Abstract
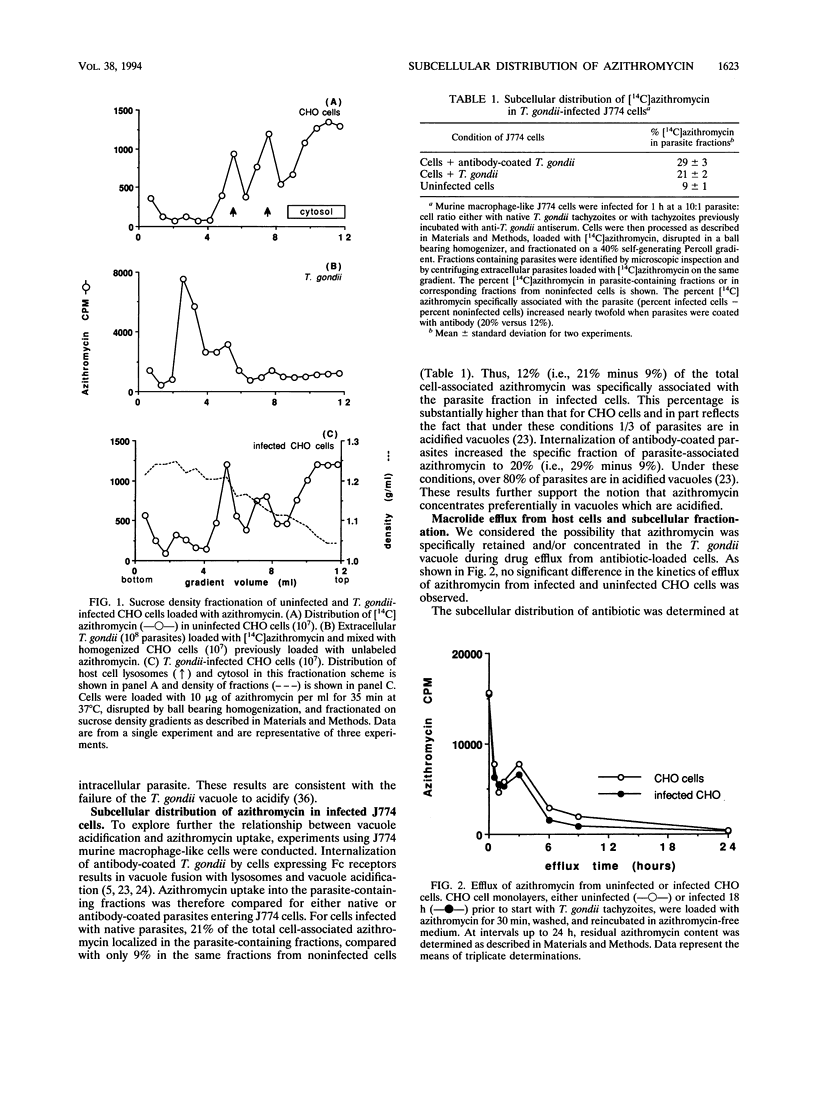
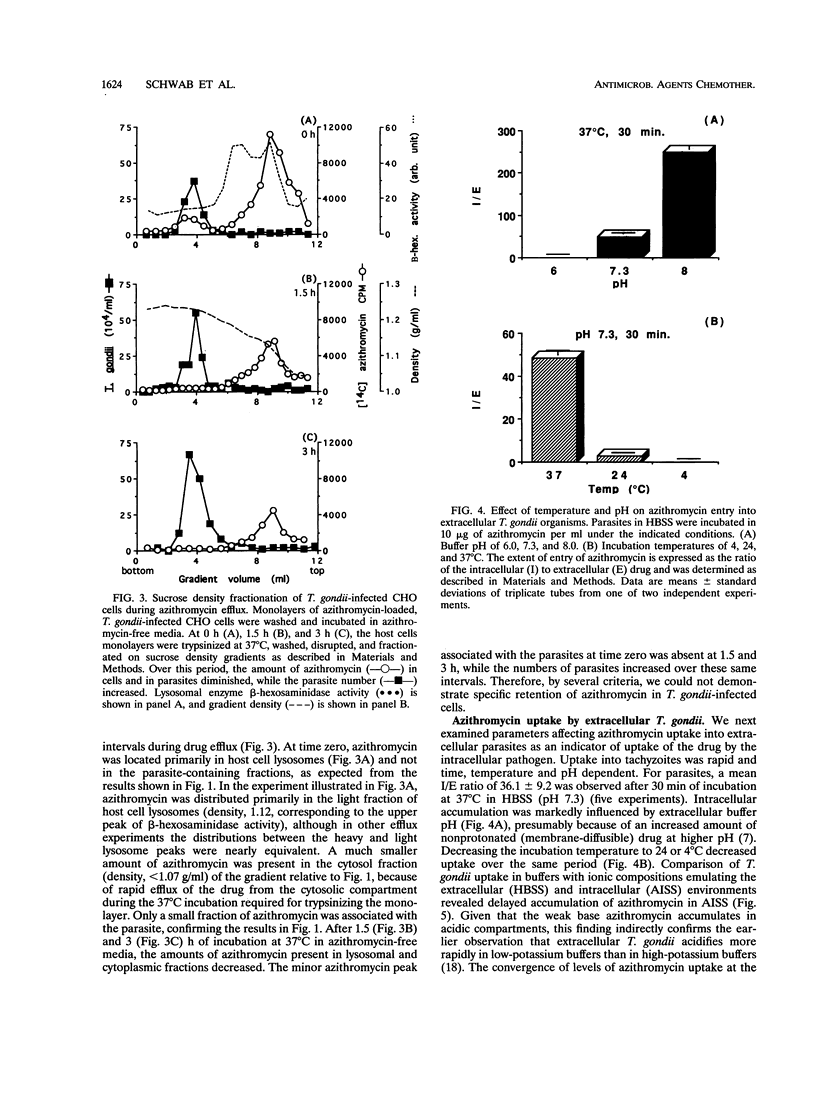
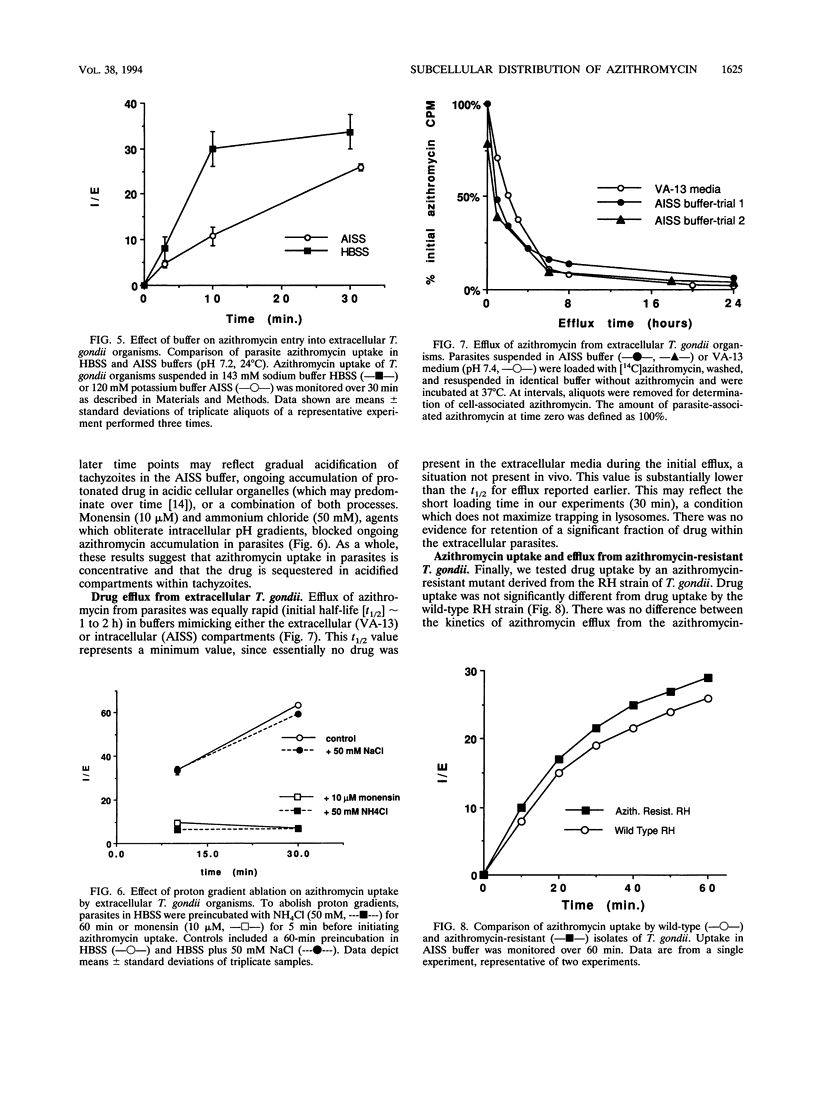
Agents effective against intracellular pathogens must enter infected cells, crossing vacuolar membranes surrounding the organisms and then penetrating into the microbe and localizing to the microbial target site. We have characterized these parameters for azithromycin entry into Toxoplasma gondii-infected Chinese hamster ovary cells and murine macrophage-like J774 cells. Azithromycin uptake into infected host cells was concentrative and was dependent upon proton gradients. Subcellular fractionation of azithromycin-loaded infected CHO cells demonstrated > 95% intracellular drug in host cell lysosomes and cytosol, with < 5% associated with the parasite. Uptake of azithromycin into the T. gondii vacuole increased if parasites were coated with antibody prior to internalization by murine J774 cells, conditions which result in the formation of acidified phagolysosomes. No redistribution or retention of azithromycin in the parasite was observed when drug efflux from antibiotic-loaded infected CHO cells was monitored. Azithromycin entry into extracellular T. gondii was concentrative, was temperature and pH dependent, and was not different when azithromycin-sensitive and -resistant parasites were compared. These results demonstrate that azithromycin concentrates primarily in acidified compartments in parasites and host cells. The high concentration of azithromycin within these compartments may not be biologically relevant to inhibition of intracellular parasite growth by this agent.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araujo F. G., Guptill D. R., Remington J. S. Azithromycin, a macrolide antibiotic with potent activity against Toxoplasma gondii. Antimicrob Agents Chemother. 1988 May;32(5):755–757. doi: 10.1128/aac.32.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W. E., Rothman J. E. Characterization of protein transport between successive compartments of the Golgi apparatus: asymmetric properties of donor and acceptor activities in a cell-free system. Arch Biochem Biophys. 1985 Jul;240(1):413–425. doi: 10.1016/0003-9861(85)90046-3. [DOI] [PubMed] [Google Scholar]
- Blais J., Garneau V., Chamberland S. Inhibition of Toxoplasma gondii protein synthesis by azithromycin. Antimicrob Agents Chemother. 1993 Aug;37(8):1701–1703. doi: 10.1128/aac.37.8.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPARAS S. D., SCHLESINGER R. W. Plaque assay of Toxoplasma on monolayers of chick embryo fibroblasts. Proc Soc Exp Biol Med. 1959 Nov;102:431–437. doi: 10.3181/00379727-102-25275. [DOI] [PubMed] [Google Scholar]
- Cantin L., Chamberland S. In vitro evaluation of the activities of azithromycin alone and combined with pyrimethamine against Toxoplasma gondii. Antimicrob Agents Chemother. 1993 Sep;37(9):1993–1996. doi: 10.1128/aac.37.9.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier M. B., Scorneaux B., Zenebergh A., Desnottes J. F., Tulkens P. M. Cellular uptake, localization and activity of fluoroquinolones in uninfected and infected macrophages. J Antimicrob Chemother. 1990 Oct;26 (Suppl B):27–39. doi: 10.1093/jac/26.suppl_b.27. [DOI] [PubMed] [Google Scholar]
- Carlier M. B., Zenebergh A., Tulkens P. M. Cellular uptake and subcellular distribution of roxithromycin and erythromycin in phagocytic cells. J Antimicrob Chemother. 1987 Nov;20 (Suppl B):47–56. doi: 10.1093/jac/20.suppl_b.47. [DOI] [PubMed] [Google Scholar]
- Chamberland S., Kirst H. A., Current W. L. Comparative activity of macrolides against Toxoplasma gondii demonstrating utility of an in vitro microassay. Antimicrob Agents Chemother. 1991 May;35(5):903–909. doi: 10.1128/aac.35.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J., Luft B. J. Activity of roxithromycin (RU 28965), a macrolide, against Toxoplasma gondii infection in mice. Antimicrob Agents Chemother. 1986 Aug;30(2):323–324. doi: 10.1128/aac.30.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H. R., Rudareanu F. C., Pechère J. C. Activity of A-56268 (TE-031), a new macrolide, against Toxoplasma gondii in mice. J Antimicrob Chemother. 1988 Sep;22(3):359–361. doi: 10.1093/jac/22.3.359. [DOI] [PubMed] [Google Scholar]
- Choi I., Mikkelsen R. B. Plasmodium falciparum: ATP/ADP transport across the parasitophorous vacuolar and plasma membranes. Exp Parasitol. 1990 Nov;71(4):452–462. doi: 10.1016/0014-4894(90)90071-j. [DOI] [PubMed] [Google Scholar]
- Darnowski J. W., Holdridge C., Handschumacher R. E. Concentrative uridine transport by murine splenocytes: kinetics, substrate specificity, and sodium dependency. Cancer Res. 1987 May 15;47(10):2614–2619. [PubMed] [Google Scholar]
- Dempster R. P. Toxoplasma gondii: purification of zoites from peritoneal exudates by eight methods. Exp Parasitol. 1984 Apr;57(2):195–207. doi: 10.1016/0014-4894(84)90080-8. [DOI] [PubMed] [Google Scholar]
- Derouin F., Almadany R., Chau F., Rouveix B., Pocidalo J. J. Synergistic activity of azithromycin and pyrimethamine or sulfadiazine in acute experimental toxoplasmosis. Antimicrob Agents Chemother. 1992 May;36(5):997–1001. doi: 10.1128/aac.36.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derouin F., Chastang C. Enzyme immunoassay to assess effect of antimicrobial agents on Toxoplasma gondii in tissue culture. Antimicrob Agents Chemother. 1988 Mar;32(3):303–307. doi: 10.1128/aac.32.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T., Tokuda H., Yagita K., Koyama T. Effects of extracellular potassium on acid release and motility initiation in Toxoplasma gondii. J Protozool. 1987 Aug;34(3):291–295. doi: 10.1111/j.1550-7408.1987.tb03177.x. [DOI] [PubMed] [Google Scholar]
- Girard A. E., Girard D., English A. R., Gootz T. D., Cimochowski C. R., Faiella J. A., Haskell S. L., Retsema J. A. Pharmacokinetic and in vivo studies with azithromycin (CP-62,993), a new macrolide with an extended half-life and excellent tissue distribution. Antimicrob Agents Chemother. 1987 Dec;31(12):1948–1954. doi: 10.1128/aac.31.12.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladue R. P., Bright G. M., Isaacson R. E., Newborg M. F. In vitro and in vivo uptake of azithromycin (CP-62,993) by phagocytic cells: possible mechanism of delivery and release at sites of infection. Antimicrob Agents Chemother. 1989 Mar;33(3):277–282. doi: 10.1128/aac.33.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladue R. P., Snider M. E. Intracellular accumulation of azithromycin by cultured human fibroblasts. Antimicrob Agents Chemother. 1990 Jun;34(6):1056–1060. doi: 10.1128/aac.34.6.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris C., Salgo M. P., Tanowitz H. B., Wittner M. In vitro assessment of antimicrobial agents against Toxoplasma gondii. J Infect Dis. 1988 Jan;157(1):14–22. doi: 10.1093/infdis/157.1.14. [DOI] [PubMed] [Google Scholar]
- Joiner K. A., Fuhrman S. A., Miettinen H. M., Kasper L. H., Mellman I. Toxoplasma gondii: fusion competence of parasitophorous vacuoles in Fc receptor-transfected fibroblasts. Science. 1990 Aug 10;249(4969):641–646. doi: 10.1126/science.2200126. [DOI] [PubMed] [Google Scholar]
- Jones T. C., Len L., Hirsch J. G. Assessment in vitro of immunity against Toxoplasma gondii. J Exp Med. 1975 Feb 1;141(2):466–482. doi: 10.1084/jem.141.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. C., Yeh S., Hirsch J. G. The interaction between Toxoplasma gondii and mammalian cells. I. Mechanism of entry and intracellular fate of the parasite. J Exp Med. 1972 Nov 1;136(5):1157–1172. doi: 10.1084/jem.136.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug E. C., Marr J. J., Berens R. L. Purine metabolism in Toxoplasma gondii. J Biol Chem. 1989 Jun 25;264(18):10601–10607. [PubMed] [Google Scholar]
- Luft B. J., Remington J. S. Toxoplasmic encephalitis in AIDS. Clin Infect Dis. 1992 Aug;15(2):211–222. doi: 10.1093/clinids/15.2.211. [DOI] [PubMed] [Google Scholar]
- Moulder J. W. Comparative biology of intracellular parasitism. Microbiol Rev. 1985 Sep;49(3):298–337. doi: 10.1128/mr.49.3.298-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby R., Lindholm L., Lycke E. Lysosomes of Toxoplasma gondii and their possible relation to the host-cell penetration of toxoplasma parasites. J Bacteriol. 1968 Oct;96(4):916–919. doi: 10.1128/jb.96.4.916-919.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn E. R., Borotz S. E. Comparison of mutants of Toxoplasma gondii selected for resistance to azithromycin, spiramycin, or clindamycin. Antimicrob Agents Chemother. 1994 Jan;38(1):31–37. doi: 10.1128/aac.38.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn E. R., Nothnagel R. F., Borotz S. E. Parasiticidal effect of clindamycin on Toxoplasma gondii grown in cultured cells and selection of a drug-resistant mutant. Antimicrob Agents Chemother. 1992 May;36(5):1091–1096. doi: 10.1128/aac.36.5.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn E. R., Pfefferkorn L. C. Specific labeling of intracellular Toxoplasma gondii with uracil. J Protozool. 1977 Aug;24(3):449–453. doi: 10.1111/j.1550-7408.1977.tb04774.x. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn E. R., Pfefferkorn L. C. Toxoplasma gondii: isolation and preliminary characterization of temperature-sensitive mutants. Exp Parasitol. 1976 Jun;39(3):365–376. doi: 10.1016/0014-4894(76)90040-0. [DOI] [PubMed] [Google Scholar]
- Schwab J. C., Mandell G. L. The importance of penetration of antimicrobial agents into cells. Infect Dis Clin North Am. 1989 Sep;3(3):461–467. [PubMed] [Google Scholar]
- Sibley L. D., Weidner E., Krahenbuhl J. L. Phagosome acidification blocked by intracellular Toxoplasma gondii. 1985 May 30-Jun 5Nature. 315(6018):416–419. doi: 10.1038/315416a0. [DOI] [PubMed] [Google Scholar]
- de Duve C., de Barsy T., Poole B., Trouet A., Tulkens P., Van Hoof F. Commentary. Lysosomotropic agents. Biochem Pharmacol. 1974 Sep 15;23(18):2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]
- van den Broek P. J. Antimicrobial drugs, microorganisms, and phagocytes. Rev Infect Dis. 1989 Mar-Apr;11(2):213–245. doi: 10.1093/clinids/11.2.213. [DOI] [PubMed] [Google Scholar]



