Abstract
Although many drugs act by indirectly stimulating multiple receptors (e.g., reuptake inhibitors), relatively little is known about interactions between agonism at different receptors. This study compared the effect of serotonin (5-HT)1A receptor agonists with the discriminative stimulus effects of the 5-HT2A receptor agonist 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane (DOM) in rats and rhesus monkeys. Eight rats discriminated 0.56 mg/kg i.p. DOM and responded under a fixed ratio (FR) 10 schedule of food presentation, whereas three rhesus monkeys discriminated 0.32 mg/kg s.c. DOM and responded under an FR 5 schedule of stimulus shock termination. DOM and the 5-HT2A receptor agonists 2,5-dimethoxy-4-n-propylthiophenethylamine (2C-T-7) and dipropyltryptamine (DPT), but not the 5-HT1A receptor agonists 8-hydroxy-2-(di-n-propylamino) tetralin hydrochloride (8-OH-DPAT) and 3-chloro-4-fluorophenyl-(4-fluoro-4-([(5-methyl-6-methylaminopyridin-2-ylmethyl) amino) methyl] piperidin-1-yl) methanone (F13714), occasioned responding on the DOM-associated lever in rats and monkeys. Both 8-OH-DPAT and F13714 attenuated the discriminative stimulus effects of DOM in monkeys but not in rats; these effects of 8-OH-DPAT and F13714 were prevented by the 5-HT1A receptor antagonist N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridyl)cyclohexanecarboxamide (WAY 100635). DPT and 2C-T-7 enhanced the discriminative stimulus effects of DOM in rats and monkeys in an additive manner. Taken together, the results suggest that the DOM discriminative stimulus is pharmacologically similar and mediated by 5-HT2A receptors in rats and monkeys; however, the ability of 5-HT1A receptor agonists to modify the effects of DOM is markedly different between these species. These results indicate possible differences in the neurobiology of 5-HT systems that could be important for studying drugs that have multiple mechanisms of action (e.g., reuptake inhibitors that indirectly stimulate multiple receptors).
Often drugs are used in combination to enhance therapeutic effectiveness and/or reduce unwanted effects. Drugs that are used together often share a common effect (e.g., decrease blood pressure) but through different mechanisms (Oates and Brown, 2001). Other pharmacotherapeutic strategies also involve multiple mechanisms of action, including indirect-acting agonists that indirectly stimulate multiple receptors. For example, by blocking serotonin (5-HT) transport back into neurons, selective 5-HT reuptake inhibitors (SSRIs) act as indirect 5-HT receptor agonists, increasing synaptic levels of 5-HT, which acts at multiple 5-HT receptor subtypes. The therapeutic efficacy of SSRIs is well established; however, the role of different 5-HT receptor subtypes in the therapeutic effects of SSRIs and the potential importance of interactions among receptor subtypes are largely unknown.
Among 5-HT receptor subtypes, 5-HT1A and 5-HT2A receptors are thought to be important in the therapeutic (e.g., antidepressant) effects of SSRIs (Celada et al., 2004). However, the role of these (and other) receptor subtypes in therapeutic effects of SSRIs and the extent to which activity at one subtype affects activity at another subtype are not well established. There are indications of interactions between receptor subtypes; for example, the 5-HT2A receptor antagonist MDL100907 enhances antidepressant-like activity of SSRIs in rats (Marek et al., 2005), suggesting that activation of 5-HT2A receptors might attenuate therapeutic effects SSRIs that are mediated through other (e.g., 5-HT1A) receptors.
Several studies have examined interactions between drugs acting at 5-HT1A and 5-HT2A receptors. For example, 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane-induced head twitching in mice, a behavior thought to be mediated by 5-HT2A receptor activation (Green et al., 1983), is inhibited by the 5-HT1A receptor agonist 8-hydroxy-2-(di-n-propylamino) tetralin hydrochloride (8-OH-DPAT; Darmani et al., 1990); in contrast, 8-OH-DPAT increases head twitching caused by 5-methoxy-dimethyltryptamine, another drug with 5-HT2A receptor agonist activity (Darmani et al., 1990). In rats 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane increases 8-OH-DPAT-induced forepaw treading, a component of the 5-HT syndrome (Arnt and Hyttel, 1989), and it antagonizes 8-OH-DPAT-induced hypoactivity (Krebs-Thomson and Geyer, 1998). Many studies on interactions between drugs acting at 5-HT1A or 5-HT2A receptors measured changes (usually decreases) in drug-elicited behavior; the current study examined drug-induced shifts in dose-response functions and the ability of a selective antagonist to attenuate those shifts.
A strength of drug discrimination procedures is that similar effects are typically obtained with the same drug(s) across a broad range of conditions (e.g., different species). However, there are exceptions to this general finding; for example, lisuride (dopamine and 5-HT receptor agonist) and eltoprazine (5-HT mixed agonist/antagonist) occasion high levels of responding on the 8-OH-DPAT-associated manipulandum in pigeons but not in rats (Kleven and Koek, 1998a), and metaphit (phencyclidine receptor acylator) shares discriminative stimulus effects with phencyclidine in pigeons but not in rhesus monkeys (Koek et al., 1986). There also are examples where the effect of one drug on the discriminative stimulus effects of a second drug is different across species. For example, the μ opioid receptor agonist heroin enhances the discriminative stimulus effects of the cannabinoid receptor agonist Δ9-tetrahydrocannabinol in rats (Solinas and Goldberg, 2005) but not in rhesus monkeys (Li et al., 2008a). Other data indicate that 5-HT1A receptor agonists enhance the discriminative stimulus effects of 5-HT2 receptor agonists in rats (Glennon, 1991; Reissig et al., 2005; Khorana et al., 2009); however, the generality of those observations to other conditions and other species has not been evaluated despite the relevance of such studies to understanding the actions of indirect-acting 5-HT receptor agonists.
The current study examined the discriminative stimulus effects of drug combinations in rats and rhesus monkeys to investigate the generality of reported interactions between agonists acting at 5-HT1A or 5-HT2A receptors and explore whether the effects obtained in one species predict effects in other species. First, the discriminative stimulus effects of agonists that are thought to act selectively at 5-HT2A receptors [1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane (DOM); 2,5-dimethoxy-4-n-propylthiophenethylamine (2C-T-7); and dipropyltryptamine (DPT)] were studied alone and in combination in rats and monkeys. Second, agonists that are thought to act selectively at 5-HT1A receptors (8-OH-DPAT and F13714) were studied for their ability to modify the discriminative stimulus effects of DOM.
Materials and Methods
Subjects
Three adult rhesus monkeys weighing between 5 and 9 kg were housed individually in stainless-steel cages where they had unlimited access to water. Monkeys received chow (High Protein Monkey Diet; Harlan Teklad, Madison, WI), fresh fruit, and peanuts daily in amounts sufficient to maintain normal age- and gender-appropriate body weights. Monkeys were maintained on a 14:10-h light/dark cycle and trained previously to discriminate between subcutaneous injections of 0.32 mg/kg DOM and saline (Li et al., 2008b, 2009a).
Eight adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) were housed individually in plastic cages in a room maintained on a 12:12-h light/dark cycle (experiments were conducted during the light period) with unlimited access to water in the home cage. Rats were maintained at a reduced, stable body weight by providing rodent chow (Rodent sterilizable diet; Harlan Teklad) in the home cage after daily sessions. The amount of chow provided after session (5–16 g) was adjusted daily to maintain body weights near 350 g.
Animals used in these studies were maintained in accordance with the Institutional Animal Care and Use Committee, The University of Texas Health Science Center at San Antonio, and the 1996 Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animals Resources on Life Sciences, National Research Council, National Academy of Sciences).
Drug Discrimination in Rhesus Monkeys
Apparatus and Procedure.
During experimental sessions, monkeys were seated in chairs (model R001; Primate Products, Miami, FL) that provided restraint at the neck and arms and were located in ventilated, sound-attenuating chambers equipped with two stimulus lights and two response levers. The monkeys responded under a schedule of stimulus-shock termination as described previously (Li et al., 2008b, 2009a). The feet of monkeys were placed in shoes mounted to the front of the chair and equipped with brass electrodes to which a brief (250-ms; 3-mA) electric shock could be delivered from an a.c. generator. Experiments were controlled, and data were recorded with a microprocessor and commercially available interface (MED Associates Inc., East Fairfield, VT).
Daily training sessions consisted of two to six 15-min cycles, and each cycle started with a 10-min timeout period, during which the stimulus lights were extinguished and responding had no programmed consequence. The timeout was followed by a 5-min response period, during which stimulus lights above both levers were illuminated and electric shocks were scheduled to occur every 15 s. Monkeys could extinguish the stimulus lights and postpone the shock schedule for 30 s by responding five times consecutively (FR 5) on the lever designated correct by an injection administered during the first minute of the cycle (e.g., right lever, saline; left lever, DOM). Incorrect responses reset the FR requirement on the correct lever. Response periods ended after 5 min or the delivery of four shocks, whichever occurred first.
On training days, monkeys received a subcutaneous injection of 0.32 mg/kg DOM followed by one sham (no injection) cycle or a subcutaneous injection of saline followed by one to five sham cycles. Monkeys used in this study had previously satisfied the following criteria for five constitutive or six of seven sessions: at least 80% of the total responses on the correct lever and fewer than five responses (one FR) on the incorrect lever before completion of the FR on the correct lever. Tests generally occurred every 3rd day and only when the same criteria noted above were satisfied during the two training sessions immediately preceding the test.
Test sessions were similar to training sessions except that five consecutive responses on either lever postponed shock. For substitution studies, saline was administered in the first cycle followed by increasing doses of a test compound in each subsequent cycle up to doses that occasioned more than 80% DOM lever responding that resulted in delivery of an electric stimulus or up to the largest dose that could be safely studied. For drug combination studies, the procedure was the same except that a dose of pretreatment drug was administered 5 min before the start of the session.
Drug Discrimination in Rats
Apparatus and Procedure.
Experiments were conducted in commercially available chambers (model ENV-008CT; MED Associates Inc.) located within sound-attenuating, ventilated enclosures (model ENV-022M; MED Associates Inc.) that are described in detail in Carter et al. (2003). Data were collected using MED-PC IV software and an interface (MED Associates Inc.). Rats were trained to discriminate 0.56 mg/kg DOM intraperitoneally from saline according to procedures described previously (Li et al., 2007, 2009b). Before the initiation of this study, rats met the following criteria: at least 90% of the total responses on the correct lever and fewer than 10 responses (one FR) on the incorrect lever before delivery of the first food pellet (45 mg; Research Diets, New Brunswick, NJ) for five consecutive or six of seven sessions. Daily training sessions consisted of two to six 15-min cycles, and each cycle started with a 10-min timeout period, during which stimulus lights were not illuminated and responding had no programmed consequence. This time-out period was followed by a 5-min response period during which two stimulus lights were illuminated above the levers. Rats could receive food by responding 10 times consecutively on the lever designated correct by an injection administered during the 1st min of the cycle (e.g., right lever, saline; left lever, DOM). Incorrect responses reset the FR requirement on the correct lever. The response period ended when rats received five pellets or after 5 min, whichever occurred first.
Test sessions were identical to training sessions, except that 10 consecutive responses on either lever resulted in the delivery of food. For substitution studies, saline was administered in the first cycle followed by increasing doses of a test compound in subsequent cycles, up to doses that occasioned more than 90% DOM lever responding, or up to the doses that markedly decreased lever pressing. For drug combination studies, the procedure was the same except that a dose of pretreatment drug was administered 5 min before the start of the session.
Data Analyses
For monkeys and rats, drug discrimination data are expressed as the average percentage of the total responses that were made on the DOM-associated lever (±1 S.E.M.) and plotted as a function of dose. When a monkey or rat responded at a rate that was less than 20% of its vehicle control rate, discrimination data from that test were not included in the average, although the response rate data were included in the group average. Rate of lever pressing on both levers is plotted in responses per second and reported as the average (±1 S.E.M.) for all tests. For drugs that occasioned responding on the DOM-associated lever, the dose required to produce 50% DOM lever responding was estimated [ED50 (95% confidence limit)] from linear regression using the portion of the dose-effect curve spanning 50%.
The dose-effect curves of DOM, 2C-T-7, and DPT were tested for parallelism, in monkeys and in rats, using methods detailed previously (e.g., Koek et al., 2009). In brief, F ratio tests in Prism version 5.01 for Windows (GraphPad Software Inc., San Diego, CA; www.graphpad.com) were used to compare dose-effect curves with respect to their slopes. A nonsignificant F ratio for slopes indicates that the dose-effect curves do not deviate significantly from parallelism. To determine whether the effects of drug combinations were additive, supra-additive, or infra-additive, isobolograms were constructed (Gessner, 1988; Krebs-Thomson and Geyer, 1998). An isobologram plots equieffective doses (e.g., ED50) of one drug in the presence of different doses of a second drug. If the effects of the two drugs are additive, the ED values for the drug combination should lie on the diagonal line between the ED values for the two drugs alone (additivity line). If the ED values fall below the additivity line, the effects of the two drugs are considered supra-additive, i.e., in the presence of one drug, smaller doses of a second drug are needed to produce the same effect. If the ED values fall above the additivity line, the effects of the two drugs are considered infra-additive, i.e., in the presence of one drug larger doses of a second drug are needed to produce the same effect. The significance of the deviation of individual points from additivity was determined by connecting the error bars of the ED values for DOM (shown on ordinate) and DOM, DPT or 2C-T-7 alone (shown on abscissa). If the error bars of the individual points did not overlap with this area around the additivity line, the deviation from additivity was considered significant (Krebs-Thomson and Geyer 1998; Lelas et al., 2001).
Drugs
The compounds used in this study were as follows: DOM and 2C-T-7 were provided by the National Institute on Drug Abuse (Research Technology Branch, Rockville, MD); DPT and MDL100907 were synthesized as described previously (Ullrich and Rice, 2000); 8-OH-DPAT was purchased from Sigma-Aldrich (St. Louis, MO); and F13714 and WAY 100635 were gifts from Dr. Adrian Newman-Tancredi (Centre de Recherche Pierre Fabre, Castres, France). MDL100907 was dissolved in 20% dimethyl sulfoxide (v/v) and saline; other drugs were dissolved in sterile 0.9% saline. Doses are expressed as the form of the drug listed above in milligrams per kilogram of body weight. Injection volumes were 0.1 to 1.0 ml.
Results
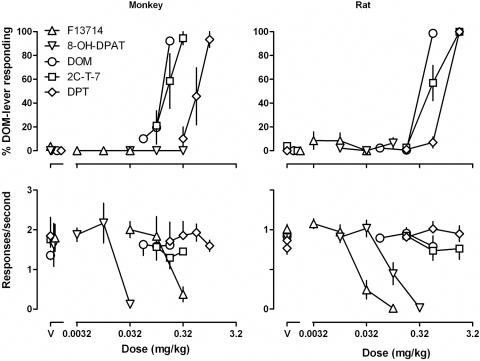
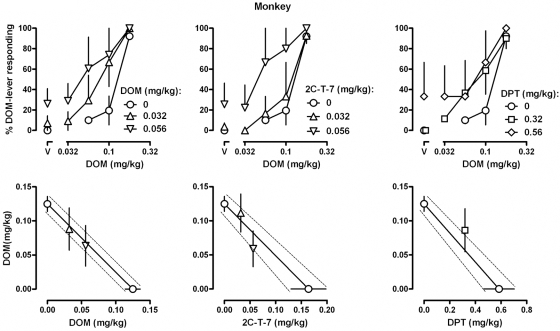
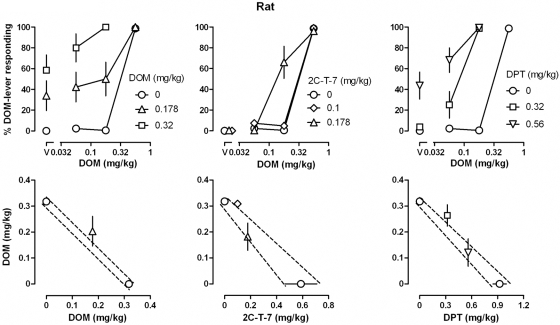
DOM, 2C-T-7, and DPT each increased responding on the DOM-associated lever in a dose-related manner in monkeys (Fig. 1, top left) and in rats (Fig. 1, top right), with the largest doses of each occasioning an average more than 90% responding on the DOM-associated lever [DOM, ED50 (95% CL) = 0.125 (0.103, 0.147) mg/kg in monkeys and 0.318 (0.316, 0.320) mg/kg in rats; 2C-T-7, 0.164 (0.097, 0.231) mg/kg in monkeys and 0.587 (0.347, 0.827) mg/kg in rats; and DPT, 0.585 (0.352, 0.817) mg/kg in monkeys and 0.922 (0.808, 1.035) mg/kg in rats]. The rank-order potency was the same in monkeys and rats, with DOM being slightly more potent than 2C-T-7 in both species and being 4.7- and 2.9-fold more potent than DPT in monkeys and rats, respectively. The slopes of the dose-effect curves for DOM, 2C-T-7, and DPT were not significantly different from each other, in monkeys [F(2, 21) = 0.13, P = 0.88] and rats [F(2, 60) = 0.007, P = 0.99]. None of the compounds markedly altered rate of lever pressing at the doses studied (Fig. 1, bottom left and right). In contrast, F13714 and 8-OH-DPAT occasioned less than 10% responding on the DOM-associated lever up to doses that markedly decreased rate of lever pressing in monkeys and in rats (Fig. 1, left and right, respectively). F13714 was 10.4- and 4.5-fold more potent than 8-OH-DPAT for decreasing responding in monkeys and rats, respectively.
Fig. 1.
Discriminative stimulus effects and effects on response rate for F13714, 8-OH-DPAT, DOM, 2C-T-7, and DPT in monkeys (n = 3; left) and rats (n = 8; right) discriminating DOM. Abscissae, dose in milligrams per kilogram of body weight; V, vehicle. Ordinates, mean (±S.E.M.) percentage of responses on the DOM-associated lever (top) and mean (±S.E.M.) rate of responding in responses per second (bottom). See Materials and Methods for other details.
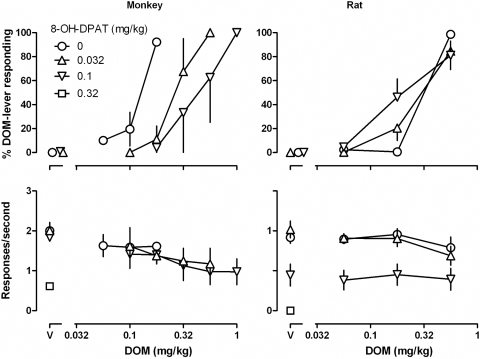
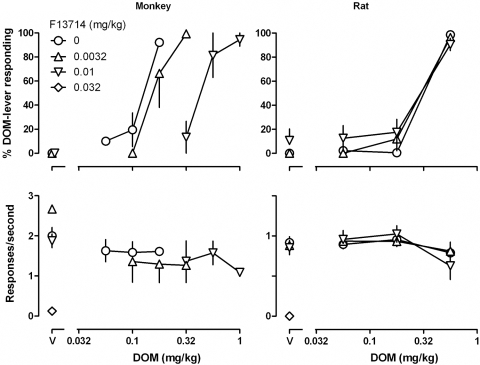
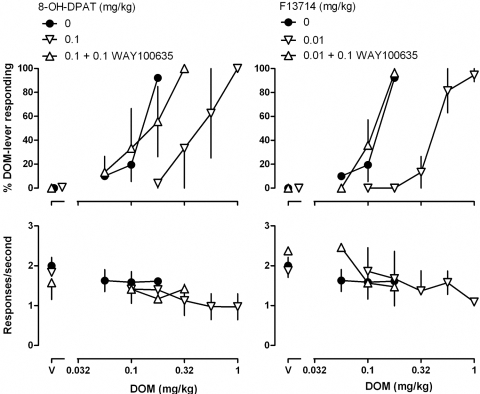
In monkeys, 8-OH-DPAT and F13714 dose-dependently attenuated the discriminative stimulus effects of DOM, as indicated by rightward shifts in the DOM dose-response curve (Figs. 2 and 3, top left). In the presence of 0.032 and 0.1 mg/kg 8-OH-DPAT, the DOM dose-response curve was shifted 2.3- and 3.6-fold rightward, respectively (Fig. 2, top left; see Table 1 for ED50 values). A larger dose of 8-OH-DPAT (0.32 mg/kg) markedly decreased responding (square above “V”; Fig. 2, bottom left). In the presence of 0.0032 and 0.01 mg/kg F13714, the DOM dose-response curve was shifted 1.3- and 3.6-fold rightward, respectively (Fig. 3, top left; Table 1). A larger dose of F13714 (0.032 mg/kg) markedly decreased responding (diamond above “V”; Fig. 3, bottom left). Attenuation of the discriminative stimulus effects of DOM in monkeys by 0.1 mg/kg 8-OH-DPAT and by 0.01 mg/kg F13714 was prevented by administration of 0.1 mg/kg WAY 100635 (Fig. 4, triangles, top).
Fig. 2.
Discriminative stimulus effects and effects on response rate for DOM administered alone and in combination with different doses of 8-OH-DPAT in monkeys (left) and rats (right). See Fig. 1 and Materials and Methods for other details.
Fig. 3.
Discriminative stimulus effects and effects on response rate for DOM administered alone and in combination with different doses of F13714 in monkeys (left) and rats (right). See Fig. 1 and Materials and Methods for other details.
TABLE 1.
ED50 values for DOM alone and in combination with 5-HT1A receptor agonists in monkeys and rats: drug discrimination
| ED50 (95% Confidence Interval) |
Dose Ratio, Monkeys/Ratsa | ||
|---|---|---|---|
| Monkeys | Rats | ||
| mg/kg | |||
| DOM alone | 0.13 (0.10, 0.15) | 0.32 (0.32, 0.32) | −/− |
| DOM + | |||
| 0.032 mg/kg 8-OH-DPAT | 0.29 (0.17, 0.41) | 0.35 (0.18, 0.52) | 2.29/1.10 |
| 0.1 mg/kg 8-OH-DPAT | 0.45 (0.20, 0.70) | 0.33 (0.09, 0.57) | 3.58/1.03 |
| + 0.1 WAY100635 | 0.16 (0.06, 0.26) | n.s. | 1.25/− |
| 0.0032 mg/kg F13714 | 0.17 (0.11, 0.23) | 0.30 (0.24, 0.36) | 1.34/0.94 |
| 0.01 mg/kg F13714 | 0.45 (0.29, 0.61) | 0.34 (0.31, 0.36) | 3.62/1.05 |
| + 0.1 WAY100635 | 0.11 (0.09, 0.14) | n.s. | 0.91/− |
n.s., not studied.
Ratio of DOM ED50 with pretreatment/DOM ED50 without pretreatment.
Fig. 4.
Discriminative stimulus effects and effects on response rate for DOM administered alone, with 8-OH-DPAT or F13714 (same data as plotted in Figs. 2 and 3, respectively), and with 8-OH-DPAT or F13714 and WAY 100635. See Fig. 1 and Materials and Methods for other details.
In contrast to the effects obtained in monkeys, neither 8-OH-DPAT nor F13714 attenuated the discriminative stimulus effects of DOM in rats. Doses of 0.032 and 0.1 mg/kg 8-OH-DPAT neither shifted the DOM dose-response curve rightward (Fig. 2, top right) nor significantly increased the DOM ED50 (Table 1). A larger dose of 8-OH-DPAT (0.1 mg/kg) markedly decreased responding (square above “V”; Fig. 2, bottom right). Similarly, in rats pretreatment with F13714 did not shift the DOM dose-response curve (Fig. 3, top right) or significantly increase the DOM ED50 (Table 1). A larger dose of F13714 (0.032 mg/kg) markedly decreased responding (diamond above “V”; Fig. 3, bottom right).
In monkeys and in rats, the DOM discrimination dose-response curves were shifted leftward (Figs. 5 and 6, top), and the DOM ED50 values decreased by pretreatment with DOM, 2C-T-7, or DPT. In monkeys, a dose of 0.56 mg/kg DPT alone occasioned more than 50% responding on the DOM-associated lever and in rats doses of 0.32 mg/kg DOM and 0.56 mg/kg DPT alone occasioned more than 50% responding on the DOM-associated lever; in each case, ED50 values could not be determined for DOM. The bottom panels of Figs. 5 and 6 show ED50 values for each drug combination plotted as an isobologram. None of the drug combinations yielded ED50 values that were significantly different from additivity. That is, the ED50 values for DOM in the presence of a pretreatment were not significantly different from the predicted line of additivity because the standard error bars fell within the field defined by the standard error bars for the two drugs alone (e.g., Lelas et al., 2001).
Fig. 5.
Top, discriminative stimulus effects of DOM administered alone and in combination with different doses of DOM (left), 2C-T-7 (center), and DPT (right) in monkeys. Bottom, same data replotted as isobolograms, with the ED50 values for DOM plotted on the ordinates and the ED50 values of the pretreatment drugs plotted on the abscissae. See Fig. 1 and Materials and Methods for other details.
Fig. 6.
Top, discriminative stimulus effects of DOM administered alone and in combination with different doses of DOM (left), 2C-T-7 (center), and DPT (right) in rats. Bottom, same data replotted as isobolograms, with the ED50 values of DOM plotted on the ordinates and the ED50 values of the pretreatment drugs plotted on the abscissae. See Fig. 1 and Materials and Methods for other details.
Discussion
Discrimination procedures are used to investigate drugs from many different classes. The popularity of these procedures results, in part, from its pharmacological selectivity (i.e., typically only drugs sharing a mechanism of action with the training drug occasion responding on the drug-appropriate lever) and the generality of results obtained (i.e., similar results are obtained across conditions in multiple species). As measured by substitution studies (i.e., does a test agonist occasion responding on the DOM-associated lever?), antagonism studies (i.e., does a test antagonist attenuate the effects of DOM?), and drug combination studies [i.e., are the combined effects of DOM and other agonists acting at the same (5-HT2A) receptor additive?], the DOM discriminative stimulus appears to be similar across species. However, studies with DOM in combination with drugs not acting at the 5-HT2A receptor yield different results between rats and monkeys.
This experiment first compared the pharmacological selectivity of the DOM discriminative stimulus in rats and monkeys, with the expectation that only drugs with agonist actions at 5-HT2A receptors occasion responding on the DOM-associated lever. Drugs with agonist actions at 5-HT2A receptors, including 2C-T-7 and DPT, occasion responding on the DOM-associated lever with each occasioning more than 90% responding on the DOM-associated lever. Moreover, the relative potency of DOM, 2C-T-7, and DPT is the same in rats and in monkeys, supporting the view that the same mechanism (receptor) mediates these effects in both species. Discriminative stimulus effects of DOM in rats have been shown to be pharmacologically selective insofar as drugs acting through receptors other than the 5-HT2A receptor (e.g., opioid) fail to occasion responding on the DOM-associated lever (Glennon, 1988; Li et al., 2008b). Similarly, in the current study, drugs with agonist actions at 5-HT1A receptors (F13714 and 8-OH-DPAT) fail to occasion responding on the DOM-associated lever in rats and monkeys. Together with published results, including data showing attenuation of the discriminative stimulus effects of DOM by 5-HT2A receptor antagonists (e.g., Li et al., 2008b, 2009a), the current results support the view that the DOM discriminative stimulus has similar pharmacological selectivity (5-HT2A receptors) in rats and monkeys.
Second, this study tested whether 5-HT1A receptor agonists enhance the discriminative stimulus effects of DOM and whether such an effect is blocked by a selective 5-HT1A receptor antagonist. This study was based, in part, on data showing that the 5-HT1A receptor agonist 8-OH-DPAT produces partial responding on the DOM-associated lever in rats discriminating DOM and, when administered together with DOM, 8-OH-DPAT enhances the effects of DOM (Glennon, 1991; Khorana et al., 2009). There are two notable differences between the results of the previous and current studies. First, in the previous study (Khorana et al., 2009) racemic 8-OH-DPAT (0.3 mg/kg) alone occasioned 37% responding on the DOM-associated lever and R-(+)8-OH-DPAT (0.25 mg/kg) alone occasioned 58% responding on the DOM-associated lever; in the current study, racemic 8-OH-DPAT occasioned responding predominantly on the saline-associated lever (i.e., <10% on the DOM-associated lever) up to a dose (0.32 mg/kg) that nearly eliminated responding. The high efficacy and selective 5-HT1A receptor agonist F13714 (Koek et al., 2001) also fails to occasion responding on the DOM-associated lever in rats. Second, in the previous study (Khorana et al., 2009) pretreatment with 8-OH-DPAT enhanced the discriminative stimulus effects of DOM, as evidenced by a shift leftward in the DOM dose-response curve; in the current study, neither pretreatment with 8-OH-DPAT nor with F13714 clearly shifts the DOM dose-response curve, leftward or rightward, in rats. Differences obtained with 5-HT1A receptor agonists in rats, alone and in combination with DOM, might be related to procedural differences between studies including the training dose of DOM (1.0 and 0.56 mg/kg in the previous and current studies, respectively), schedule of reinforcement (variable interval 15 s and FR10, respectively), and reinforcers (sweetened condensed milk and food pellets, respectively). Different results might also be related to the sensitivity of rats to rate-decreasing effects of DOM. In the current study, responding was nearly suppressed by a dose of 0.32 mg/kg 8-OH-DPAT, whereas in the previous study (Khorana et al., 2009) only doses of 0.3 and 0.5 mg/kg enhanced the discriminative stimulus effects of DOM. Thus, the current study fails to demonstrate enhancement of the discriminative stimulus effects of DOM in rats by 5-HT1A receptor agonists.
In contrast to 5-HT1A receptor agonists enhancing (Khorana et al., 2009) or not affecting (current study) the discriminative stimulus effects of DOM in rats, 5-HT1A receptor agonists attenuate the discriminative stimulus effect of DOM in monkeys. For example, 8-OH-DPAT shifts the DOM dose-response curve more than 3.5-fold to the right. Although 8-OH-DPAT has agonist actions at 5-HT7 receptors (Hedlund et al., 2004), it appears as though 5-HT1A receptors mediate the attention of the discriminative stimulus effects of DOM for the following reasons: 1) the high-efficacy agonist F13714, which is more than 1000-fold selective for 5-HT1A compared with 5-HT7 receptors (Assié et al., 2006), attenuates the discriminative stimulus effects of DOM, shifting the dose-response curve more than 3.5-fold to the right; and 2) attenuation of the discriminative stimulus effects of DOM by 8-OH-DPAT and by F13714 is prevented by the selective 5-HT1A receptor antagonist WAY 100635. Thus, in contrast to what is observed in rats, these data show that agonists acting at 5-HT1A receptors attenuate the discriminative stimulus effects of DOM in rhesus monkeys.
It is unclear why rats and monkeys differ in whether 5-HT1A receptor agonists affect the discriminative stimulus effects of 5-HT2A receptor agonists. Although there are a number of procedural differences among these studies, one hallmark of drug discrimination procedures is that similar effects are typically obtained under a broad range of conditions. However, there are examples of marked differences in discriminative stimulus effects across species. For example, some compounds with activity at 5-HT1A and 5-HT1B receptors mimic the discriminative stimulus effects of 8-OH-DPAT in pigeons (Barrett and Gleeson, 1992) but not in rats (Cunningham et al., 1987). Moreover, lisuride and eltoprazine occasion high levels of 8-OH-DPAT-appropriate responding in pigeons but not in rats (Kleven and Koek, 1998a), and metaphit has phencyclidine-like discriminative stimulus effects in pigeons but not in rhesus monkeys (Koek et al., 1986). In the current study, differences observed with drug combinations, in procedures that otherwise generate very similar data in rats and monkeys, might reflect fundamental species differences in the neurobiology of 5-HT systems.
Finally, this study examined combinations of 5-HT2A receptor agonists to further test whether, under each of these procedures, the discriminative stimulus effects of DOM can be enhanced and the dose-response curve shifted leftward (in contrast to the failure of 5-HT1A receptor agonists to enhance the effects of DOM in this study). Both 2C-T-7 and DPT share discriminative stimulus effects with DOM in rats and monkeys and when administered as a pretreatment, each agonist shifts the DOM dose-response curve to the left. Moreover, isobolographic analyses indicate that the interaction between each drug and DOM is additive in both species, supporting the view that these drugs act at the same receptor. A similar additivity is observed when a single injection of DOM is administered before redetermination of the DOM dose-response curve. DPT has affinity and efficacy at 5-HT1A as well as 5-HT2A receptors (Li et al., 2007); however, the interaction between DPT and DOM was additive, suggesting that any actions of DPT at 5-HT1A receptors did not affect its interaction with DOM.
This study compared the discriminative stimulus effects of DOM, alone and in combination with other drugs, in rats and monkeys. Consistent with the generality of discrimination results across different conditions, the DOM discriminative stimulus is very similar between rats and monkeys, in each case involving actions at 5-HT2A receptors (Fiorella et al., 1995; Li et al., 2009a); however, interactions between DOM and drugs acting at 5-HT1A receptors are strikingly different between rats and monkeys. Others have reported differences across species for 5-HT drugs administered in combination with cocaine. For example, in rats 8-OH-DPAT decreases the reinforcing effects of cocaine under a progressive ratio schedule of self-administration (Parsons et al., 1998), and in monkeys it enhances the effects of cocaine in a choice procedure (Czoty et al., 2005). In rats, SSRIs enhance the discriminative stimulus effects (Cunningham and Callahan, 1991; Kleven and Koek, 1998b) and not the reinforcing effects (Tella, 1995) of cocaine, whereas in monkeys SSRIs attenuate discriminative stimulus (Spealman, 1993) and reinforcing effects of cocaine (Howell and Byrd, 1995). Functional differences in the effects of 5-HT drugs between rats and monkeys are consistent with reported differences in the underlying neurobiology of 5-HT systems in these species. For example, the density and distribution of 5-HT1A and 5-HT2A receptors are markedly different between rats and monkeys (Waeber et al., 1989; López-Giménez et al., 2001; Lucaites et al., 2005); moreover, the density of 5-HT transporters in hippocampus is significantly different between rats and monkeys (Owashi et al., 2004). Collectively, functional and neuroanatomical data demonstrate marked differences in 5-HT systems between species that might be particularly relevant for studies on drugs that indirectly activate multiple mechanisms (e.g., reuptake inhibitors that indirectly stimulate multiple receptors).
Acknowledgments
We thank Christopher Cruz, Brandi Blaylock, Sonia Cano, Margarita Gardea, and Jennifer Kite for excellent technical assistance.
This work was supported in part by the Intramural Research Programs of the National Institutes of Health National Institute on Drug Abuse and the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism. C.P.F. is supported by a Senior Scientist Award from the National Institutes of Health National Institute on Drug Abuse [Grant K05-DA17918].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.163451.
- 5-HT
- serotonin
- SSRI
- selective serotonin reuptake inhibitor
- MDL100907
- (R)-(+)-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl]-4-piperidinemethanol
- 8-OH-DPAT
- 8-hydroxy-2-(di-n-propylamino) tetralin hydrochloride
- DOM
- 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane
- 2C-T-7
- 2,5-dimethoxy-4-n-propylthiophenethylamine
- WAY 100635
- N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridyl) cyclohexanecarboxamide
- DPT
- dipropyltryptamine
- F13714
- 3-chloro-4-fluorophenyl-(4-fluoro-4-([(5-methyl-6-methylaminopyridin-2-ylmethyl) amino) methyl] piperidin-1-yl) methanone
- FR
- fixed ratio
- CL
- confidence limit.
References
- Arnt J, Hyttel J. (1989) Facilitation of 8-OH-DPAT-induced forepaw treading of rats by the 5-HT2 agonist DOI. Eur J Pharmacol 161:45–51 [DOI] [PubMed] [Google Scholar]
- Assié MB, Lomenech H, Ravailhe V, Faucillon V, Newman-Tancredi A. (2006) Rapid desensitization of somatodendritic 5-HT1A receptors by chronic administration of the high-efficacy 5-HT1A agonist, F13714: a microdialysis study in the rat. Br J Pharmacol 149:170–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JE, Gleeson S. (1992) Discriminative stimulus effects of 8-OH-DPAT in pigeons: antagonism studies with the putative 5-HT1A receptor antagonists BMY 7378 and NAN-190. Eur J Pharmacol 217:163–171 [DOI] [PubMed] [Google Scholar]
- Carter LP, Flores LR, Wu H, Chen W, Unzeitig AW, Coop A, France CP. (2003) The role of GABAB receptors in the discriminative stimulus effects of gamma-hydroxybutyrate in rats: time course and antagonism studies. J Pharmacol Exp Ther 305:668–674 [DOI] [PubMed] [Google Scholar]
- Celada P, Puig M, Amargós-Bosch M, Adell A, Artigas F. (2004) The therapeutic role of 5-HT1A and 5-HT2A receptors in depression. J Psychiatry Neurosci 29:252–265 [PMC free article] [PubMed] [Google Scholar]
- Cunningham KA, Callahan PM. (1991) Monoamine reuptake inhibitors enhance the discriminative state induced by cocaine in the rat. Psychopharmacology (Berl) 104:177–180 [DOI] [PubMed] [Google Scholar]
- Cunningham KA, Callahan PM, Appel JB. (1987) Discriminative stimulus properties of 8-hydroxy-2-(di-n-propylamino) tetralin (8-OHDPAT): implications for understanding the actions of novel anxiolytics. Eur J Pharmacol 138:29–36 [DOI] [PubMed] [Google Scholar]
- Czoty PW, McCabe C, Nader MA. (2005) Effects of the 5-HT(1A) agonist (+/−)-8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) on cocaine choice in cynomolgus monkeys. Behav Pharmacol 16:187–191 [DOI] [PubMed] [Google Scholar]
- Darmani NA, Martin BR, Pandey U, Glennon RA. (1990) Do functional relationships exist between 5-HT1A and 5-HT2 receptors. Pharmacol Biochem Behav 36:901–906 [DOI] [PubMed] [Google Scholar]
- Fiorella D, Rabin RA, Winter JC. (1995) The role of the 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs: I. Antagonist correlation analysis. Psychopharmacology (Berl) 121:347–356 [DOI] [PubMed] [Google Scholar]
- Gessner PK. (1988) A straightforward method for the study of drug interactions: an isobolographic analysis primer. J Am Coll Toxicol 7:987–1012 [Google Scholar]
- Glennon RA. (1988) Site-selective serotonin agonists as discriminative stimuli. Psychopharmacol Ser 4:15–31 [DOI] [PubMed] [Google Scholar]
- Glennon RA. (1991) Discriminative stimulus properties of hallucinogens and related designer drugs. NIDA Res Monogr 116:25–44 [PubMed] [Google Scholar]
- Green AR, O'Shaughnessy K, Hammond M, Schächter M, Grahame-Smith DG. (1983) Inhibition of 5-hydroxytryptamine-mediated behaviour by the putative 5-HT2 antagonist pirenperone. Neuropharmacology 22:573–578 [DOI] [PubMed] [Google Scholar]
- Hedlund PB, Kelly L, Mazur C, Lovenberg T, Sutcliffe JG, Bonaventure P. (2004) 8-OH-DPAT acts on both 5-HT1A and 5-HT7 receptors to induce hypothermia in rodents. Eur J Pharmacol 487:125–132 [DOI] [PubMed] [Google Scholar]
- Howell LL, Byrd LD. (1995) Serotonergic modulation of the behavioral effects of cocaine in the squirrel monkey. J Pharmacol Exp Ther 275:1551–1559 [PubMed] [Google Scholar]
- Khorana N, Young R, Glennon RA. (2009) Effect of 8-hydroxy-2-(N,N-di-n-propylamino)tetralin and MDMA on the discriminative stimulus effects of the classical hallucinogen DOM in rats. Pharmacol Biochem Behav 91:385–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleven MS, Koek W. (1998a) Discriminative stimulus effects of 8-hydroxy-2-(di-n-propylamino)tetralin in pigeons and rats: species similarities and differences. J Pharmacol Exp Ther 284:238–249 [PubMed] [Google Scholar]
- Kleven MS, Koek W. (1998b) Discriminative stimulus properties of cocaine: enhancement by monoamine reuptake blockers. J Pharmacol Exp Ther 284:1015–1025 [PubMed] [Google Scholar]
- Koek W, Mercer SL, Coop A, France CP. (2009) Behavioral effects of γ-hydroxybutyrate, its precursor γ-butyrolactone, and GABA(B) receptor agonists: time course and differential antagonism by the GABAB receptor antagonist 3-aminopropyl(diethoxymethyl)phosphinic acid (CGP35348). J Pharmacol Exp Ther 330:876–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koek W, Vacher B, Cosi C, Assié MB, Patoiseau JF, Pauwels PJ, Colpaert FC. (2001) 5-HT1A receptor activation and antidepressant-like effects: F 13714 has high efficacy and marked antidepressant potential. Eur J Pharmacol 420:103–112 [DOI] [PubMed] [Google Scholar]
- Koek W, Woods JH, Jacobson AE, Rice KC, Lessor RA. (1986) Metaphit, a proposed phencyclidine receptor acylator: phencyclidine-like behavioral effects and evidence of absence of antagonist activity in pigeons and in rhesus monkeys. J Pharmacol Exp Ther 237:386–392 [PubMed] [Google Scholar]
- Krebs-Thomson K, Geyer MA. (1998) Evidence for a functional interaction between 5-HT1A and 5-HT2A receptors in rats. Psychopharmacology 140:69–74 [DOI] [PubMed] [Google Scholar]
- Lelas S, Rowlett JK, Spealman RD. (2001) Isobolographic analysis of chlordiazepoxide and triazolam combinations in squirrel monkeys discriminating triazolam. Psychopharmacology 158:181–189 [DOI] [PubMed] [Google Scholar]
- Li JX, McMahon LR, Gerak LR, Becker GL, France CP. (2008a) Interactions between delta9-tetrahydrocannabinol and mu opioid receptor agonists in rhesus monkeys: discrimination and antinociception. Psychopharmacology 199:199–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, Rice KC, France CP. (2007) Behavioral effects of dipropyltryptamine in rats: evidence for 5-HT1A and 5-HT2A agonist activity. Behav Pharmacol 18:283–288 [DOI] [PubMed] [Google Scholar]
- Li JX, Rice KC, France CP. (2008b) Discriminative stimulus effects of 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane in rhesus monkeys. J Pharmacol Exp Ther 324:827–833 [DOI] [PubMed] [Google Scholar]
- Li JX, Rice KC, France CP. (2009a) Discriminative stimulus effects of 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane in rhesus monkeys: antagonism and apparent pA2 analyses. J Pharmacol Exp Ther 328:976–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, Unzeitig A, Javors MA, Rice KC, Koek W, France CP. (2009b) Discriminative stimulus effects of 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane (DOM), ketanserin, and (R)-(+)-{alpha}-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl]-4-pipidinemethanol (MDL100907) in rats. J Pharmacol Exp Ther 331:671–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Giménez JF, Vilaró MT, Palacios JM, Mengod G. (2001) Mapping of 5-HT2A receptors and their mRNA in monkey brain: [3H]MDL100,907 autoradiography and in situ hybridization studies. J Comp Neurol 429:571–589 [DOI] [PubMed] [Google Scholar]
- Lucaites VL, Krushinski JH, Schaus JM, Audia JE, Nelson DL. (2005) [3H]LY334370, a novel radioligand for the 5-HT1F receptor. II. Autoradiographic localization in rat, guinea pig, monkey and human brain. Naunyn Schmiedebergs Arch Pharmacol 371:178–184 [DOI] [PubMed] [Google Scholar]
- Marek GJ, Martin-Ruiz R, Abo A, Artigas F. (2005) The selective 5-HT2A receptor antagonist M100907 enhances antidepressant-like behavioral effects of the SSRI fluoxetine. Neuropsychopharmacology 30:2205–2215 [DOI] [PubMed] [Google Scholar]
- Oates JA, Brown NJ. (2001) Antihypertensive agents and the drug therapy of hypertension, in Goodman & Gilman's The Pharmacological Basis of Therapeutics, 10th Ed (Hardman JG, Limbird LE, Gilman AG. eds) pp 871–900, McGraw-Hill, New York: [Google Scholar]
- Owashi T, Iritani S, Niizato K, Ikeda K, Kamijima K. (2004) The distribution of serotonin transporter immunoreactivity in hippocampal formation in monkeys and rats. Brain Res 1010:166–168 [DOI] [PubMed] [Google Scholar]
- Parsons LH, Weiss F, Koob GF. (1998) Serotonin 1B receptor stimulation enhances cocaine reinforcement. J Neurosci 18:1078–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissig CJ, Eckler JR, Rabin RA, Winter JC. (2005) The 5-HT1A receptor and the stimulus effects of LSD in the rat. Psychopharmacology 182:197–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Goldberg SR. (2005) Involvement of mu-, delta- and kappa-opioid receptor subtypes in the discriminative-stimulus effects of delta-9-tetrahydrocannabinol (THC) in rats. Psychopharmacology 179:804–812 [DOI] [PubMed] [Google Scholar]
- Spealman RD. (1993) Modification of behavioral effects of cocaine by selective serotonin and dopamine uptake inhibitors in squirrel monkeys. Psychopharmacology (Berl) 112:93–99 [DOI] [PubMed] [Google Scholar]
- Tella SR. (1995) Effects of monoamine reuptake inhibitors on cocaine self-administration in rats. Pharmacol Biochem Behav 51:687–692 [DOI] [PubMed] [Google Scholar]
- Ullrich T, Rice KC. (2000) A practical synthesis of the serotonin 5-HT2A receptor antagonist MDL 100907, its enantiomer and their 3-phenolic derivatives as precursors for [11C]labeled PET ligands. Bioorg Med Chem 8:2427–2432 [DOI] [PubMed] [Google Scholar]
- Waeber C, Dietl MM, Hoyer D, Palacios JM. (1989) 5HT1 receptors in the vertebrate brain. Regional distribution examined by autoradiography. Naunyn Schmiedebergs Arch Pharmacol 340:486–494 [DOI] [PubMed] [Google Scholar]