Abstract
Endotoxins in grain dust, household dust, and animal bedding may induce respiratory symptoms in rodents and humans. We assayed the endotoxin, coliform, and dust levels in 20 types of rodent bedding. Endotoxin concentrations were measured by using a commercial test kit, coliform counts were determined by using conventional microbiologic procedures, and dust content was evaluated by using a rotating–tapping shaker. Paper bedding types contained significantly less endotoxin than did other bedding types; the highest levels of endotoxin were detected in hardwood and corncob beddings. The range of endotoxin content for each bedding type was: corncob bedding, 1913 to 4504 endotoxin units per gram (EU/g); hardwood bedding, 3121 to 5401 EU/g; corncob–paper mixed bedding, 1586 to 2416 EU/g; and paper bedding, less than 5 to 105 EU/g. Coliform counts varied from less than 10 to 7591 cfu/g in corncob beddings, 90 to 4010 cfu/g in corncob–paper mixed beddings, less than 10 to 137 cfu/g in hardwood beddings, and less than 10 cfu/g in paper beddings. Average dust content was less than 0.15% in all commercial bedding types. We conclude that paper bedding is the optimal bedding type for conducting LPS inhalation studies and that rodent bedding containing high levels of endotoxin may alter the results of respiratory and immunologic studies in rodents.
Abbreviation: EU, endotoxin units
Bacterial endotoxin (that is, LPS) is derived from the outer cell wall of gram-negative coliform bacteria, is heat-stable, and is ubiquitous in the environment. The innate immune system recognizes both viable microorganisms and nonviable parts of these organisms (including LPS), which are found in varying concentrations in many indoor and outdoor environments.1 Exposure to LPS during early life induces inflammation and potentially tolerance to subsequent LPS exposure.10 Endotoxins are present in household dust,1,19 grain dust,6,11,15,16 and rodent bedding,3,7 which can be a source of airborne contaminants such as fungi, bacteria, and endotoxins for humans working with laboratory animals.7 A previous study7 compared the percentages of airborne dust and endotoxin in clean (aspen, spruce or fir, birch, and straw) and soiled bedding used for 4 d from rat or mouse cages. The results showed that the bedding of laboratory animals may contain biologically active compounds that can be distributed into ambient air, depending on the bedding material used. The authors concluded that the percentages of dust and endotoxin in different types of rodent bedding can be important factors affecting the occupational exposure of personnel working with laboratory animals as well as having harmful effects on the animals themselves.7 Additional reports indicate that endotoxin plays an important role in inducing respiratory problems in humans.1,4,6,7,15,16,19 For example, personnel working with dried wood demonstrated shortness of breath and cough, and endotoxin levels in wood samples were strongly correlated with the amount of dust generated, suggesting that endotoxin could be a significant problem for humans when working with dried wood associated with high dust levels.4 In another study,1 endotoxin levels in samples of dust collected from the mattresses of school-age children were inversely related to the occurrence of hay fever, atopic asthma, and atopic sensitization. The authors concluded that environmental exposure to endotoxins may play a key role in the development of tolerance to ubiquitous allergens found in the environment.1 The endotoxins in corn dust extract exhibited a dose-related correlation between the amount of inhaled endotoxin and the inflammatory response exhibited by mice.6,15,16 Sprague–Dawley rats housed on paper-crumb bedding and exposed to aerosolized endotoxin (4 ng/animal daily) and (1,3)-β-D-glucans (1.6 and 16 ng/animal daily) developed inflammatory lung responses and pulmonary lesions.3 The previously cited study also showed that wood bedding contained more than twice the amount of endotoxins and (1,3)-β-D-glucans than did paper bedding.3 This difference in endotoxin content between wood and paper bedding may be attributed to differences in processing procedures and the types of wood used.6,16
The (1,3)-β-D-glucans are found in the cell walls of fungi, yeasts, algae, some bacteria, and plants.13 Instructions provided with a commercial kit for blocking these compounds (β-G-Blocker Kit, Lonza, Walkersville, MD) state that when found in sufficient amounts in a sample, (1,3)-β-D-glucans may act synergistically with endotoxin, falsely elevating or enhancing endotoxin levels. Amebocytes from the horseshoe crab on which the kinetic Limulus amebocyte lysate test is based contain 2 independent coagulation pathways; both endotoxin and (1,3)-β-D-glucans can mediate these pathways, resulting in the transformation of coagulogen to coagulin and synergistically increasing expected levels of endotoxin.8 The incidence of intestinal Peyer patches was increased in mice housed on hardwood bedding that contained a mixture of birch, beech, and maple woods compared with those in mice on cotton bedding; the LPS levels of these beddings was not reported.14 This supports the idea that the intestinal immune system of mice may be affected by bedding type. These reports suggest that the endotoxin contained in various types of rodent bedding may play an important role in the development and incidence of respiratory syndromes and immunologic responses in mice and rats.3,6,15,16 Only a few reports3,6,7,14 have evaluated the effects of endotoxin and dust content in rodent bedding on immunologic and inflammatory studies using rodents. In addition, investigators are using aerosolized LPS in various mouse models to better understand the role of endotoxins in immunologic responses, respiratory syndromes such as asthma, and inflammation.15,16,19 The optimal choice of bedding for conducting such studies and the immunologic consequences of the level of endotoxin exposure from bedding is unclear. Whether differences in endotoxin concentrations in bedding affect immunologic response in rodents used in LPS studies is unknown and needs to be ascertained. In addition, determining the dust and coliform content of rodent beddings is important because microbes are the source of endotoxins, and dust may be the primary exposure route. The objective of the present study was to survey various types of commercially available rodent bedding to determine the endotoxin concentration, coliform counts, and dust content in an effort to determine the optimal bedding type for use during inhalation studies that may be altered by endotoxins.
Materials and Methods
Bedding types.
We obtained 20 types of commercially available rodent bedding: The Andersons (Maumee, OH) Bed-o'cobs 1/8-in. (CC1), Bed-o'cobs 1/4-in. (CC2), and Enrich-o'cobs (CP1); Northeastern Products (Warrensburg, NY) Beta Chip (HW1); Shepherd Specialty Papers (Milford, NJ) Shepherd's Cob 1/8-in. (CC3), Shepherd's Cob 1/4-in. (CC4), Shepherd's Specialty Blend (CP2), and Alpha-dri (PP1); Green Products (Conrad, IA) 1/8-in. Grade Corncob (CC5), 1/4-in. Grade Corncob (CC6), and PureLite Corncob 1/4-in. (CC7); Harlan Teklad (Indianapolis, IN) Corncob 1/8-in. (CC8), Corncob 1/4-in. (CC9), Soft Cobs Enrichment (CP3), Diamond Soft (PP2), Certified Irradiated Diamond Soft (PP3), Pelleted Paper (PP4), Tek-Fresh (PP5; produced by Absorption Corporation [Ferndale, WA] as CareFresh), Omega-dri (PP6; produced by Omni BioResources [Cherry Hill, NJ]); and PJ Murphy (Montville, NJ) Sani-Chips (HW2). Bedding types were assigned to 1 of 4 categories: corncob (CC), corncob–paper mixed (CP), hardwood (HW), and paper (PP). At least 3 different lots per type of bedding were analyzed for endotoxin, coliform, and dust levels. Samples were stored at 40 to 45 °F prior to being assayed.
Preprocessed hardwood bedding.
Because most hardwood bedding samples contained very low coliform counts and high endotoxin content, samples of preprocessed hardwood bedding were obtained from vendors to determine whether preprocessed samples contained high levels of coliforms. Samples of noncommercial, raw materials consisting of birch, beech, and maple used to prepare hardwood bedding were tested to determine the endotoxin, coliform, and dust levels prior to undergoing processing and bagging procedures. Preprocessed corncob bedding was not tested for endotoxins or coliforms because high levels of coliforms were detected in most commercially available corncob bedding samples.
Endotoxin assays.
At our institution, all bedding is autoclaved prior to animal exposure; therefore, all bedding samples were autoclaved before conducting endotoxin assays. Endotoxin levels in bedding samples were determined by using a commercially available kit (LAL Kinetic-QCL Test Kit, Lonza, Walkersville, MD), and results were collected by using an automated plate reader (ELx808 Absorbance Microplate Reader, Bio-Tek, Winooski, VT). We selected this particular commercial kit because it is 1,000-fold more sensitive for detecting endotoxins than reactive glucans.13 Samples of bedding (10 g each) were added to 90 mL sterile pyrogen-free water in glass beakers that were rendered pyrogen-free by heating to 250 °C for 30 min. The samples were mixed and allowed to soak for 1 h before proceeding with further dilutions. Each dilution was run in duplicate, along with duplicate endotoxin-spiked (50 endotoxin units [EU]/mL) positive product control samples to verify the lack of product inhibition. As stated in the manufacturer's instructions, positive and negative controls were run on each 96-well plate, and positive control values were within the acceptable range (50% to 200%), indicating valid assays. Assays were carried out according to test kit instructions. Endotoxin levels (EU/g) were calculated by averaging the results of at least 3 different lots for each bedding type.
Endotoxin levels after blocking of β-glucans.
Several samples from each bedding type were assayed again after β-1,3-glucans were blocked by using a commercial kit (β-G-Blocker Kit, Lonza) in an effort to further increase the specificity for endotoxin. Samples were run in duplicate, at the same dilution, with and without blocking, on 2 separate occasions (as described in the manufacturer's instructions) to determine whether β-1,3-glucans were falsely enhancing the endotoxin content of some bedding types. Positive and negative controls were run on each 96-well plate, and positive control values were within the acceptable range (50% to 200%), indicating valid assays. Results were analyzed by direct comparison, and the average was reported for each category of bedding.
Coliform counts.
Total coliform counts in nonautoclaved bedding samples were determined by using standard microbiologic procedures.5,9,20 Samples (11 g each) of nonautoclaved bedding were diluted in 99 mL phosphate-buffered water by dissolving 38 g KH2PO4 in 500 mL distilled water, adjusting the pH to 7.2 with 1 N NaOH, and diluting to 1,000 mL. Serial 10-fold dilutions were immediately plated on Violet Red agar to determine total coliform counts. Plates were inverted and incubated at 35 to 37 °C for approximately 48 h before coliform counts were determined.
Dust content.
Dust is defined as particles that measure less than 300 µm, pass through a no. 50 sieve, and are small enough to remain in an aerosol state for several minutes, depending on air flow.18 The average percentage dust content was determined as previously described.18 Preweighed US standard sieves were stacked with the coarsest sieve (no. 8) on top and progressing down (nos. 20, 30, and 50) to the dust catch pan. The catch pan was lined with preweighed aluminum foil to facilitate weighing small amounts of dust. Samples (50 g each) of each bedding type were placed on sieve no. 8 and shaken for 3 min in a portable rotating–tapping shaker (Meinzer II Sieve Master Rotap Shaker, Labtronics, Ontario, Canada). Dust content was determined by subtracting foil weight from the weight of the dust-bearing foil. Average percentage dust was calculated and recorded per bedding type.18
Statistical analysis.
Because endotoxin levels were not normally distributed, statistical analyses were conducted on the log-transformed values. Log-transformed values of the mean endotoxin levels from different bags underwent ANOVA to compare the endotoxin levels for each bedding category. A 2-sample t test was used to compare the 1/4-in. and 1/8-in. corncob beddings, and paired t tests were used to compare endotoxin levels measured with and without blocking of glucans. Two-sided P values less than 0.05 were considered significant. All data are presented as mean ± SEM.
Results
Endotoxin assays.
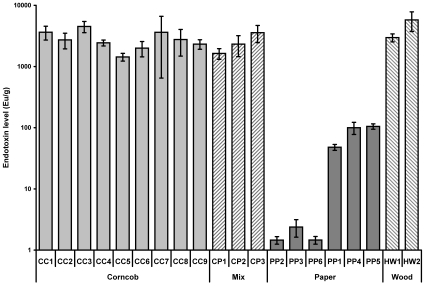
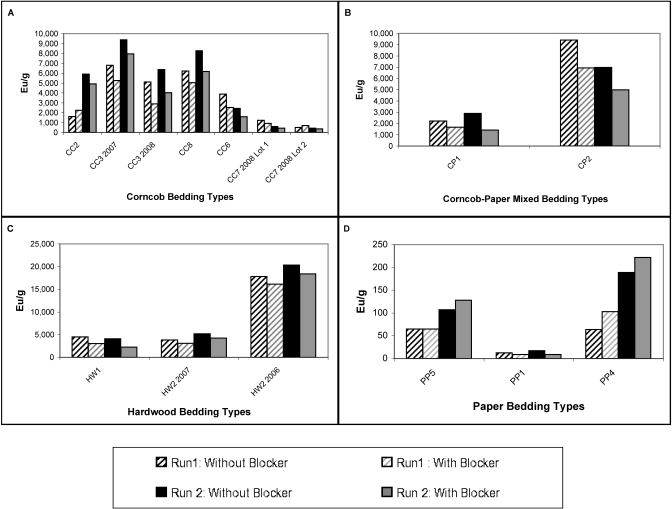
Endotoxin content (determined without blocking any contribution from glucans) was evaluated for each type of bedding (Figure 1). All types of bedding assayed contained endotoxin at some level; the concentration of endotoxin exceeded 1500 EU/g in 14 of the 20 commercially available bedding samples tested. The endotoxin level of all paper bedding types (mean ± SE, 38 ± 8 EU/g) was significantly (P < 0.0001) lower than that for corncob, hardwood, and corncob–paper mixed bedding types. The amount of endotoxin obtained from hardwood bedding (4925 ± 1443 EU/g) was significantly higher than from corncob (2749 ± 260 EU/g) and corncob–paper mixed beddings (2417 ± 458 EU/g; P < 0.05). Endotoxin levels did not differ significantly between the 1/4-in. and 1/8-in. corncob bedding types. Preprocessed hardwood bedding (raw materials) contained both high endotoxin content (39,568 EU/g) and high coliform counts (greater than 10,000 cfu/g). In contrast, the processed commercially available hardwood bedding contained high endotoxin content and low coliform counts. Blocking the contribution of glucans to the assayed concentration of endotoxin in the different bedding types (Figure 2) did not significantly alter the data for any of the bedding types (P > 0.12 for all paired t tests).
Figure 1.
Endotoxin levels (mean ± SE) in rodent bedding. Corncob bedding, n = 62 bags; corncob–paper mixed bedding, n = 21 bags; paper bedding, n = 40 bags; hardwood bedding, n = 23 bags. Paper bedding contained significantly (P < 0.0001, ANOVA and Fisher least significant difference test) less endotoxin than did other types of bedding.
Figure 2.
Endotoxin levels from individual samples with and without blocking of β-(1,3)-glucans. (A) Corncob bedding. (B) Corncob–paper mixed bedding. (C) Hardwood bedding. (D) Paper bedding. Endotoxin levels with and without blocking did not differ, according to paired t tests within each bedding type.
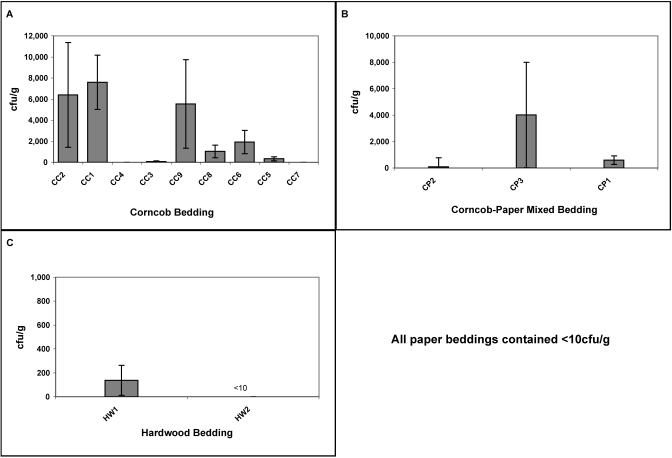
Coliform counts.
The highest coliform counts were found in corncob beddings followed by corncob–paper mixed beddings (P < 0.0001; Figure 3). Bedding CC1 contained the highest number of viable coliform counts (7591 cfu/g) followed by CC2 (6399 cfu/g), CC9 (5535 cfu/g), and finally CP3 (4010 cfu/g; P = 0.002). Coliform counts from hardwood bedding HW1 were 137 cfu/g whereas HW2 contained less than 10 cfu/g (P = 0.012). Preprocessed hardwood bedding contained greater than 10,000 cfu/g. All paper beddings were negative for coliforms, significantly lower than corncob and corncob-paper mixed beddings (P < 0.001), but similar to processed hardwood bedding (P > 0.05).
Figure 3.
Coliform counts (mean ± SE) by bedding type. (A) Corncob bedding. (B) Corncob/Paper Mix bedding. (C) Hardwood bedding. All paper beddings were culture-negative for coliforms.
Dust content.
The average dust content was less than 0.15% in all commercially available bedding types. The highest dust content was 0.13%, found in beddings HW1 and HW2. Lowest levels were contained in CC5, CC8, and PP4 beddings, which all yielded less than 0.01% dust. Preprocessed hardwood bedding contained 2.23% dust.
Discussion
Endotoxin is derived from the cell wall of gram-negative bacteria and is ubiquitous in the environment. Experimental animals may be exposed to endotoxin from the bedding, bedding dust, and feces by the oral or aerosol route and from endogenous gram-negative bacteria in the gastrointestinal tract. Previous reports do not clearly indicate which source of endotoxin - bedding dust or animal feces - would have a greater effect on LPS inhalation studies, but a reasonable hypothesis is that endotoxin from the bedding dust would be more detrimental in this regard. Corncob, and corncob–paper mixed beddings contained significantly (P < 0.05) higher levels of endotoxins, whereas paper bedding contained the lowest levels (Figure 1). Endotoxin levels measured in corncob–paper mixed bedding were similar to those measured in corncob beddings. Our results suggest that the corncob component is the primary source of endotoxin in the corncob–paper mixed bedding types; for example, the paper component of CP3 corncob–paper mixed bedding (PP2), contained less than 5 EU/g. The low endotoxin values in the paper bedding may reflect the source and type of raw materials used and differences in processing procedures.
The endotoxin content in various types of corncob, corncob–paper mixed, and hardwood bedding may vary depending on differences in harvesting, storage, and processing of raw materials and in processing procedures for the finished product. Different testing procedures and variations in the sensitivity and specificity of endotoxin test kits and extraction methods make it difficult to accurately reproduce endotoxin results between different testing labs.2,4,11 Differences in the microbial quality of the pre- and postprocessed bedding types also can affect the endotoxin content of the finished bedding. For example, the high endotoxin content in some commercially available corncob and corncob–paper mixed bedding types can be explained by the high viable coliform counts in these bedding types. In contrast, the high endotoxin content in the commercially available hardwood bedding types (Figure 1) cannot be explained by the low viable coliform counts in the hardwood bedding. The low levels of coliforms in the hardwood bedding (Figure 3) reflect the high temperatures and drying times used to reduce the moisture level in the finished bedding. The high endotoxin content and absence of coliforms in nonautoclaved hardwood bedding were confirmed when preprocessed, raw materials (that is, not exposed to the temperatures used for drying just prior to bagging) were shown to contain both high total coliform counts and high endotoxin content. Heat also is used to reduce the moisture content in corncob bedding. Beddings CC4 and CC7 contained fewer than 10 cfu/g whereas their average endotoxin levels were 2437 and 3629 EU/g, respectively. One explanation for the different endotoxin levels may be that different manufacturers heat corncobs to different temperatures for different times to achieve different moisture levels in the finished products.
A study3 addressing the content of endotoxins and (1,3)-β-D-glucans in wood shavings and paper crumb bedding did not state the actual products assayed but reported that the amount of (1,3)-β-D-glucans was higher than the endotoxin content in the paper and wood beddings. However, paper crumbs had lower amounts of endotoxin and (1,3)-β-D-glucans than did wood chips.3 Our current study supports these results in that the paper bedding contained less endotoxin than did hardwood beddings. In addition, nonautoclaved corncob beddings yielded the highest levels of viable coliforms in our study (Figure 3). These results are consistent with previous reports,15,16 which show that extracts of corn dust contain sufficiently high levels of endotoxin that induced lung inflammation in mice.
People working with dried wood containing very high dust and endotoxin levels have been reported to experience significant respiratory problems.4 Although no country has established official endotoxin exposure limits, the recommended limit of 50 EU/m3 is used in the Netherlands.4 Even less information exists on the level of endotoxin in rodent bedding that would induce respiratory symptoms or affect respiratory or immunologic studies in rodents. The threshold level for endotoxins is not known and is difficult to determine because previous studies3,6,14-16 did not report the endotoxin content of the bedding used. To further complicate the situation, some inhalation studies measured aerosolized endotoxin rather than the endotoxin content of the bedding.6,14-16 Previous studies do not clearly indicate which bedding type is optimal for conducting LPS inhalation studies in mice to determine the role of endotoxin in the inflammatory response.3,6,15,16 Our results suggest that the amount of endotoxin in corncob, corncob–paper mixed, and hardwood beddings is a factor that investigators should consider when conducting sensitive studies such as LPS inhalation or respiratory physiology studies using laboratory rodents. Additional comparative controlled studies using rodents exposed to bedding containing variable levels of endotoxin would be helpful in establishing no-effect or threshold levels for conducting LPS studies in rodents.
The commercial test kit for the photometric detection of endotoxin that we used was selected because its reagents are 1000 times more sensitive for detecting endotoxins than β-(1,3)-D-glucans. Our results suggest that this test kit primarily measured the endotoxin content of the bedding samples and that the presence of β-(1,3)-D-glucans did not lead to falsely increased endotoxin levels, as can occur in samples containing cellulose or fungal products.12 Similarly, blocking of β-(1,3)-D-glucans before endotoxin analysis did not alter measured endotoxin levels in any of the samples tested, regardless of the bedding type (Figure 2).
Our results indicate that commercially available rodent beddings, especially corncob and corncob–paper mixes with high bacterial counts including coliforms or pathogenic agents, should be autoclaved or irradiated prior to use in strict barrier animal facilities. Although autoclaving kills viable bacteria, we confirmed that some types of autoclaved bedding still contain high levels of endotoxins, consistent with the known heat-stability of these compounds. In addition, proper storage of processed bedding can decrease the risk of bacterial contamination and subsequent endotoxin levels in rodent bedding, thereby decreasing animal exposure to these agents.
The percentage dust in all types of commercially available beddings was low (less than 0.15%). Whereas preprocessed hardwood bedding contained approximately 2.0% dust, dust levels in commercially available hardwood beddings were reduced to 0.13% through processing. The percentages of dust in the commercially available hardwood beddings we tested in the current study were lower than those previously reported for hardwood bedding.18 This reduction in the dust content of hardwood bedding may reflect our institution's improved specifications regarding particle size and dust content for HW1 and HW2.17,18 For example, in past years, our animal facility filled caging with HW1 or HW2 bedding by using an automated dispensing system, which generated both total and respirable dust levels in the work area that exceeded the threshold limit value of 1 mg/m3 for an 8-h work shift for unprotected human exposure to hardwood dust as recommended by the American Conference of Governmental Industrial Hygenists.17 Studies performed to address this issue17,18 led to improvements in our contract specifications for dust levels in hardwood bedding, and current dust levels are within the recommended exposure limits for humans.17
The main objective of the current study was to determine the amount of endotoxin in various types of animal bedding in an effort to identify the optimal bedding for use in LPS inhalation, respiratory, and immunologic studies in rodents. Additional studies are needed to determine the lowest concentration of endotoxin in rodent bedding that produces significant biologic effects in LPS studies involving rodents. Both corncob and hardwood bedding types commonly used in rodent research studies contained high concentrations of endotoxins. The potential effect of endotoxin exposure in animals should be considered when conducting studies involving LPS or assessing respiratory or immunologic endpoints. As compared with the other bedding types, the paper beddings tested contained significantly (P < 0.0001) lower levels of endotoxins. Furthermore, paper beddings contained significantly (P < 0.001) lower levels of coliforms than did corncob and corncob-paper mixed beddings. Taken together, these findings suggest that paper bedding may be preferable when endotoxins are a concern, such as during immunologic, respiratory, or inhalation studies. To our knowledge, this is the first study to survey the major types of rodent bedding for endotoxin, coliform, and dust content.
Acknowledgments
The authors thank Drs John Hollingsworth, Michael Fessler, and Donald Cook for their assistance in reviewing and editing the manuscript and Gordon Caviness, Jacqueline Locklear, Patricia Deese, and Margaret Fender for their technical support. This research was supported by the Intramural Research Program of the National Institute of Environmental Health Sciences, NIH.
References
- 1.Braun-Fahrlander C, Riedler J, Herz U, Eder W, Waser M, Grize L, Maisch S, Carr D, Gerlach F, Bufe A, Lauener RP, Schierl R, Renz H, Nowak D, von Mutius E. 2002. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med 347:869–877 [DOI] [PubMed] [Google Scholar]
- 2.Chun DT, Chew V, Bartlett K, Gordon T, Jacobs R, Larsson BM, Lewis DM, Liesivuori J, Michel O, Rylander R, Thorne PS, White EM, Gunn VC, Wurtz H. 2002. Second interlaboratory study comparing endotoxin assay results from cotton dust. Ann Agric Environ Med 9:49–53 [PubMed] [Google Scholar]
- 3.Ewaldsson B, Fogelmark B, Feinstein R, Ewaldsson L, Rylander R. 2002. Microbial cell wall product contamination of bedding may induce pulmonary inflammation in rats. Lab Anim 36:282–290 [DOI] [PubMed] [Google Scholar]
- 4.Harper M, Andrew ME. 2006. Airborne endotoxin in woodworking (joinery) shops. J Environ Monit 8:73–78 [DOI] [PubMed] [Google Scholar]
- 5.Helper O. 1966. Manual of clinical laboratory methods. Springfield (IL): Charles C. Thomas [Google Scholar]
- 6.Jagielo PJ, Thorne PS, Kern JA, Quinn TJ, Schwartz DA. 1996. Role of endotoxin in grain dust-induced lung inflammation in mice. Am J Physiol 270:L1052–L1059 [DOI] [PubMed] [Google Scholar]
- 7.Kaliste E, Linnainmaa M, Meklin T, Torvinen E, Nevalainen A. 2004. The bedding of laboratory animals as a source of airborne contaminants. Lab Anim 38:25–37 [DOI] [PubMed] [Google Scholar]
- 8.Morita T, Tanaka S, Nakamura T, Iwanaga S. 1981. A new (1 → 3)-[β]-D-glucan-mediated coagulation pathway found in limulus amebocytes. FEBS Lett 129:318–321 [Google Scholar]
- 9.Murray P, Baron E, Pfaller M, Tenover F, Yolken R. 1995. Manual of clinical microbiology. Washington (DC): American Society of Microbiology Press [Google Scholar]
- 10.Natarajan S, Kim J, Remick DG. 2008. Acute pulmonary LPS tolerance decreases TNFα without reducing neutrophil recruitment. J Immunol 181:8402–8408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reynolds SJ, Milton DK, Heederik D, Thorne PS, Donham KJ, Croteau EA, Kelly KM, Douwes J, Lewis D, Whitmer M, Connaughton I, Koch S, Malmberg P, Larsson BM, Deddens J, Saraf A, Larsson L. 2005. Interlaboratory evaluation of endotoxin analyses in agricultural dusts–comparison of LAL assay and mass spectrometry. J Environ Monit 7:1371–1377 [DOI] [PubMed] [Google Scholar]
- 12.Roslansky PF, Dawson ME, Novitsky TJ. 1991. Plastics, endotoxins, and the Limulus amebocyte lysate test. J Parenter Sci Technol 45:83–87 [PubMed] [Google Scholar]
- 13.Roslansky PF, Novitsky TJ. 1991. Sensitivity of Limulus amebocyte lysate (LAL) to LAL-reactive glucans. J Clin Microbiol 29:2477–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanford AN, Clark SE, Talham G, Sidelsky MG, Coffin SE. 2002. Influence of bedding type on mucosal immune responses. Comp Med 52:429–432 [PubMed] [Google Scholar]
- 15.Schwartz DA. 1996. Grain dust, endotoxin, and airflow obstruction. Chest 109:57S–63S [DOI] [PubMed] [Google Scholar]
- 16.Schwartz DA, Thorne PS, Jagielo PJ, White GE, Bleuer SA, Frees KL. 1994. Endotoxin responsiveness and grain dust-induced inflammation in the lower respiratory tract. Am J Physiol 267:L609–L617 [DOI] [PubMed] [Google Scholar]
- 17.Shropshire VD, Hunt CL, Merkle SE. An industrial hygiene evaluation of hardwood dust exposure during the dispensing of animal bedding. American Industrial Hygiene Conference and Exposition. 18-24 May 1996. Washington, DC [Google Scholar]
- 18.Thigpen JE, Lebetkin EH, Dawes ML, Clark JL, Langley CL, Amyx HL, Crawford D. 1989. A standard procedure for measuring rodent bedding particle size and dust content. Lab Anim Sci 39:60–62 [PubMed] [Google Scholar]
- 19.Thorne PS, Kulhankova K, Yin M, Cohn R, Arbes SJ, Jr, Zeldin DC. 2005. Endotoxin exposure is a risk factor for asthma: the national survey of endotoxin in United States housing. Am J Respir Crit Care Med 172:1371–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walter W. 1967. Standard methods for the examination of dairy products. New York (NY): American Public Health Association [Google Scholar]