Abstract
An adult female squirrel monkey (Saimiri sciureus) presented with a 3.0 × 2.5 cm firm mass palpable within the caudal abdomen. Differentiation of the organs or structures involved with the mass could not be achieved with radiography or ultrasonography. Exploratory laparotomy revealed a mass within the lumen of the uterus; the mass was removed by partial hysterectomy. On gross examination, the mass was a focally extensive, unencapsulated, firm, solitary tumor. Histologic examination revealed that the mass was composed of interlacing bundles of smooth muscle cells with little fibrous stroma. The cells were elongated with poorly delineated borders and cigar-shaped nuclei, each containing a single, small nucleolus. Fewer than 1 mitosis per 20 high-power (magnification, × 400) fields were present. These gross and histologic findings supported a diagnosis of uterine leiomyoma. Although leiomyomas are the most common tumor of the reproductive tract in nonhuman primates, to our knowledge the current lesion is the first uterine leiomyoma reported to occur in a squirrel monkey.
Leiomyomas are discrete, benign neoplasms of smooth muscle which frequently occur in the viscera of the gastrointestinal tract and uterus.2 They have also been reported to occur in the ovary,13 vulva,7 and cervix.7 Leiomyomas of the female genitalia are among the most frequently encountered tumors of the reproductive system in almost all domestic animal species.7 In humans, they are the most common gynecologic neoplasm, clinically affecting as many as 30% of women of reproductive age.12 Although most uterine leiomyomas are asymptomatic and do not require therapy, signs that can be seen in affected patients include abnormal uterine bleeding,12 anemia,23 bowel dysfunction,12 increased urinary frequency and urgency,12 and decreased efficiency of subsequent assisted-reproduction cycles.15 The effect of uterine leiomyomas on overall fertility in women is under debate. Although most patients remain asymptomatic, it is important to differentiate leiomyomas from their malignant counterparts, leiomyosarcomas. The differentiation between the 2 types of smooth muscle tumors can generally be made with reasonable certainty according to gross and light microscopic features. More specifically, mitotic index and determination of nucleolar organizer regions have both been shown to be useful in differentiating leiomyomas from leiomyosarcomas.7
Few neoplasms of the reproductive tract of nonhuman primates have been reported. In a worldwide literature review of 783 spontaneous neoplasms that occurred in nonhuman primates, only 1.0% were cervical, 1.6% involved the ovaries, and 2.0% were uterine tumors (94% of which were leiomyomas).3,5 In the current report, we describe the first documented case of a uterine leiomyoma in a squirrel monkey (Saimiri sciureus).
Case Report
History.
A 12-y-old, intact, female Guyanese squirrel monkey (Saimiri sciureus sciureus) presented to the Veterinary Service Center of our institution (Stanford University, Stanford, CA) with acute swelling of the caudal abdomen. This monkey had been at our facility since May 2005 when it was received from a commercial primate vendor (Osage Research Primates, Osage Beach, MO). The animal tested negative for Mycobacterium tuberculosis at the beginning and end of a 31-d quarantine period and annually thereafter. For the past 3.5 y, the subject had been group-housed with 3 other female squirrel monkeys in an indoor fenced enclosure measuring 1.2 m wide × 0.9 m deep × 1.9 m high within an AALAC-accredited facility. The monkey was enrolled in a research protocol that was approved by the Institutional Animal Care and Use Committee of Stanford University and that studied hormonal and behavioral adaptations to social variability in female squirrel monkeys. Diet consisted of daily commercial primate chow (5040 New World Primate Diet, PMI Nutrition International, Brentwood, MO) and various fruits and vegetables; water was provided ad libitum by means of lixits. Room conditions included a 12:12-h light:dark cycle, temperature of 24.6 to 29 °C, relative humidity of 30% to 70%, and 10 to 15 air changes per hour.
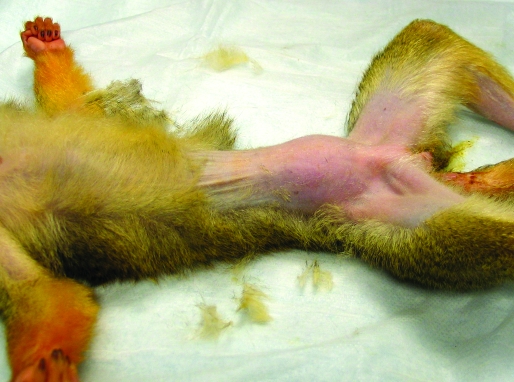
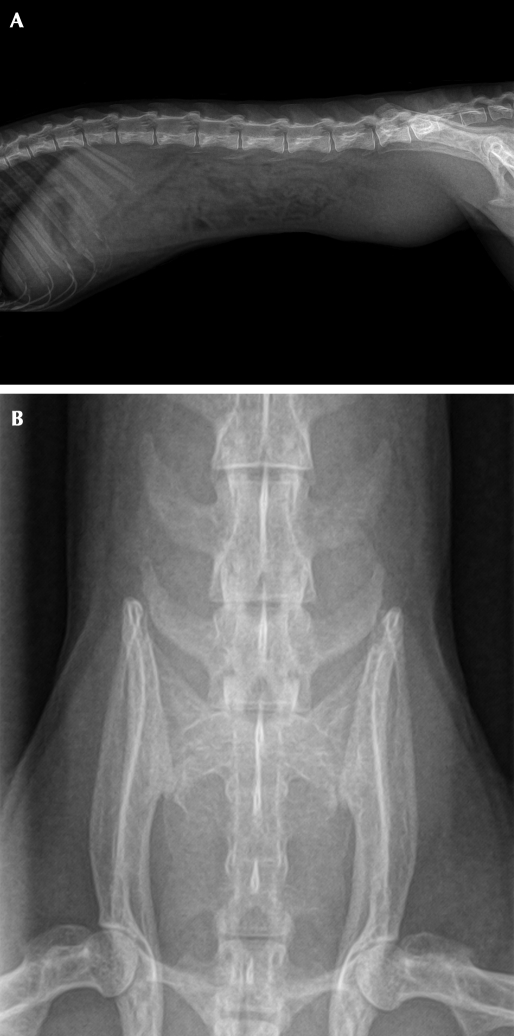
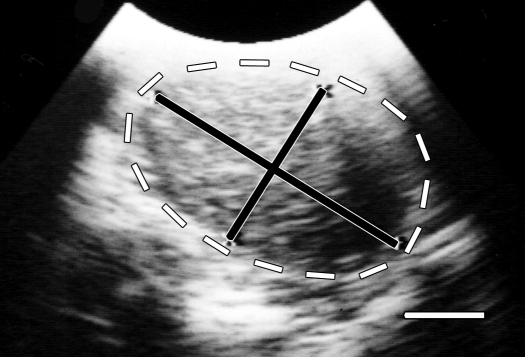
On examination, the monkey weighed 739 g and was in good body condition with normal vital signs. No abnormalities had been noted during previous yearly examinations, the last one occurring 12 mo earlier. Palpation of the ventral aspect of the caudal abdomen revealed a smooth-surfaced, firm, slightly mobile, approximately 3.0 × 2.5 cm mass (Figure 1); no vaginal discharge was present. The animal's behavior, appetite, urination, and feces were normal. The monkey was anesthetized with 1% to 2% isoflurane, and 2-view abdominal radiographs were made by using a direct flat-panel digital radiography system (TruDR, Sound Technologies, Carlsbad, CA). Radiographically the mass appeared as a round opacity within the caudal abdomen with ill-defined borders (Figure 2 A, B). An abdominal ultrasound exam (6.5-MHz probe; Sonovet 600, Universal Medical Systems, Bedford Hills, NY) was performed while the animal was still anesthetized and revealed an elliptical mass that measured approximately 3.5 × 2.25 cm directly beneath the ventral surface of the caudal abdominal midline (Figure 3). The mass was characterized as having heterogeneously hyperechoic cranial and middle portions with a caudal anechoic portion. However, the organs or structures involved with the mass could not be differentiated definitively by imaging. A subsequent CBC and serum biochemistry profile revealed no abnormalities, but because of the acute presentation and large size of the mass relative to the total body size of the squirrel monkey, abdominal exploratory surgery was performed 6 d after presentation.
Figure 1.
Female squirrel monkey. A palpable, smooth-surfaced, firm, slightly mobile, approximately 3.0 × 2.5 cm mass is centered on the ventral midline of the caudal abdomen.
Figure 2.
(A) Right lateral radiographic view of squirrel monkey. (B) Ventrodorsal view of the caudal abdomen of squirrel monkey. In both views, the caudal abdominal mass appears as a round opacity with ill-defined borders; it is superimposed over the sixth and seventh lumbar vertebrae in the ventrodorsal view. Whether surrounding structures within the caudal abdomen were involved could not be determined by imaging.
Figure 3.
Abdominal ultrasound examination of squirrel monkey. An elliptical, heterogeneously hyperechoic mass measuring approximately 3.25 × 2.25 cm (black lines) with an anechoic caudal portion can be seen within the lumen of uterus (white lines) just beneath the ventral surface of the caudal abdominal midline. Bar, 1.0 cm.
Surgery.
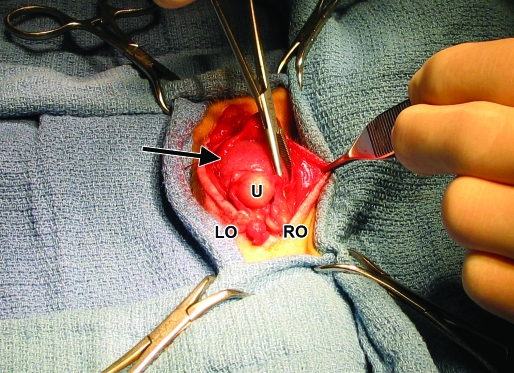
Atropine (0.04 mg/kg SC), cefazolin (25 mg/kg SC), and ketamine (10 mg/kg IM) were administered before surgery. The monkey was intubated and maintained on isoflurane anesthesia. A ventral midline incision was made from the caudal umbilicus to the pelvic inlet. Visual inspection of the abdominal contents revealed that the urinary bladder was pressed tightly against the ventral abdominal wall, but its surface was grossly normal. The body of the uterus was dorsal to the urinary bladder and contained an approximately 3.0 × 2.5 cm intraluminal mass just proximal to the cervix (Figure 4). All other organs within the abdominal cavity were unremarkable. Partial hysterectomy was performed, with careful ligation of each fallopian tube at the junction of the uterine body with 2 circumferential ligatures. A 360° pursestring suture was placed distal to the mass but proximal to the cervix, and a circumferential ligature was placed distal to the pursestring suture. The abdomen was packed with sterile gauze, and the uterine body was transected below the mass (proximal to the pursestring suture); no fluid or discharge was noted within the lumen of the uterus, and the uterine stump was closed in a Parker–Kerr suture pattern. The monkey received buprenorphine (0.01 mg/kg SC) at abdominal closure and twice more at 12-h intervals. Meloxicam (0.05 mg/kg PO) was administered every 24 h for 2 d. The animal recovered unremarkably from surgery, skin sutures were removed 7 d postsurgery, and the animal continues to be asymptomatic 6 mo after surgery.
Figure 4.
Uterus with intraluminal mass. The middle and distal aspects of the uterine body contain an approximately 3.0 × 2.5 cm intraluminal mass (black arrow). U, normal proximal uterus; RO, right ovary; LO, left ovary. The head of the animal is toward the bottom of the frame.
Pathology.
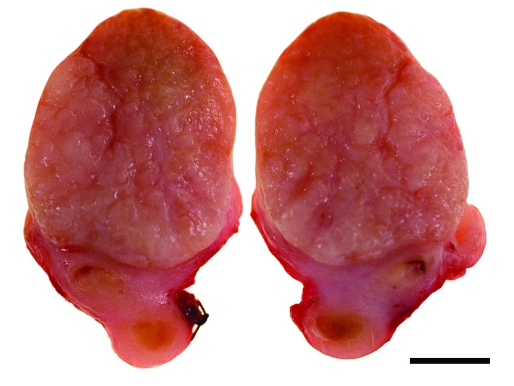
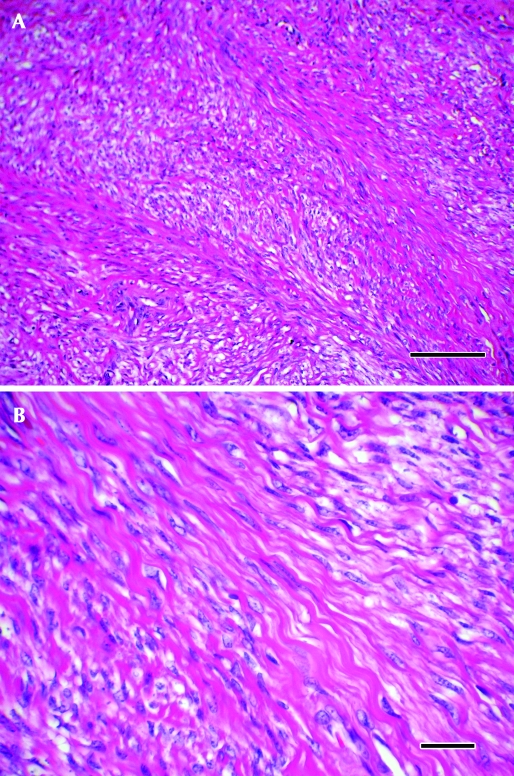
Grossly, the uterine mass was 3.0 × 2.5 × 2.5 cm in size, focally extensive, unencapsulated, pedunculated, smooth, dull pink to red, and firm and projected into the lumen of the uterus and bulged along its cut surface (Figure 5). The entire mass was fixed in 10% neutral-buffered formalin for 48 h; tissues were trimmed, paraffin-embedded, sectioned to 5 µm, and stained with hematoxylin and eosin. On histologic analysis, the mass was composed of interlacing bundles and fascicles of smooth muscle cells with little fibrous stroma (Figure 6 A). Cells were elongated and wavy, with poorly delineated cell borders surrounding moderate amounts of eosinophilic cytoplasm (Figure 6 B). Nuclei were ovoid or cigar-shaped, with finely stippled, dispersed chromatin and a single, small nucleolus. Fewer than 1 mitoses per 20 400× field (mean; range, 0 to 1) were noted. Anisocytosis and anisokaryosis were mild (less than 1.5-fold). Overlying nonkeratinized stratified squamous epithelium showed multifocal erosions, with minimal associated inflammation. Endometrium in the sections showed moderate physiologic hyperplasia. A diagnosis of uterine myometrial leiomyoma was made in light of these gross and histologic findings.
Figure 5.
Gross characteristics of mass. A focally extensive, unencapsulated, pedunculated, smooth, dull pink to red, firm tumor measuring 2.5 × 2.5 × 3.0 cm projects into the distal lumen of the uterus and bulges along its cut surface. Bar, 1.0 cm.
Figure 6.
Histology. (A) The mass was composed of interlacing bundles and fascicles of cells with little fibrous stroma. Hematoxylin and eosin stain; magnification, ×10; bar, 200 µm. (B) The cells were elongated and wavy, with poorly delineated cell borders surrounding moderate amounts of eosinophilic cytoplasm. The nuclei were ovoid or cigar-shaped, with finely stippled, dispersed chromatin and a single, small nucleolus. Hematoxylin and eosin stain; magnification, ×600; bar, 25 µm.
Discussion
Leiomyomas are discrete, nonencapsulated, noninvasive tumors composed primarily of smooth muscle. On gross inspection, they are sharply circumscribed, discrete, round, firm, gray-white tumors that vary in size from barely visible nodules to massive tumors that fill the pelvis.8 Histologically, leiomyomas are comprised of elongated, spindle-shaped cells containing pink cytoplasm with indistinct boundaries. The nuclei of the cells are characterized as elongate and vesicular with blunted ends and often contain inconspicuous nucleoli.14 Mitoses are uncommon with leiomyomas.7,8 Well-differentiated leiomyomas may be difficult to differentiate from normal smooth muscle; however, the characteristic whirled pattern of smooth muscle bundles and disorientation of cells make these lesions identifiable on gross and microscopic inspection8.
The differential diagnosis for a uterine leiomyoma includes leiomyosarcoma, endometrial adenocarcinoma, endometriosis, uterine or cervical sarcoma, pregnancy, and pelvic masses from tumors originating from other abdominal organs, such as the large bowel and urinary bladder. Clinically, in postmenopausal women a uterine mass increasing in size suggests leiomyosarcoma rather than leiomyoma. In addition, compared with leiomyomas, leiomyosarcomas tend to be singular, larger uterine masses that are softer in texture due to tissue necrosis and internal cystic degeneration.12 In contrast to leiomyomas, leiomyosarcomas are frequently invasive.7 Histologically, leiomyosarcomas contain a wide range of atypia, from those that are well-differentiated, with densely packed, relatively homogenous spindle cells to those that contain ovoid to round cells with variable histologic patterns and that resemble invasive sarcomas.8 Binucleate, multinucleate, and bizarre cells are common in poorly differentiated leiomyosarcomas, and mitoses may be numerous.7 When trying to distinguish between benign and malignant smooth muscle tumors, the following features suggest malignancy: mitotic index of 1 per 10 high-power (magnification, ×400) fields or more,7 nuclear atypia,8 and evidence of invasion.7 Because both types of smooth muscle tumors can display extensive necrosis, this feature is not necessarily indicative of malignancy.14 Table 1 summarizes the immunohistochemical markers that can be used to differentiate smooth muscle tumors (leiomyomas and leiomyosarcomas) from striated muscle tumors (rhabdomyomas and rhabdomyosarcomas) and other mesenchymal tumors.
Table 1.
Expected immunophenotypes of muscle tumors after staining of formalin-fixed tissues
| Vimentina | Desmin | α-Smooth muscle actin | Myoglobin | |
| Leiomyoma and leiomyosarcoma | + | + | + | – |
| Rhabdomyoma and rhabdomyosarcoma | + | + | – | + |
| Other mesenchymal tumors | + | – | – | – |
Vimentin might not be expressed in benign muscle tumors or well-differentiated or low-grade malignant muscle tumors.
In human patients with asymptomatic uterine leiomyomas of the uterus, hysterectomy is indicated only in the rare situation where rapidly enlarging masses (particularly after menopause) raise concerns of leiomyosarcoma.12 Definitive treatment for symptomatic uterine leiomyomas or leiomyosarcomas can usually be accomplished with hysterectomy, which leaves the patient infertile. For women who wish to preserve fertility, other treatment options include myomectomy, uterine artery occlusion, and medical management with such agents as gonadotropin-releasing hormone agonists.12 Although our squirrel monkey patient had no clinical signs related to the uterine mass, hysterectomy was performed because of the mass's large size and rapid growth, which suggested a potentially malignant neoplasm. In addition, the animal was not part of a breeding colony, and preserving fertility was not a requirement of the experimental study.
In general, few neoplasms of the female reproductive tract have been reported in nonhuman primates. However, leiomyomas are the most common tumor of the reproductive tract in nonhuman primates6 and have been reported in the uterus of a rhesus macaque,17,18 black-handed spider monkey,10 chimpanzee,19,23 and cynomolgus monkey.11,18 In addition to those in the uterus, leiomyomas have been reported in the colon of a dwarf galago and cotton-top tamarin,4 ovary of a baboon,13 and esophagus of a cynomolgus monkey18.
Squirrel monkeys are the most common neotropical primates used in biomedical research in the United States. Squirrel monkeys have been used to study the biomechanics of labor and delivery, because fetal rotation in these animals is similar to that seen in women. Squirrel monkeys also have been used in the study of pelvic organ prolapse, because multiparous squirrel monkeys develop lesions resembling this syndrome in women.1 In addition, studies involving the pathogenesis of malaria,1,20 Creutzfeldt–Jakob disease,1,21 and cardiovascular research1,16 have relied on squirrel monkeys as research models. Several features make them appealing as animal research subjects, including their easy adaptability to the laboratory setting, need for smaller and less-expensive cages, and lower risk of zoonotic disease transmission as compared with those of macaques and Old World primates.1 A disadvantage to the use of squirrel monkeys in research is their poor reproductive efficiency. In a study of 41 term pregnancies in squirrel monkeys, 41% were stillbirths or abortions, and only 54% resulted in livebirths.1,9 In the breeding colony at the University of South Alabama, average squirrel monkey fetal mortality was 27% during a 5-y period (1987 to 1991), and only 33% of first pregnancies in Saimiri boliviensis boliviensis resulted in live births.22
In conclusion, although few neoplasms of the female reproductive tract have been reported in nonhuman primates, leiomyomas are the most common. This benign smooth muscle tumor must be differentiated from other tumors of the reproductive tract, including its malignant counterpart, leiomyosarcoma. To our knowledge, this is the first reported case of uterine leiomyoma in squirrel monkeys, which are often used as research models in comparative medicine. Therefore, leiomyoma should be included in the differential diagnosis for adult female squirrel monkeys that present with a uterine mass.
Acknowledgment
This case report was presented at the 2009 AALAS National Meeting (8–12 November, Denver, CO).
References
- 1.Abee CR. 2000. Squirrel monkey (Saimiri spp.) research and resources. ILAR J 41:2–9 [DOI] [PubMed] [Google Scholar]
- 2.Allison JG, Dodds HM. 1989. Leiomyoma of the male nipple. Am Surg 55:501–502 [PubMed] [Google Scholar]
- 3.Beniashvili DS. 1989. An overview of the world literature on spontaneous tumors in nonhuman primates. J Med Primatol 18:423–437 [PubMed] [Google Scholar]
- 4.Brack M. 1998. Gastrointestinal tumors observed in nonhuman primates at the German Primate Center. J Med Primatol 27:319–324 [DOI] [PubMed] [Google Scholar]
- 5.Cline M. 2004. Neoplasms of the reproductive tract: the role of hormone exposure. ILAR J 45:179–188 [DOI] [PubMed] [Google Scholar]
- 6.Cook AL, Rogers TD, Sowers M. 2004. Spontaneous uterine leiomyosarcoma in a rhesus macaque. Contemp Top Lab Anim Sci 43:47–49 [PubMed] [Google Scholar]
- 7.Cooper BJ, Valentine BA. 2002. Tumors of muscle, p 319–363 : Meuten DJ. Tumors in domestic animals. Ames (IA): Iowa State Press [Google Scholar]
- 8.Crum CP. 1994. Female genital tract, p 1033–1088 : Cotran RS, Kumar V, Robbins SL, Schoen FJ. Pathologic basis of disease. Philadelphia (PA): WB Saunders [Google Scholar]
- 9.Goss CM, Popejoy LT, Fusiler JL, Smith TM. 1968. Observations on the relationship between embryological development, time of conception, and gestation, p 171–191 : Rosenblum RA, Cooper RW. The squirrel monkey. New York (NY): Academic Press [Google Scholar]
- 10.Hernández-López L, Cerda-Molina A, Páez-Ponce D, Rojas-Maya S, Mondragón-Ceballos R. 2007. Artificial insemination in black-handed spider monkey (Ateles geoffroyi). Theriogenology 67:399–406 [DOI] [PubMed] [Google Scholar]
- 11.Kaspareit J, Friderichs-Gromoll S, Buse E, Habermann G. 2007. Spontaneous neoplasms observed in cynomolgus monkeys (Macaca fascicularis) during a 15-year period. Exp Toxicol Pathol 59:163–169 [DOI] [PubMed] [Google Scholar]
- 12.Lefebvre G, Vilos G, Allaire C, Jeffrey J, Arneja J, Birch C, Fortier M, Wagner S, Clinical Practice Gynaecology Committee, Society for Obstetricians and Gynaecologists of Canada 2003. The management of uterine leiomyomas. J Obstet Gynaecol Can 25:396–418 [PubMed] [Google Scholar]
- 13.Moore CM, Hubbard GB, Leland MM, Dunn BG, Barrier BF, Siler-Khodr TM, Schlabritz-Loutsevitch NE. 2006. Primary amenorrhea associated with ovarian leiomyoma in a baboon (Papio hamadryas). J Am Assoc Lab Anim Sci 45:58–62 [PubMed] [Google Scholar]
- 14.Solleveld HA. 1987. Leiomyoma and leiomyosarcoma, uterus, rat, p 116–120 : Jones TC, Mohr U, Hunt RD. Genital system. New York (NY): Springer-Verlag [Google Scholar]
- 15.Stovall DW, Parrish SB, Van Voorhis BJ, Hahn SJ, Sparks AE, Syrop CH. 1998. Uterine leiomyomas reduce the efficacy of assisted reproduction cycles: results of a matched follow-up study. Hum Reprod 13:192–197 [DOI] [PubMed] [Google Scholar]
- 16.Strickland HL, Clarkson TB. 1985. Use of squirrel monkeys in cardiovascular research, p 295–311 : Rosenblum RA, Coe CL. Handbook of squirrel monkey research. New York (NY): Plenum Press [Google Scholar]
- 17.Takayama S, Renwick AG, Johansson SL, Thorgeirsson UP, Tsutsumi M, Dalgard DW, Sieber SM. 2000. Long-term toxicity and carcinogenicity study of cyclamate in nonhuman primates. Toxicol Sci 53:33–39 [DOI] [PubMed] [Google Scholar]
- 18.Takayama S, Sieber SM, Dalgard DW, Thorgeirsson UP, Adamson RH. 1999. Effects of long-term oral administration of DDT on nonhuman primates. J Cancer Res Clin Oncol 125:219–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toft JD, 2nd, MacKenzie WF. 1975. Endometrial stromal tumor in a chimpanzee. Vet Pathol 12:32–36 [DOI] [PubMed] [Google Scholar]
- 20.Whiteley HE, Everitt JI, Kakoma I, James MA. 1987. Pathologic changes associated with fatal Plasmodium falciparum infection in the Bolivian squirrel monkey (Saimiri sciureus boliviensis). Am J Trop Med Hyg 37:1–8 [DOI] [PubMed] [Google Scholar]
- 21.Williams L, Brown P, Ironside J, Gibson S, Will R, Ritchie D, Kreil TR, Abee C. 2007. Clinical, neuropatholgical and immunohistochemical features of sporadic and variant forms of Creutzfeldt–Jakob disease in the squirrel monkey (Saimiri sciureus). J Gen Virol 88:688–695 [DOI] [PubMed] [Google Scholar]
- 22.Williams LE, Brady AG, Gibson SV, Abee CR. 2002. The squirrel monkey breeding and research resource: a review of Saimiri reproductive biology and behavior and breeding performance. Primatologie 5:303–334 [Google Scholar]
- 23.Young LA, Lung NP, Isaza R, Heard DJ. 1996. Anemia associated with lead intoxication and uterine leiomyoma in a chimpanzee (Pan troglodytes). J Zoo Wildl Med 27:96–100 [Google Scholar]