.3 | |
| BCF (Hz) | 26.4 ± 1.4 | 24.7 ± 1.3 | 22.5 ± 0.8 | 24.5 ± 0.8 | 25.6 ± 1.3 | |
| Acrosomal integrity (%) | 75.9 ± 0.5 | 71.7 ± 4.0 | 73.6 ± 5.9 | 75.1 ± 0.5 | 71.8 ± 1.8 | |
| Frozen–thawed sperm | Motility (%) | 11.3 ± 2.2 | 17.5 ± 3.5 | 19.7 ± 2.8 | 22.3 ± 4.0a | 12.6 ± 4.3 |
| VSL (μm/s) | 3.5 ± 0.6 | 2.8 ± 0.3 | 3.6 ± 0.2 | 3.8 ± 0.1 | 4.9 ± 0.5 | |
| VCL (μm/s) | 78.6 ± 8.2 | 75.0 ± 3.5 | 78.5 ± 4.2 | 95.0 ± 14.1 | 126.9 ± 5.1a | |
| ALD (μm) | 3.4 ± 0.6 | 3.3 ± 0.1 | 3.5 ± 0.2 | 4.5 ± 0.6 | 5.5 ± 0.4a | |
| BCF (Hz) | 44.8 ± 1.5 | 36.7 ± 1.8 | 39.8 ± 0.8 | 36.5 ± 2.3 | 42.9 ± 2.1 | |
| Acrosomal integrity (%) | 69.3 ± 2.9 | 71.6 ± 0.4 | 69.0 ± 0.2 | 69.2 ± 0.2 | 61.5 ± 1.8 | |
ALD, amplitude of lateral head displacement; BCF, beat cross frequency; VCL, curvilinear velocity; VSL, straight-line velocity.
Data are presented as mean ± SEM (n = 3).
Value significantly (P < 0.05) different from control value.
Effect of ATP in raffinose–mKRB–egg yolk extender containing 32.37 mM lactate on fresh and frozen–thawed sperm (experiment 1c).
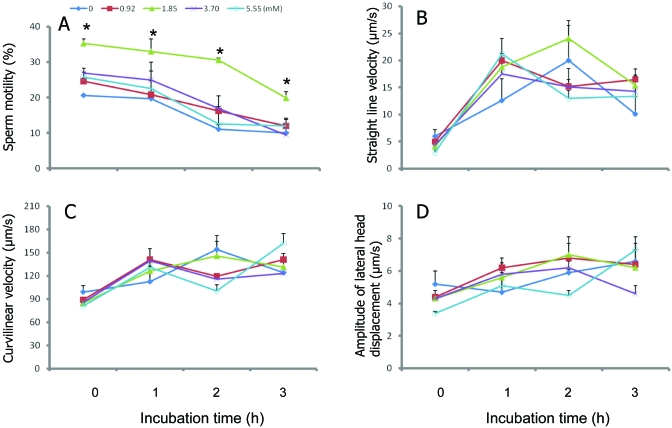
The effect of adding 0, 0.92, 1.85, 3.70, or 5.55 mM ATP to raffinose–mKRB–egg yolk extender containing 32.37 mM lactate on the cryosurvival of the sperm are summarized in Table 3. Sperm frozen in extender containing 32.37 mM lactate and 1.85 mM ATP exhibited significantly (P < 0.05) higher motility than that of sperm frozen in ATP-free extender. The sperm frozen and thawed in extender supplemented with 1.85 mM ATP maintained significantly (P < 0.05) higher motility throughout the 3-h incubation at 37 °C than did sperm frozen and thawed in the ATP-free extender (Figure 1). The addition of ATP to the extender increased the proportion of intact acrosomes in after collected sperm, and among all concentrations of ATP tested, the percentage of intact acrosomes was highest at 1.85 mM ATP. Similar results were obtained for the acrosome status of frozen–thawed sperm.
Table 3.
Effect of ATP in raffinose–mKRB–egg yolk extender containing 32.37 mM lactate on fresh and frozen–thawed sperm
| ATP concentration (mM) |
||||||
| 0 | 0.92 | 1.85 | 3.70 | 5.55 | ||
| Fresh sperm | Motility (%) | 74.7 ± 1.8 | 72.8 ± 8.1 | 79.2 ± 3.3 | 73.8 ± 6.6 | 55.6 ± 4.2a |
| VSL (μm/s) | 9.1 ± 1.7 | 10.1 ± 0.5 | 14.7 ± 3.3 | 7.9 ± 0.6 | 8.4 ± 1.4 | |
| VCL (μm/s) | 110.2 ± 9.9 | 116.9 ± 8.5 | 123.6 ± 5.2 | 101.5 ± 8.8 | 104.9 ± 7.9 | |
| ALD (μm) | 6.0 ± 0.1 | 7.6 ± 0.6 | 6.3 ± 0.6 | 6.8 ± 1.0 | 5.7 ± 0.4 | |
| BCF (Hz) | 29.9 ± 2.8 | 34.0 ± 1.7 | 29.7 ± 2.7 | 32.9 ± 2.6 | 31.2 ± 1.5 | |
| Acrosomal integrity (%) | 75.5 ± 6.3 | 78.9 ± 6.5 | 83.2 ± 1.6 | 82.5 ± 7.2 | 77.1 ± 7.1 | |
| Frozen–thawed sperm | Motility (%) | 20.6 ± 0.3 | 24.6 ± 0.9 | 35.3 ± 1.3a | 26.9 ± 1.4 | 25.7 ± 7.2 |
| VSL (μm/s) | 6.0 ± 1.2 | 5.0 ± 0.2 | 4.1 ± 0.6 | 4.4 ± 0.6 | 3.0 ± 0.4 | |
| VCL (μm/s) | 99.0 ± 8.3 | 89.3 ± 4.5 | 84.5 ± 4.1 | 85.8 ± 2.7 | 80.9 ± 3.9 | |
| ALD (μm) | 5.2 ± 0.8 | 4.4 ± 0.4 | 4.3 ± 0.1 | 4.3 ± 0.1 | 3.4 ± 0.1 | |
| BCF (Hz) | 37.1 ± 2.0 | 34.4 ± 0.6 | 35.0 ± 1.6 | 34.6 ± 5.0 | 37.2 ± 2.2 | |
| Acrosomal integrity (%) | 67.7 ± 2.3 | 68.4 ± 8.8 | 70.6 ± 4.6 | 61.7 ± 3.5 | 66.5 ± 3.8 | |
ALD, amplitude of lateral head displacement; BCF, beat cross frequency; VCL, curvilinear velocity; VSL, straight-line velocity.
Data are presented as mean ± SEM (n = 3).
Value significantly (P < 0.05) different from control value.
Figure 1.
Effect of ATP in raffinose–mKRB–egg yolk medium containing 32.37 mM lactate on the (A) motility, (B) straight line velocity, (C) curvilinear velocity, and (D) amplitude of lateral head displacement of frozen–thawed sperm during incubation at 37 °C for 3 h. Data are presented as mean ± SEM (n = 3). *, Value significantly (P < 0.05) different from control value.
Effect of glucose, pyruvate, and lactate in raffinose–mKRB–egg yolk medium on the oxygen consumption of sperm (experiment 2a).
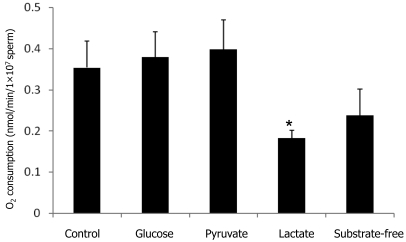
In the next series of experiments, we examined the effect of the substrates glucose, pyruvate, and lactate in the raffinose–mKRB–egg yolk medium on the rate of oxygen consumption of sperm. Incubation of the sperm suspension with lactate-free medium resulted in a significant (P < 0.05) decline in the rate of oxygen consumption during incubation as compared with the incubation of the sperm suspension with medium containing glucose, pyruvate, and lactate (Figure 2). The oxygen consumption of sperm in medium lacking any added substrates tended to be decreased compared with that of sperm in the complete medium. In contrast, oxygen consumption did not differ significantly between sperm in glucose- or pyruvate-free media and those in the medium containing glucose, pyruvate, and lactate.
Figure 2.
Effect of glucose, pyruvate, and lactate in raffinose–mKRB–egg yolk medium on the oxygen consumption of fresh sperm during incubation at 37 °C for 10 min. Data are presented as mean ± SEM (n = 5). *, Value significantly (P < 0.05) different from control value.
Effect of lactate in raffinose–mKRB–egg yolk medium on the oxygen consumption of sperm (experiment 2b).
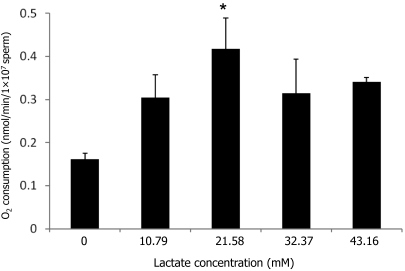
The respiration capacity of sperm was tested after their incubation in a lactate-free raffinose–mKRB–egg yolk medium or in a medium supplemented with 10.79, 21.58, 32.37, or 43.16 mM lactate (Figure 3). Oxygen uptake was significantly (P < 0.05) higher in sperm incubated in medium containing 32.37 mM lactate than in sperm incubated in lactate-free medium.
Figure 3.
Effect of lactate in raffinose–mKRB–egg yolk medium on oxygen consumption of fresh sperm during incubation at 37 °C for 10 min. Data are presented as mean ± SEM (n = 5). *, Value significantly (P < 0.05) different from control value.
Effect of ATP in the raffinose–mKRB–egg yolk extender containing 32.37 mM lactate on the oxygen consumption of sperm (experiment 2c).
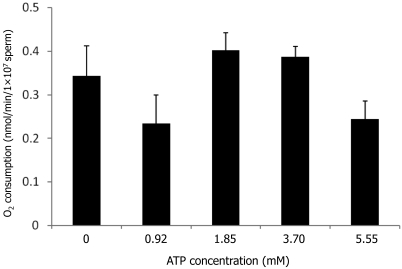
This experiment evaluated the influence of supplementation of the raffinose–mKRB–egg yolk medium containing 32.37 mM lactate with various concentrations of ATP (0, 0.92, 1.85, 3.70, and 5.55 mM) on the oxygen consumption of sperm during incubation at 37 °C for 10 min (Figure 4). When the medium was supplemented with 1.85 mM ATP, the rate of oxygen consumption tended to be increased compared with that in ATP-free medium, but difference is not significant.
Figure 4.
Effect of ATP in raffinose–mKRB–egg yolk medium containing 32.37 mM lactate on oxygen consumption of fresh sperm during incubation at 37 °C for 10 min. Data are presented as mean ± SEM (n = 5). *, Value significantly (P < 0.05) different from control value.
Discussion
The present study demonstrated that an extender of raffinose–mKRB–egg yolk containing 32.37 mM lactate enhanced the metabolic capacity and survival of rat sperm after cryopreservation. The cryosurvival of rat sperm was further improved by the addition of 1.85 mM exogenous ATP to the freezing extender.
When the oxidizable substrate lactate was not added to the raffinose–mKRB–egg yolk extender, the motility, viability, and rate of oxygen consumption decreased considerably in both fresh and frozen–thawed sperm. In contrast, sperm frozen and thawed in extender supplemented with 32.37 mM lactate exhibited higher motility than those frozen and thawed in lactate-free extender. This finding indicates that exogenous lactate in the freezing extender is a potent inducer that enhances the oxygen consumption of rat sperm and their motility after collection and freezing–thawing.
The sperm-specific enzyme lactate dehydrogenase isozyme C4 is located in the cytosol and the matrix of the mitochondria in the midpiece of rat sperm. Further, a study9 has revealed that both a shuttle involving the redox couple lactate–pyruvate and lactate dehydrogenase isozyme C4 are active in rat sperm mitochondria. In another study,12 the lactate concentration in oviductal fluids was 10-fold higher than the glucose concentration, and the lactate concentration in the uterine fluids was 15-fold higher than the glucose concentration during the murine estrous cycle. Therefore, it is very likely that lactate is used by rat sperm as an essential substrate to maintain highly regulated ATP production and dissipation: lactate in the cytosol and mitochondrial matrix is oxidized to pyruvate by mitochondrial lactate dehydrogenase isozyme C4, and pyruvate is oxidized through the Krebs cycle and electron transport chain.4,5,23,24 To our knowledge, our findings are the first evidence showing that rat sperm can use exogenous lactate in the cryodiluent as an essential substrate to maintain highly regulated metabolic capacity and that this lactate acts as an energy substrate for mitochondria to the mobilization of fresh and frozen–thawed sperm.
Mitochondria, the site of ATP generation due to oxidative phosphorylation, are localized solely in the midpiece of sperm.22 The oxidative production of ATP through the Krebs cycle is an essential function of the midpiece mitochondria for motility.31 The mitochondrial biochemical pathways of oxidative phosphorylation are 15 times more efficient than is anaerobic glycolysis for ATP production.7,28 These findings also support our arguments that the energy production and dissipation in rat sperm are highly dependent on the mitochondria.
The present study showed that supplementation of raffinose–mKRB–egg yolk extender with 32.37 mM lactate and 1.85 mM exogenous ATP considerably increases sperm motility before freezing, thus improving the survivability of sperm after cryopreservation. Exogenous ATP in the freezing medium may be responsible for the generation of multiple metabolic signals that appear to be related to the sperm motility through a rise in calcium levels;10,17,18,20,25,27 this reaction increases de novo ATP synthesis before freezing and may contribute to the remobilization of sperm after freezing–thawing. The motility of ram sperm was restored by exogenous ATP that crossed plasma membrane when the membrane was damaged by cryopreservation.13 In light of that finding,13 we cannot discount that our result is caused by the facultative transport of ATP across plasma membrane because of damage during freezing, thereby allowing substrates to directly access ATP and allowing adenosine triphosphatase to use ATP directly to generate energy for the mobilization of rat sperm.
In conclusion, the current study demonstrated that the addition of lactate and ATP to the raffinose–mKRB–egg yolk extender before freezing increases the number of motile sperm and mediates the energy-dependent synthetic processes of rat epididymal sperm. In turn, these effects may increase the cryosurvival of rat sperm. Further investigation of species-specific differences in the energy-dependent synthetic processes in sperm may prove valuable in defining the ideal components of a cryodiluent, which interact to regulate the cryosurvival of rat sperm, and in clarifying the adaptations needed for cryopreservation of sperm from other species.
Acknowledgment
This work was supported in part by grants 19658102 and 16108003 (to ES) from the Japan Society for the Promotion of Science.
References
- 1.Aboagla EM, Terada T. 2003. Trehalose enhanced fluidity of the goat sperm membrane and its protection during freezing. Biol Reprod 69:1245–1250 [DOI] [PubMed] [Google Scholar]
- 2.Barbas JP, Mascarenhas RD. 2009. Cryopreservation of domestic animal sperm cells. Cell Tissue Bank 10:49–62 [DOI] [PubMed] [Google Scholar]
- 3.Brokaw CJ. 1972. Flagellar movement: a sliding filament model. Science 178:455–462 [DOI] [PubMed] [Google Scholar]
- 4.Brooks GA, Dubouchaud H, Brown M, Sicurello JP, Butz CE. 1999. Role of mitochondrial lactate dehydrogenase and lactate oxidation in the intracellular lactate shuttle. Proc Natl Acad Sci USA 96:1129–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks GA. 2002. Lactate shuttles in nature. Biochem Soc Trans 30:258–264 [DOI] [PubMed] [Google Scholar]
- 6.Cancel AM, Lobdell D, Mendola PP, Perreault SD. 2000. Objective evaluation of hyperactivated motility in rat spermatozoa using computer-assisted sperm analysis. Hum Reprod 15:1322–1328 [DOI] [PubMed] [Google Scholar]
- 7.Cardullo RA, Baltz JM. 1991. Metabolic regulation in mammalian sperm: mitochondrial volume determines sperm length and flagellar beat frequency. Cell Motil Cytoskeleton 19:180–188 [DOI] [PubMed] [Google Scholar]
- 8.Ford WCL. 2006. Glycolysis and sperm motility: does a spoonful of sugar help the flagellum go round? Hum Reprod Update 12:269–274 [DOI] [PubMed] [Google Scholar]
- 9.Gallina FG, Deburgos NMG, Burgos C, Coronel CE, Blanco A. 1994. The lactate–pyruvate shuttle in spermatozoa: operation in vitro. Arch Biochem Biophys 308:515–519 [DOI] [PubMed] [Google Scholar]
- 10.Gibbons IR. 1963. Studies on the protein components of cilia from Tetrahymena pyriformis. Proc Natl Acad Sci USA 50:1002–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halang KW, Bohnensack R, Kunz W. 1985. Interdependence of mitochondrial ATP production and extramitochondrial ATP utilization in intact spermatozoa. Biochim Biophys Acta 808:316–322 [DOI] [PubMed] [Google Scholar]
- 12.Harris SE, Gopichandran N, Picton HM, Leese HJ, Orsi NM. 2005. Nutrient concentrations in murine follicular fluid and the female reproductive tract. Theriogenology 64:992–1006 [DOI] [PubMed] [Google Scholar]
- 13.Holt WV, Head MF, North RD. 1992. Freeze-induced membrane damage in ram spermatozoa is manifested after thawing: observations with experimental cryomicroscopy. Biol Reprod 46:1086–1094 [DOI] [PubMed] [Google Scholar]
- 14.Jones AR. 1997. Metabolism of lactate by mature boar spermatozoa. Reprod Fertil Dev 9:227–232 [DOI] [PubMed] [Google Scholar]
- 15.Kamp G, Busselmann G, Lauterwein J. 1996. Spermatozoa: models for studying regulatory aspects of energy metabolism. Experientia 52:487–494 [DOI] [PubMed] [Google Scholar]
- 16.Klingenberg M. 1979. The ADP–ATP shuttle of the mitochondrion. Trends Biochem Sci 4:249–252 [Google Scholar]
- 17.Kinukawa M, Oda S, Shirakura Y, Okabe M, Ohmuro J, Baba SA, Nagata M, Aoki F. 2006. Roles of cAMP in regulating microtubule sliding and flagellar bending in demembranated hamster spermatozoa. FEBS Lett 580:1515–1520 [DOI] [PubMed] [Google Scholar]
- 18.Litvin TN, Kamenetsky M, Zarifyan A, Buck J, Levin LR. 2003. Kinetic properties of ‘soluble’ adenylyl cyclase: synergism between calcium and bicarbonate. J Biol Chem 278:15922–15926 [DOI] [PubMed] [Google Scholar]
- 19.Long JA. 2006. Avian semen cryopreservation: what are the biological challenges? Poult Sci 85:232–236 [DOI] [PubMed] [Google Scholar]
- 20.Luria A, Rubinstein S, Lax Y, Breitbart H. 2002. Extracellular adenosine triphosphate stimulates acrosomal exocytosis in bovine spermatozoa via P2 purinoceptor. Biol Reprod 66:429–437 [DOI] [PubMed] [Google Scholar]
- 21.Medrano A, Fernández-Novell JM, Ramió L, Alvarez J, Goldberg E, Rivera MM, Guinovart JJ, Rigau T, Rodríguez-Gil JE. 2006. Utilization of citrate and lactate through a lactate-dehydrogenase- and ATP-regulated pathway in boar spermatozoa. Mol Reprod Dev 73:369–378 [DOI] [PubMed] [Google Scholar]
- 22.Millette CF, Spear PG, Gall WE, Edelman GM. 1973. Chemical dissection of mammalian spermatozoa. J Cell Biol 58:662–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montamat EE, Vermouth NT, Blanco A. 1988. Subcellular localization of branched-chain amino acid aminotransferase and lactate dehydrogenase C4 in rat and mouse spermatozoa. Biochem J 255:1053–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poole RC, Halestrap AP. 1993. Transport of lactate and other monocarboxylates across mammalian plasma membranes. Am J Physiol 264(4Pt 1):C761–C782 [DOI] [PubMed] [Google Scholar]
- 25.Ren D, Navarro B, Perez G, Jackson AC, Hsu SQ, Shi Q, Tilly JL, Clapham DE. 2001. A sperm ion channel required for sperm motility and male fertility. Nature 413:603–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reynafarje B, Costa LE, Lehninger AL. 1985. O2 solubility in aqueous media determined by a kinetic method. Anal Biochem 145:406–418 [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Miranda E, Buffone MG, Edwards SE, Ord TS, Lin K, Sammel MD, Gerton GL, Moss SB, William CJ. 2008. Extracellular adenosine 5′-triphosphate alters motility and improves the fertilizing capability of mouse sperm. Biol Reprod 79:164–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruiz-Pesini E, Díez-Sánchez C, López-Pérez MJ, Enríquez JA. 2007. The role of the mitochondrion in sperm function: is there a place for oxidative phosphorylation or is this a purely glycolytic process? Curr Top Dev Biol 77:3–19 [DOI] [PubMed] [Google Scholar]
- 29.Storey BT, Kayne FJ. 1978. Energy metabolism of spermatozoa. VII. Interactions between lactate, pyruvate and malate as oxidative substrates for rabbit sperm mitochondria. Biol Reprod 18:527–536 [DOI] [PubMed] [Google Scholar]
- 30.Storey BT. 2008. Mammalian sperm metabolism: oxygen and sugar, friend and foe. Int J Dev Biol 52:427–437 [DOI] [PubMed] [Google Scholar]
- 31.Suarez SS, Marquez B, Harris TP, Schimenti JC. 2007. Different regulatory systems operate in the midpiece and principal piece of the mammalian sperm flagellum. Soc Reprod Fertil Suppl 65:331–334 [PubMed] [Google Scholar]
- 32.Turner RM. 2003. Tales from the tail: what do we really know about sperm motility? J Androl 24:790–803 [DOI] [PubMed] [Google Scholar]
- 33.Yamashiro H, Han YJ, Sugawara A, Tomioka I, Hoshino Y, Sato E. 2007. Freezability of rat epididymal sperm induced by raffinose in modified Krebs–Ringer bicarbonate (mKRB) based extender solution. Cryobiology 55:285–294 [DOI] [PubMed] [Google Scholar]