Abstract
Collection of blood from the submandibular vein allows simple and rapid processing of many animals without anesthesia and facilitates rapid recovery with no signs of pain and discomfort in the mice. Here we compared the submandibular vein and retroorbital plexus blood collection methods, to determine the potential effect of the sampling technique on several clinical biochemistry parameters in C57BL/6J mice. We found statistically significant differences for 8 of the 9 biochemical parameters studied between the 2 blood sampling techniques. Compared with samples collected from the retroorbital plexus, blood obtained from the submadibular vein had higher levels of AST, ALT, protein, albumin, triglycerides, total cholesterol, and creatinine. Glucose values of retroorbital blood were higher than those from the submandibular vein. Urea levels were similar for both sampling techniques. Our results demonstrate that the technique used to obtain blood samples affects parameters commonly used to assess animal health. We recommend caution when comparing results of biochemical analysis of blood obtained from the submandibular vein in mice with reference values obtained by other blood sampling techniques.
Blood for biochemical analysis can be obtained in mice by various techniques, including bleeding from the retroorbital plexus (also described as the retrobulbar venous plexus and periorbital sinus), by the tailclip technique, by cardiocentesis, and by saphenous venipuncture.7,9,10 Each method can affect the outcome of serum biochemistry analysis, due to differences in handling, restraining, anesthesia, invasiveness, and animal discomfort.1,13,19,20 The retroorbital blood-collection method is widely used in mice.8,9,13 Although this method consistently yields a reasonable blood volume when the investigator is experienced in the procedure, retroorbital blood collection is controversial because it may cause pain, distress, or even blindness when performed incorrectly.21,22 A joint working group on refinement does not recommend retroorbital sampling because of the risk of tissue damage12 and states that this method is acceptable only as a terminal procedure while the animal is anesthetized.17
Recently, new blood sampling methods considered more humane and less aggressive than the retroorbital technique, such submandibular venipuncture, have been developed in mice.8 Although several reports16,19 address the retroorbital sampling method, detailed information regarding submandibular venipuncture is scarce in the published literature. The goal of our study was to compare the retroorbital technique of blood collection with submandibular venipuncture to determine the effect of this new bleeding method on several clinical biochemistry parameters in C57BL/6J mice.
Materials and Methods
Animals.
All the animal procedures were carried out at our AAALAC-accredited animal facility (CIC bioGUNE, Biscay, Spain) and conducted in accordance with the Guide for the Care and Use of Laboratory Animals11 and European policies.5 The animal colony was screened quarterly and tested negative for: minute virus of mice, mouse parvovirus, mouse hepatitis virus, pneumonia virus of mice, reovirus 3, Sendai virus, mouse rotavirus, mouse norovirus, mouse thymic virus, mouse cytomegalovirus, Hantaan virus, lymphocytic choriomeningitis virus, Theiler virus, mouse adenovirus, K virus, ectromelia virus, polyoma virus, lactic dehydrogenase virus, Clostridium piliforme, Bordetella bronchiseptica, Citrobacter rodentium, Corynebacterium kutscheri, Mycoplasma pulmonis, Pasteurella spp., Salmonella spp., Streptobacillus moniliformis, β-hemolytic Streptococcus, Streptococcus pneumoniae, Helicobacter spp., cilia-associated respiratory bacillus, ectoparasites, Encephalitozoon cuniculi, and pathogenic protozoa and helminthes. C57BL/6J male mice (age, 6 wk; n = 20) were obtained from Charles River Laboratories (L'Arbresle, France) and housed in groups of 5 in polycarbonate cages containing woodchip bedding (Lignocel, J Rettenmaier and Söhne, Rosenberg, Germany) in a room with controlled temperature (20 to 24 °C) and relative humidity (50% to 65%) and a 12:12-h dark:light cycle (lights on 0800 to 2000). Mice were fed rodent maintenance diet (2014, Harlan Teklad, Barcelona, Spain) and provided with water ad libitum. The protocol was approved by the Bioethical and Animal Welfare Committee of CIC bioGUNE (code P-CBG-CBBA-0307).
Experimental design.
Mice were assigned randomly to 2 groups (SM and RO) of 10 each and were acclimated for 2 wk before the first blood extraction. When mice were 8 and 16 wk old, blood samples were obtained by the retroorbital method from each animal in the RO group first and then by submandibular venipuncture from each animal of the SM group. When mice were 22 wk old, retroorbital samples were obtained from the mice that previously had undergone submandibular venipuncture and vice versa. This approach was used to control for possible intrinsic variations in the parameters under study among the mice.
Blood sampling method and sample handling.
Blood samples were always collected during the same time interval in the afternoon (1500 to 1530) after a fasting period of 5 h. Submandibular blood samples were obtained by incising the right submandibular vein of unanesthetized mice with a sterile 4-mm lancet (MediPoint, Mineola, NY). Retroorbital blood samples were collected from the right retroorbital plexus of anesthetized mice. Anesthesia was induced by placing each mouse in an inhalation chamber with 4% isoflurane (IsoFlo, Abbott Laboratories, Berkshire, UK) regulated with a calibrated vaporizer. Blood samples were deposited in serum separator gel tubes (Microtainer, Becton–Dickinson, Franklin Park, NJ) and centrifuged (9,300 × g, 30 min, 4 °C) for serum separation. Serum hemolysis was evaluated by direct observation and recorded as follows: 0, no hemolysis; 1, slight; 2, moderate; and 3, severe hemolysis. The volume of each blood sample was approximately 300 µL, and at no time did this volume exceed that recommended for mice in regard to body weight and recovery time.7 Mice were allowed to recover completely on a regulated thermal blanket after each bleeding session and were observed daily for signs of pain or discomfort.
Clinical chemistry parameters.
Serum activity AST and ALT and concentrations of triglycerides, glucose, total cholesterol, total protein, albumin, urea, and creatinine were determined by using an automated analyzer (Selectra Junior Spinlab 100, Vital Scientific, Dieren, Netherlands; Spinreact, Girona, Spain) according to the manufacturers' instructions. Standard controls were run before each determination, and the values obtained for the different biochemical parameters were always within the expected ranges. The intraassay variability of biochemical assays was relative to 12 repeated determinations of the control serum in the same analytical session, whereas interassay variability for each parameter was calculated on the mean values of control sera measured during 6 analytical sessions.
Statistical analysis.
To identify the optimal number of mice, a power analysis for determining sample size (SPSS 10.0, SPSS, Chicago, IL) was conducted with an alpha value of 0.05 and a power of 90%. Our laboratory's historical data were used to establish an expected difference in means and an expected SD for each biochemical parameter to perform this calculation.
For each biochemical parameter and time point, the group mean, SD, and 95% confidence interval were calculated for both blood sampling methods. Statistical analyses were performed (SPSS 10.0, SPSS) and included tests of normality (Kolmogorov–Smirnov) and equal variance (Levene median). The Grubb test (GraphPad Software, La Jolla, CA) was run for outlier detection. Outliers were removed, and group means, SD, and CI were recalculated.
To compare data between groups (SPSS 10.0, SPSS), the Student t test (parametric) was used when conditions of normality and equal variance were met. The Student t test was used with Welch correction when unequal variances were detected. When the normality test failed, a 2-tailed and exact Mann–Whitney rank sum test (nonparametric) was used. Differences were considered statistically significant at a P value of less than 0.05.
Results
Hemolysis level.
A mean hemolysis value was calculated for the SM and RO groups at each time point. Hemolysis did not differ significantly between SM and RO groups at 8 wk (0.6 and 0.2, respectively), 16 wk (0.6 and 0.3, respectively), or 22 wk (0.4 and 0.1, respectively).
Clinical chemistry parameters.
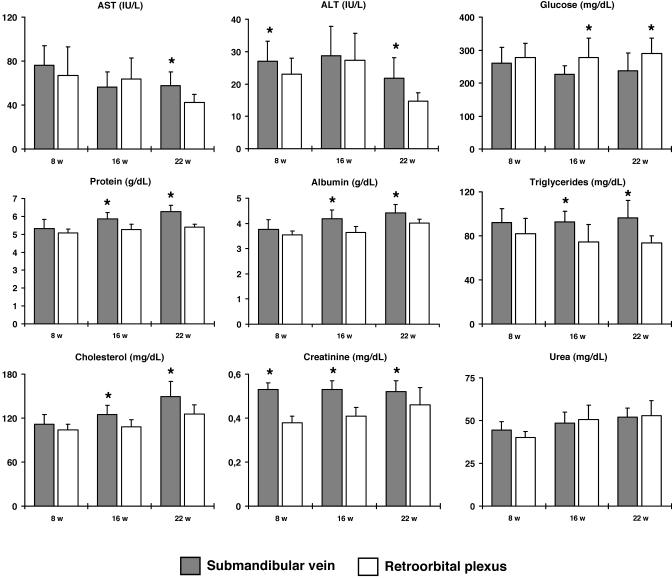
Intra- and interassay variability of analytical assays (expressed as coefficients of variation) were always less than 4% for all biochemical parameters (data not shown). Age-related statistical data for biochemical parameters derived from both blood sampling techniques are presented in Table 1. At 8 wk, blood obtained by submandibular venipuncture had significantly (P < 0.05) higher mean levels of ALT and creatinine compared with samples collected by retroorbital technique (Figure 1). Identical analyses performed at 16 wk revealed that blood collected from the SM site had significantly (P < 0.05) higher levels of protein, albumin, triglycerides, total cholesterol, and creatinine and significantly lower (P < 0.05) glucose than did blood collected from the RO site.
Table 1.
Serum biochemistry parameters in mice by age and collection method
| 8 wk |
16 wk |
22 wk |
|||||||||||
| Parameter | Collection method | n | Mean | SD | 95% Confidence interval | n | Mean | SD | 95% Confidence interval | n | Mean | SD | 95% Confidence interval |
| AST (IU/L) | |||||||||||||
| SM | 10 | 76.19 | 17.71 | 65.21–87.17 | 9a | 56.07 | 14.14 | 47.31–64.84 | 9a | 57.73b | 12.55 | 49.95–65.51 | |
| RO | 10 | 67.13 | 25.81 | 51.13–83.13 | 10 | 63.49 | 19.33 | 51.51–75.47 | 10 | 42.27 | 7.45 | 37.65–46.89 | |
| ALT (IU/L) | |||||||||||||
| SM | 9a | 27.00b | 6.16 | 23.18–30.82 | 9a | 28.79 | 9.00 | 23.21–34.37 | 9a | 21.78bc | 6.36 | 17.84–25.72 | |
| RO | 10 | 23.00 | 4.92 | 19.95–26.05 | 10 | 27.38 | 8.34 | 22.21–32.55 | 10 | 14.67 | 2.65 | 13.03–16.31 | |
| Glucose (mg/dL) | |||||||||||||
| SM | 10 | 260.61 | 48.02 | 230.85–290.37 | 10 | 225.66bc | 26.49 | 209.24–242.08 | 10 | 237.08b | 54.65 | 203.21–270.95 | |
| RO | 10 | 277.98 | 42.58 | 251.59–304.37 | 10 | 276.90 | 58.91 | 240.39–313.41 | 10 | 290. 14 | 45.96 | 261.66–318.62 | |
| Protein (g/dL) | |||||||||||||
| SM | 10 | 5.326 | 0.514 | 5.007–5.644 | 10 | 5.865b | 0.352 | 5.647–6.083 | 10 | 6.255b | 0.353 | 6.036–6.473 | |
| RO | 10 | 5.077 | 0.207 | 4.949–5.205 | 10 | 5.267 | 0.306 | 5.078–5.457 | 10 | 5.402 | 0.167 | 5.299–5.506 | |
| Albumin (g/dL) | |||||||||||||
| SM | 10 | 3.769 | 0.379 | 3.534–4.004 | 10 | 4.181b | 0.353 | 3.962–4.400 | 10 | 4.419b | 0.327 | 4.216–4.621 | |
| RO | 10 | 3.549 | 0.145 | 3.459–3.639 | 10 | 3.642 | 0.241 | 3.493–3.792 | 10 | 4.012 | 0.154 | 3.917–4.108 | |
| Triglycerides (mg/dL) | |||||||||||||
| SM | 10 | 92.25 | 12.22 | 84.67–99.83 | 9a | 92.74b | 9.56 | 86.82–98.67 | 10 | 96.28b | 15.87 | 86.45–106.11 | |
| RO | 10 | 82.08 | 13.75 | 73.56–90.60 | 10 | 74.34 | 15.87 | 64.51–84.17 | 10 | 73.39 | 6.40 | 69.43–77.35 | |
| Total cholesterol (mg/dL) | |||||||||||||
| SM | 10 | 111.53 | 13.17 | 103.37–119.69 | 10 | 124.57b | 12.88 | 116.59–132.55 | 10 | 149.37b | 20.76 | 136.50–162.24 | |
| RO | 10 | 103.92 | 7.79 | 99.09–108.75 | 10 | 108.05 | 9.93 | 101.90–114.20 | 10 | 125.79 | 12.47 | 118.06–133.52 | |
| Creatinine (mg/dL) | |||||||||||||
| SM | 10 | 0.53bd | 0.03 | 0.51–0.55 | 10 | 0.53b | 0.04 | 0.50–0.56 | 10 | 0.52b | 0.05 | 0.49–0.55 | |
| RO | 10 | 0.38 | 0.03 | 0.36–0.40 | 10 | 0.41 | 0.04 | 0.39–0.44 | 10 | 0.46 | 0.08 | 0.41–0.51 | |
| Urea (mg/dL) | |||||||||||||
| SM | 10 | 44.47 | 4.96 | 41.39–47.54 | 10 | 48.41 | 6.43 | 44.42–52.39 | 9a | 52.04 | 5.35 | 48.72–55.35 | |
| RO | 10 | 40.14 | 3.34 | 38.07–42.21 | 9a | 50.61 | 8.47 | 45.36–55.85 | 10 | 52.86 | 8.83 | 47.39–58.33 | |
SM, submandibular venipuncture; RO, retroorbital collection method.
1 outlier removed.
Significantly different (P < 0.05) from RO value.
Statistical comparison based on Welch test.
Statistical comparison based on Mann–Whitney rank sum test.
Figure 1.
Comparison of clinical biochemistry parameters in mice by age and collection method. Values (mean ± SD) for 9 parameters measured in the serum of C57BL/6J mice at 8, 16, and 22 wk of age by using submandibular (dark bars) and retroorbital (white bars) collection methods. Statistically significant differences (*, P < 0.05) observed among means are indicated.
At 22 wk of age (when mice were sampled from the alternate site), blood obtained from the SM site had higher (P < 0.05) levels of AST, ALT, protein, albumin, triglycerides, total cholesterol, and creatinine and lower glucose than did blood from the RO site. However, no difference between methods was found at any time point for urea.
Discussion
Many sources of variation can affect the results of clinical biochemistry assays.2,4,6,15,16,18,23 These alterations can result from events that occur prior to sample collection, such as the fasting condition or environmental stress. The blood sampling process itself is a source of variation, including the collection site, anesthesia used, level of hemolysis, and the skill of the technician performing the procedure. Finally, factors during the period that follows blood extraction may influence biochemical parameters and include tube selection, processing delay, and the analytical procedure itself. Additional factors, such as gender, age, and strain, also are known sources of variation in biochemical values. In light of the ability of the sampling method to alter assay results, here we focus on the effect of the new submandibular blood collection method on 9 biochemistry parameters in C57BL/6J mice.1,13,19,20 The 3 time points considered in this study (8, 16, and 22 wk of age) were chosen because most of published data about mice biochemical physiology refer to these ages.3,14
We found statistically significant differences between the 2 blood sampling techniques for 8 of the 9 biochemical parameters studied. Seven biochemical parameters (AST, ALT, triglycerides, total cholesterol, protein, albumin, and creatinine) showed small but statistically significant higher mean values in serum obtained by submandibular venipuncture compared with retroorbital blood collection. In contrast, serum collected from the retroorbital demonstrated significantly higher mean glucose values than that obtained from the submandibular vein. Urea concentration was unaffected by sampling technique at the 3 time points studied.
The blood sampling in this work was performed by the same skilled technician for all samples and time points, and all the actions performed before and after blood collection were standardized, so that variability due to these reasons could be excluded from our results. Therefore, we assume that the differences in the values assessed reflect factors directly associated with the blood sampling method, including handling stress, anesthesia, hemolysis, and tissue damage. That changing the bleeding technique between groups yielded similar results strongly suggests that the mean differences observed are associated directly with the blood collection technique used, rather than due to intrinsic variation of biochemical parameters among animals. Stress associated with the handling of mice during the bleeding process increases the glucose serum concentrations proportionally to the handling time.2 This situation may explain the substantial increase in glucose concentration in the RO group during our study: retroorbital blood sampling involves extensive handling, primarily because of anesthesia. In addition, the lower protein and albumin concentrations obtained after retroorbital blood collection may reflect effects of the isoflurane anesthesia used for this bleeding method. In line with this argument, total protein and albumin levels were lower in rats anesthetized with isoflurane in comparison with animals that did not receive anesthesia.6
In the present study, the level of hemolysis in all serum samples was scored by direct observation. Increased hemolysis has been proposed to contribute to differences in some biochemical parameters, including AST and ALT.16 The tissue damage that may occur during the sampling process is another factor that may contribute to differences between values obtained by different collection techniques. Additional studies should address the effects that these and other variables, including as mouse gender and strain, exert on samples obtained by submandibular venipuncture.
After 2 y of experience with the technique in our facility, we consider submandibular venipuncture much easier to perform than retroorbital blood collection. The submandibular bleeding method allows relatively rapid processing of many animals without anesthesia, thereby eliminating the potential effects of an anesthetic on animal physiology and the potential risk of ocular trauma. In addition, submandibular venipuncture accommodates the use of the same animal at multiple time points, facilitates rapid recovery, and is not associated with signs of pain and discomfort in mice after blood collection. In light of these observations, we now use submandibular venipuncture when repeated blood samples from mice are needed. We recommend the retroorbital method only as a terminal procedure.
The differences observed between the 2 sampling techniques we assessed suggest the necessity for individual laboratories to establish their own reference ranges for clinical biochemical parameters in mice, according to their routine analytical procedures. We recommend caution when biochemical parameter results obtained by submandibular venipuncture in mice must be compared with reference values obtained through other blood sampling techniques. Submandibular venipuncture clearly contributes to refinement of experimental technique, in accordance with ethical and legal regulations regarding the use of animals in biomedical research.5
Acknowledgments
This work was supported by the ETORTEK program (Industry Department of the Basque Government). We thank Drs Luis A Parada and James Sutherland for critical reading of the manuscript.
References
- 1.Abatan OI, Welch KB, Nemzek JA. 2008. Evaluation of saphenous venipuncture and modified tailclip blood collection in mice. J Am Assoc Lab Anim Sci 47:8–15 [PMC free article] [PubMed] [Google Scholar]
- 2.Balcombe JP, Barnard ND, Sandusky C. 2004. Laboratory routines cause animal stress. Contemp Top Lab Anim Sci 43:42–51 [PubMed] [Google Scholar]
- 3.Bogue MA, Grubb SC, Maddatu TP, Bult CJ. 2007. Mouse phenome database (MPD). Nucleic Acids Res 35:D643–D649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Champy MF, Selloum M, Piard L, Zeitler V, Caradec C, Chambon P, Auwerx J. 2004. Mouse functional genomics requires standardization of mouse handling and housing conditions. Mamm Genome 15:768–783 [DOI] [PubMed] [Google Scholar]
- 5.Council of the European Communities 1986. Council Directive 86/609/EEC on the approximation of laws, regulations, and administrative provisions of the member states regarding the protection of animals used for experimental and other scientific purposes. Off J Eur Commun L358: 1–29 [Google Scholar]
- 6.Deckardt K, Weber I, Kaspers U, Hellwig J, Tennekes H, van Ravenzwaay B. 2007. The effects of inhalation anesthetics on common clinical pathology parameters in laboratory rats. Food Chem Toxicol 45:1709–1718 [DOI] [PubMed] [Google Scholar]
- 7.Diehl KH, Hull R, Morton D, Pfister R, Rabemampianina Y, Smith D, Vidal JM, van de Vorstenbosch C. 2001. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J Appl Toxicol 21:15–23 [DOI] [PubMed] [Google Scholar]
- 8.Golde WT, Gollobin P, Rodriguez LL. 2005. A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab Anim (NY) 34:39–43 [DOI] [PubMed] [Google Scholar]
- 9.Hem A, Smith AJ, Solberg P. 1998. Saphenous vein puncture for blood sampling of the mouse, rat, hamster, gerbil, guinea pig, ferret, and mink. Lab Anim 32:364–368 [DOI] [PubMed] [Google Scholar]
- 10.Hoff J. 2000. Methods of blood collection in the mouse. Lab Anim (NY) 29:47–53 [Google Scholar]
- 11.Institute of Laboratory Animal Resources 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press; [DOI] [PubMed] [Google Scholar]
- 12.Le Net JL, Abbot DP, Mompon RP, Leblanc B. 1994. Repeated orbital sinus puncture in rats induced damage to optic nerve and retina. Vet Pathol 31:621 [Google Scholar]
- 13.Mahl A, Heining P, Ulrich P, Jakubowski J, Bobadilla M, Zeller W, Bergmann R, Singer T, Meister L. 2000. Comparison of clinical pathology parameters with two different blood sampling techniques in rats: retrobulbar plexus versus sublingual vein. Lab Anim 34:351–361 [DOI] [PubMed] [Google Scholar]
- 14.Mallon AM, Blake A, Hancock JM. 2008. EuroPhenome and EMPReSS: online mouse phenotyping resource. Nucleic Acids Res 36Database issue:D715–D718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuzawa T, Hayashi Y, Nomura M, Unno T, Igarashi T, Furuya T, Sekita K, Ono A, Kurokawa Y, Hayashi Y. 1997. A survey of the values of clinical chemistry parameters obtained for a common rat blood sample in 98 Japanese laboratories. J Toxicol Sci 22:25–45 [DOI] [PubMed] [Google Scholar]
- 16.Mazzaccara C, Labruna G, Cito G, Scarfò M, De Felice M, Pastore L, Sachetti L. 2008. Age-related reference intervals of the main biochemical and haematological parameters in C57BL/6J, 129SV/EV, and C3H/HeJ Mouse strains. PLoS One 3:e3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morton DB, Abbot D, Barclay R, Close BS, Ewbank R, Gask D, Heath M, Mattic S, Poole T, Seamer J, Southee J, Thompson A, Trussel B, West C, Jennings M. 1993. Removal of blood from laboratory mammals and birds. Lab Anim 27:1–22 [DOI] [PubMed] [Google Scholar]
- 18.Riley JH. 1992. Clinical pathology: preanalytical variation in preclinical safety assessment studies—effect on predictive value of analyte tests. Toxicol Pathol 20:490–500 [DOI] [PubMed] [Google Scholar]
- 19.Schnell MA, Hardy C, Hawley M, Propert KJ, Wilson JM. 2002. Effect of blood collection technique in mice on clinical pathology parameters. Hum Gene Ther 13:155–162 [DOI] [PubMed] [Google Scholar]
- 20.van Herck H, Baumans V, Brandt CJWM, Boere HAG, Hesp APM, van Lith HA, Schurink M, Beynen AC. 2001. Blood sampling from the retroorbital plexus, the saphenous vein, and the tail vein in rats: comparative effects on selected behavioural and blood variables. Lab Anim 35:131–139 [DOI] [PubMed] [Google Scholar]
- 21.van Herck H, Baumans V, Brandt CJWM, Hesp APM, Sturkenboom JH, van Lidth HA, van Tintelen G, Beynen AC. 1998. Orbital sinus blood sampling in rats as performed by different animal technicians: the influence of technique and expertise. Lab Anim 32:377–386 [DOI] [PubMed] [Google Scholar]
- 22.van Herck H, Baumans V, van der Craats NR, Hesp APM, Meijer GW, van Tintelen G, Walvoort HC, Beynen AC. 1992. Histological changes in the orbital region of rats after orbital bleeding. Lab Anim 26:53–58 [DOI] [PubMed] [Google Scholar]
- 23.Zhou X, Hansson GK. 2004. Effect of sex and age on serum biochemical reference ranges in C57BL/6J mice. Comp Med 54:176–178 [PubMed] [Google Scholar]