Abstract
Monitoring of sanitation is an essential function of laboratory animal facilities. The purpose of the current study was to assess the ability of an ATP-based system to detect microbes and organic contaminants. Serial dilutions of Escherichia coli, Staphylococcus aureus, Toxocara canis eggs, Toxoplasma gondii tachyzoites, epithelial cells, and rodent blood, urine, and feces were analyzed according to the manufacturer's recommendations. The limit of E. coli detection was 104 organisms; sonication of E. coli significantly improved detection, indicating incomplete bacterial lysis in the detection system. Detection of S. aureus was significantly greater than that of E. coli with a limit of detection of 102; sonication did not alter results. In contrast, detection of T. canis, T. gondii, RBC, and epithelial cells was robust and ranged from 2 T. canis eggs to 10 epithelial cells. Urine was weakly detected, with a limit of detection at 1:10 dilution. Detection of all cell types except epithelia had a strong linear correlation to total cell number. In addition, our data demonstrate that the efficacy of the detection system can be affected adversely by residual disinfectants and that sample-bearing swabs are stable for more than 7 h after swabbing. These data demonstrate that this ATP based system sensitively detects pure cells and organic contaminants with a strong degree of linear predictability. A limitation of the system is its inability to detect gram-negative bacteria efficiently because of incomplete cell lysis.
Abbreviations: RLU, relative light units; LOD, limit of detection
Monitoring of sanitation and cleaning is an essential component of contemporary laboratory animal facilities. Primary and secondary animal housing units must be sanitized appropriately to ensure that pathogens and opportunistic agents are minimized or excluded. Further, sensitive areas within the laboratory animal facility, including cage, food, and bedding storage areas and surgical suites, require adequate sanitation to ensure that they are free of microbial contaminants. For these reasons, monitoring methods must be reliable, sensitive, and able to detect a wide variety of pathogens and other unwanted microbes.
The Institute for Laboratory Animal Research recommends that sanitized equipment located within barrier facilities be free of all gram-negative organisms.3,8 Historically, this recommendation is the basis for most microbiologic monitoring programs, with agar contact methods being the primary technique used to test surfaces for growth of organisms after cleaning and sanitization.5,8 An important limitation of this method is that it does not detect a variety of unwanted microbes but merely detects easily cultivatable, aerobic bacteria and fungi. Therefore, a wide variety of pathogenic and opportunistic agents are not evaluated. The utility of contact plates is limited further because they require several days of incubation for colony growth,2,5 and they cannot detect parasites and other unwanted nonbacterial contaminants.6
Recently, ATP-based microbiologic monitoring methods were developed to monitor the cleaning and sanitization of equipment and materials.2,6 These monitoring systems currently are used in food production facilities, state health laboratories, and drug companies.4,7,8,10 The systems immediately detect the presence or absence of organic material (live or dead) on solid surfaces.8,9 Most ATP detection devices use bioluminescence to indicate the level of residual ATP present on swabbed surfaces. Once the surface is swabbed, the sample is exposed to an ATP-releasing agent (lysis buffer) and an ATP-activated light-producing substrate and enzyme (luciferin and luciferase). The amount of ATP present on the surfaces tested can then be quantified by the amount of light emitted during the enzymatic reaction (relative light units, RLU).6 If the ATP detection device discovers contamination, the surface can be resanitized immediately and retested. The rapid nature of these devices combined with their ability to detect a wide range of common laboratory animal facility contaminants make them an attractive alternative to the current contact agar monitoring practices. However, to date, rigorous assessment of the efficacy of these automated readers in detecting potential contaminants in laboratory animal facilities has not been reported.
The focus of this study was to determine the benefits and limitations of an ATP-based detection system. We hypothesized that the use of an ATP-based detection system would provide important advantages over culture-based microbiologic methods currently used in laboratory animal facilities. Therefore we first determined the level of sensitivity of the system to its intended substrate (that is, ATP). Second, we assessed the ability of the system to detect bacterial contaminants and compared these data to those from standard microbial counting methods. Third, we investigated the ability of the system to detect a variety of common contaminants found in a laboratory animal setting, and we analyzed the ability of the system to detect ATP in the presence of residual cleaning solutions. Finally, we explored the system's capability to function over prolonged sampling times. Our data provide the first systematic analyses of an ATP-based biomonitoring system in the context of laboratory animal medicine. Our findings likely will be valuable in establishing updated microbiologic monitoring protocols in animal facilities.
Materials and Methods
Animal use.
All tissues were collected from animals maintained according to institutionally approved protocols. Animals were housed in AAALAC-accredited facilities, and tissues were collected after euthanasia.
RLU determination.
A 10-µL aliquot of each test substance (3 to 5 replicates per substance) was micropipetted into the microtube of the sample collection system (PocketSwab Plus, Charm Sciences, Lawrence, MA) prior to activation of the swab. All swabs were analyzed by using an automated ATP detection device (novaLUM, Charm Sciences) according to the manufacturers’ instructions.
Clean cages.
To determine whether residual ATP remained on clean surfaces, we swabbed 3 cages newly emerged from an automatic cage wash. These swabs were performed according to the manufacturer's (Charm Sciences) recommendations.
ATP.
Purified ATP (Sigma-Aldrich, St Louis, MO) was used to determine the automated device's sensitivity of detection. The manufacturer supplied kit contained lyophilized standard disodium salt trihydrate ATP, and dilutions were prepared according to the manufacturer's recommendation.
Bacteria.
Escherichia coli (American Type Culture Collection strain 25922) and Staphylococcus aureus (American Type Culture Collection strain 29213) were cultured in Luria–Bertani broth and grown to an optical density of approximately 0.8 at 600 nm wavelength. Serial 10-fold dilutions of these pure cultures were prepared in sterile saline; comparable aliquots of each dilution were used as samples for ATP detection (with and without sonication before analysis). For sonication of bacteria, samples were placed on ice for 10 min and then were sonicated (Sonifier 250, Branson, Danbury, CT) for 2 min at a 50% duty cycle and level 3 output. After sonication, 10 µL of each dilution was used to determine the amount of ATP detected by the automated device.
Toxocara canis.
T. canis eggs were collected from gravid adults and stored in sterile water overnight at 4 °C1 to allow the eggs to settle. The following morning, the supernatant was removed, eggs were pipetted into 100 μL sterile saline, and 10-fold dilutions of this stock solution were prepared. After ATP detection, 10 μL from each dilution was placed (in triplicate) onto microscope slides, and the eggs counted manually for comparison with the RLU data.
Toxoplasma gondii.
T. gondii strain GT1 tachyzoites were maintained by serial passage on HS27 monolayers in DMEM. A 3-mL stock solution containing 1.92 × 107 T. gondii tachyzoites was used to prepare 10-fold serial dilutions in PBS, and 10 µL from each dilution was used for ATP detection. For each dilution, the ATP detected was compared with the actual number of T. gondii tachyzoites.
Laboratory contaminants.
Blood was collected from a freshly euthanized Sprague–Dawley rat, placed into an EDTA blood-collection tube, and serially diluted into sterile saline; 10 μL from each dilution was used for ATP detection. Hemogram counts were used for comparison with the RLU data.
Fresh rat feces were diluted 1:1 (w:v) with sterile saline and homogenized. Serial dilutions were made, and 10 µL from each dilution was analyzed with the detection device.
Approximately 150 µL urine was collected from a sterile cage bottom after the euthanasia of 6 CD1 mice. The pooled urine sample was serially diluted, and 10 µL from each dilution was analyzed by using the ATP detection system.
Mammalian cells.
Cultured cervical epithelial (HeLa) cells (3.0 × 106) were serially diluted in HBSS; 10 µL from each dilution was used for ATP analysis.
Residual disinfectant.
Recommended working dilutions of sodium hypochlorite (10% bleach), a hydrogen peroxide–peracetic acid mixture (Spor-klenz, Steris, Mentor, OH), and a quaternary ammonium chloride compound (Quatricide, Pharmaceutical Research Laboratories, Naugatuck, CT) were combined with ATP (Sigma) to determine whether exposure to residual disinfectant altered the level of bioluminescence. To this end, we combined 5 µL of disinfectant (or saline as a control) with 5 µL (20 fmole) ATP in the microtube of the sample swab unit.
Time-course study.
To evaluate the manufacturer's claim that sample-bearing swabs can be held for as long as 6 h before activation and analysis, 10-µL aliquots of a stationary-phase culture of E. coli were added to sterile tubes, into which swabs were placed. Swabs (n = 3 per time-point) were activated and read each hour until 7 h after activation.
Statistical analysis.
The Student t test was used to compare E. coli and S. aureus data before and after sonication. Linear regressions were performed on all other data sets collected. All analyses were conducted by using GraphPad Prism (version 5.02 for Windows, GraphPad Software, San Diego, CA). Data were deemed significant at P < 0.05.
Results
ATP and clean cages.
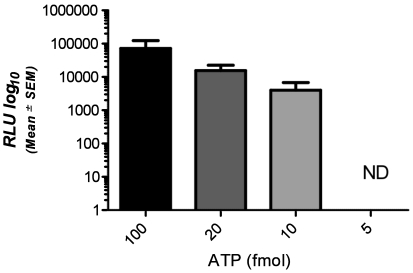
To ensure that the ATP detection system was working appropriately and as described by the manufacturer, we first tested its ability to detect pure ATP. The limit of detection (LOD) for the ATP standard was 10 fmol; RLU values did not correlate with ATP concentration (R2 = 0.4034; Figure 1). To verify that the system was not yielding false-positive results, we swabbed 3 freshly cleaned cages. In all cases the swab produced a reading of zero (data not shown).
Figure 1.
Signals (RLU, mean ± SEM) detected by the device for known concentrations of pure ATP. ND, no detection of ATP at this concentration.
Bacteria.
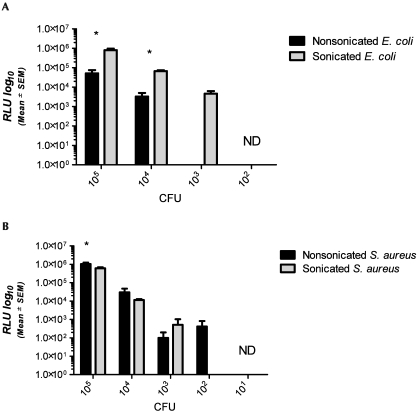
The ability of the automated device to detect representative gram-negative (E. coli) and gram-positive (S. aureus) bacteria was determined and compared with standard colony counts. We also tested whether disruption of bacterial membranes by sonication affected the device's sensitivity. The LOD for intact E. coli was 104 cfu; sonication of E. coli increased the LOD to 103 (Figure 2 A). In addition, sonication significantly (P < 0.05) increased the RLU detected from E. coli at both 105 and 104 cfu. S. aureus yielded a significantly (P < 0.05) higher signal at 105 cfu and a lower LOD (102 cfu; Figure 2 B) than did E. coli. Sonication of S. aureus prior to analysis did not significantly affect detection (Figure 1 B). There was strong linear correlation between bacterial colony counts and ATP readings (RLU) for unsonicated (R2 = 0.9993) and sonicated (R2 = 0.9983) E. coli and unsonicated (R2 = 0.9995) and sonicated (R2 = 0.9992) S. aureus.
Figure 2.
Signals (RLU, mean ± SEM) detected by the device for nonsonicated and sonicated (A) E. coli and (B) S. aureus at 105,104, 103, 102, and 101 cfu. Detection of E. coli organisms (panel A) was significantly greater (*, P < 0.05) at 105 and 104 cfu when the sample was sonicated before analysis. At 105 cfu, detection of S. aureus (panel B) was significantly (*P < 0.05) greater than that of E. coli. ND, no detection of ATP at this concentration.
Parasites.
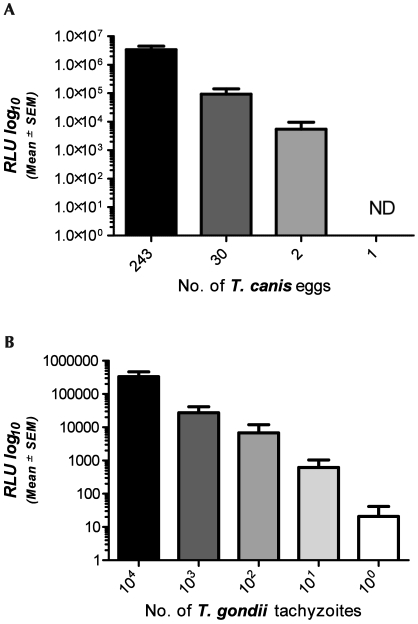
Parasitic organisms may contaminate laboratory animal facilities. To test the device's ability to effectively detect these agents, we analyzed a helminth (T. canis) and a protozoan (T. gondii) parasite, and compared the RLU readings to those of known quantities of eggs and tachyzoites. The limit of RLU detection was 2 Toxocara canis eggs (Figure 3 A), and 2 Toxoplasma gondii tachyzoites (Figure 3 B). RLU readings from both organisms exhibited a strong linear correlation with both egg (R2 = 0.9933) and tachyzoite (R2 = 0.9995) burden.
Figure 3.
Signals (RLU, mean ± SEM) detected by the device for (A) Toxocara canis eggs or (B) Toxoplasma gondii tachyzoites are plotted against T. canis egg counts and known tachyzooite concentrations, respectively, at various dilutions. Detection of both organism exhibited a strong linear correlation with the dilution (R2 = 0.9933 and R2 = 0.9995, respectively). ND, no detection of ATP at this concentration.
Rodent blood, feces, and urine.
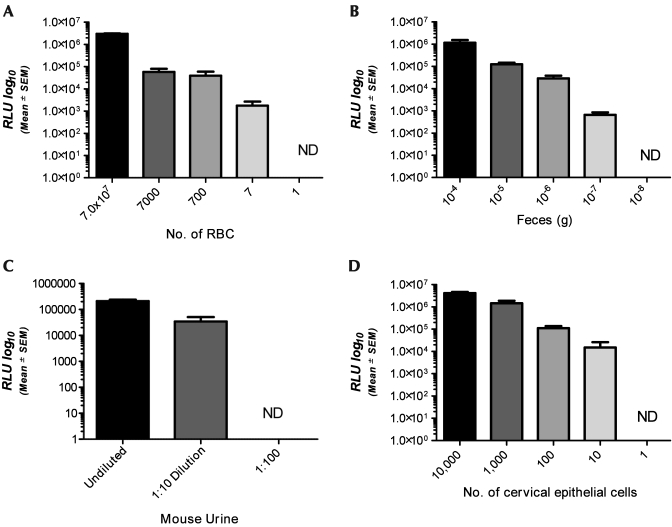
Trace amounts of unwanted animal biologics and waste products, such as blood, urine, and feces, may be present in animal facilities and procedure rooms. To investigate the device's capacity to detect these unwanted contaminants, we tested small aliquots of each organic material and compared these readings to quantifiable references or dilutions of each substrate. The LOD for rat blood was 7 RBC and ATP values showed strong linear correlation with the RBC values of the rat hemogram (R2 = 0.9943; Figure 4 A). The LOD for rat feces was the 105 dilution (1 × 10−7 g feces), and fecal ATP units were linearly correlated with dilution (R2 = 0.7629; Figure 4 B). Mouse urine was detected only to a dilution of 1:10, and in this small dataset the ATP values exhibited linear correlation with dilution levels (R2 = 0.8770; Figure 4 C).
Figure 4.
Signals (RLU, mean ± SEM) detected by the device for (A) rat RBC, (B) rat feces, (C) mouse urine, and (D) cervical epithelial cells. There was a strong linear correlation for detection of all rodent samples (R2 = 0.9943, R2 = 0.7629, and R2 = 0.8870, respectively). In contrast, the signal from epithelial cells did not correlate with cellularity. ND, no detection of ATP at this concentration.
Epithelial cells.
Human and animal tissue may also serve as contaminants in animal facilities. This is particularly true of epithelial cells, which are routinely shed from humans and animals. To test the system's capability to detect epithelial cells we analyzed known quantities of a human cervical epithelial cell line (HeLa) and compared them to the mean RLU generated by the ATP detection device. The system was able to detect as few as 10 epithelial cells; however, unlike the other substrates examined, epithelial cell number did not exhibit a linear correlation with RLU value (R2 = 0.4221, Figure 4 D).
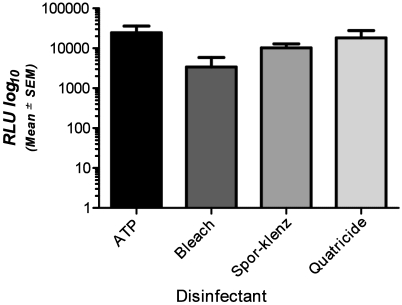
Disinfectants.
Previous reports indicate that several standard surface disinfectants may alter ATP detection in other systems.6,7 To investigate these findings in relation to the detection device we used, we tested a variety of common disinfecting agents. Compared with the signal associated with pure ATP (24,583 ± 11,529 RLU), the mean RLU for ATP in the presence of disinfectant was decreased (10% bleach, 3414 ± 2438 RLU; hydrogen peroxide–peracetic acid mixture, 10,218 ± 2710 RLU; quaternary ammonium product, 18,149 ± 9568 RLU). Although ATP detection did not differ significantly between disinfectants (Figure 5), the ATP in 2 of the 5 bleach replicates was undetectable and yielded a value of 0 RLU, indicating that residual bleach has the potential to provide a false-negative reading.
Figure 5.
Signals (RLU, mean ± SEM) detected by the device for ATP diluted in various disinfectant solutions at their appropriate working concentrations compared with that from the same amount of undiluted ATP. There was no significant difference between detection of pure ATP and the amount of ATP detected with each disinfecting solution.
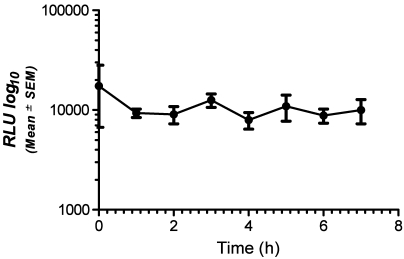
Time-course study.
To verify the manufacturer's claim that sample-bearing swabs are stable for 6 h before activation and analysis, we analyzed swabs containing equal concentrations of E. coli at various time points (0 to 7 h). The device was able to detect ATP at all time points tested without a significant change in the mean signal level, even at 7 h after bacterial exposure (Figure 6).
Figure 6.
Signals (RLU, mean ± SEM) detected by the device at 0, 1, 2, 3, 4, 5, 6, and 7 h after activation of the sample swab. There were no statistical differences in detection at any of the time points.
Discussion
Our results demonstrate that the ATP-based monitoring system we evaluated is able to detect a wide variety of prokaryotic and eukaryotic organisms that may be found in a laboratory animal environment. However, the sensitivity of detection varied widely among substrates. Importantly, pure bacteria are detected fairly weakly with an LOD of 104 for representative gram-negative and 102 for representative gram-positive bacteria. Although this drawback might be viewed as a significant limitation of this testing modality, pure bacteria would actually be encountered only rarely in a laboratory animal setting. Rather, bacteria would more commonly be encountered together with other organic contaminants (for example, feces), a situation that significantly improves the detection limit.
Sonication of gram-negative bacteria improves the detection limits of the ATP-reading device. The microtube of the swab contains a compartmentalized releasing–buffering agent, which is intended to lyse the cell walls of microorganisms rapidly and release their ATP, allowing its detection by the detection device. The sonication data suggest that the lysis buffer provided incompletely breaks down gram-negative bacteria. Conversely, sonication did not alter the detection of S. aureus, indicating that the provided reagents readily lyse gram-positive organisms.
Previous reports have confirmed that replicate organism direct agar contact (RODAC) plates and other common methods of microbiologic monitoring are limited by their ability to only detect cultivatable, aerobic organisms.4,5,10 We aimed to test a variety of common noncultivatible eukaryotic agents to investigate the device's ability to detect their presence. Among these eukaryotic cells, we chose 2 varieties of parasites that are relevant to laboratory animal facilites, Toxocara canis and Toxoplasma gondii. During the collection of the T. canis eggs, the eggs were carefully dissected from the uteri of the adult parasites and completely isolated. This process minimized the amount of nonegg organic matter available for detection. We found that the system was very sensitive, detecting as few as 2 T. canis eggs and 1 T. gondii tachyzoite. These results are relevant to a laboratory animal setting because they demonstrate that ATP detection systems can detect pathogenic agents that would be missed by standard testing with replicate organism direct agar contact plates. Specifically, the incorporation of an ATP-based detection system could prove valuable when dealing with a parasitic outbreak (for example, mouse pinworms) or when decontaminating a facility after purposeful infection with parasites.
In addition, the system robustly detected rodent red blood cells (LOD, 7 cells), and human epithelial cells (LOD, 10 cells). Although neither of these substrates is likely to pose a direct risk to laboratory animals, the presence of either of these substrates would indicate incomplete cleaning of surfaces. In addition, both animal blood and tissue may pose a risk to laboratory animal workers (for example, primate blood, tissue from BSL2 studies). Feces could pose a direct risk to animal health, either through pathogen transmission or providing an excellent environment for microbial growth. The detection of rodent feces by the automated device was very strong (to a 1:105 dilution). Because feces is a richly cellular substrate containing both living and dead eukaryotic and prokaryotic cells, it is unsurprising that dilute fecal solutions could be detected so readily. In contrast, rodent urine was more difficult to detect (LOD, 1:10 dilution), likely related to its 95% water makeup and overall lack of cellularity.
Various disinfectants have been reported to alter quantification when using ATP-based monitoring systems.6,7 After surfaces within a laboratory animal facility have been cleaned, residual chemicals may remain after insufficient rinsing. Some disinfecting solutions lower RLU readouts, whereas others increase them.6 In our studies, all of the mean RLU readings for the disinfectant–ATP solutions were lower than those obtained for the control ATP preparations. However, none of these readings achieved statistical significance. However, 2 of the 5 replicate bleach-containing samples failed to yield an ATP reading (0 RLU), perhaps suggesting that residual bleach-based disinfectants are capable of producing a false-negative reading.
The final component of the ATP detection system tested in this experiment was the amount of time that sample-bearing swabs could be stored prior to activation and RLU determination. Samples are activated by stabbing the swab into the microtube containing the releasing–buffering agent, luciferin, and luciferase. Swabs must be read in the detection device within 1 min after activation. Realistically, the detection device may not be present when samples are collected on swabs. For example, to avoid cross-contamination, one likely would not bring the detection device into areas where it could come in contact with potential pathogens (for example, quarantine and BSL2 facilities). We tested the stability of sample-bearing swabs by using purified E. coli bacteria and found consistent detection of equivalent numbers of organisms over a 7-h time period. This flexible time-frame allows personnel to screen a number of rooms and facilities during the day while maintaining the detection system in a centralized facility. This arrangement makes the system very functional for programs with a limited number of detection devices and with facilities of differing health status.
One puzzling aspect of the present study was the lack of linear correlation between RLU and pure ATP concentration. One likely explanation is that we sampled a narrow range of ATP concentrations (5 to 100 fmole). Despite this finding, virtually all other substrates examined displayed a strong linear tendency between ATP concentration and RLU value. This finding suggests that the RLU values obtained can be used not only in a strictly bimodal (yes or no) fashion but also to accurately predict the relative contamination level of a given substrate.
Overall, the current data demonstrate that the ATP system we evaluated sensitively detects pure cells and organic contaminants with a strong degree of linear predictability, and does so within minutes of swabbing. The primary limitation of the system is its relatively poor detection of gram-negative bacteria, likely due to incomplete cell lysis. A further consideration of this method is the possible interference of detection with residual bleach solutions. One key component of an ATP-based detection system is the establishment of RLU cutoffs, which need to be established at an institutional level according to the needs of the institution and the system being used. Our data for the present ATP detection system suggest that a cutoff value of 1,000 RLU would be reasonable, because lower values showed high variability. In conclusion, the ATP system we tested could serve as a suitable replacement or an excellent adjunct to standard colony-count analysis. Ultimately, the choice between these 2 systems will depend largely on the needs and constraints of the institution and animal facility in question. Certain areas of an animal facility that require impeccable cleaning (for example, dedicated surgical suite, barrier mouse facility) may be monitored most appropriately by using a combination of these 2 monitoring methods, thereby taking advantage of their relative strengths and minimizing their respective weaknesses.
Acknowledgments
The authors thank Alice Che Yu Lee and Dwight Bowman for their kind collection and donation of Toxocara canis eggs, Chia-Hsin Ju and Cynthia Leifer for their donation of T. gondii, Ursula Krotscheck for her donation of rat blood and feces, Robert Weiss for his donation of HeLa cells, and Ethan Hildebrand for help with sample collection.
References
- 1.Bowman DD, Mika-Grieve M, Grieve RB. 1987. Circulating excretory–secretory antigen levels and specific antibody responses in mice infected with Toxocara canis. Am J Trop Med Hyg 36:75–82 [DOI] [PubMed] [Google Scholar]
- 2.Colquhoun KO, Timms S, Fricker CR. 1998. A simple method for the comparison of commercially available ATP hygiene-monitoring systems. J Food Prot 61:499–501 [DOI] [PubMed] [Google Scholar]
- 3.Council NR. 1991. Long-term holding of laboratory rodents. ILAR News 19:L1–L25 [Google Scholar]
- 4.Davidson CA, Griffith CJ, Peters AC, Fielding LM. 1999. Evaluation of 2 methods for monitoring surface cleanliness—ATP bioluminescence and traditional hygiene swabbing. Luminescence 14:33–38 [DOI] [PubMed] [Google Scholar]
- 5.Douglas L, Ednie RPW, Max Lang C. 1998. Comparison of 2 sanitation monitoring methods in an animal research facility. Contemp Top Lab Anim Sci 37:71–7412456174 [Google Scholar]
- 6.Green TA, Russell SM, Fletcher DL. 1999. Effect of chemical cleaning agents and commercial sanitizers on ATP bioluminescence measurements. J Food Prot 62:86–90 [DOI] [PubMed] [Google Scholar]
- 7.Lappalainen J, Loikkanen S, Havana M, Karp M, Sjoberg AM, Wirtanen G. 2000. Microbial testing methods for detection of residual cleaning agents and disinfectants—prevention of ATP bioluminescence measurement errors in the food industry. J Food Prot 63:210–215 [DOI] [PubMed] [Google Scholar]
- 8.Schondelmeyer CW, Dillehay DL, Webb SK, Huerkamp MJ, Mook DM, Pullium JK. 2006. Investigation of appropriate sanitization frequency for rodent caging accessories: evidence supporting less-frequent cleaning. J Am Assoc Lab Anim Sci 45:40–43 [PubMed] [Google Scholar]
- 9.Sherlock O, O'Connell N, Creamer E, Humphreys H. 2009. Is it really clean? An evaluation of the efficacy of 4 methods for determining hospital cleanliness. J Hosp Infect 72:140–146 [DOI] [PubMed] [Google Scholar]
- 10.Vilar MJ, Rodriguez-Otero JL, Dieguez FJ, Sanjuan ML, Yus E. 2008. Application of ATP bioluminescence for evaluation of surface cleanliness of milking equipment. Int J Food Microbiol 125:357–361 [DOI] [PubMed] [Google Scholar]