Abstract
Here we describe diagnosis of concurrent infection with Aeromonas hydrophila, Mycobacterium spp., and Batrachochytrium dendrobatidis in a wild female Xenopus laevis captured in Chile and transported to the United States. After approximately 130 d in the laboratory, the frog was presented for dysecdysis and obtundation. After euthanasia, tissues were submitted for histopathologic evaluation and PCR analysis for B. dendrobatidis and Ranavirus. Clinically significant gross lesions included cutaneous ulcerations on the lip, right forelimb, and ventral chest. Microscopic findings included regionally extensive splenic necrosis, diffuse pneumonia, and fibrinous coelomitis all containing intralesional bacteria. PCR analysis yielded positive results for B. dendrobatidis only. Bacterial culture of the ulcerated skin and liver yielded A. hydrophila. Infection with Contracaecum spp. was diagnosed as an incidental finding. To our knowledge, this case is the first report of simultaneous infection with Aeromonas hydrophila, Mycobacterium spp., and Batrachochytrium dendrobatidis in a laboratory-maintained X. laevis captured from the wild.
The African clawed frog, Xenopus laevis, is likely the most widely used amphibian research model.22,30 This species is an aquatic anuran and is readily suited for the research environment due to year-round gametogenesis, brief generation time, longevity in captivity, and ability to adjust to various laboratory conditions.11 Xenopus oocytes historically have been used in human pregnancy assays,22 and their recent popularity is attributable to their widespread use in cell and molecular biology research22,26 and developmental toxicology investigations.22,30 Because clawed frogs are present in virtually all vivaria supporting the aforementioned research endeavors,22,30 accurate diagnosis of clinical conditions is paramount for the laboratory animal practitioner and other research personnel.
Bacterial infections have long been a challenge to investigators using frogs for research.4,6,9,13,15 Mycobacterium spp. are implicated frequently as the cause of anuran disease. Species of Mycobacterium isolated from X. laevis include M. marinum, M. chelonae, M. xenopi, and M. liflandii.10 Mycobacterium spp. are potential zoonotic pathogens and thereby raise additional concerns for laboratory personnel. Aeromonas hydrophila has been reported to cause one of the most devastating infectious diseases in laboratory amphibians.3 Historically called red-leg disease, A. hydrophila infections have been cited for widespread amphibian mortality in wild and captive populations.13,22 Recent evidence suggests multiple pathogens may cause signs similar to A. hydrophila. As a result, epizootics attributed to red-leg disease prior to the 1990s may have been misdiagnosed and over-reported. Newly recognized pathogens with similar clinical presentations include ranaviruses and the chytrid fungus Batrachochytrium dendrobatidis.5 As evidence of their world-wide significance, both Ranavirus and B. dendrobatidis have recently been listed as diseases notifiable to the World Organisation for Animal Health.36 Since the mid1990s, amphibian mass mortalities and population declines in the wild have coincided with the sudden appearance of chytridiomycosis and its etiologic agent B. dendrobatidis.20,34,35 Although predominately a disease of wild amphibians, chytridiomycosis is also problematic in captive colonies.5,7,19,23,24,35 To date, a single report has described B. dendrobatidis infection in laboratory-maintained frogs (X. tropicalis and X. laevis).23 Here we discuss diagnosis of concurrent infection with A. hydrophila, Mycobacterium spp., and B. dendrobatidis in a female X. laevis.
Case Report
A colony (average daily census, 14) of female, pigmented ‘wildtype’ X. laevis was maintained and used at our institution (University of Tennessee, Knoxville, TN) pursuant to an IACUC-approved protocol for aseptic oocyte collection. All frogs were acquired from a single supplier (Xenopus Express, Brooksville, FL) at various times and were housed on arrival in shipment pairs. Frogs were examined visually for gross lesions and injuries prior to colony introduction but were neither prophylactically treated nor quarantined. The frog we describe here was received with 3 other frogs and was cohoused on arrival with another frog received in the same shipment. According to the supplier, all frogs were captured in Santiago, Chile, and were acclimated for 4 to 6 wk prior to shipment.17
Frogs were housed in approximately 25 L water in static polypropylene tanks (18 in. × 12 in. × 12 in.) supported by a bileveled metal frame. Water was received from a common municipal source, treated with a probiotic (Koi Care Kennel, Westminster, CA) and 2 chlorine and heavy metal removers (Aquarium Pharmaceuticals, Chalfont, PA, and Novalek, Hayward, CA), and aged for 48 h prior to use. Frogs were maintained in a single room on a 12:12-h light:dark cycle at 72 ± 1 °F and fed a pelleted chow (Nasco, Modesto, CA) on alternate days. Water temperature and pH were maintained at 21.2 ± 2 °C and 7.4 to 7.8, respectively. Tanks were changed weekly, and prior to frog introduction, water quality was assessed by use of commercially available reagents (Aquarium Pharmaceuticals) and test strips (Jungle Laboratories, Cibolo, TX). At tank change, animals were captured in their hometank polyvinyl-chloride enrichment tube and transferred to a new enclosure. Enrichment tubes were sanitized weekly at the time of tank change in a mechanical washer (model SW6700, Scientek Technology Corporation, Delta, BC, Canada). Waste water was discarded down facility drains, and soiled tanks were hand-washed with a detergent (Proctor and Gamble, Cincinnati, OH), filled with undiluted bleach (The Clorox Company, Oakland, CA), and allowed to sit for 3 d. Tanks were emptied, filled with tap water, and allowed to sit for an additional day. Tanks then were rinsed with municipal water and air-dried. All frogs were housed, cared for, and used in compliance with the Guide for the Care and Use of Laboratory Animals14 in an AAALAC-accredited program.
A 234-g mature adult female frog was presented for dysecdysis and obtundation. Large desquamated skin flakes were observed throughout the water column. The frog was received approximately 4 mo prior and had been used for 2 separate, intracoelomic oocyte collection procedures. The surgeries were performed approximately 60 d apart, with the last procedure occurring 43 d prior to the frog's presentation. The frog was euthanized by overdose of buffered tricaine methanesulfonate (1.5 g/L) solution followed by double pithing. After euthanasia, a single digit was excised and submitted along with sloughed skin to a commercial laboratory (Pisces Molecular, Boulder, CO) for PCR analysis for B. dendrobatidis and Ranavirus. The remaining carcass was submitted for necropsy evaluation. A water sample was collected and submitted for analysis by a local aquarium hobbyist store (Aquarium, Knoxville, TN). Water quality parameters measured included ammonia (0.5 ppm), pH (7.4), and general hardness (300 ppm).
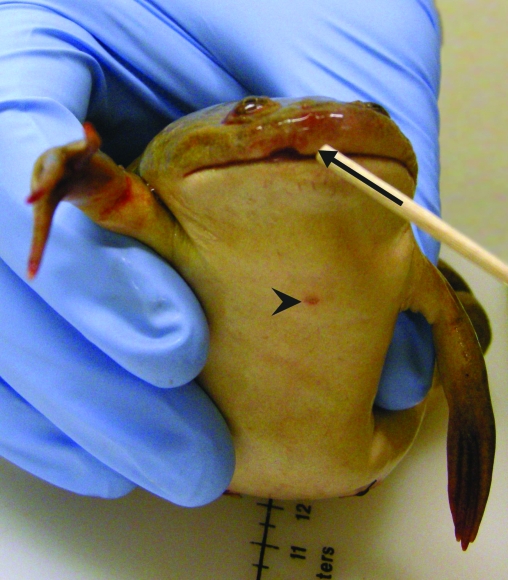
On gross examination, 3 cutaneous ulcerations including a 3-mm-diameter focus on the upper lip (Figure 1), a 1.5-mm-diameter focus on the dorsal aspect of the right forelimb, and a 2-mm-diameter focus located on the ventral chest were identified (Figure 1). The coelom contained 35 mL serosanguineous effusion and innumerable gelatinous eggs. In addition, along the serosal surface of the stomach, 5 coiled nematodes formed a 3-mm-diameter nodule.
Figure 1.
Cutaneous ulcerations on the upper lip (diameter, 3 mm; arrow) and ventral chest (diameter, 2 mm; arrowhead)
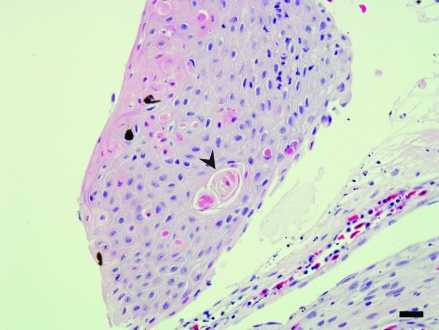
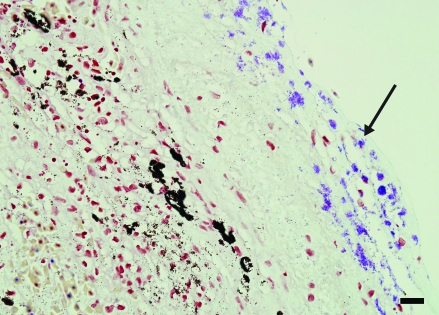
Sections of lesional and nonlesional skin, lung, liver, kidney, heart, spleen, stomach, ovary, and small intestine were collected, fixed in 10% buffered formalin, routinely processed, and stained with hematoxylin and eosin. Despite examination of multiple sections of skin, much of the ulcerated regions were lost during sectioning. The skin adjacent to the ulcers showed multifocal single-cell apoptosis and necrosis of epidermal cells and accompanying superficial dermal fibrosis (Figure 2). Gram staining revealed moderate numbers of gram-positive bacilli, and Ziehl–Neilson staining revealed few acid-fast bacilli within the deep dermis of lesional skin. The pulmonary parenchyma was focally necrotic and infiltrated by neutrophils. In addition, eosinophilic fluid, fibrin, and minimal cellular debris were present within alveolar lumens. Fluid within the alveolar interstitium and alveolar macrophages contained both gram-positive and acid-fast bacilli. Along the capsular surface of the liver, a thin layer of fibrin interspersed with necrotic and viable neutrophils and red blood cells was present (Figure 3). Both gram-positive and acid-fast bacilli were present in the fibrinous exudate. Few lymphocytes were seen in portal triads. Extramedullary hematopoiesis was noted within sinusoids in the periportal region. Gram and acid-fast staining failed to reveal organisms within the hepatic parenchyma.
Figure 2.
Skin. This section shows disorganization of the surface epithelium, and dyskeratotic cells (arrowhead) are noted within the stratum granulosum. There is a layer of fibrin and a few inflammatory cells beneath the epidermis. Hematoxylin and eosin stain; bar, 70 µm.
Figure 3.
Liver. A layer of fibrin and few inflammatory cells are present along the capsular surface (arrow). Large melanomacrophage centers are noted within the otherwise normal parenchyma (arrowhead). Hematoxylin and eosin stain; bar, 35 µm.
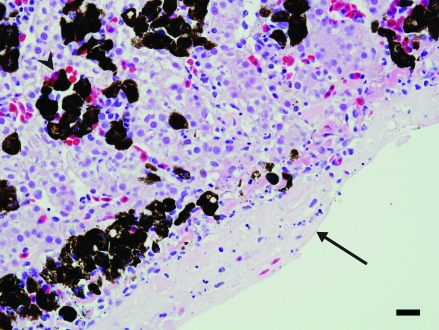
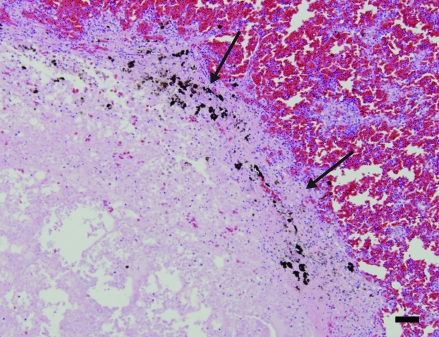
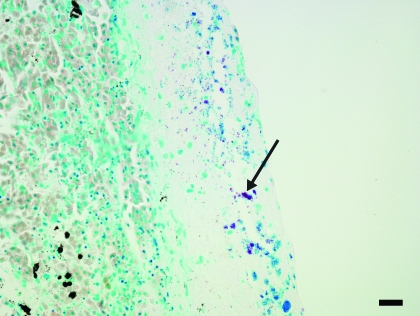
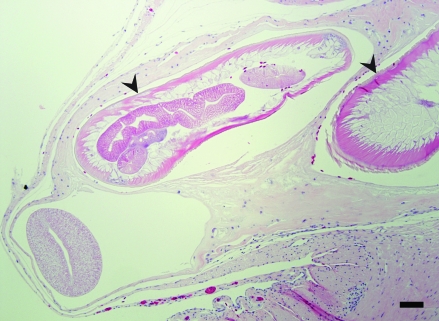
Within the renal section, a solitary focus of acute tubular degeneration and necrosis of tubular epithelial cells was present. Inflammatory infiltrates were minimal. Spleen contained a large focus and several smaller foci of necrosis affecting approximately 30% of the section. The necrosis was characterized by loss of cellular detail, pyknosis, karyorrhexis, and deposition of fibrin (Figure 4). Faint bacteria outlines similar to those in the lung were scattered throughout the fibrin. Gram staining demonstrated the presence of myriad gram-positive coccobacilli (Figure 5) both within the necrotic region and within the capsular fibrin mat. Acid-fast staining demonstrated the presence of few organisms within the splenic parenchyma and within the fibrinous exudate (Figure 6). The capsular surface of the spleen was coated with fibrin, into which the bacteria infiltrated. Lastly, a nodular proliferation within the serosa and outer muscularis of the stomach contained multiple cross-sections of nematodes (Figure 7). These nematodes were approximately 1 mm in width and had a ridged cuticle. The cuticle was lined internally by coelomyarian musculature, and there were prominent lateral cords. Within the body cavity was a muscular esophagus adjacent to a muscular cecum. In addition, the organism contained an immature gonad. No significant lesions were identified in nonlesional skin, heart, ovary, or small intestine.
Figure 4.
Spleen. Approximately 30% of the organ is necrotic (arrows). Within the region of pallor, all architectural detail is lost and replaced by eosinophilic cell debris. Hematoxylin and eosin stain; bar, 120 µm
Figure 5.
Spleen. Within the necrotic region, multiple gram-positive cocci are present (arrow). Gram stain; bar, 35 µm.
Figure 6.
Spleen. A layer of fibrin and few inflammatory cells are present along the capsular surface. Acid-fast–positive bacteria are admixed within the exudate (arrow). Ziehl–Neilson stain; bar, 120 µm.
Figure 7.
Stomach serosa. Within the serosa, there is a fibrous-walled nodule in which several cross-sections of nematodes, characterized as Contracaecum spp., are present. Hematoxylin and eosin stain; bar, 300 µm.
Ancillary tests included aerobic culture of the liver and the ulcerated skin of the lip. No other tissues or fluids were cultured. Although A. hydrophila was isolated from broth only from the liver sample, more than 30 colonies were recovered from the ulcerated lip. In addition, 4 colonies of a gram-positive cocci (Micrococcus-like) were recovered. Parasite identification yielded a diagnosis of Contracaecum spp. for the gastric serosal worm.
This frog had multiple bacterial infections. A. hydrophila was isolated from both lesional skin and liver. The animal was infected concurrently with a second uncultured, gram-positive coccal bacteria that was present in the necrotizing lesions in the spleen and the lung. In addition, acid-fast bacilli most consistent with Mycobacterium spp. were present in the celomic fibrin, lung, spleen, and lesional skin. Fungi were not detected in any examined tissue section. Moreover, intracytoplasmic inclusions consistent with Ranavirus inclusion bodies were not observed.
The excised digit and sloughed skin submitted for PCR analysis were assayed for the presence of the B. dendrobatidis ribosomal RNA intervening transcribed sequence region by 45-cycle single-round PCR amplification1 that was modified for increased specificity and sensitivity at the testing laboratory (Pisces Molecular). The signal from the submitted sample was very strongly positive for B. dendrobatidis infection. Tissue also was assayed for the presence of the Ranavirus major capsid protein gene with single-round PCR amplification.18 No signal was detected from the submitted sample.
Discussion
Histologic, microbiologic, and molecular findings resulted in a diagnosis of a bacterial septicemia with A. hydrophila and Mycobacterium spp. and concomitant B. dendrobatidis infection. To our knowledge, this report is the first description of concurrent infections with Mycobacterium spp., A. hydrophila, and B. dendrobatidis in a laboratory-maintained X. laevis. Another recent report19 described concurrent infection with ranavirus, B. dendrobatidis, and A. hydrophila in a captive colony of Dendrobates auratus (green and black poison dart frog), Phyllobates terribilis (golden poison frog), Pyxicephalus adspersus (African bullfrog), and Rhacophorus dennysi (Chinese gliding frog).
A. hydrophila is a waterborne, gram-negative bacillus that is a commensal inhabitant of the gastrointestinal tract of clinically healthy frogs.13 Stress and consequent immunomodulation predispose amphibians to A. hydrophila colonization and clinical disease.22 Aeromoniasis is a communicable disease of teleost fishes, amphibians, and reptiles.8 The skin and visceral organs are common sites of A. hydrophila colonization in amphibians, and clinical presentation may include cutaneous petechiation and ulceration, lethargy, anorexia, edema, and neurologic signs.3,8,13,15,31 A. hydrophila infection in the frog we describe here was determined by bacterial culture of skin and liver and was associated with cutaneous ulceration. A. hydrophila was likely an opportunistic pathogen in this frog and may have been resulted from environmental inoculation through ulcerated skin or colonization of normal intestinal flora. Other visceral organs including the lung, heart, celomic cavity and spleen were not cultured. Gram stains of the lung and spleen failed to detect noteworthy numbers of gram-negative bacilli but rather identified concurrent gram-positive coccobacilli. Because the bacteria from these organs were not cultured, an etiologic agent was not identified; potential pathogens include Staphylococcus and Streptococcus spp.
The necrotic and inflammatory lesions noted in the lungs, liver, and spleen initially were considered to be part of a septicemia due to A. hydrophila. The presence of concurrent acid-fast bacteria was suggestive of concurrent primary infection by presumptive Mycobacterium spp. (no cultures were performed). Mycobacteria are aerobic, nonmotile, acid-fast organisms that are found commonly in aquatic environments.10 Both A. hydrophila and Mycobacterium spp. were present in sections of lung, liver, and spleen present as revealed by Gram and Zeihl–Neilson staining, respectively. Amphibian mycobacteriosis often is characterized by the formation of cutaneous and visceral granulomas or cutaneous ulcers.33 Mycobacteriosis does not appear to be highly communicable from animal to animal and most likely is transmitted through environmental inoculation of traumatized skin.22 No organisms were noted within the epithelium of the lesional skin although preservation of ulcerated regions was poor. Accumulations of A. hydrophila and Mycobacterium spp. were present within the deep dermis, suggesting that skin was colonized by both organisms.
Reported clinical signs of B. dendrobatidis infection in anurans include obtundation, dysecdysis, and erythema and ulceration of the skin.20,23,24 Because the frog we report here presented with similar signs, chytridiomycosis was a likely differential diagnosis. B. dendrobatidis is a member of the phylum Chytridiomycota and is the only known member of the phylum to parasitize a vertebrate host.28 B. dendrobatidis colonizes the keratinized skin of postmetamorphic amphibians.21 The pathogen is thought to be spread by waterborne zoospores that attach, enter, and replicate within host epithelial cells. Once inside the cell, the zoospore transforms into a thallus and then a zoosporangium. Zoosporangia release zoospores from the host cell through a membranous discharge tube, and the cycle is repeated.29 Despite repeated sectioning and evaluation, fungi were not detected in any examined skin section. B. dendrobatidis infection was confirmed by PCR analysis. The infection was deemed subclinical because zoosporangia were not identified on histopathologic skin sections. The subclinical B. dendrobatidis infection in this frog corresponds to other reported cases of chytrid infection among captive colonies of X. laevis.23,27 Moreover, wild X. laevis do not exhibit clinical signs, nor has the species experienced the sudden die-offs reported in other amphibians.35 Three purified peptides secreted by X. laevis skin have been shown to exert in vitro antimicrobial activity against B. dendrobatidis. Secretion of these peptides—caerulein precursor fragment family members, peptide with aminoterminal glycine and carboxyterminal leucinamide, and magainin II—may explain the nonclinical presentation in this species.28 The subclinical chytrid infection we observed differs remarkably from the fulminant disease reported in X. tropicalis.23 Our evidence supports other findings35 and suggests that X. laevis could be a natural carrier of B. dendrobatidis and argues for strict separation of the 2 species in the laboratory environment. Moreover, due to the asymptomatic presentation of B. dendrobatidis in X. laevis, the species has been implicated in international translocation of the pathogen.35
The serosanguineous celomic effusion may have been due to any number of reasons and likely is best explained by leakage of high-protein fluid from associated inflamed vessels. Although lymphatic blockage, hypoproteinemia, and increased oncotic pressure can contribute to such change, there is little evidence to support these as underlying pathogeneses in this case. The finding of Contracaecum spp. in this frog is thought to be incidental but demonstrates the pathogens that may be present in wild-caught laboratory frogs. The genus Contracaecum includes nematodes parasitic in fish-eating birds and mammals as definitive hosts, fishes as intermediate hosts, and snails and slugs as paratenic hosts.32 A single report describes Contracaecum spp. infection in wild X. laevis.16 We concur with the conclusion of other colleagues16—that due to the completely aquatic life-history of X. laevis, the species may serve as a paratenic host for Contracaecum spp.
The source of Mycobacterium spp. and B. dendrobatidis was not determined. The pathogens were likely present in the frog on arrival at our facility. The frog we describe here was wild-caught in Chile and may have acquired the infections in the native habitat. To our knowledge, chytridiomycosis has not been reported in Chile, but there are 2 reports of chytridiomycosis affecting anurans from Argentina.2,12 Moreover, the temperate forest in Chile has been identified as a region suitable for B. dendrobatidis infection.29 These facts coupled together make the existence of B. dendrobatidis in wild Chilean fauna highly plausible. Alternatively, the frog may have acquired infections by cohabitation with diseased frogs at the supplier and prior to shipment our facility.
Factors associated with the occurrence of clinical A. hydrophila infection in this frog may have included water temperatures exceeding 22 °C, infrequent water changes, postsurgical stress, trauma,13 and Mycobacterium spp. infection. In addition, water temperature may have affected the growth of B. dendrobatidis, given that the fungus grows optimally at temperatures of 17 to 25 °C.25 Water temperatures of 18 to 24 °C are considered acceptable for adequate Xenopus growth.22 The pH of our tanks (7.5 to 7.8) exceeded the pH optimum (6.7) for B. dendrobatidis.25
The frog we describe here was 1 of 4 recent imports into our colony. At the time of the outbreak, no specific tank service order was established, and instruments and gloves were shared between tanks. Moreover, we declined to treat unaffected frogs prophylactically. Despite these practices, clinical signs were not observed in additional frogs in the 6 mo after the presentation of the frog we describe here. To prevent future outbreaks, import and husbandry practices were reevaluated. Importation of frogs into the colony has been restricted to purpose-bred stock. All newly acquired frogs are housed on bottom shelves and serviced last. Furthermore, complete tank changes and water quality assessments were increased to biweekly, and strict requirements for change of gloves and restriction of equipment movement between tanks was instituted. In addition, all waste water is treated with sodium hypochlorite at a ratio of 1:32 prior to disposal.
In summary, we have documented concurrent infection with A. hydrophila, Mycobacterium spp., and B. dendrobatidis in X. laevis. The results of this diagnostic investigation demonstrate continued relevance of A. hydrophila and Mycobacterium spp. and the emergence of B. dendrobatidis as pathogens capable of causing or precipitating morbidity in Xenopus spp. Institutions using Xenopus spp. should exercise caution when importing wildcaught frogs of unknown health status, and decisions to procure wild fauna should only be made after careful risk–benefit analysis. Moreover, husbandry practices should be developed and implemented to reduce environmental stress, minimize pathogen growth, and avoid cross-contamination between species and conspecifics.
Acknowledgments
The authors acknowledge the assistance of Shelley Gentry in formatting this manuscript. We also thank Debra Lee Miller (Veterinary Diagnostic and Investigational Laboratory, University of Georgia), Matthew Gray (Center for Wildlife Health; Department of Forestry, Wildlife, and Fisheries; University of Tennessee Institute of Agriculture), and Juergen Schumacher, DVM, DACZM (Department of Small Animal Clinical Sciences, University of Tennessee Institute of Agriculture) for their review of preliminary versions of this manuscript. In addition, Deborah Floyd is commended for her commitment to animal care.
References
- 1.Annis SL, Dastoor FP, Ziel H, Daszak P, Longcore J. 2004. A DNA-based assay identifies Batrachochytrium dendrobatidis in amphibians. J Wildl Dis 40:420–428 [DOI] [PubMed] [Google Scholar]
- 2.Barrionuevo S, Mangione S. 2006. Chytridiomycosis in two species of Telmatobius (Anura: Leptodactylidae) from Argentina. Dis Aquat Organ 73:171–174 [DOI] [PubMed] [Google Scholar]
- 3.Boyer CI, Blackler K, Delanney LE. 1971. Aeromonas hydrophila infection in the Mexican axolotl, Siredon mexicanum. Lab Anim Sci 21:372–375 [PubMed] [Google Scholar]
- 4.Cromeens DM, Robbins VW, Stephens LC. 1987. Diagnostic exercise: cutaneous lesions in frogs. Lab Anim Sci 37:58–59 [PubMed] [Google Scholar]
- 5.Densmore CL, Green DE. 2007. Diseases of amphibians. ILAR J 48:235–254 [DOI] [PubMed] [Google Scholar]
- 6.Emerson H, Norris C. 1905. “Red-Leg”—an infectious disease of frogs. J Exp Med 7:32–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forzan MJ, Gunn H, Scott P. 2008. Chytridiomycosis in an aquarium collection of frogs: diagnosis, treatment, and control. J Zoo Wildl Med 39:406–411 [DOI] [PubMed] [Google Scholar]
- 8.Frye FL. 1985. An unusual epizootic of anuran aeromoniasis. J Am Vet Med Assoc 187:1223–1224 [PubMed] [Google Scholar]
- 9.Gibbs EL. 1963. An effective treatment for red-leg disease in Rana pipiens. Lab Anim Care 13:781–783 [PubMed] [Google Scholar]
- 10.Godfrey D, Williamson H, Silverman J, Small PLC. 2007. Newly identified Mycobacterium species in a Xenopus laevis colony. Comp Med 57:97–104 [PubMed] [Google Scholar]
- 11.Green SL. 2002. Factors affecting oogenesis in the South African clawed frog (Xenopus laevis). Comp Med 52:307–312 [PubMed] [Google Scholar]
- 12.Herrera RA, Steciow MM, Natale GS. 2005. Chytrid fungus parasitizing the wild amphibian Leptodactylus ocellatus (Anura: Leptodactylidae) in Argentina. Dis Aquat Organ 64:247–252 [DOI] [PubMed] [Google Scholar]
- 13.Hubbard GB. 1981. Aeromonas hydrophila infection in Xenopus laevis. Lab Anim Sci 31:297–300 [PubMed] [Google Scholar]
- 14.Institute of Laboratory Animal Resources 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press; [DOI] [PubMed] [Google Scholar]
- 15.Koivastik T. 1950. An outbreak of red-leg disease in Xenopus laevis controlled with Penicillin G. Am J Clin Pathol 20:71–74 [DOI] [PubMed] [Google Scholar]
- 16.Kuperman BI, Matey VE, Fisher RN, Ervin EL, Warburton ML, Bakhireva L, Lehman CA. 2004. Parasites of the African clawed frog, Xenopus laevis, in southern California, USA. Comparative Parasitology 71:229–232 [Google Scholar]
- 17.Lilley B.2009. Personal communication.
- 18.Mao J, Hedrick RP, Chinchar VG. 1997. Molecular characterization, sequence analysis, and taxonomic position of newly isolated fish iridoviruses. Virology 229:212–220 [DOI] [PubMed] [Google Scholar]
- 19.Miller DL, Rajeev S, Brookins M, Cook J, Whittington L, Baldwin CA. 2008. Concurrent infection with ranavirus, Batrachochytrium dendrobatidis, and Aeromonas in a captive anuran colony. J Zoo Wildl Med 39:445–449 [DOI] [PubMed] [Google Scholar]
- 20.Nolen RS. 2009. New guidelines intended to guard amphibians against deadly fungus. J Am Vet Med Assoc 234:1000. [PubMed] [Google Scholar]
- 21.Olsen V, Hyatt AD, Boyle DG, Mendez D. 2004. Co-localisation of Batrachochytrium dendrobatidis and keratin for enhanced diagnosis of chytridiomycosis in frogs. Dis Aquat Organ 61:85–88 [DOI] [PubMed] [Google Scholar]
- 22.O'Rourke DP, Schultz TW. 2002. Biology and diseases of amphibians, p 793–826 : Fox JG, Anderson LC, Lowe FM, Quimby FW. Laboratory animal medicine, 2nd ed San Diego (CA): Academic Press [Google Scholar]
- 23.Parker JM, Mikaelian I, Hahn N, Diggs HE. 2002. Clinical diagnosis and treatment of epidermal chytridiomycosis in African clawed frogs (Xenopus tropicalis). Comp Med 52:265–268 [PubMed] [Google Scholar]
- 24.Pessier AP, Nichols DK, Longcore JE, Fuller MS. 1999. Cutaneous chytridiomycosis in poison dart frogs (Dendrobates spp.) and White's tree frogs (Litoria caerulea). J Vet Diagn Invest 11:194–199 [DOI] [PubMed] [Google Scholar]
- 25.Piotrowski JS, Annis SL, Longcore JE. 2004. Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycologia 96:9–15 [PubMed] [Google Scholar]
- 26.Pritchett KR, Sanders GE. 2007. Epistylididae ectoparasites in a colony of African clawed frogs (Xenopus laevis). J Am Assoc Lab Anim Sci 46:86–91 [PubMed] [Google Scholar]
- 27.Reed KD, Ruth GR, Meyer JA, Shukla SK. 2000. Chlamydia pneumonia infection in a breeding colony of African clawed frogs (Xenopus tropicalis). Emerg Infect Dis 6:196–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rollins-Smith LA, Ramsey JP, Reinert LK, Woodhams DC, Livo LJ, Carey C. 2009. Immune defenses of Xenopus laevis against Batrachochytrium dendrobatidis. Front Biosci (Schol Ed) 1:68–91 [DOI] [PubMed] [Google Scholar]
- 29.Ron SR. 2005. Predicting the distribution of the amphibian pathogen Batrachochytrium dendrobatidis in the new world. Biotropica 37:209–221 [Google Scholar]
- 30.Schultz TW, Dawson DA. 2003. Housing and husbandry of Xenopus for oocyte production. Lab Anim (NY) 32:34–39 [DOI] [PubMed] [Google Scholar]
- 31.Smith SW. 1950. Chloromycetin in the treatment of “red leg”. Science 112:274–275 [DOI] [PubMed] [Google Scholar]
- 32.Thiengo SC, Santos SB, Vicente JJ, Pinto RM. 2000. Occurrence of Contracaecum sp. larvae (Nematoda, Anisakidae) in Gundlachia radiate (Guilding, 1828) (Mullusca, Gastropoda, Ancylidae) in Brazil. J Invertebr Pathol 75:178–179 [DOI] [PubMed] [Google Scholar]
- 33.Trott KA, Stacy BA, Lifland BD, Diggs HE, Harland RM, Khokha MK, Grammer TC, Parker JM. 2004. Characterization of a Mycobacterium ulcerans-like infection in a colony of African tropical clawed frogs (Xenopus tropicalis). Comp Med 54:309–317 [PubMed] [Google Scholar]
- 34.Voyles J, Berger L, Young S, Spears R, Webb R, Warner J, Rudd D, Campbell R, Skerratt LF. 2007. Electrolyte depletion and osmotic imbalance in amphibians with chytridiomycosis. Dis Aquat Organ 77:113–188 [DOI] [PubMed] [Google Scholar]
- 35.Weldon C, du Preez LH, Hyatt AD, Muller R, Speare R. 2004. Origin of the amphibian chytrid fungus. Emerg Infect Dis 10:2100–2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Organisation for Animal Health. [Internet]. OIE listed diseases: 01 May 2009 update. [Cited 30 Jun 2009]. Available at http://www.oie.int/eng/maladies/en_classification2009.htm?e1d7.