Abstract
African dormice (Graphiurus spp.) are small nocturnal rodents that currently are uncommon in laboratory settings. Their use may increase as they have recently been shown to develop an infection with monkeypox virus and may prove to be a valuable animal model for infectious disease research. Because African dormice are not commercially available, an extensive breeding colony is required to produce the animals needed for research use. Husbandry modifications that increased the production of offspring were the use of a high-protein diet, increased cage enrichment, and decreased animal density. To optimize consumption of a high-protein diet, we tested the palatability of several high-protein foods in a series of preference trials. Dormice preferred wax worm larva, cottage cheese, roasted soy nuts, and canned chicken. Issues related to medical management of Graphiurus kelleni include potential complications from traumatic injury. The development of a program for the husbandry and care of African dormice at our institution typifies the experiences of many laboratory animal facilities that are asked to support the development of animal models using novel species.
African dormice (Graphiurus spp.) are small nocturnal rodents. Much of the current research involving dormice revolves around field studies and the ongoing taxonomic characterization of the family.2,3,5-7,9 Because visual speciation of Graphiurus is difficult, speciation usually is accomplished by using karyotypic and anatomic variation.5 African dormice are the only members of the Gliridae family that are located solely in subSaharan Africa.5 The other members of the Gliridae family, the Glirinae and Leithiinae, are widely distributed geographically and more commonly used in research.7,9
The few publications about African dormice are primarily the result of field studies. Studies involving captive Graphiurus provide minimal detail on feeding or housing.4,8,11,12 Food preference testing of captive G. murinus has shown these rodents to be omnivorous, with a marked preference for arthropods in addition to consumption of shelled eggs, nuts, and soft fruits.8 This pattern is similar to that seen in the field in which stomach contents of Graphiurus species comprise primarily insects but also other animals and fruits.10,13 This diet correlates well with the lack a cecum capable of supporting the digestion of fibrous plant matter. Although studies have suggested that the preferred foods of dormice typically are those found within their natural habitat,8 these items are not practical for colonies in captivity.
Graphiurus spp. imported from Ghana were associated with the human monkeypox outbreak in 2003. Eight of the 40 African dormice from this shipment had infectious virus in visceral tissues and other indications of a productive viremia.4 Apart from the spontaneous fatality of several infected animals, the dormice did not display clinical signs or lesions indicative of monkeypox.4 To minimize the potential for human disease from infected African dormice, the Centers for Disease Control and Prevention has banned the importation of African dormice.1 Dormice are being used currently to study monkeypox virus.11
We have developed a colony of African dormice Graphiurus kelleni (Figure 1) to be used for research on orthopoxviruses. Here we describe the housing and breeding of G. kelleni at our institution and discuss health and welfare concerns of this unique species. Although our emphasis was on adapting our experience with Mus spp. to this novel species rather than preference testing for optimization, we have produced a viable colony that is capable of reproduction. In addition, we conducted a study identifying a selection of highly palatable, high-protein foods with which to supplement the dormice's standard diet.
Figure 1.
African dormouse (Graphurius kelleni).
Materials and Methods
Animal husbandry.
Dormice were housed in individually ventilated, sterile, microisolation caging (Allentown Caging, Allentown, NJ) with paper bedding (Diamond Soft, Harlan Teklad, Madison, WI), 2 nesting structures (Mouse Igloo or Tunnel, Bioserve, Frenchtown, NJ), and nesting material (Nestlet, Ancare, Bellmore, NY). Breeder cages were provided with a high-fat, high-protein autoclaved pelleted rodent diet (2019S, Harlan Teklad). Adult offspring were provided with an autoclaved pelleted rodent diet (2018SX, Harlan Teklad) and sterilized water. Dormice received protein enrichment consisting of 3 to 5 live mealworms 3 times weekly or 1 tablespoon of diced hard-boiled egg weekly. Cages were changed weekly by using aseptic technique within a class II biosafety cabinet. Animal holding room temperatures were maintained between 20 °C and 23 °C, and relative humidity was maintained between 30% to 70%. Dormice were housed on a 14:10-h light:dark cycle with the light intensity at cage level less than 325 lx. All facility personnel wore dedicated shoes and scrubs and donned a surgical mask, hair bonnet, gloves, and disposable shoe covers. All animals were on protocols approved by our animal care and use committee in accordance with applicable federal regulations.
Sentinel procedures.
Outbred NIH Swiss Webster sentinels were exposed to dirty bedding from cages containing experimental dormice weekly for at least 6 wk before being submitted for serology, parasitology, and necropsy. New sentinel mice were placed into the sentinel cage at the same time that the oldest sentinel was removed for testing. The viruses monitored were mouse hepatitis virus, Theiler murine encephalitis virus, mouse rotavirus, pneumonia virus of mice, Sendai virus, lymphocytic choriomeningitis virus, ectromelia virus, mouse cytomegalovirus, polyoma virus, reovirus 3, murine norovirus, mouse adenovirus, Hantaan virus, and murine parvoviruses. In addition, the following murine pathogenic bacteria were not found in the facility: cilia-associated respiratory bacillus, Citrobacter rodentium, Clostridium piliforme, Corynebacterium kutscheri, Mycoplasma spp., Salmonella spp., and Streptobacillus moniliformis. With the exception of Helicobacter spp. and murine norovirus, all sentinels from this facility had been free from all mouse pathogens since the facility was first populated in 2006.
Breeding and weaning of offspring.
Dormice used for breeding were initially established as permanent pairs or trios of 2 female and 1 male animals. Dormice were mated by using animals that were at least 4 mo old without a history of aggression or obesity. Offspring were weaned at least 28 d after birth. Weaned offspring were housed as littermates until they were sexed at 4 to 8 wk of age, at which point they were housed in unisex groups of 5 or fewer dormice per cage. Litters of 1 or 2 dormice or small dormice were kept in the breeder cage until they were able to be sexed.
Anesthesia.
Isoflurane anesthesia was administered at 3% in an induction chamber. Dormice were transferred to a nose cone for anesthesia maintenance (1.5% to 2.5% isoflurane) once they had begun to close their eyes and lose the toe-pinch reflex.
Phlebotomy.
All blood collections were performed in isoflurane-anesthetized dormice. Mandibular blood collection was performed by using a 23-gauge needle to pierce the skin approximately 0.5 to 1 cm ventral from the ear base. Additional collection attempts were made when blood was not obtained or the site clotted.
Diet supplementation.
To identify a readily consumed protein supplement, several high-protein foods were tested for palatability (Table 1). Food was aliquoted at 2 g protein per dormouse per cage, placed on culture dishes, distributed to experimental animals in the late afternoon, and weighed after the completion of a single light:dark cycle. Food remained in the cage for less than 16 h. This process was repeated 4 times with a maximum of 3 trials per week. Cages contained 2 to 5 dormice with an even gender distribution. Rodent diet pellets and water were available ad libitum. Standard enrichment was suspended during the trials.
Table 1.
Preference scores.
| Food item | Category | % Protein | Preference scorea |
| Waxworm larvae | arthropod | 14.1 | 4 |
| Soy nuts, dry-roasted | nuts | 42.9 | 3 |
| Canned chicken, boneless | other | 21.8 | 3 |
| Cottage cheese, lowfat (1%) | dairy | 11.5 | 3 |
| Eggs, hard-boiled | dairy | 12.0 | 2 |
| Tofu, firm | other | 7.1 | 2 |
| Parmesan cheese | dairy | 32.1 | 1 |
| Sunflower seeds | nuts | 21.4 | 1 |
| Pistachio nuts | nuts | 20.0 | 0 |
| Peanut butter | nuts | 18.8 | 0 |
| Black walnuts | nuts | 16.7 | 0 |
4, 100% consumed within 16 h; 3, 90% to 100%; 2, 75% to 90%; 1, 50% to 75%; 0, less than 50%
To evaluate the suitability of each food option as means of protein enrichment, we adapted a published preference score system.8 Suitability was measured on a 5-point scale (0 to 4, least to most preferable) determined by the percentage of an item that was consumed during the 16-h feeding period. Results were analyzed by using a 2-tailed t test and a general linear model (SAS version 9.1, SAS Institute, Cary, NC). Once a clear preference was evident, the top 5 items were retested in a series of comparison trials during which the dormice were presented with 2 food options per cage. The 10 pairings were offered to 6 cages each for a feeding period of one light-cycle.
Results
Colony management.
The average daily census was 400 dormice. The weight of mature dormice ranged from 15 to 42 g, with an average weight of 24.4 g. Cyclical vaginal swellings occurred in group-housed females (at least 3 dormice per cage) that were at least 5 mo old (Figure 2).
Figure 2.
Vaginal swelling in grouphoused female dormice.
Fighting occurred in all the breeder trios (2 female mice with 1 male), with the subsequent removal of 1 female mouse and establishment of a permanent pair. Of the 70 mating pairs that were established, 44 pairs (63%) weaned at least one litter. The average litter size was 3 pups born and 2 pups weaned. Sexing was performed by palpation of the os penis: if a penis was not palpable by 8 wk after birth, the dormouse was considered female. The female breeder was 16 to 19 mo old when her first litter was weaned and the male breeder was 8 to 11 mo of age. Although dormice were included as part of the routine sentinel program, no murine pathogens have been detected.
Phlebotomy.
Although visual observation was a good indicator of anesthetic depth in dormice, a toe pinch was used to determine whether an animal had reached an adequate plane of anesthesia. This reflex was usually lost after 3 min at 3% isoflurane. Blood (maximum, 300 µL) could be collected from the mandibular region on a monthly basis without obvious resultant clinical abnormalities. More than 1 mL was collected from the cranial vena cava of dormice or by cardiocentesis as a terminal phlebotomy. Collection of blood from the retroorbital sinus or tail vein was unsuccessful. Shaving the tail fur did not enhance visualization of the tail vein nor improve the ability to collect blood from this vein. Depending on the depth and width of the puncture, dormice often hemorrhaged from the ear or cheek. During anesthesia recovery, many dormice developed tremors of large muscle groups. Return of the blink reflex frequently was the final stage of anesthetic recovery.
Protein enrichment.
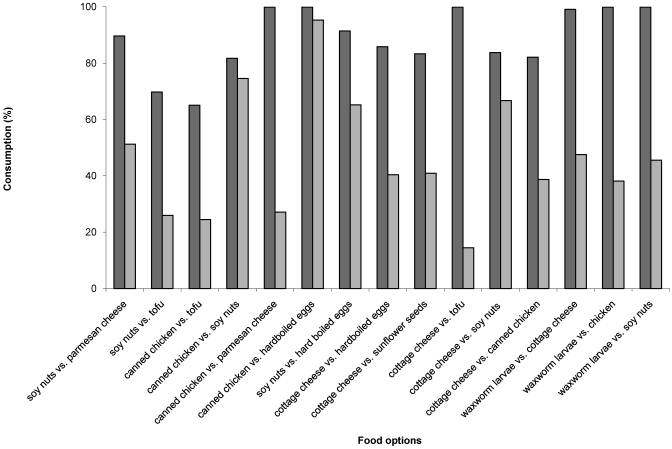
The most palatable foods identified in the diet trial were wax worm larvae, cottage cheese, soy nuts, and chicken. Of the 11 food options offered, wax worm larvae proved significantly (P < 0.05) more palatable than the other food options (Table 1). All food options with a score of 3 or 4 were consumed in entirety within 48 h, unlike hard-boiled eggs, tofu, parmesan cheese, sunflower seeds, pistachio nuts, peanut butter, and black walnuts. Direct comparison trials demonstrated that ‘unsuitable’ food items (score, 0 to 2) were less palatable than items with a score of 3 or 4 (Figure 3). Low-fat cottage cheese proved significantly (P < 0.005) more palatable than canned chicken, soy beans, and tofu. Both fat and fiber were significant (P < 0.0001) predictors of consumption. Lowfat foods (less than 0.2 g fat/g) were highly preferable. As fat content increased, there was a significant (P < 0.0001) decline in preference. However, high-fat foods (0.5 to 0.7 g fat/g) gained preference when accompanied by an increase in fiber content.
Figure 3.
Comparison trials between food options. Dark bar, consumption of first item listed; light bar, consumption of second item listed.
Clinical problems.
Of a total colony of 700 dormice, 122 (fewer than 20% of the colony) dormice presented with clinical problems over a period of nearly 2 y, 77 (63%) of the cases were traumatic injuries in group-housed animals. The remaining 46 dormice displayed either lethargy or dehydration. Dehydrated dormice tended to be younger than 3 mo (43%), and no sex predilection was noted. Treatment for dehydration consisted of twice-daily administration of subcutaneous warm lactated Ringers solution (1 mL/10 g) and feed supplementation on the cage floor; 37 of the 46 dormice responded to the treatment. The nonresponsive animals were euthanized for failure to respond to the initial treatment or for a relapse after successfully responding to treatments given for the first 24 to 48 h.
Injuries incurred from fighting occurred predominantly in breeder cages within 72 h before parturition. The most common injury was traumatic amputation of the tail and resulting myositis and cellulitis in male breeders. Wounds were treated with a variety of topical antibiotics, including twice-daily application of topical antibiotic ointment (Bacitracin, Perrigo, Allegan, MI) and continuously supplied oral amoxicillin (0.5 mg/mL in drinking water; Axoxil, GlaxoSmithKline, Research Park, NC) and required an average of 40 d for resolution. Minor wounds to the remainder of the body were responsive to treatment with twice-daily application of topical povidone–iodine ointment (10% Povidone–Iodine Ointment, Qualitest Pharmaceuticals, Huntsville, AL) or triple-antibiotic ophthalmic ointment (Neomycin and Polymyxin B Sulfates and Bacitracin Zinc Ophthalmic Ointment, Bausch and Lomb, Tampa, FL) within 3 wk.
Discussion
This report is the first description of optimization of housing and production of Graphiurus kelleni. The husbandry we provided for African dormice initially was based on what was feasible within the parameters of the animal facility. This plan was refined as we learned more about the requirements for captive Gliridae from the scientific literature and websites describing the care of pet dormice.
We hypothesized that a high-protein diet would best mimic the food options available to Graphiurus in their natural habitat. Initially, hard-boiled eggs were provided to the dormice as standard protein enrichment. Because the eggs required additional preparation time and rarely were consumed in entirety during the first 16 h, we sought a more palatable, easily prepared, high-protein, lowfat supplement. Of the 11 foods tested, the 2 most preferred—waxworm larvae and soy nuts—are relatively low in fat and high in fiber, similar to the foods preferred by dormice in the wild (that is, insects, berries, plants). Although comparison trials judged waxworm larvae significantly (P < 0.02) more preferable than soy nuts, consideration of cost, ease of preparation, and nutritional analysis deemed soy nuts a more suitable alternative. Soy nuts are cost-efficient and contain a relatively low amount of fat. They can be distributed easily, are nonperishable, and necessitate minimal preparation. Furthermore, dormice find soy nuts more palatable than hard-boiled eggs.
Although the husbandry for dormice could be optimized by using available sources, the basic research manipulations and veterinary care of dormice had not been described previously. We found that blood was best collected from anesthetized dormice, because awake young dormice were difficult to restrain by hand and collect blood concurrently. We selected isoflurane over intraperitoneal injection of ketamine and xylazine (125 mg/kg and 20 mg/kg, respectively) based on speed of recovery in domestic Mus species, and the same proved true for dormice. In comparison with recovery times seen in mice housed in the facility, dormice appear to have prolonged recovery from isoflurane; the application of a heat source and physical stimulation shortened the recovery time. The weight of adult dormice averaged 24.4 g, which is comparable to the average of 24.9 g, SD = 3.9, presented by Webb and Skinner.12 Veterinary care for dormice was adapted from that provided to Mus spp., with most of the care directed at recovery from traumatic injuries and dehydration in young animals. Because dormice had a pronounced tendency to develop myositis from wounds, aggressive treatment with local and systemic antibiotics was required to minimize this condition. Traumatic injury was most prevalent in harem breeder cages. Once this method of production was halted, the incidence of traumatic injury dropped to less than 5% of the colony.
As the first description of optimization of housing and production of Graphiurus kelleni, this report illustrates many of the considerations that arise when seeking to adapt a novel research species to a defined laboratory environment.
Acknowledgments
We would like to thank Dr Gerald Shea for statistical analysis and the staff of the Comparative Medicine Branch for their care of the animals. This research was supported by the Intramural Research Program of the NIH, National Institute for Allergy and Infectious Diseases.
References
- 1. African rodents and other animals that may carry the monkeypox virus. 2003. 42 CFR §71.56.
- 2.Bentz S, Montgelard C. 1999. Systematic position of the African dormouse Graphurius (Rodentia, Gliridae) assessed from cytochrome b and 12S rRNA mitochondria genes. J Mamm Evol 6:67–83 [Google Scholar]
- 3.Fitzherbert E, Gardner T, Caro T, Jenkins P. 2007. Habitat preferences of small mammals in the Katavi ecosystem of western Tanzania. Afr J Ecol 45:249–257 [Google Scholar]
- 4.Hutson CL, Lee KN, Abel J, Carroll DS, Montgomery JM, Olson VA, Li Y, Davidson W, Hughes C, Dillon M, Spurlock P, Kazmierczak JJ, Austin C, Miser L, Sorhage FE, Howell J, Davis JP, Reynolds MG, Braden Z, Karem KL, Damon IK, Regnery RL. 2007. Monkeypox zoonotic associations: insights from laboratory evaluation of animals associated with the multistate US outbreak. Am J Trop Med Hyg 76:757–767 [PubMed] [Google Scholar]
- 5.Krystufek B, Haberl W, Baxter RM, Zima J. 2004. Morphology and karyology of 2 populations of the woodland dormouse Graphurius murinus in Eastern Cape, South Africa. Folia Zool (Brno) 53:339–350 [Google Scholar]
- 6.Maier W, Klingler P, Ruf I. 2002. Ontogeny of the medial masseter muscle, pseudomyomorphy, and the systematic position of the Gliridae (Rodentia, Mammalia). J Mamm Evol 9:253–269 [Google Scholar]
- 7.Montgelard C, Matthee CA, Robinson TJ. 2003. Molecular systematics of dormice (Rodentia: Gliridae) and the radiation of Graphurius in Africa. Proc R Soc Lond 270:1947–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nowakowski WK, Remisiewicz M, Kosowska J. 2006. Food preferences of Glis glis (L.), Dryomys nitedula (Pallas), and Graphiurus murinus (Smuts) kept in captivity. Pol J Ecol 54:369–378 [Google Scholar]
- 9.Nunome M, Yasuda SP, Sato JJ, Vogel P, Suzuki H. 2007. Phylogenetic relationships and divergence times among dormice (Rodentia, Gliridae) based on 3 nuclear genes. Zool Scr 36:537–546 [Google Scholar]
- 10.Perrin MR. 1981. Notes on the activity patterns of 12 species of southern African rodents and a new design of activity monitor. S Afr J Zool 16:248–258 [Google Scholar]
- 11.Schultz DA, Sagartz JE, Huso DL, Buller RML. 2009. Experimental infection of an African dormouse (Graphiurus kelleni) with monkeypox virus. Virology 383:86–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Webb PI, Skinner JD. 1996. Summer torpor in African woodland dormice Graphurius murinus (Myoxidae: Graphiurinae). J Comp Physiol [B] 166:325–330 [DOI] [PubMed] [Google Scholar]
- 13.Wirminghaus JO, Perrin MR. 1992. Diets of small mammals in a southern African temperate forest. Isr J Zool 38:353–361 [Google Scholar]