Abstract
The angiotensin (Ang) IV analog norleual [Nle-Tyr-Ile-ψ-(CH2-NH2)3-4-His-Pro-Phe] exhibits structural homology with the hinge (linker) region of hepatocyte growth factor (HGF) and is hypothesized to act as a hinge region mimic. Norleual competitively inhibited the binding of HGF to its receptor c-Met in mouse liver membranes, with an IC50 value of 3 pM. Predictably, norleual was able to inhibit HGF-dependent signaling, proliferation, migration, and invasion in multiple cell types at concentrations in the picomolar range. Ex vivo studies demonstrated that norleual exhibited potent antiangiogenic activity, an attribute that would be predicted for a HGF/c-Met antagonist. Furthermore, norleual suppressed pulmonary colonization by B16-F10 murine melanoma cells, which are characterized by an overactive HGF/c-Met system. Together, these data suggest that AngIV analogs exert at least some of their biological activity through interference with the HGF/c-Met system and may have utility as therapeutic agents in disorders that are dependent on an intact HGF/c-Met system. Finally, the ability of norleual to induce marked biological responses in human embryonic kidney cells, which do not express insulin-responsive aminopeptidase (IRAP), coupled with the observed effects of norleual on the HGF/c-Met system, casts doubt on the physiological significance of AngIV-dependent inhibition of IRAP.
Angiotensin II (DRVYIHPF) and angiotensin III (RVYIHPF) have long been recognized as important regulators of blood pressure and body water balance. In 1992, a specific membrane binding site was identified for angiotensin IV (AngIV; VYIHPF; Swanson et al., 1992), a shorter angiotensin fragment that was at the time considered biologically inactive (Wright et al., 1989). This AngIV binding site, termed the AT4 receptor, was found to be concentrated in brain, heart, kidney, adrenals, and blood vessels (Wright et al., 2008). The identification of a specific AngIV binding site subsequently led to the demonstration that AngIV and AngIV analogs induced several marked biological effects in central nervous, renal, pulmonary, and vascular tissues (Handa, 2001; Li et al., 2002; Vinh et al., 2008; Wright et al., 2008). Nevertheless, the molecular identity of the AngIV target responsible for these observed activities has remained elusive.
One proposed target for AngIV-related molecules is the insulin-responsive aminopeptidase (IRAP; Albiston et al., 2001), a membrane-associated aminopeptidase. In this view, the multiple physiological actions of AngIV-related molecules are due to their ability to competitively inhibit IRAP, thus potentiating the actions of endogenous peptides that would normally be degraded by IRAP (Lew et al., 2003). However, there are several conceptual problems with this idea. First, this notion is difficult to reconcile with the existence of both agonist and antagonist AngIV-like compounds that exhibit opposing physiological actions (Wright et al., 1999; Kramár et al., 2001; Esteban et al., 2005; Vinh et al., 2008). Second, under this scenario, the onset of the physiological effects of AngIV would be expected to be slow because accumulation of endogenous IRAP substrates would be required. This prediction does not agree with the observation that AT4 ligands have very rapid effects on downstream signaling molecules (Chen et al., 2001; Handa, 2001; Li et al., 2002). Similarly, in vivo studies indicate rapid AT4 receptor-mediated changes in blood flow (Kramár et al., 1997), renal oxygen consumption (Handa et al., 1998), and long-term potentiation (LTP; Kramár et al., 2001), typically manifesting in less than 1 min. Finally, the concentrations of AT4 ligands required to affect changes in physiological function are subpicomolar or subnanomolar (Chen et al., 2001; Handa, 2001; Li et al., 2002), well below those reported for most peptide-based enzyme inhibitors. This concern is particularly relevant for IRAP given that the reported Ki values of various AngIV analogs for IRAP are several orders of magnitude higher than the biologically effective doses of the same ligands (Lew et al., 2003).
Given the discordance between the IRAP-inhibitor model and the observations described above, we hypothesized that an as yet undiscovered molecular target must be responsible for the biological activity exhibited by AngIV analogs. This hypothesis led us to search for naturally occurring ligands with 1) structural homology to AngIV and 2) physiological actions reminiscent of those initiated by AngIV analogs. Using this approach, we discovered a partial match to the antiangiogenic protein angiostatin and the related plasminogen family member hepatocyte growth factor (HGF; Fig. 1). HGF is a powerful mitogenic, morphogenic, and motogenic growth factor that acts via the type I tyrosine kinase receptor c-Met (Ma et al., 2003). Classically, c-Met is known for its ability to direct stem cell proliferation and differentiation, induce tubular morphogenesis in many organs (including kidney), and support angiogenesis by activating vascular endothelial cells. More recently, c-Met has attracted attention because of its role in multiple cancers (Comoglio et al., 2008), its ability to blunt neurodegenerative changes, and its potential involvement in learning and memory consolidation (Shimamura et al., 2006). The demonstrated ability of AngIV analogs to alter cognitive function, augment neurite outgrowth, influence renal tubular function, and activate vascular endothelial cells (Wright et al., 2008) led us to hypothesize that AngIV-like analogs exert their biological activity through the HGF/c-Met system.
Fig. 1.
Norleual and HGF each compete for high-affinity 125I-HGF binding to mouse liver membranes. a, sequence comparison of the hinge region (HG) of the plasminogen family precursors to AngIV. b and c, mouse liver plasma membranes were incubated with 50 pM 125I-HGF and indicated concentrations of HGF (■) or norleual (●). Results are presented as percentage of specific 125I-HGF binding, and error bars indicate ±S.E.M., n = 4. Specific binding was defined as total binding minus nonspecific binding, which was consider binding in the presence of 10−6 M HGF. Competition experiments each included quadruplicate data points, and each experiment was repeated in quadruplicate with an average HGF IC50 value of 29 ± 14.7 pM and norleual IC50 value of 3.1 ± 2.1 pM.
Here, we demonstrate that picomolar concentrations of the AngIV analog norleual [Nle-Tyr-Leu-ψ-(CH2-NH2)3-4-His-Pro-Phe; Kramár et al., 2001; Davis et al., 2006] are capable of inhibiting HGF binding to c-Met, and HGF-dependent signaling, proliferation, invasion, and scattering. In total, these studies suggest that the biological effects of AngIV and AngIV-like molecules are mediated through the HGF/c-Met system and that c-Met or HGF is the molecular target of norleual and other AngIV analogs. Furthermore, these data posit that AngIV analogs may have therapeutic utility in multiple pathologies.
Materials and Methods
Animals.
C57BL/6 mice (Taconic Farms, Germantown, NY) were used in these studies. Mice were housed and cared for in accordance with National Institutes of Health guidelines as described in the Guide for the Care and Use of Laboratory Animals.
Compounds.
Norleual was synthesized by Syngene (Bangalore, India) and purified in the Harding laboratory by reverse-phase high-performance liquid chromatography. d-Nle-Tyr-Ile-(6)aminohexanoic amide was synthesized by solid-phase methods in the Harding laboratory and purified by reverse-phase high-performance liquid chromatography. Purity and structure were verified by liquid chromatography/mass spectrometry. HGF (294-HGN) was purchased from R&D Systems (Minneapolis, MN).
Antibodies.
Antibodies against c-Met (DO-24, 07-283, and DQ-13) were purchased from Millipore (Billerica, MA). Anti-phosphotyrosine, HAM1676, was purchased from R&D Systems, and 06-427 was purchased from Millipore. Anti-Gab1, 06-579, was also purchased from Millipore. The phospho-Met antibody (44887G) was purchased from Invitrogen (Carlsbad, CA), and the phospho-Erk antibody was purchased from Cell Signaling Technology Inc. (Danvers, MA).
Collagen I.
Rat tail tendons were dissected out from adult rats immediately after sacrifice and sterilized in 70% ethanol for 30 min. After sterilization, 1 g of tendons was solubilized in 100 ml of 0.5 M acetic acid with stirring for 48 h at 4°C. The dissolved tendons were centrifuged at 2000g for 20 min to remove any insoluble material. The clarified supernatant was then dialyzed against 4 l of water for 4 days at 4°C. The collagen I gelling solution consisted of a 1:1:1 mixture of 10× minimal essential medium, 0.1 N NaOH, and 1 M NaHCO3. To induce gelatinization, a 1:5 dilution of gelling solution to collagen was made and incubated at 37°C for 30 min.
Cell Culture.
Human embryonic kidney (HEK) 293 cells and Madin-Darby canine kidney (MDCK) cells were grown in DMEM containing 10% fetal bovine serum (FBS). Human umbilical vein endothelial cells (HUVECs) were grown in EGM-2 (Lonza Walkersville Inc., Walkersville, MD). Cells were grown to 100% confluence before use. HEK and MDCK cells were serum-starved for 2 to 24 h before the initiation of drug treatment.
Iodination of 125I-HGF.
Carrier-free HGF was iodinated using the chloramine-T method described by Higuchi and Nakamura (1991). We placed 10 μl of 1.5 M Na3(PO4), pH 7.4, 4 μl of ∼1 mCi Na125I (PerkinElmer Life and Analytical Sciences, Boston, MA), and 10 μl of 1 ng/ml HGF in a 500-μl microcentrifuge tube. The reaction was started by adding 5 μl of 0.1 mg/ml chloramine-T, which was added a total of four times at 90-s intervals. The reaction was halted by the addition of 50 μl of 50 mM N-acetyl-l-tyrosine, 200 μl of 60 mM NaI, and 200 μl of 1.2 mg/ml urea. 125I-HGF was separated from free iodine using a G-25 Sephadex (1.5 × 20-cm) gel filtration column, a mobile phase of 20 mM sodium phosphate, 75 mM NaCl, and 0.1% BSA and a flow rate of 0.5 ml/min. Fractions between 18 and 36 min were collected and combined.
125I-HGF Binding Assay.
Mouse liver was homogenized in hypotonic buffer (50 mM Tris, pH 7.4) fortified with 0.1% BSA. Then, 600 μg of membrane protein was incubated with 50 pM 125I-HGF and various concentrations of HGF or norleual in a total volume of 100 μl of phosphate-buffered saline (PBS) fortified with 0.1% BSA. Binding was carried out for 1 h on ice. Preliminary studies indicated that 1 h was sufficient to reach equilibrium. Membranes were pelleted by centrifugation, and unbound label was removed by washing the pellet twice with ice-cold incubation buffer. Pellets were resuspended, and radioactivity was counted with a gamma counter (Isomedic 10/880; MP Biomedicals, Solon, OH). Nonspecific binding was defined as the radioactivity remaining in the pellet in the presence of 1 μM HGF and typically represented ∼67% of the total binding.
Immunoprecipitation and Western Blotting.
Cells were washed twice with PBS and serum-starved for 2 h in DMEM before HGF/compound treatment. Cells were harvested using radioimmunoprecipitation assay lysis buffer (Millipore) and fortified with phosphatase inhibitor cocktails 1 and 2 (Sigma-Aldrich, St. Louis, MO). Protein concentrations were determined using the bicinchoninic acid total protein assay (Pierce Chemical, Rockford, IL). Lysates were incubated with antibody overnight at 4°C with gentle agitation. Immunoprecipitation controls received mouse nonspecific IgG (Millipore). Proteins were captured with protein A-agarose beads (Millipore) and washed three times with phosphate-buffered saline. After heating with reducing Laemmli buffer for 10 min at 95°C, proteins were resolved using SDS-polyacrylamide gel electrophoresis (Criterion; Bio-Rad Laboratories, Hercules, CA), and transferred to a nitrocellulose membrane. Membranes were incubated with the appropriate primary antibodies and washed in water and PBS, 0.05% Tween 20. Membranes were then incubated with the appropriate secondary antibody (Pierce Chemical) and washed following Millipore protocols. Proteins were visualized using the SuperSignal West Pico Chemiluminescent Substrate system (Pierce Chemical). Molecular weights were determined by comparison with protein ladders (BenchMark, Invitrogen; and Kaleidoscope, Bio-Rad Laboratories). The film images were digitized and analyzed using Totallab software (ADInstruments Inc., Colorado Springs, CO) or a phosphorimaging device (UVP, Inc., Upland, CA).
Phospho-Met and Phospho-Erk Western Blots.
MDCK cells were seeded in 100-mm tissue culture plates and grown to 95% confluence in DMEM containing 10% FBS. The cells were serum-deprived for 24 h before the treatment to reduce the basal levels of phospho-Met and phospho-Erk. After serum starvation, cocktails made up of vehicle, HGF alone, and/or norleual were prepared and preincubated for 90 min at room temperature. The cocktail was then added to the cells for 10 min to stimulate the c-Met receptor and downstream proteins. The cells were lysed in ice-cold radioimmunoprecipitation assay buffer with protease and phosphatase inhibitors. The lysate was clarified by centrifugation at 15,000g for 15 min, diluted with 2× reducing Laemmli buffer, and heated for 10 min at 95°C. Then, 20 μl of lysate was resolved using SDS-polyacrylamide gel electrophoresis, transferred to nitrocellulose, and blocked in Tris-buffered saline (TBS) containing 5% milk for 1 h at room temperature. The phospho-Met antibody and phospho-Erk antibody were added to the blocking buffer at a final concentration of 1:1000 and incubated at 4°C overnight with gentle agitation. The membranes were washed several times with TBS, a 1:5000 concentration of horseradish peroxidase-conjugated goat anti-rabbit was added to the blocking buffer, and the membranes were incubated for 1 h at room temperature. The membranes were washed several times with TBS before being developed by chemiluminescence, and the bands were detected and quantitated using a phosphorimaging device (UVP, Inc.).
Cell Proliferation.
We seeded 5000 MDCK cells into the wells of a 96-well plate in DMEM containing 10% FBS. To induce cellular quiescence, the cells were serum-deprived for 24 h before initiating the treatments. After serum starvation, 10 ng/ml HGF alone and with various concentrations of norleual or PBS vehicle were added to the media. The cells were allowed to grow under these conditions for 4 days, with a daily addition of norleual. On the 4th day, 1 mg/ml 1-(4,5-dimethylthiazol-2-yl)-3,5-diphenylformazan (MTT) reagent (Sigma-Aldrich) prepared in PBS was added to the cells and incubated for 4 h. Dimethyl sulfoxide diluted in a 0.1 M glycine buffer was added to solubilize the cell membranes, and the absorbance of reduced MTT in the buffer was quantitated at 590 nm on a plate reader (BioTek Instruments, Winooski, VT). Control values were subtracted from all values to determine the increase in proliferation due to HGF treatment.
Cell Invasion.
The insides of the BD Falcon cell culture inserts for 24-well plates (BD Biosciences, San Jose, CA) were coated with 50 μl of 2 mg/ml neutralized collagen I. The collagen I was allowed to gel at 37°C. Then, 50,000 MDCK cells were added to the inside of the inserts and allowed to attach to the collagen for 2 h before adding 20 ng/ml HGF alone or in combination with norleual to the bottom chamber to stimulate migration. Norleual or the PBS vehicle was also added to the top chamber. The cells were allowed to invade the collagen and migrate to the underside of the chamber for 8 h. The cells that had invaded the collagen and migrated to the underside of the membrane were fixed with 100% methanol for 10 min at room temperature. The nonmigrated cells remaining on the inside of the insert were wiped away with a cotton swab, and the cells on the bottom of the insert were stained with Diff-Quik (Dade Behring, Inc., Newark, DE). The cells that invaded the collagen and migrated to the underside of the membrane were counted in five random fields at 20× objective on a light microscope.
Urokinase-Type Plasminogen Activator Activity Assay.
We seeded 5000 MDCK cells in 96-well plates in 100 μl of DMEM containing 1% FBS to induce quiescence. The MDCK cells were stimulated with 10 ng/ml HGF in the presence or absence of various concentrations of norleual. After 24 to 48 h of treatment, uPA activity was quantitated using an activity assay kit (Millipore) according to the manufacturer's instructions. After 24 to 48 h of treatment, 10 μl of assay buffer and 10 μl of the chromogenic tripeptide substrate were added to the media and incubated for 4 h at 37°C. Absorbance was quantitated at 405 nm on a plate reader (BioTek). Control values were subtracted from all values to determine HGF-induced uPA activity.
Scattering Assay.
Using aseptic technique, coverslips were placed in six-well plates. MDCK cells were grown to 100% confluence and washed twice with PBS. The confluent coverslips were then transferred to new six-well plates containing 900 μl of serum-free DMEM. Norleual, d-Nle-Tyr-Ile-(6)aminohexanoic amide, and/or HGF (20 ng/ml) were added to the appropriate wells. Control wells received PBS vehicle. Plates were incubated at 37°C with 5% CO2 for 48 h. Media were removed, and cells were fixed with methanol. Cells were stained with Diff-Quik Wright-Giemsa (Dade Behring, Inc.), and digital images were taken. Coverslips were removed with forceps, and more digital images were captured. Pixel quantification of images was achieved using ImageJ (National Institutes of Health, Bethesda, MD), and statistics were performed by Prism (GraphPad Software Inc., San Diego, CA).
Aortic Ring Assay.
Forty-eight well plates were coated with growth factor reduced Matrigel according to the manufacturer's protocol (thick gel method; BD Biosciences). Under microscopic dissection, the thoracic aorta of a 6-week-old C57 mouse was removed and cut into 0.5-mm sections under aseptic conditions, washed in PBS, and placed in the Matrigel-coated wells. We then added 100 μl of Matrigel and allowed the preparation to solidify for 30 min at 37°C to embed the rings. The rings were then incubated for 24 h in growth factor-containing EGM-2 (Lonza Walkersville Inc.) at 37°C in 5% CO2, air. The medium was then replaced with basal medium; endothelial basal medium (Lonza Walkersville Inc.) was supplemented with 2% FBS and 10 mg/ml gentamicin with or without norleual at 10−10 M. Aortic rings were then incubated for 4 days at 37°C in 5% CO2. Digital images were taken, and angiogenic sprout area was measured using ImageJ (National Institutes of Health). Areas were normalized to aortic diameters.
Scratch Assay.
Confluent B16-F10 cells in 48-well plates were scratched with a 200-μl pipette tip. Wells were treated with vehicle, HGF (20 ng/ml), and/or 10−10 M norleual. Photos were taken at various time points. Photos were graded independently by two of us in a blinded manner to determine the extent of wound closure.
Lung Colony Formation.
Six- to 8-month-old C57BL/6 mice were injected with 400,000 B16-F10 cells in 200 μl of PBS by tail-vein injection and subsequently received daily intraperitoneal injections of either norleual (50 μg/kg) or a PBS vehicle control. Two weeks later, mice were anesthetized, and the lungs were perfused with PBS and removed. Photos were taken and lungs were solubilized in 1% Triton X-100, 20 mM Tris, 0.15 M NaCl, 2 mM EDTA, and 0.02% sodium azide. Samples were disrupted by sonication (Mixonix, Farmingdale, NY) and spun. The supernatant was transferred to a 96-well plate, and melanin absorbance at 410 nm was measured using a plate reader (Synergy 2; BioTek).
Statistics.
Independent one-way analyses of variance (InStat version 3.05; GraphPad Software Inc.) were used to determine differences among groups. Tukey-Kramer or Bonferroni's multiple comparison post hoc tests were performed as necessary. Statistical comparisons of two groups were determined using the two-tailed Student's t test (InStat and Prism; GraphPad Software Inc.).
Results
The working hypothesis underlying these studies is that AngIV and its analogs possess a level of structural homology with an as yet unidentified ligand that is independent of and not derived from angiotensinogen. We further posited that the biological activity initiated by AngIV analogs is the result of the interaction between AngIV analogs and the unknown ligand or its cognate receptor. Thus, the starting point in this investigation was to perform a homologous sequence-conservation screen against all possible transcripts that were independent of and not derived from angiotensinogen looking for similarities to AngIV. This effort identified partial homology with the hinge region (Wajih and Sane, 2003) of the plasminogen family of proteins, which include plasminogen itself, its antiangiogenic degradation product, angiostatin, and the protein hormones HGF and macrophage-stimulating protein (MSP), which both share a tyrosine-branched chain amino acid-basic amino acid motif with AngIV (Fig. 1a).
Norleual Blocks Binding of HGF to c-Met with High Affinity.
To begin to probe for a potential interaction between AngIV analogs and the HGF/c-Met system, radioligand binding methods were used to determine whether the AngIV analog and AT4 receptor antagonist norleual (Davis et al., 2006) could compete with HGF for binding to c-Met in mouse liver membranes. Both norleual and HGF were observed to compete with the high-affinity binding of 125I-HGF to liver membranes (Fig. 1, b and c). The IC50 values for norleual and HGF were 3.1 ± 2.1 and 29.4 ± 14.7 pM (mean ± S.E.M.; n = 4), respectively; the later value for HGF was similar to that reported previously (Higuchi and Nakamura, 1991). These data indicated a competitive interplay between norleual and HGF, and they suggest either a direct interaction between norleual and c-Met or between norleual and HGF itself.
Norleual Attenuates HGF-Dependent c-Met Signaling.
After establishing that norleual could interact with a component of the HGF/c-Met system with high affinity, we next asked whether the interaction of norleual had functional consequences regarding c-Met signaling. Typical of type I receptor tyrosine kinase receptors, activation of c-Met can induce tyrosine residue autophosphorylation and the recruitment of various signaling proteins. Critical to c-Met signaling is its interaction with growth factor receptor bound protein-2-associated binder Gab1, a multifunctional scaffolding adapter (Corso et al., 2005). Gab1 association provides c-Met with multiple docking sites for a variety of intracellular signal transducers.
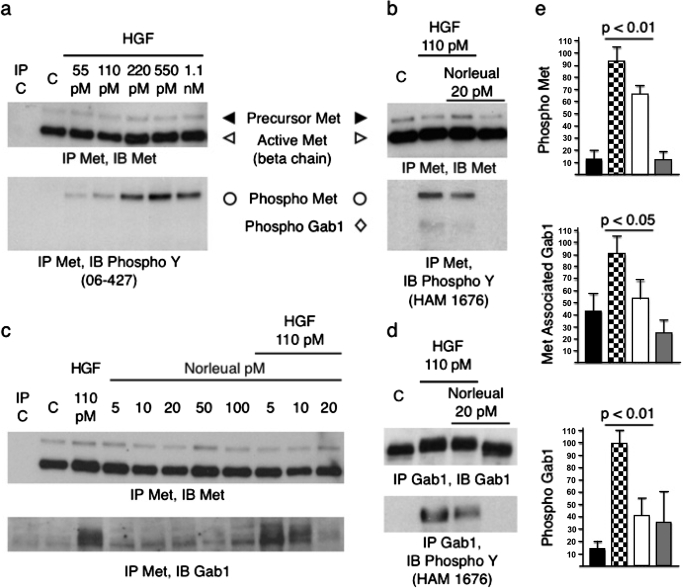
HEK293 cells are known to contain high levels of c-Met (Sakkab et al., 2000) but do not express the putative AT4 receptor IRAP (Laustsen et al., 2001). Thus, to avoid any possible confounds related to an interaction with IRAP, we determined whether norleual could modulate c-Met and Gab1 phosphorylation and c-Met/Gab1 association in HGF-activated HEK293 cells. Solubilized membranes were immunoprecipitated (IP) with an anti-c-Met antibody and immunoblotted for total c-Met, anti-phosphotyrosine to detect the activated forms of c-Met and Gab1, and anti-Gab1 to detect c-Met/Gab1 association. Fig. 2a demonstrates that c-Met autophosphorylation can be induced in a dose-dependent manner by HGF with saturation occurring at 220 pM. Although norleual applied alone did not alter c-Met phosphorylation (Fig. 2b), norleual at 20 and 50 pM significantly reduced HGF-dependent c-Met and Gab1 phosphorylation (Fig. 2, b and e). In addition, 20 pM norleual dramatically reduced HGF-initiated association between Gab1 and c-Met (Fig. 2, c and e). Similar results were seen with other AngIV analogs, including d-Nle-Tyr-Ile-(6)aminohexanoic amide (Supplemental Fig. 1). To confirm these findings, treated HEK293 cell lysates were immunoprecipitated with an anti-Gab1 antibody and immunoblotted for total Gab1 and phosphotyrosine to detect the activated form of Gab1. Even though norleual was found to have no impact on total Gab1, again it was observed to significantly reduce Gab1 phosphorylation (Fig. 2, d and e). Norleual was additionally found to suppress activation of the nonreceptor tyrosine kinase c-Src by HGF (data not shown). Collectively, these data indicate that the AngIV analog and AT4 antagonist norleual markedly attenuated HGF-dependent c-Met activation and downstream signaling.
Fig. 2.
Norleual inhibits HGF-dependent Met phosphorylation and Gab1 association and phosphorylation in vitro. a, HEK293 cells were treated for 10 min with HGF at the indicated concentrations. Lysates were IP with anti-Met (DO-24) and immunoblotted (IB) with anti-Met or anti-phosphotyrosine (06-427) b, HEK293 cells were treated for 10 min with HGF and/or norleual at the indicated concentrations. Lysates were IP with anti-Met (DO-24) and IB with anti-Met (DQ-13) or anti-phosphotyrosine (HAM 1676, lot JLB03). c, HEK293 lysates were IP with anti-Met (DO-24) and IB with anti-Gab1. d, HEK293 lysates were IP with anti-Gab1 and IB with anti-Gab1 or anti-phosphotyrosine (HAM 1676). e, relative amounts (normalized to percentage of total Met in IP) of quantified IP/IB band intensities for control (black bars), 110 pM HGF (checkered bars), 110 pM HGF plus 20 pM norleual (white bars), and 20 pM norleual (gray bars)-treated HEK293 cells for phospho-Met (n = 4), phospho-Gab1 (n = 4), and Met associated Gab1 (n = 3). Error bars are standard deviation, and p values were determined by one-way analysis of variance followed by Tukey-Kramer multiple comparison test.
Norleual Attenuates HGF/c-Met-Stimulated MDCK Cell Proliferation and Erk Activation.
c-Met activation initiates multiple cellular responses, including increased proliferation/survival, motility, and differentiation (Zhang and Vande Woude, 2003). Together, these cellular responses contribute to the hallmark effect of scattering by c-Met, a process characterized by decreased cell adhesion, increased motility, and increased proliferation. To explore the physiological significance of the ability of norleual to depress c-Met signaling, we evaluated the effect of norleual on classic c-Met-dependent changes in cell behavior using MDCK cells, the standard cellular model for investigating the HGF/c-Met system (Stella and Comoglio, 1999).
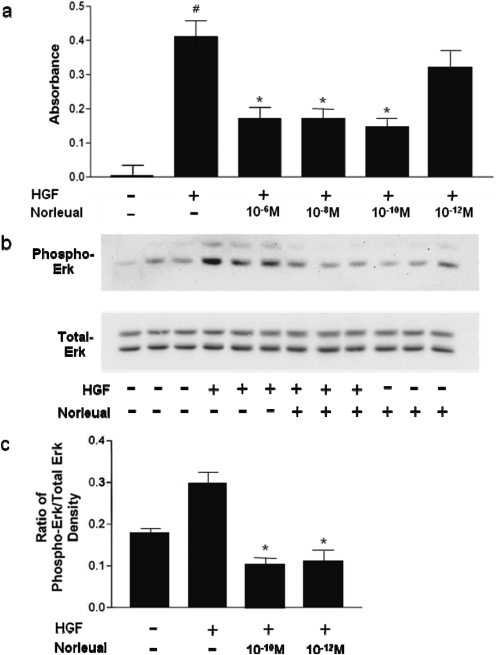
As seen in Fig. 3a, norleual was effective at attenuating the ability of HGF to stimulate MDCK cell proliferation at concentrations as low as 10−10 M. Increased proliferation during HGF treatment is known to be dependent on the activation of the Ras/MEK/Erk pathway downstream of c-Met activation (Zhang and Vande Woude, 2003). Because of this known involvement of the Erk pathway in the proproliferative effects of HGF, norleual was expected to attenuate the ability of HGF to activate Erk in MDCK cells. To test this idea, we monitored changes in phospho-Erk levels induced by HGF in the absence and presence of norleual. MDCK cells were treated for 10 min with HGF and phospho-Erk as well as total Erk was quantified by Western blotting. As shown in Fig. 3b, 100 pM norleual effectively blocked HGF-dependent Erk activation. Concentrations as low as 10−12 M (Fig. 3c) were found to suppress Erk activation, whereas total Erk expression was unaffected at all concentrations of norleual evaluated (Fig. 3b).
Fig. 3.
Norleual attenuates HGF/c-Met proliferation and Erk activation in MDCK cells. a, MDCK cells were treated with PBS vehicle (controls), HGF, or HGF in combination with norleual. The cells were allowed to grow under these treatment conditions for 4 days. Cell numbers were estimated on the 4th day with an MTT assay by measuring absorbance at 590 nm. Control values were subtracted from all values to determine the HGF-induced increase in cell proliferation. Data are expressed as mean ± S.E.M., n = 6. ∗, p < 0.05 versus control as determined by Tukey's post hoc analysis. b and c, MDCK cells were plated in 100-mm plates and grown to 95% confluence. After serum deprivation, the cells were stimulated with 10 ng/ml HGF alone and with 10−10 M norleual. Data are expressed as mean ± S.E.M., n = 3. ∗, p < 0.01 versus control by Tukey's post hoc analysis. Immunoblots for phospho-Erk and total Erk are shown in b, and the ratios of phospho-Erk to total Erk are indicated in c for each experimental group.
Norleual Is a Potent Inhibitor of HGF-Dependent MDCK Cell Invasion.
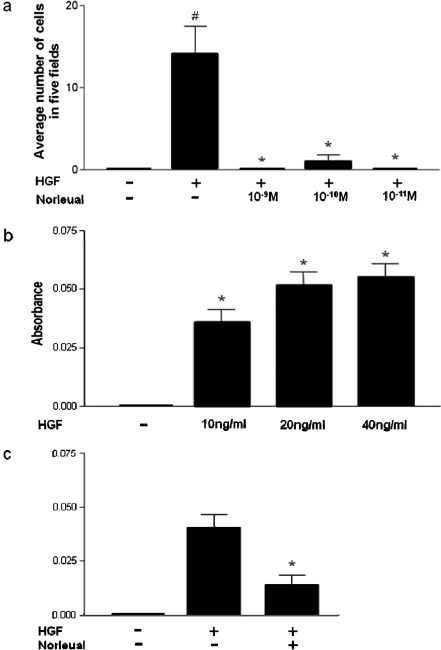
In addition to its proproliferative effect on MDCK cells, HGF causes the cells to transition from their flattened morphology to an invasive phenotype characterized by a decrease in cell-to-cell and cell-to-extracellular matrix adhesion and an increase in pseudopodia. This increase in the invasive potential of the cells provides a mechanistic basis for the ability of c-Met to support tumor metastasis. For tumor cells to metastasize, they must detach from their substrate, stay viable in suspension, and reattach to a distant organ. The cell is then stimulated by chemotactic cues from the surrounding stroma to invade the organ. HGF is one such chemotactic agent secreted from the mesenchyme, which upon activating c-Met, promotes migration and invasion. As shown in Fig. 4a, picomolar concentrations of norleual almost completely blocked HGF-dependent MDCK cell invasion.
Fig. 4.
Norleual inhibits MDCK cell invasion and HGF/c-Met-dependent uPA induction. a, MDCK cells were seeded on top of collagen gels in the top compartment of a transwell chamber and stimulated to migrate to the underside of the membrane with PBS (controls), 10 ng/ml HGF, or 10 ng/ml HGF in combination with norleual. Nearly total inhibition of invasion was evident at 10−8, 10−10, and 10−11 M norleual. Data are expressed as mean ± S.E.M., n = 3. ∗, p < 0.05 versus control by Tukey's post hoc analysis. b, MDCK cells in 96-well plates were stimulated with either 10, 20, or 40 ng/ml HGF, yielding a dose-dependent induction of uPA. uPA activity was assessed by following the degradation of a chromogenic substrate as evidenced by an increase in absorbance at 405 nm. c, norleual attenuated the induction of uPA resulting from the addition of 40 ng/ml HGF. Data are expressed as mean ± S.E.M., n = 6. #, p < 0.01, HGF versus nontreated control; ∗, p < 0.05, norleual-treated versus HGF-treated cells by Tukey's post hoc analysis.
It has been demonstrated that c-Met-driven migration and invasion require the activation of the c-Met-uPA signaling axis. uPA induction has been shown to be indispensable for c-Met-mediated proliferation and invasion; application of the uPA inhibitor B428 blocks these c-Met-dependent activities (Webb et al., 2000). Furthermore, activation of the MEK/Erk pathway by HGF, which was demonstrated above to be sensitive to norleual inhibition (Fig. 3, a and b), seems to be downstream of c-Met activation and upstream of uPA induction, because MEK inhibitors block uPA induction and subsequent cellular functions (Lee et al., 2006b). The requisite involvement of uPA in c-Met-dependent cell invasion and the apparent ability of norleual to block this process implied that norleual should attenuate uPA activity in HGF-stimulated MDCK cells. To evaluate this prediction, a dose-response curve for HGF-dependent uPA induction was generated (Fig. 4b), thus establishing the minimal dose required for maximal induction. The data in Fig. 4c demonstrate that 100 pM norleual dramatically inhibited the activation of uPA at saturating concentrations of HGF. Additional studies using nonsaturating concentrations of HGF revealed similar results (data not shown).
Norleual Inhibits HGF/c-Met-Mediated Cell Scattering in MDCK Cells.
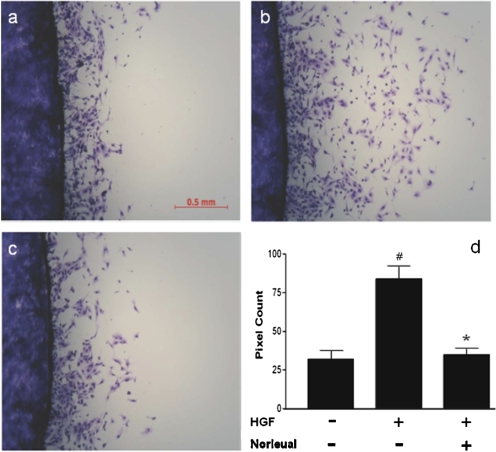
Cell scattering is the hallmark effect of HGF/c-Met signaling. MDCK cells grown at a low cell density form colonies and demonstrate a “cobblestone” morphology, which is characterized by tight intercellular junctions. Application of HGF initiates a scattering response that occurs in two stages. First, the cells lose their cell-to-cell adhesion and become polarized. Second, they separate completely and migrate away from each other. If norleual is indeed capable of disrupting HGF/c-Met-initiated cellular activities, then it would be expected to attenuate scattering in MDCK cells stimulated with HGF.
We developed a quantitative assay to analyze cell scattering in which cells were plated on plastic coverslips in six-well plates and allowed to grow to 100% confluence. In the absence of HGF, the MDCK cells grow as a compact monolayer on the coverslips. Once confluent, the coverslips were transferred to a clean dish and treated with HGF. This stimulated the cells to scatter off of the coverslips onto the plate, leaving a ring of cells on the plate after coverslip removal. The plate area covered by cells that had scattered off of the coverslip was then quantitated by densitometry. Figure 5a illustrates the typical level of scattering observed in nontreated control cells. Figure 5b demonstrates the dramatic effect that HGF has on the scattering process, whereas Fig. 5c highlights the ability of 100 pM norleual to block the HGF effect. Quantitative data describing this marked inhibition is included in the accompanying graph (Fig. 5d). Similar effects of norleual were observed at concentrations as low as 1 pM (data not shown). Other AngIV analogs have exhibited even higher potencies than norleual with regard to inhibition of HGF-dependent scattering. Data presented in Supplemental Fig. 2 for d-Nle-Tyr-Ile-(6)aminohexanoic amide, which exhibits activity at 0.1 pM, illustrate the potential potency of one such AngIV analog in this regard.
Fig. 5.
Norleual inhibits MDCK cell scattering. MDCK cells were grown to 100% confluence on coverslips and placed in a clean plate. The cells were stimulated to scatter off of the coverslip by adding 20 ng/ml HGF to the media. After 24 h of scattering, the cells were fixed with methanol and stained with Diff-Quik. The coverslips were removed to reveal the ring of cells that had scattered off of the coverslip and onto the plate. The effect of HGF on scattering was quantitated by determining by densitometry of the digital images from scattered cells. a, control scattering. b, effect of HGF (20 ng/ml). c, blockade of HGF-induced scattering by norleual (10−10 M). Quantitative data are shown in d. Data are expressed as mean ± S.E.M., n = 4. #, p < 0.05, HGF versus nontreated control; ∗, p < 0.05, norleual-treated versus HGF-treated cells by Tukey's post hoc analysis.
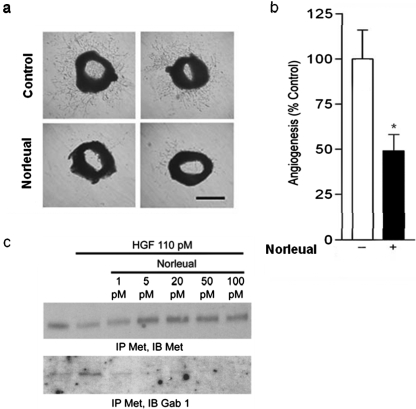
Norleual Inhibits Angiogenesis and Attenuates HGF/c-Met Signaling in Endothelial Cells.
The c-Met receptor is enriched on vascular endothelial cells in which it plays a crucial role in the regulation of angiogenesis (Zhang and Vande Woude, 2003). The large-molecule c-Met inhibitor NK4 (Fig. 1a), has been shown previously to inhibit angiogenesis (Kuba et al., 2000). Because norleual seems to act as a small-molecule c-Met inhibitor, we hypothesized that norleual should exhibit similar antiangiogenic activity. To test this prediction, angiogenesis was evaluated with the ex vivo mouse aortic ring assay in the presence and absence of norleual. After 4 days in culture, robust angiogenic sprouts were observed in control rings (Fig. 6a, top). Angiogenic growth was significantly attenuated in norleual-treated wells (Fig. 6a, bottom). The area covered by angiogenic sprouts was quantitated and the data are shown in Fig. 6b. These data underestimate the effectiveness of the norleual inhibition because differences in the density of sprouts were not considered.
Fig. 6.
Norleual inhibits angiogenesis and Gab1/c-Met association in human umbilical vein endothelial cells. a, norleual inhibits angiogenesis in the ex vivo mouse aortic ring assay. Mouse aortas were cut into 0.5-mm sections and imbedded in Matrigel. Rings were incubated in EGM-2 media with (n = 8) or without (n = 6) 10−10 M norleual for 4 days. Digital photos of the rings were taken on day 4. Scale bar, approximately 2 mm. b, quantification of aortic ring angiogenesis. Areas covered by angiogenic sprouts were quantified from day 4 ring photos for control- (white bar) and norleual (black bar)-treated rings. Data are expressed as mean ± S.E.M. A p value of 0.012 was obtained from a two-tailed t test. c, HUVECs were treated for 5 min with HGF and/or norleual at indicated concentrations. Lysates were IP with anti-Met and immunoblotted (IB) with anti-Met and Gab1.
Because c-Met activation in endothelial cells is a critical enabler of angiogenesis, we further anticipated that norleual should inhibit c-Met signaling in endothelial cells. In support of this idea, norleual was observed to inhibit HGF-induced c-Met/Gab1 association in HUVECs in vitro (Fig. 6c). Together, these observations indicate that norleual is a potent inhibitor of angiogenesis and suggest that this inhibitory activity results from the attenuation of c-Met signaling in endothelial cells.
Norleual Inhibits B16-F10 Murine Melanoma Cell Migration and Lung Colony Formation.
To evaluate the potential utility of norleual (and AT4 receptor antagonists in general) as potential therapeutics, we examined the ability of norleual to suppress the migratory and lung colony-forming capacity of B16-F10 murine melanoma cells. B16 melanoma cells overexpress c-Met (Ferraro et al., 2006) and were chosen for these studies because c-Met signaling is critical for their migration, invasion, and metastasis. To further demonstrate the antimigratory activity of norleual, norleual was applied to HGF-treated B16-F10 melanoma cells and the effects on migration (and to some extent, proliferation) were assessed in a scratch wound assay. In this assay, a confluent monolayer of cells was scratched and wound closure was monitored over time. As expected, HGF accelerated the time to wound closure, whereas norleual was able to attenuate this HGF-dependent acceleration of closure at both 4 and 6 h after wounding (Fig. 7, a and b). As indicated above for MDCK cells, Erk signaling plays an important role in c-Met-dependent migration. Consistent with this, we found that norleual not only inhibited wound closure of B16-F10 cells but also attenuated activation of Erk that occurs in response to the combined effect of the wounding process and the presence of HGF (Fig. 7c).
Fig. 7.
Norleual inhibits proliferation/migration and Erk phosphorylation in B16-F10 cells. a, confluent B16-F10 cells were scratched and the denuded area was allowed to heal for 6 h with or without HGF (312 pM) and HGF/norleual (10−11 M). Images at time of scratch and 6 h are shown. b, distance of scratch closure was measured in a blinded manner (n = 6). The difference between HGF and HGF + norleual groups was significant at 4 and 6 h. Data are expressed as mean ± S.E.M., n = 6. ∗, p < 0.01, norleual-treated versus HGF-treated cells. c, Western blot analysis of phospho-Erk1/2 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in B16-F10 cells. Cell lysates that were analyzed for phospho-Erk and GAPDH content were cells that were stimulated with and without scratching in the presence of HGF (312 pM) and/or norleual (10−11 M) for 2 h.
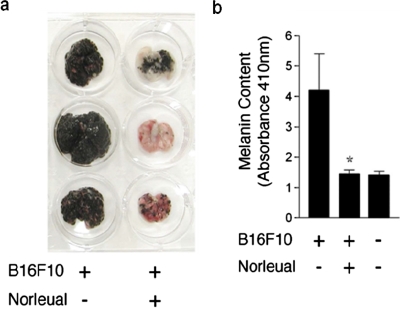
As a final test of the physiological relevance of blockade by norleual of c-Met-dependent proliferation, migration, and angiogenesis, we evaluated the ability of norleual to inhibit the formation of pulmonary colonies by B16-F10s after their introduction into the tail vein of mice. Figure 8a illustrates the range of inhibitory responses that was observed with daily intraperitoneal injections of norleual, and Figure 8b provides quantitative data regarding pulmonary melanin content, which reflects the level of melanoma colonization. Together, these data demonstrate that treatment of melanoma cells with low concentrations of norleual dramatically suppressed lung colonization and highlight the potential utility of norleual as an anticancer agent.
Fig. 8.
Norleual inhibits B16-F10 melanoma lung colonization. B16-F10 murine melanoma cells (400,000), treated in suspension with norleual at 10−11 M or PBS vehicle, were injected into the tail vein of C57BL/6 mice. Mice received daily intraperitoneal injections of norleual (50 μg/kg) or PBS vehicle. a, after 14 days, the lungs from norleual-treated mice exhibited an obvious reduction in melanoma colonies compared with untreated controls. b, after removal, lungs were homogenized and total melanin content was determined spectrophotometrically and used to quantify total pulmonary melanoma colonization in vehicle-treated and norleual-treated cells. Ungrafted age-matched control lungs exhibited a background absorbance at 410 nm. Data are expressed as mean ± S.E.M., n = 5. ∗, p < 0.05.
Discussion
Studies have shown that AngIV and selected analogs are capable of binding to and competitively inhibiting the activity of insulin-responsive aminopeptidase (Albiston et al., 2001). These findings led to the hypothesis that the biological activity of AngIV and related analogs could be attributable to the accumulation of bioactive peptides that are normally degraded by IRAP. Although this idea seems to be a logical extension of the above-mentioned findings, it has never been challenged by a direct test. That is, no biological effect initiated by AngIV, or related molecules, has ever been demonstrated to require IRAP inhibition. This lack of critical confirmation of the “IRAP inhibition” hypothesis coupled with multiple inconsistencies between the hypothesis and available data (see Introduction) strongly suggests that the observed biological effects of AngIV-like molecules results from a non-IRAP-dependent mechanism of action. This thinking led us to consider the possibility that another unidentified protein(s) might be responsible for the action of AT4 receptor ligands (Wright et al., 2008). Data presented here indicate that the HGF/c-Met system represents a likely target for AngIV-like compounds and that the mechanism of action of norleual is at least partly dependent on its ability to inhibit c-Met function. These studies demonstrate that norleual competes with HGF for binding to c-Met, antagonizes HGF-dependent c-Met signaling activity in vitro; blocks HGF-induced proliferation, invasion, cell scattering, and wound closure in vitro; inhibits ex vivo angiogenesis; and attenuates melanoma lung colonization in vivo. These results using norleual are consistent with behaviors predicted for a HGF/c-Met antagonist. We have examined many additional AngIV analogs relative to their ability to interact with the HGF/c-Met system. Although no data set for any one compound is as complete as the data set for norleual, the compiled results indicate that this ability is widespread. As an illustration, data with d-Nle-Tyr-Ile(6)aminohexanoic amide that demonstrate its ability to block HGF/c-Met signaling as well as characteristic scattering has been included.
These studies, however, do not delineate precisely how norleual disrupts the interaction of HGF and c-Met. If our speculation is accurate and norleual is indeed a hinge (linker) region mimic, there are at least two potential mechanisms of inhibition one could envision. First norleual could directly compete for the high-affinity binding of the α-chain of HGF to the immunoglobulin-like region of c-Met (Basilico et al., 2008). It is well established that this interaction between HGF and c-Met involves the N-terminal and first kringle domains of HGF, including the intervening hinge or linker region, a c-Met fragment known as NK1 (Michieli et al., 2004; Tolbert et al., 2007; Fig. 1a). The crystal structure of NK1 reveals that that the linker region is presented on the surface of HGF, connects the two tightly packed N and K1 domains (Chirgadze et al., 1999), and therefore would be exposed and available for direct interaction with c-Met. It is interesting to note that longer fragments of HGF, NK2-NK4, exhibit varying biological effects despite originating from a common parent molecule, HGF. It is possible that the addition or subtraction of kringle domains results in differential ligand-induced receptor or ligand/ligand conformational states. This idea is supported by proteins such as angiostatin and its fragments (plasminogen derivatives), which share hinge region homology with HGF and interact with c-Met (Fig. 1a; Wajih and Sane, 2003). AngIV analogs such as norleual may also mimic the conformation of the hinge region of HGF. In norleual, the reduced peptide bond increases the rotatability around the 3-4-position, making the molecule more flexible. This may permit norleual to emulate hinge conformations in HGF that are normally induced by tertiary structure (kringles) to facilitate its interaction with the c-Met.
A second potential mechanism of inhibition could involve the blockade of HGF dimerization. In this scenario, norleual could bind to HGF precluding its binding and dimerization to a second HGF molecule. Small-angle X-ray scattering data of the HGF/c-Met complex indicate that the predominant stoichiometry of this complex is equimolar mixtures of two-chain HGF and c-Met with a 2:2 ratio (Gherardi et al., 2006). These investigators further demonstrated that HGF is capable of spontaneous dimerization and indeed polymerization to form higher order oligomers. Moreover, this observed dimerization could be disrupted by mutations to the hinge region of HGF (Gherardi et al., 2006). Most importantly, however, was the determination that dimerization-deficient mutants supported little or no biological activity, suggesting that HGF dimerization is requisite for c-Met-dependent activation. Recent attempts to develop NK1-based inhibitors (Fig. 1a) have confirmed the importance of the hinge region in determining both the binding to and activation of c-Met by HGF (Youles et al., 2008). Hinge region mutants again failed to form 2:2 HGF:c-Met complexes in the presence of heparin and exhibited no ability to induce scattering in MDCK cells. We are currently investigating whether norleual is capable of binding directly to c-Met or to HGF and thus disrupting dimerization.
Although the results of this study indicate that norleual interacts in some way to disrupt the HGF/c-Met system, they do not preclude the possibility that norleual may interact with other molecular targets, which may contribute to the observed biological activities of norleual. One potential target of AngIV analogs that warrants investigation is the MSP/Ron receptor system, which is similar to the HGF/c-Met system in terms of ligand and receptor structure, intracellular signaling mechanisms, functionality, and even pathophysiological involvement (Wagh et al., 2008). The striking homology between the hinge regions of HGF and MSP (Fig. 1a) suggests that if norleual (and other AngIV analogs) are mimics of the hinge region of HGF it would likewise be expected to mimic the hinge region of MSP. Ron like c-Met, which is also highly expressed in MDCK cells, has been shown to promote cell migration and invasion and is known to participate in cancer metastasis (Wagh et al., 2008). The homology of the hinge regions of HGF and MSP and the similar biological profiles of the c-Met and Ron systems supports the likely possibility that the MSP/Ron system may represent a second target of AngIV-like molecules. In addition to the MSP/Ron system, other potential norleual interactors include multiple coreceptors of c-Met, including Fas, CD44, integrin α6β4, Plexin B, and ErbB2E, which modulate and amplify the signaling response of c-Met (Corso et al., 2005). Future studies from our laboratory will examine the potential involvement of these coreceptors in the mechanism of action of norleual.
The c-Met system is known to be dysregulated in many diseases, including human cancers in which it is involved in tumorigenesis, metastasis, and tumor-associated angiogenesis (Corso et al., 2005). HGF/c-Met signaling activates several cellular responses, such as proliferation, migration, invasion, and scattering, which are essential for transition to a metastatic phenotype. Many studies have shown that HGF/c-Met activation augments mitogenic and invasive activity in ovarian, glioma, gastric, and lung carcinomas (Ma et al., 2003; Lee et al., 2006a). Elevated c-Met expression and/or activation are generally correlated with a poor prognosis in many malignancies. Highly invasive cancers, such as metastatic breast cancer, express c-Met at levels well above those observed in less aggressive cancers (Parr and Jiang, 2001). In addition to elevated expression, multiple mutations of c-Met contribute to malignancy. These include mutations to the tyrosine kinase domain of c-Met, which confers constitutive phosphorylation of the receptor. However, gain-of-function mutations to c-Met are not exclusive to the tyrosine kinase domain. The juxtamembrane domain is critical for the metabolism of c-Met because mutations in this domain inhibit ubiquitylation, ultimately increasing receptor insertion into the cell membrane (Lee et al., 2006a). The ability of c-Met siRNA treatment of tumor cells to suppress growth and metastasis further highlights the critical role played by c-Met in cancer cell behavior (Corso et al., 2008).
The anticancer potential of inhibiting the HGF/c-Met system has been clearly recognized as evidenced by the recent explosion of relevant reviews on the subject (Knudsen and Vande Woude, 2008) and the concerted efforts of the pharmaceutical community to develop HGF/c-Met-based therapeutics (Comoglio et al., 2008). The demonstrated ability of norleual to inhibit c-Met signaling and consequent biological activities suggests that norleual and other AngIV analogs could represent a novel treatment option for aggressive cancers where c-Met is overexpressed or mutated. This notion is directly supported by the observation that low concentrations of norleual are capable of blocking HGF-dependent proliferation/migration of melanoma cells in vitro and dramatically attenuating their pulmonary colonization in vivo. Moreover, the observed antiangiogenic activity of norleual predicts that its potential anticancer activity may manifest both as a direct effect on cancer cells and as an indirect effect on the angiogenic response of the host to the tumor. Historically, AngIV and its analogs, including norleual, have been best recognized for their dramatic ability to alter cognitive function and related processes such as LTP (Wright et al., 2008). Recognition of this activity raised the question as to whether the therapeutic potential of molecules like norleual, which interfere with learning and LTP (Davis et al., 2006), might be diminished by this confounding anticognitive activity. Fortunately, the majority of AngIV analogs that have been examined are not expected to pass the blood-brain barrier, thus reducing this concern.
This investigation documents the ability of norleual to interfere with signaling through the HGF/c-Met axis and further suggests that AngIV-related analogs may have clinical utility for the treatment of disorders that are characterized by either an overactivation of or an absolute dependence on the HGF/c-Met system. Broad interpretation of these data encourages further evaluation of the hypothesis that HGF hinge region mimics may possess important therapeutic potential. Moreover, the data presented in this study demonstrate that the AT4 receptor antagonist and AngIV analog norleual, which is known to block the activity of the procognitive AngIV analog Nle1-AngIV (Davis et al., 2006), exhibits cellular and biological activity independently of IRAP. These findings thus cast doubt on the physiological significances of AngIV-dependent IRAP inhibition.
Supplementary Material
Acknowledgments
We thank Jeanne Jensen and Laureen Poesy for help preparing this manuscript.
This work was supported by the state of Washington and Pacific Northwest Biotechnology, LLC and the National Institutes of Health National Eye Institute [Grant EY012836] (to M.D.V.).
Portions of the work described here can be found in Yamamoto BJ (2006) Norleual, an Angiotensin IV Receptor Ligand and c-Met Antagonist, Ph.D. thesis, Washington State University, Pullman, WA and Elias JD (2008) On the Development of the Angiotensin IV Ligands, Norleual, and Nle′-angiotensin IV as Anticancer and Wound Healing Agents, Ph.D. thesis, Washington State University, Pullman, WA.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.161711.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- AngIV
- angiotensin IV
- IRAP
- insulin-responsive aminopeptidase
- LTP
- long-term potentiation
- HGF
- hepatocyte growth factor
- norleual
- Nle-Tyr-Leu-ψ-(CH2-NH2)3-4-His-Pro-Phe
- Erk
- extracellular signal-regulated kinase
- EGM
- endothelial growth medium
- HEK
- human embryonic kidney
- MDCK
- Madin-Darby canine kidney
- DMEM
- Dulbecco's modified Eagle's medium
- FBS
- fetal bovine serum
- HUVEC
- human umbilical vein endothelial cell
- BSA
- bovine serum albumin
- PBS
- phosphate-buffered saline
- TBS
- Tris-buffered saline
- MTT
- 1-(4,5-dimethylthiazol-2-yl)-3,5-diphenylformazan
- uPA
- urokinase-type plasminogen activator
- MSP
- macrophage-stimulating protein
- MEK
- mitogen-activated protein kinase kinase
- Gab1
- growth factor receptor bound protein-2-associated binder
- B428
- 4-iodo[b]thiophene-2-carboxamidine
- IP
- immunoprecipitated
- IB
- immunoblotted.
References
- Albiston AL, McDowall SG, Matsacos D, Sim P, Clune E, Mustafa T, Lee J, Mendelsohn FA, Simpson RJ, Connolly LM, et al. (2001) Evidence that the angiotensin IV (AT(4)) receptor is the enzyme insulin-regulated aminopeptidase. J Biol Chem 276:48623–48626 [DOI] [PubMed] [Google Scholar]
- Basilico C, Arnesano A, Galluzzo M, Comoglio PM, Michieli P. (2008) A high affinity hepatocyte growth factor-binding site in the immunoglobulin-like region of Met. J Biol Chem 283:21267–21277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JK, Zimpelmann J, Harris RC, Burns KD. (2001) Angiotensin IV induces tyrosine phosphorylation of focal adhesion kinase and paxillin in proximal tubule cells. Am J Physiol Renal Physiol 280:F980–F988 [DOI] [PubMed] [Google Scholar]
- Chirgadze DY, Hepple JP, Zhou H, Byrd RA, Blundell TL, Gherardi E. (1999) Crystal structure of the NK1 fragment of HGF/SF suggests a novel mode for growth factor dimerization and receptor binding. Nat Struct Biol 6:72–79 [DOI] [PubMed] [Google Scholar]
- Comoglio PM, Giordano S, Trusolino L. (2008) Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat Rev Drug Discov 7:504–516 [DOI] [PubMed] [Google Scholar]
- Corso S, Comoglio PM, Giordano S. (2005) Cancer therapy: can the challenge be MET? Trends Mol Med 11:284–292 [DOI] [PubMed] [Google Scholar]
- Corso S, Migliore C, Ghiso E, De Rosa G, Comoglio PM, Giordano S. (2008) Silencing the MET oncogene leads to regression of experimental tumors and metastases. Oncogene 27:684–693 [DOI] [PubMed] [Google Scholar]
- Davis CJ, Kramár EA, De A, Meighan PC, Simasko SM, Wright JW, Harding JW. (2006) AT4 receptor activation increases intracellular calcium influx and induces a non-N-methyl-D-aspartate dependent form of long-term potentiation. Neuroscience 137:1369–1379 [DOI] [PubMed] [Google Scholar]
- Esteban V, Ruperez M, Sánchez-López E, Rodríguez-Vita J, Lorenzo O, Demaegdt H, Vanderheyden P, Egido J, Ruiz-Ortega M. (2005) Angiotensin IV activates the nuclear transcription factor-kappaB and related proinflammatory genes in vascular smooth muscle cells. Circ Res 96:965–973 [DOI] [PubMed] [Google Scholar]
- Ferraro D, Corso S, Fasano E, Panieri E, Santangelo R, Borrello S, Giordano S, Pani G, Galeotti T. (2006) Pro-metastatic signaling by c-Met through RAC-1 and reactive oxygen species (ROS). Oncogene 25:3689–3698 [DOI] [PubMed] [Google Scholar]
- Gherardi E, Sandin S, Petoukhov MV, Finch J, Youles ME, Ofverstedt LG, Miguel RN, Blundell TL, Vande Woude GF, Skoglund U, et al. (2006) Structural basis of hepatocyte growth factor/scatter factor and MET signalling. Proc Natl Acad Sci USA 103:4046–4051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa RK. (2001) Characterization and signaling of the AT(4) receptor in human proximal tubule epithelial (HK-2) cells. J Am Soc Nephrol 12:440–449 [DOI] [PubMed] [Google Scholar]
- Handa RK, Krebs LT, Harding JW, Handa SE. (1998) Angiotensin IV AT4-receptor system in the rat kidney. Am J Physiol 274:F290–F299 [DOI] [PubMed] [Google Scholar]
- Higuchi O, Nakamura T. (1991) Identification and change in the receptor for hepatocyte growth factor in rat liver after partial hepatectomy or induced hepatitis. Biochem Biophys Res Commun 176:599–607 [DOI] [PubMed] [Google Scholar]
- Knudsen BS, Vande Woude G. (2008) Showering c-MET-dependent cancers with drugs. Curr Opin Genet Dev 18:87–96 [DOI] [PubMed] [Google Scholar]
- Kramár EA, Armstrong DL, Ikeda S, Wayner MJ, Harding JW, Wright JW. (2001) The effects of angiotensin IV analogs on long-term potentiation within the CA1 region of the hippocampus in vitro. Brain Res 897:114–121 [DOI] [PubMed] [Google Scholar]
- Kramár EA, Harding JW, Wright JW. (1997) Angiotensin II- and IV-induced changes in cerebral blood flow. Roles of AT1, AT2, and AT4 receptor subtypes. Regul Pept 68:131–138 [DOI] [PubMed] [Google Scholar]
- Kuba K, Matsumoto K, Date K, Shimura H, Tanaka M, Nakamura T. (2000) HGF/NK4, a four-kringle antagonist of hepatocyte growth factor, is an angiogenesis inhibitor that suppresses tumor growth and metastasis in mice. Cancer Res 60:6737–6743 [PubMed] [Google Scholar]
- Laustsen PG, Vang S, Kristensen T. (2001) Mutational analysis of the active site of human insulin-regulated aminopeptidase. Eur J Biochem 268:98–104 [DOI] [PubMed] [Google Scholar]
- Lee JH, Gao CF, Lee CC, Kim MD, Vande Woude GF. (2006a) An alternatively spliced form of Met receptor is tumorigenic. Exp Mol Med 38:565–573 [DOI] [PubMed] [Google Scholar]
- Lee KH, Choi EY, Hyun MS, Jang BI, Kim TN, Kim SW, Song SK, Kim JH, Kim JR. (2006b) Hepatocyte growth factor/c-met signaling in regulating urokinase plasminogen activator in human stomach cancer: a potential therapeutic target for human stomach cancer. Korean J Intern Med 21:20–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew RA, Mustafa T, Ye S, McDowall SG, Chai SY, Albiston AL. (2003) Angiotensin AT4 ligands are potent, competitive inhibitors of insulin regulated aminopeptidase (IRAP). J Neurochem 86:344–350 [DOI] [PubMed] [Google Scholar]
- Li YD, Block ER, Patel JM. (2002) Activation of multiple signaling modules is critical in angiotensin IV-induced lung endothelial cell proliferation. Am J Physiol Lung Cell Mol Physiol 283:L707–L716 [DOI] [PubMed] [Google Scholar]
- Ma PC, Maulik G, Christensen J, Salgia R. (2003) c-Met: structure, functions and potential for therapeutic inhibition. Cancer Metastasis Rev 22:309–325 [DOI] [PubMed] [Google Scholar]
- Michieli P, Mazzone M, Basilico C, Cavassa S, Sottile A, Naldini L, Comoglio PM. (2004) Targeting the tumor and its microenvironment by a dual-function decoy Met receptor. Cancer Cell 6:61–73 [DOI] [PubMed] [Google Scholar]
- Parr C, Jiang WG. (2001) Expression of hepatocyte growth factor/scatter factor, its activator, inhibitors and the c-Met receptor in human cancer cells. Int J Oncol 19:857–863 [PubMed] [Google Scholar]
- Sakkab D, Lewitzky M, Posern G, Schaeper U, Sachs M, Birchmeier W, Feller SM. (2000) Signaling of hepatocyte growth factor/scatter factor (HGF) to the small GTPase Rap1 via the large docking protein Gab1 and the adapter protein CRKL. J Biol Chem 275:10772–10778 [DOI] [PubMed] [Google Scholar]
- Shimamura M, Sato N, Waguri S, Uchiyama Y, Hayashi T, Iida H, Nakamura T, Ogihara T, Kaneda Y, Morishita R. (2006) Gene transfer of hepatocyte growth factor gene improves learning and memory in the chronic stage of cerebral infarction. Hypertension 47:742–751 [DOI] [PubMed] [Google Scholar]
- Stella MC, Comoglio PM. (1999) HGF: a multifunctional growth factor controlling cell scattering. Int J Biochem Cell Biol 31:1357–1362 [DOI] [PubMed] [Google Scholar]
- Swanson GN, Hanesworth JM, Sardinia MF, Coleman JK, Wright JW, Hall KL, Miller-Wing AV, Stobb JW, Cook VI, Harding EC. (1992) Discovery of a distinct binding site for angiotensin II (3-8), a putative angiotensin IV receptor. Regul Pept 40:409–419 [DOI] [PubMed] [Google Scholar]
- Tolbert WD, Daugherty J, Gao C, Xie Q, Miranti C, Gherardi E, Woude GV, Xu HE. (2007) A mechanistic basis for converting a receptor tyrosine kinase agonist to an antagonist. Proc Natl Acad Sci USA 104:14592–14597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinh A, Widdop RE, Drummond GR, Gaspari TA. (2008) Chronic angiotensin IV treatment reverses endothelial dysfunction in ApoE-deficient mice. Cardiovasc Res 77:178–187 [DOI] [PubMed] [Google Scholar]
- Wagh PK, Peace BE, Waltz SE. (2008) Met-related receptor tyrosine kinase Ron in tumor growth and metastasis. Adv Cancer Res 100:1–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajih N, Sane DC. (2003) Angiostatin selectively inhibits signaling by hepatocyte growth factor in endothelial and smooth muscle cells. Blood 101:1857–1863 [DOI] [PubMed] [Google Scholar]
- Webb CP, Hose CD, Koochekpour S, Jeffers M, Oskarsson M, Sausville E, Monks A, Vande Woude GF. (2000) The geldanamycins are potent inhibitors of the hepatocyte growth factor/scatter factor-met-urokinase plasminogen activator-plasmin proteolytic network. Cancer Res 60:342–349 [PubMed] [Google Scholar]
- Wright JW, Jensen LL, Roberts KA, Sardinia MF, Harding JW. (1989) Structure-function analyses of brain angiotensin control of pressor action in rats. Am J Physiol 257:R1551–R1557 [DOI] [PubMed] [Google Scholar]
- Wright JW, Stubley L, Pederson ES, Kramár EA, Hanesworth JM, Harding JW. (1999) Contributions of the brain angiotensin IV-AT4 receptor subtype system to spatial learning. J Neurosci 19:3952–3961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JW, Yamamoto BJ, Harding JW. (2008) Angiotensin receptor subtype mediated physiologies and behaviors: new discoveries and clinical targets. Prog Neurobiol 84:157–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youles M, Holmes O, Petoukhov MV, Nessen MA, Stivala S, Svergun DI, Gherardi E. (2008) Engineering the NK1 fragment of hepatocyte growth factor/scatter factor as a MET receptor antagonist. J Mol Biol 377:616–622 [DOI] [PubMed] [Google Scholar]
- Zhang YW, Vande Woude GF. (2003) HGF/SF-met signaling in the control of branching morphogenesis and invasion. J Cell Biochem 88:408–417 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.