Abstract
The commonly used general anesthetic isoflurane induces widespread neurodegeneration in the developing mammalian brain through poorly understood mechanisms. We have investigated whether excessive Ca2+ release from the endoplasmic reticulum via overactivation of inositol 1,4,5-trisphosphate receptors (InsP3Rs) is a contributing factor in such neurodegeneration in rodent primary cultured neurons and developing rat brain. We also investigated the correlation between isoflurane exposure and cognitive decline in rats at 1 month of age. Our results show that isoflurane increases cytosolic calcium in the primary cortical neurons through release from the endoplasmic reticulum and influx from the extracellular space. Pharmacological inhibition of InsP3R activity and knockdown of its expression nearly abolishes the isoflurane-mediated elevation of the cytosolic calcium concentration and cell death in rodent primary cortical and hippocampal neurons. Inhibition of InsP3R activity by its antagonist xestospongin C significantly inhibits neurodegeneration induced by isoflurane at clinically used concentration in the developing brain of postnatal day 7 rats. Moreover, our results show that isoflurane activates β-site amyloid β precursor protein-cleaving enzyme via activation of the InsP3R. We also noted that mice exposed to isoflurane during early postnatal development showed transient memory and learning impairments, which did not correlate well with the noted neuropathological defects. Taken together, our results suggest that Ca2+ dysregulation through overactivation of the InsP3R may be a contributing factor in the mechanism of isoflurane-induced neurodegeneration in rodent neuronal cell culture and during brain development.
More than 200 million people undergo surgery annually worldwide, most of which is carried out under general anesthesia using inhalational anesthetics such as isoflurane, sevoflurane, or desflurane. Although these anesthetics are considered to be generally safe and effective, several studies have implicated them in postoperative cognitive decline. For example, cognitive decline has been documented in both middle-aged and older patients several months after anesthesia and surgery, and cognitive decline at 1 week after surgery is correlated with the duration of anesthesia (Moller et al., 1998). Likewise, isoflurane alone causes learning and memory deficits in older rodents several weeks after exposure (Culley et al., 2004; Bianchi et al., 2008). At the other extreme of age, isoflurane has been associated with a massive increase in neonatal apoptosis and subsequent learning impairments in the rodent (Jevtovic-Todorovic et al., 2003; Ma et al., 2007; Wang et al., 2009). Recent retrospective work suggests a greater incidence of learning disabilities in children having anesthesia and surgery before the age of 4, especially with accumulated anesthesia and surgeries (Kalkman et al., 2009; Wilder et al., 2009). These results suggest that isoflurane may induce neurotoxicity and subsequent cognitive decline, but the cellular and molecular mechanisms of these effects are not clear. More importantly, it is not clear whether anesthetic-mediated neuropathological defects are the direct cause of the cognitive decline seen in many animal models.
Isoflurane exposure has been shown to cause cell damage in various neuronal and non-neuronal tissues and cells, including hippocampal slices (Wise-Faberowski et al., 2005), lymphocytes (Wei et al., 2008; Yang et al., 2008), neuroglioma cells (Xie et al., 2007; Zhang et al., 2008), hepatocytes (Malledant et al., 1990), gingival fibroblasts (Chang and Chou, 2001), the PC12 neurosecretory cell line (Wei et al., 2007; Liang et al., 2008), and striatal neurons (Wei et al., 2008). Interestingly, these cells have different intrinsic vulnerability to anesthetic toxicity, with lymphocytes generally being more vulnerable than neurons (Wei et al., 2005, 2008; Liang et al., 2008). The InsP3R on the endoplasmic reticulum (ER) membrane and calcium release from the ER is a key element of an important, prevalent, and highly conserved second messenger system (Berridge, 1993), but its prolonged activation may initiate apoptosis and thereby contribute to altered neurogenesis or synaptogenesis in the neonate (Sedlak and Snyder, 2006). In our previous studies (Wei et al., 2008; Yang et al., 2008), we showed that inositol 1,4,5-trisphosphate receptor (InsP3R) null lymphocytes were resistant to isoflurane-mediated calcium release from the ER and cell death by apoptosis, suggesting a critical role for overactivation of InsP3R in anesthetics-mediated cell death in these cells. However, it is not clear whether overactivation of InsP3Rs and the subsequent excessive Ca2+ release from the ER play a role in neurodegeneration in both primary neuronal cultures and the developing brain. Moreover, it is not clear whether such excessive Ca2+ release from the ER is a contributing factor in cognitive decline in rats exposed to isoflurane during early postnatal development. In this study, we examined whether isoflurane-induced neurodegeneration in rodent primary neuronal cell cultures and the developing brain occurs through disruption of intracellular calcium homeostasis via overactivation of the InsP3R. Isoflurane has been previously implicated in increasing the activity of the β-site amyloid β precursor protein (APP)-cleaving enzyme (BACE) (Xie et al., 2007) and apoptosis regulatory proteins (Bcl-xl, Bax) (Wei et al., 2005). Thus, we investigated the relationship between the levels of these proteins and InsP3R activation.
Materials and Methods
The Institutional Animal Care and Use Committee at the University of Pennsylvania (Philadelphia, PA) approved the use of all animals and experimental procedures and protocols in this study, and the study conforms to National Institutes of Health Guidelines.
In Vitro Studies
Cell Culture.
We used primary neuronal cell cultures from both rat and mouse because they are vulnerable to anesthetic-induced neurodegeneration during brain development (Ma et al., 2007; Wang et al., 2009). Specifically, we used primary hippocampal and cortical neuronal cell cultures, given the importance of these brain regions in learning and memory. Rat and/or mouse primary cortical or hippocampal neurons were cultured as previously described (Wei et al., 2005, 2007). In brief, the cortices and hippocampus were dissected from embryonic brain (E16–17 mice or E18 rats), and the meninges were removed. Cells were dissociated by trypsinization and trituration, followed by DNase treatment. The dissociated cells were resuspended in serum-free B27/neurobasal medium and plated at a density of 150 to 300 cells/cm2 on poly(d-lysine)-coated 24-well plates. Cultures were maintained in serum-free B27/neurobasal medium in a humidified atmosphere (5% CO2, 95% air) at 37°C. More than 95% of the mixed cells present on day 5 in vitro (DIV 5) differentiate into neurons, as characterized by the appearance of long neurites expressing neurofilament protein. Half of the medium was changed every 4th day. Primary, mixed cortical (cortex and hippocampal), or hippocampal neurons (DIV 5–8) were used in all in vitro studies.
siRNA Transfection and Knockdown of InsP3R Expression.
We used the small interfering RNA (siRNA) against rat InsP3R type 1 (GenBank accession number: NM_001007235; target sequence: AAG GCC TAC ATG CAA GGC GAA) and control siRNA: AllStars Neg. siRNA AF 488 (QIAGEN, Valencia, CA). Rat cortical neurons at DIV 5 were transfected in either 96-well plates with 10 pmol of siRNA or six-well plates with 25 pmol of siRNA by using Lipofectamine 2000 Transfection Reagent (Invitrogen, Carlsbad, CA). siRNA and Lipofectamine each were diluted in the culture medium and then mixed together for 20 min. Cells were incubated with transfection mixture for 6 h at 37°C in a culture incubator. Thereafter, the media in the wells were changed to that without transfection mixture. Total RNA was then harvested 7 days after transfection (DIV 12) for real-time polymerase chain reaction (PCR) to determine the InsP3R knockdown efficiency.
Anesthetic Exposures.
Primary cortical or hippocampal neurons cultured from rat or mice pups were exposed to 2.4% isoflurane for 24 h in a gas-tight chamber inside the culture incubator, with humidified 5% CO2/21% O2/balance N2 going through a calibrated agent-specific vaporizer as described previously (Wei et al., 2005, 2008). Gas-phase concentrations in the gas chamber and anesthetic concentrations in the medium were monitored and maintained throughout experiments as described previously (Wei et al., 2005). We chose to expose our neuronal cultures to isoflurane for 24 h because our previous studies indicated that they require much longer exposure than lymphocytes for isoflurane-mediated cell death (Wei et al., 2005, 2008).
Cytotoxicity Assays.
To determine the effects of suppression of the InsP3R caused by isoflurane-induced neurotoxicity, we treated rat primary cortical neurons at DIV 11 (6 days after InsP3R siRNA transfection or its negative control) with 2.4% isoflurane or carrying gas for 24 h. Immediately after treatment, 3-(4,5-dimethyithiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) reduction (early cell damage) and lactate dehydrogenase (LDH) release (late cell damage) were performed to determine the cytotoxicity of isoflurane in the presence or absence of InsP3R knockdown. The inhibition of isoflurane-induced cytotoxicity by the InsP3R blocker, xestospongin C, was also assessed by the MTT reduction and LDH release assays and propidium iodide (PI) staining as described previously (Wei et al., 2005, 2008). Primary cortical or hippocampal neurons at DIV 8 from rats or mice were exposed to 2.4% isoflurane for 24 h in the presence or absence of InsP3R siRNA or xestospongin C. Immediately after treatment, the extent of cell damage was assessed by MTT reduction, LDH release, or PI staining. Results are expressed as a percentage of the control without anesthetic treatment.
Cytosolic Calcium Concentration Measurements.
Cytosolic calcium concentration ([Ca2+]c) was measured by using fura-2 fluorescence as described previously (Wei et al., 2008; Yang et al., 2008). In brief, mixed mouse hippocampal and cortical neurons grown on 25-mm round glass coverslips were washed three times with Hanks' balanced salt solution buffer with 1.8 mM CaCl2 and 0.8 mM MgCl2 and loaded with 2.5 μM fura-2/AM for 30 min in the same buffer at room temperature. Coverslips were mounted in a sealed chamber and constantly perfused through an inflow tube with Krebs-Ringer buffer either with or without calcium, depending on the experimental design. After baseline acquisitions, cells were exposed to isoflurane (0.7 mM at room temperature) dissolved in Hanks' balanced salt solution via a separate inflow tube driven by a syringe pump. The fluorescence signals were measured with alternate excitation at 340 and 380 and emission at 510 nm for up to 18 min for each treatment. The data are given in terms of 340/380 fluorescence ratio or real concentrations as previously described (Grynkiewicz et al., 1985).
Brain Tissue Preparation.
For Western blot analyses, experimental and control rats at postnatal day 7 (P7) were anesthetized with sodium pentobarbital (100 mg/kg i.p.) 2 h after the end of isoflurane exposure and transcardially perfused with cold saline. The cortex was immediately dissected, frozen in liquid nitrogen, and stored at −80°C. For caspase-3 immunohistochemistry, experimental and control rats were anesthetized with an intraperitoneal injection of sodium pentobarbital 2 h after isoflurane exposure, transcardially perfused with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) at 4°C overnight, and cryoprotected in 30% (w/v) sucrose in 0.1 M phosphate buffer (pH 7.4) at 4°C for 24 h, then frozen in isopentane on dry ice and embedded in optimal cutting temperature compound. The embedded brains were kept at −80°C until serially sectioned at 10 μm with a cryostat, mounted on gelatin-coated slides, and stored at −80°C.
Immunohistochemistry.
Caspase-3-positive cells were detected by using immunohistochemical methods as described previously (Li et al., 2007; Wang et al., 2009). In brief, brain sections from the P7 rat pups were first incubated in 3% hydrogen peroxide in methanol for 20 min to quench endogenous peroxidase activity. Sections were then incubated with blocking solution containing 10% normal goat serum in 0.1% phosphate-buffered saline with 0.1% Tween 20 (PBST) for 1 h at room temperature after washing with 0.1% PBST. The activated caspase-3 primary antibody was then applied in blocking solution and incubated at 4°C overnight. Tissue sections were biotinylated with goat anti-rabbit antibody in 0.1% PBST for 40 min, followed by incubation with the avidin-biotinylated peroxidase complex for 40 min. Tissue sections were then colorized with diaminobenzidine for 8 min and counterstained with modified hematoxylin. Negative control sections were incubated in blocking solution that did not contain primary antibody. Images were acquired and assessed at 200× using IP lab 4.0 (IP Lab 4.0, Biovision Technologies, Exton, PA) software linked to an Olympus (Tokyo, Japan) IX70 microscope equipped with a Cooke SensiCam camera (Applied Scientific Instrumentation, Eugene, OR). At least two sections were counted from each animal, and 10 animals were used in each group. Immunoreactive cells were counted by at least two different individuals blinded to the experimental treatments. The data are given in terms of density of caspase-3-positive cells in the hippocampal CA1 region, which was calculated by dividing the number of caspase-3-positive cells by the area of that brain region.
Western Blot Analysis.
Western blot analysis was done as previously described (Wei et al., 2000; Li et al., 2007). Frozen cortical samples from the P7 rat brains were thawed and lysed with Cell-Lytic MT Mammalian Tissue lysis reagent (Sigma-Aldrich, St. Louis, MO) in the presence of a protease inhibitor cocktail (Sigma-Aldrich). Proteins from these cortical lysates were separated by 12% gel electrophoresis and transferred to a nitrocellulose membrane. The blots were incubated with a monoclonal antibody against cleaved caspase-3, BACE, Bcl-XL, or Bax and then probed with horseradish peroxidase-conjugated secondary antibody. Detection was performed by using the ECL-Plus system (GE Healthcare, Chalfont St. Giles, UK) and photographed. Actin protein was used as a loading control, and the changes of targeted proteins were normalized to the level of β-actin. Western blots were from three different animals, and the density was measured by Quantity One software (version 4.5.0; Bio-Rad Laboratories, Hercules, CA) and a GS-800 Densitometer (Bio-Rad Laboratories).
Animal Studies
Anesthetic Exposure and Intracerebroventricular Injection.
For anesthetic exposure in the animal studies, we first administered xestospongin C to 7-day-old rats by intracerebroventricular (5 μl of 100 ppm) injection into the right lateral ventricle 30 min before isoflurane treatment (Galeotti et al., 2006). Xestospongin C was diluted in normal saline, and injection was carried out under isoflurane-induced (1%) general anesthesia. The injection point was approximately 1 mm to the right of a line drawn through to the anterior roots of the ears, with the injection needle inserted to a depth of 2 mm through the skull. A pilot study in 7-day-old rats (n = 20) confirmed a success rate of intracerebroventricular injection of 100% (data not shown). After xestospongin C administration, rats were exposed to 1% (n = 8) or 1.5% (n = 10) isoflurane for 6 h in a chamber as described previously (Li et al., 2007; Bianchi et al., 2008). Control rats at P7 in the vehicle-only group (n = 10) received the same amount of normal saline but without the addition of xestospongin C. The isoflurane concentration in the chamber was monitored and maintained by using a vaporizer. The exposure chambers were kept on a slightly elevated platform, which was in a water bath set at 40°C. Under those conditions, the mean rectal temperature of the animals in those chambers was 37 ± 0.5°C throughout the exposure time. Isoflurane treatment of up to 1.5% for 6 h had been shown not to alter arterial blood gas significantly in a previous study (Jevtovic-Todorovic et al., 2003), so we did not measure the arterial blood gas in this study. Blood glucose level was monitored during and 2 h after isoflurane treatment with a glucometer using mixed blood obtained from rat tails in a pilot study.
Morris Water Maze.
Rats exposed at day P7 were allowed to mature before Morris water maze (MWM). Testing of spatial learning and memory was performed in the MWM with protocols and paradigms as previously described (Li et al., 2007; Bianchi et al., 2008). In brief, experimental and control P35 rats were trained to locate an escape platform (1.5 cm in diameter) within a fiberglass pool (1.5 m in diameter) by distant visual cues located on the walls around the pool and a pole with flags on the escape platform. The pool was filled with opaque water with the moveable escape platform submerged 1.5 cm below the surface of the water. Rats underwent four daily trials at four distinct starting locations for 5 consecutive days. Each trial allowed for a 60-s search time, and once an animal located the platform it was allowed to remain on it for 15 s before the next trial. Animals that failed to find the escape platform during the search period were gently guided to it. The training or cued trials were performed to train the rats to swim to the platform to be removed from the pool and assess noncognitive impairments (e.g., visual impairments and/or swimming difficulties), which might affect performance during place or probe trials. After completion of the cued trials, we assessed the performance of these rats in place trials or reference memory starting at P42 (1%, 1.5% isoflurane) and again at P102 (1.5%) to determine their ability to learn the spatial relationship between distant cues and the escape platform (no rod or flags), which remained in the same location for all place trials. The time to reach the platform (escape latency), an indicator of learning and spatial memory, was recorded for each trial. After reference memory, memory retention capability by the control and experimental animals was assessed by the probe test. For the probe test, the platform was removed from the pool and the rats were placed in the quadrant opposite to the one where the platform had been previously located. The rats were allowed to swim for 60 s during each probe trial, and the time spent in each quadrant was recorded. The percentage of time spent in the target quadrant compared with other quadrants was an indication of memory retention. Probe trials commenced at P46 (1%, 1.5% isoflurane) and P106 (1.5% isoflurane) after the last place trials. The water in the pool was maintained at room temperature, and all experiments were carried out in a dimly lit room.
Analysis and Statistics.
Data are expressed as mean ± S.E.M. and statistical significance between means. All data except for the behavior was assessed by one-way analysis of variance (ANOVA) followed by the Tukey multiple comparison tests. The behavioral data were analyzed by two-way ANOVA. The significance level for all of our analyses was set at 95% (P < 0.05). We used GraphPad Prism software (GraphPad Software Inc.) for all statistical analyses.
Results
Knockdown and Pharmacological Inhibition of InsP3R Activity in Cultured Neurons Suppressed Isoflurane-Mediated Ca2+ Dysregulation and Neurodegeneration.
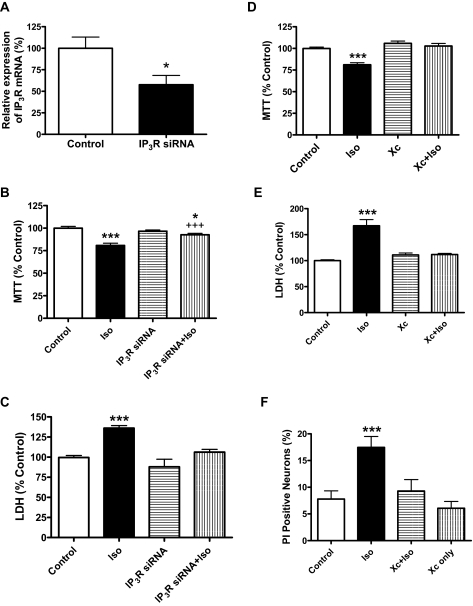
To investigate the role of InsP3R activation in isoflurane-mediated calcium dysregulation and apoptosis, we used xestospongin C to inhibit the function of InsP3Rs or siRNA to knock down the expression of the InsP3R message in primary cortical and/or hippocampal neurons. Transfection of InsP3R siRNA in rat primary cortical neurons suppressed the expression of InsP3R mRNA by 42% as determined by real-time PCR (Fig. 1A). As a result, the suppression of the InsP3R message significantly inhibited both the early (Fig. 1B, MTT assay) and late (Fig. 1C, LDH release assay) neurodegeneration induced by 2.4% isoflurane exposure for 24 h. Suppression of InsP3R expression with InsP3R siRNA alone did not affect neuronal cell viability (Fig. 1, B and C). Consistent with these findings, pharmacological inhibition of these receptors with xestospongin C significantly inhibited isoflurane-mediated neurodegeneration in rat primary cortical (Fig. 1D) and hippocampal neurons (Fig. 1E) as measured by MTT reduction and LDH release assays, respectively. Similarly, xestospongin C prevented isoflurane-mediated neurodegeneration in mouse primary cortical neurons as measured by PI assay (Fig. 1F).
Fig. 1.
Suppression of InsP3R expression or activity significantly inhibited isoflurane-induced neurotoxicity. A–C, transfection of rat primary cortical neurons with InsP3 receptor siRNA significantly suppressed the relative expression of InsP3 receptor mRNA determined with real-time PCR and neurotoxicity determined by MTT reduction and LDH release assay. A, n ≥ 7 for each condition, * P < 0.05, Student's t test. Suppression of InsP3 receptor expression (siRNA) significantly inhibited the isoflurane-induced neuronal damage determined with both MTT (B, n ≥ 31 for each condition) and LDH (C, n ≥ 15 for each condition) assays. * or ***, P < 0.05 or 0.001, respectively compared with control. +++, P < 0.001 compared with isoflurane (Iso) treatment alone. ANOVA was followed by Tukey multiple comparison tests. D and E, InsP3 receptor antagonist xestospongin C (Xc) nearly abolished the isoflurane-induced neuronal damage in rat primary cortical (D) and hippocampal neurons (E). n ≥ 35 for each condition.***, P < 0.001 compared with control; +++, P < 0.001 compared with isoflurane (Iso) treatment alone. ANOVA followed by Tukey multiple comparison tests. F, Xc produced such a significant decline that it nearly abolished the isoflurane-induced late cell damage in mouse primary cortical neurons. n ≥ 22 for each condition. ***, P < 0.001 compared with control with ANOVA followed by Tukey multiple comparison tests. All data were collected from at least three different preparations.
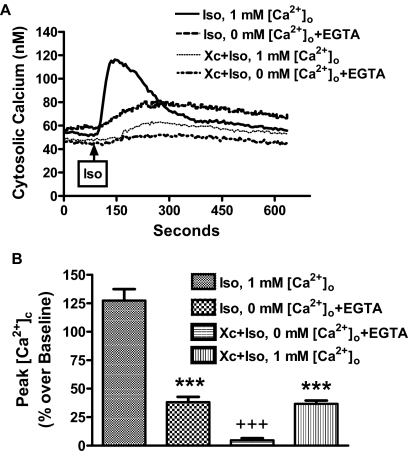
To study the mechanism for the neurodegeneration caused by isoflurane, we found that mouse primary cortical neurons exposed to isoflurane increased the cytosolic calcium signal in the absence of extracellular calcium, an effect that was blocked by xestospongin C (Fig. 2). This increase in intracellular calcium was significantly greater in the presence of extracellular calcium (Fig. 2), suggesting that calcium influx from the extracellular space contributed to the isoflurane-mediated calcium increase in primary cortical neurons. Interestingly, the greater increase in calcium concentration mediated by extracellular calcium was remarkably inhibited by xestospongin C (Fig. 2), suggesting that the store-operated calcium channels on the plasma membrane may be responsible for the isoflurane-mediated calcium increase in the cytosolic space. It is known that voltage-gated Ca2+ channels (VGCCs) are important pathways for the influx of Ca2+ from the extracellular space during synaptic transmission. Therefore, we studied the effects of specific inhibitors, nicardipine and nimodipine, of the L-type VGCCs on the isoflurane-mediated Ca2+ increase in mixed, mouse hippocampal, and cortical neurons. Nicardipine and nimodipine had no effect on the isoflurane-mediated Ca2+ (Supplemental Methods and Supplemental Fig. 1), suggesting that the L-type VGCCs play no role in isoflurane-mediated calcium influx from extracellular space in our in vitro culture systems.
Fig. 2.
Suppression of InsP3R activity abolished isoflurane-mediated calcium release in mouse primary cortical neurons. A, representative changes in isoflurane (0.7 mM at room temperature)-mediated [Ca2+]c in the presence or absence of extracellular calcium or InsP3R antagonist xestospongin C (1 μM). B, xestospongin C remarkably inhibited the isoflurane-induced elevation of the [Ca2+]c in the presence of extracellular calcium and nearly abolished the calcium release from intracellular stores in the virtual absence of extracellular calcium. Data are expressed as ± S.E.M. n ≥ 55 for each condition. ***, P < 0.001 compared with isoflurane (Iso) treatment alone in the presence of 1 mM extracellular Ca2+. +++, P < 0.001 compared with xestospongin C (Xc) plus isoflurane (Iso) in the presence of 1 mM extracellular Ca2+. All data from Ca2+ measurements were collected from at least four different preparations.
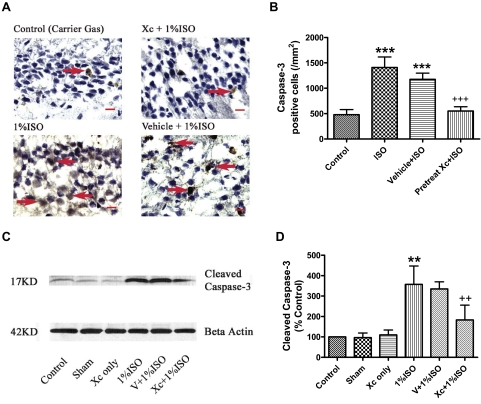
Pharmacological Inhibition of InsP3R Activity Inhibited Isoflurane-Induced Apoptosis in P7 Developing Brain.
To translate our in vitro calcium dysregulation and neurodegeneration findings to in vivo, we asked whether inhibition of InsP3R activity by xestospongin C could inhibit previously reported neuronal apoptosis in neonatal rats (Jevtovic-Todorovic et al., 2003). In that previous report, isoflurane alone (up to 1.5%) or in combination with other anesthetics (nitrous oxide and midazolam) did not significantly affect arterial blood gases, but caused widespread neurodegeneration in different brain regions in P7 rats and subsequent impairment in learning and memory. In addition to the detection of caspase-3 activation, anesthetic-induced apoptosis in neuronal cultures and the developing brain have been confirmed by various methods [e.g., terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling, ultra-structural studies, poly(ADP-ribose)polymerase, etc.] in previous studies (Jevtovic-Todorovic et al., 2003; Dong et al., 2009; Wang et al., 2009; Zhen et al., 2009), so we did not use other methods than the caspase-3 assay to further confirm apoptosis in the current study. Using the same conditions and exposures, we reproduced the previously reported increase in apoptosis after anesthetic exposure (Jevtovic-Todorovic et al., 2003). We found that the number of caspase-3-positive neurons increased in the hippocampal CA1 region of 7-day-old rats exposed to isoflurane (Fig. 3B, P < 0.001). Administration of xestospongin C (100 ppm in normal saline, 5 μl) via intracerebroventricular injection into the right lateral ventricle of P7 rats 30 min before the 6-h isoflurane treatment (Galeotti et al., 2006) nearly abolished the isoflurane-mediated neuronal apoptosis in the hippocampal CA1 region (Fig. 3, A and B) and significantly inhibited the activation of the apoptotic marker caspase-3 in the cortex (Fig. 3, C and D).
Fig. 3.
Intracerebroventricular pretreatment of xestospongin C significantly inhibited isoflurane-induced apoptosis in hippocampus CA1 and cerebral cortex in 7-day-old rat brains. A, representative image of caspase-3-positive immunohistological staining (arrows) in the hippocampal CA1 region. Blue cells are normal cells counterstained by hematoxylin. Scale bar, 10 μm. B, intracerebroventricular pretreatment of Xc significantly inhibited the increase in the isoflurane (ISO)-induced caspase-3-positive cells in the hippocampus. n = 10 for each condition. *** or +++, P < 0.001compared with control and ISO treatment alone, respectively, using ANOVA followed by Tukey multiple comparison tests. C, representative changes of cleaved caspase-3 in cerebral cortex by Western blot using specific antibody targeted to cleaved caspase-3. D, the quantified cleaved caspase-3 bands were normalized to the loading control β-actin. Intracerebroventricular pretreatment of Xc significantly inhibited the isoflurane (ISO)-induced activation of caspase-3 in the cerebral cortex. n = 3 for each condition. ** or ++, P < 0.01 compared with control and ISO treatment alone, respectively, by ANOVA followed by Tukey multiple comparison tests.
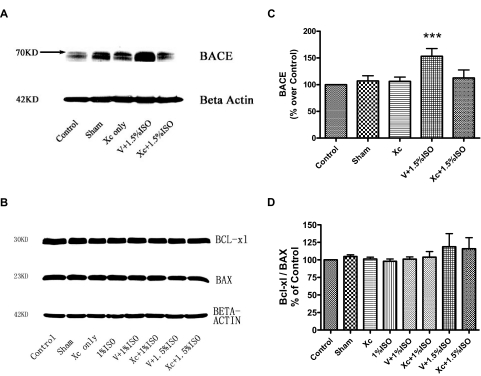
It has been previously reported that isoflurane exposure, up to 1.5% for 6 h, did not affect arterial blood gases significantly (Jevtovic-Todorovic et al., 2003). Thus, we did not measure arterial blood gases because our treatment protocol was similar to that of Jevtovic-Todorovic et al. (2003). A decrease in blood sugar levels has been proposed as a mechanism for anesthesia-mediated neurodegeneration in rodents (Loepke et al., 2006), but we found no discernible differences in blood sugar levels in animals treated with vehicle gas and isoflurane (Supplemental Methods and Supplemental Fig. 2). Given that isoflurane has been previously implicated in increasing the activity of BACE (Xie et al., 2007), Bcl-xl, and Bax (Wei et al., 2005), we investigated the effects of isoflurane treatment on the expression levels of these proteins and its relation with activation of InsP3Rs. As in previous studies (Xie et al., 2007; Zhen et al., 2009), isoflurane significantly increased levels of BACE, an enzyme that contributes to the production of β-amyloid and neuropathology in Alzheimer's disease. Interestingly, intracerebroventricular injection of xestospongin C before isoflurane exposure prevented this increase in BACE expression level (Fig. 4, A and C). In contrast to our previous report in cell cultures (Wei et al., 2005), isoflurane did not change expression levels of the regulatory apoptotic proteins Bcl-XL, Bax, Bcl-XL/Bax ratio in vivo in these animals (Fig. 4 B, and D).
Fig. 4.
Effects of isoflurane on BACE and Bcl-xl/Bax ratio in cerebral cortex. A and B, representative changes of BACE (A) and Bcl-xl and Bax (B) in cerebral cortex by Western blot. C, intracerebroventricular pretreatment of IP3R antagonist xestospongin C (Xc) abolished isoflurane-induced elevation of BACE in cerebral cortex. D, isoflurane (1.5%) for 6 h in rats at P7 did not significantly change the levels of the apoptotic regulatory proteins Bcl-xl, Bax, or the Bcl-xl/Bax ratio in the cerebral cortex. n = 3 for each condition.
Pharmacological Inhibition of InsP3R Activity in Vivo Ameliorated the Transient and Dose-Dependent Isoflurane-Induced Learning Disability.
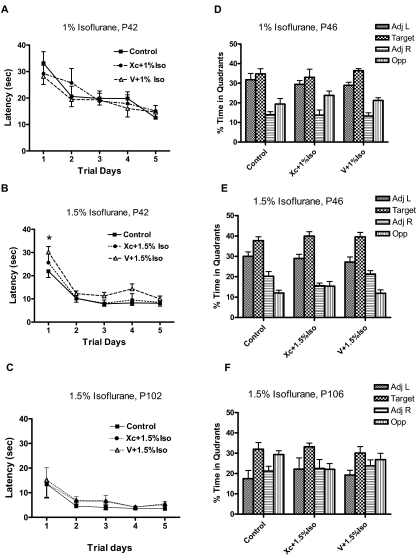
The relevance of neonatal apoptosis to brain development remains unclear. Thus, we first asked whether there is a direct correlation between the noted apoptosis in our study with learning and memory impairments. According to our in vitro and in vivo data, any correlation between apoptosis and learning and memory impairments should be significantly reduced by xestospongin C. To this end, P7 rat pups, with or without intracerebroventricular xestospongin C pretreatment, were exposed to either 1% or 1.5% isoflurane for 6 h and then allowed to grow and develop like the unexposed littermates. Learning and memory were first assessed by place and probe trials in the MWM beginning at P42 and P46, respectively (Fig. 5) and then again at P102 and P106, respectively (Fig. 5). We found a small impairment in the P42 group that was exposed to 1.5% isoflurane during the place trials (Fig. 5B). Pre-exposure to xestospongin C by intracerebroventricular injection blocked this impairment (Fig. 5B). No significant learning and memory impairments were noted in control or experimental groups during place trials in animals older than P42 (Fig. 5, D–F).
Fig. 5.
Intracerebroventricular pretreatment of InsP3R antagonist xestospongin C ameliorated isoflurane-mediated transient and dose-dependent cognitive dysfunction. A–C, learning and memory were tested by place trials with the MWM beginning at P42 after a P7 exposure to either 1% (A) or 1.5% isoflurane (B) and repeated again at P102 for the 1.5% exposure group (C). These studies were carried out in the presence or absence of intracerebroventricular pretreatment with 100 pmol of InsP3R antagonist xestospongin C (Xc) or vehicle control (V) 30 min before isoflurane exposures. n = 10 for control; n = 8 for 1% isoflurane; n = 10 for 1.5% isoflurane. *, P < 0.05 compared with vehicle + 1.5% isoflurane (V + 1.5% iso), two-way ANOVA. D–F, after completion of the place trials, spatial memory was determined by probe trials in the same group of animals initially exposed to 1% isoflurane (D) or 1.5% isoflurane (E and F).
Discussion
Our data clearly show that exposure of the commonly used general anesthetic isoflurane to cortical and hippocampal rodent cultures results in an increase in cytosolic calcium. This increase in cytosolic Ca2+ seems to be a consequence of both release from the ER and influx from the extracellular space. Furthermore, prolonged exposure of primary cultured neurons and developing rats (P7) to isoflurane results in neurodegeneration in both in vitro and in vivo neuronal systems. Interestingly, this neurodegeneration is reversed mostly by the specific InsP3R antagonist, xestospongin C, suggesting a role for ER-mediated Ca2+-dysregulation in neurodegeneration. To our knowledge, this is the first evidence for a mechanism directly linking isoflurane exposure in neonates to apoptosis. Given that these changes in calcium and the subsequent apoptosis could be largely suppressed by xestospongin C, our data strongly suggest that InsP3Rs may be the initial source of calcium and an upstream trigger for apoptosis. Furthermore, the up-regulation of BACE by isoflurane described in our results may contribute to the isoflurane-induced vicious cycle of apoptosis (Xie et al., 2007). Thus, our data suggest that the entire cascade of InsP3R-mediated calcium release and/or calcium influx and neurodegeneration can be prevented by the inhibition of InsP3Rs activity.
Our results provide strong evidence in support of a mechanism by which isoflurane, and perhaps other general anesthetics, causes neurodegeneration by apoptosis via excessive activation of InsP3Rs. According to this model, overactivation of InsP3Rs would result in the elevation of cytosolic and mitochondrial Ca2+ levels and depletion in the ER. This dysregulation of cytosolic Ca2+ by isoflurane would then be expected to activate neurodegenerative and apoptotic pathways. Given that calcium release from the ER via InsP3Rs plays important roles in many cellular functions (e.g., cell excitation, growth, death etc.), the results of this article may lead to future studies into the pharmacological or side effects of general anesthetics.
The results of this article suggest a clear effect of isoflurane on Ca2+ dysregulation by overactivation of InsP3Rs, but it is not yet clear whether this effect is caused by direct or indirect interaction with these receptors. Thus, it would be interesting to know whether isoflurane and other commonly used inhalational anesthetics (sevoflurane and desflurane) can directly activate the InsP3R. Our calcium imaging experiments suggest that Ca2+ influx from the extracellular space contributes to the isoflurane-mediated increase in cytosolic Ca2+ in cortical and hippocampal neurons. Several membrane receptors could theoretically serve as the pathways for such influx, but our data clearly rule out the L-type VGCCs as a contributing pathway. Although we cannot rule out the possibility of other VGCC types playing a role in mediating this Ca2+ influx from the extracellular space, we propose that isoflurane may cause Ca2+ release from the ER, which then induces Ca2+ influx from the extracellular space via capacitive calcium entry (Hewavitharana et al., 2007). Ca2+ dysregulation may be a general mechanism of neurodegeneration induced by general anesthetics via interactions with various calcium pathways. It has long been known that anesthetics like halothane activate the other major ER calcium release pathway, the ryanodine receptor channel complex (Akata et al., 2001), and this is thought to be the basis for a dangerous clinical occurrence called malignant hyperpyrexia (Denborough, 1998). Like InsP3Rs, ryanodine receptors are expressed throughout the nervous system and play important roles in both normal cell function and various neurodegenerative diseases (Paschen and Frandsen, 2001). In support of this notion, we previously showed a role for ryanodine receptor activation in isoflurane-induced apoptosis in cultured neurons (Wei et al., 2005). Both InsP3Rs and ryanodine receptors contribute to the regulation of intracellular calcium homeostasis and may interact with each other in a deleterious manner to mediate isoflurane-induced neurodegeneration. Thus, it would be interesting to investigate the possible interaction between ryanodine and InsP3Rs functions during prolonged exposures to anesthetics.
Our in vitro and in vivo data suggest that activation of InsP3Rs contributes to isoflurane-induced calcium elevation and neurodegeneration in the neonatal rat brain. Excessive calcium release from the ER, via the InsP3Rs, could cause calcium overload in mitochondria and depletion of ER calcium, which are two of the most common contributors to apoptosis (Hanson et al., 2004). Indeed, Ca2+ overload in mitochondria has been shown to cause cytochrome c release (Hanson et al., 2004). This cytochrome c release is associated with the removal of a negative feedback inhibition of InsP3Rs by cytosolic calcium, which then initiates a vicious cycle of ER calcium release (Hanson et al., 2004). Furthermore, cytosolic cytochrome c activates caspase-3, which in turn cleaves the InsP3Rs, resulting in a permanent leak of calcium from the ER (Assefa et al., 2004). Thus, excessive and/or prolonged activation of InsP3R by isoflurane may set into motion a cascade of events resulting in apoptosis. In support of this notion, our data clearly suggest that prolonged exposure of neonatal rats and cultured cortical/hippocampal neurons causes neurodegeneration via direct or indirect activation of InsP3Rs. We believe that these results are evidence that InsP3Rs may be targets for therapy toward the prevention of neurodegeneration associated with exposure to inhalational anesthetics. Toward that end, we carried out a pilot study to assess the ability of xestospongin C in crossing the blood-brain barrier (BBB) after intraperitoneal injection, but our results suggest that this drug did not readily cross the BBB. Nonetheless, these results provide greater impetus to develop soluble analogs that can readily cross the BBB so that patients undergoing surgical procedures that require inhalational anesthetics can be protected from the possible deleterious side effects of such prolonged exposure.
The InsP3R is emerging as a contributor to neurodegenerative disorders such as Alzheimer's disease (Cheung et al., 2008) and Huntington disease (Tang et al., 2003). Anesthetics have been implicated as contributing to these forms of neurodegeneration as well (Eckenhoff et al., 2004; Xie et al., 2007; Liang et al., 2008; Wei et al., 2008). For example, in cells and animals, anesthetics increase the levels of BACE, amyloid β (Xie et al., 2007), plaques, and cognitive impairment (Bianchi et al., 2008). Although the underlying mechanisms for these effects are still uncertain, recent reports seem to support a role for Ca2+ dysregulation and activation of InsP3Rs in neurodegenerative conditions such as Alzheimer's and Huntington diseases (Liang et al., 2008; Wei et al., 2008; Bezprozvanny, 2009). In support of those studies, our study shows that inhibition of InsP3Rs nearly abolishes the isoflurane-mediated elevation of BACE. It is not clear whether the amyloid β pathways contribute to apoptosis in the neonate, but it is known that some members of this pathway are essential for normal central nervous system development, making it difficult to interpret the meaning of the noted isoflurane-mediated increase in BACE expression. Because BACE contributes to the production of β-amyloid and neuropathology in Alzheimer's disease, the ability of xestospongin C to inhibit the BACE makes this compound a potential therapeutic agent for treatment of Alzheimer's disease.
Although neonatal apoptosis has been previously linked with subsequent learning and memory deficits (Jevtovic-Todorovic et al., 2003), another study could not corroborate these findings (Stratmann et al., 2009). Likewise, the lack of significant learning and memory dysfunctions despite significant increases in neonatal apoptosis in our study is inconsistent with such a link. It is possible that alternate mechanisms, possibly cell-cycle arrest, are responsible for learning and memory defects later in life after exposures to anesthetics. It is intriguing that we noted a small impairment in place trials in only one group (P42) of animals exposed to 1.5% isoflurane. However, our data cannot confirm an anesthesia-induced cognitive dysfunction at the selected exposures in normal neonatal rats.
In summary, our results indicate that exposure to the commonly used inhalational anesthetic isoflurane increases cytosolic calcium via activation of InsP3Rs in rodent primary cultured neurons, resulting in apoptosis. More importantly, pharmacological inhibition of these receptors in vitro and in vivo mostly prevents this isoflurane-mediated neurodegeneration. This study provides a novel mechanism to explain the anesthesia-mediated neurodegeneration in neonatal rodents and identifies InsP3Rs as potential therapeutic targets for the prevention of the deleterious effects of prolonged exposures to anesthetics.
Supplementary Material
Acknowledgments
We thank Dr. Randall Pittman (Department of Pharmacology, University of Pennsylvania, Philadelphia, PA) for valuable discussions.
This work was supported by the National Institutes of Health National Institute of General Medical Science [Grants 1-K08-GM073224-03, 1R01-GM084979-01, 3R01-GM084979-02S1] (to H.W.); and March of Dimes Birth Defects Foundation Research [Grant 12-FY08-167] (to H.W.).
An abstract related to part of this manuscript was submitted to the annual meeting of the American Society of Anesthesiology: Wei H, Liang G, Yang H, and Li J (2008) Suppression of IP3 receptor inhibited isoflurane neurotoxicity in rat primary cortical neurons, Annual Meeting of the American Society of Anesthesiology, 2008 18–22 Oct, Orlando, FL (American Society of Anesthesiology, Park Ridge, IL).
An abstract related to part of this manuscript was submitted to the annual meeting of the Society for Neuroscience: Liang G, Yang H, Li J, Eckenhoff RG, and Wei H (2008) Suppression of IP3 receptor expression inhibited isoflurane-induced neurotoxicity in rat primary cortical neurons, Annual Meeting of the Society for Neuroscience, 2008 15–19 Nov, Washington, DC (Society for Neuroscience, Washington, DC).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.161562.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- ER
- endoplasmic reticulum
- InsP3
- inositol 1,4,5-trisphosphate
- InsP3R
- InsP3 receptor
- LDH
- lactate dehydrogenase
- MTT
- 3-(4,5-dimethyithiazol-2-yl)-2,5-diphenyl-tetrazolium bromide
- MWM
- Morris water maze
- ANOVA
- analysis of variance
- DIV
- day in vitro
- siRNA
- small interfering RNA
- PCR
- polymerase chain reaction
- PI
- propidium iodide
- [Ca2+]c
- cytosolic calcium concentration
- PBST
- phosphate-buffered saline with 0.1% Tween 20
- VGCC
- voltage-gated Ca2+ channel
- BBB
- blood-brain barrier.
References
- Akata T, Nakashima M, Izumi K. (2001) Comparison of volatile anesthetic actions on intracellular calcium stores of vascular smooth muscle: investigation in isolated systemic resistance arteries. Anesthesiology 94:840–850 [DOI] [PubMed] [Google Scholar]
- Assefa Z, Bultynck G, Szlufcik K, Nadif KN, Vermassen E, Goris J, Missiaen L, Callewaert G, Parys JB, De Smedt H. (2004) Caspase-3-induced truncation of type 1 inositol trisphosphate receptor accelerates apoptotic cell death and induces inositol trisphosphate-independent calcium release during apoptosis. J Biol Chem 279:43227–43236 [DOI] [PubMed] [Google Scholar]
- Berridge MJ. (1993) Inositol trisphosphate and calcium signaling. Nature 361:315–325 [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I. (2009) Calcium signaling and neurodegenerative diseases. Trends Mol Med 15:89–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi SL, Tran T, Liu C, Lin S, Li Y, Keller JM, Eckenhoff RG, Eckenhoff MF. (2008) Brain and behavior changes in 12-month-old Tg2576 and nontransgenic mice exposed to anesthetics. Neurobiol Aging 29:1002–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Chou MY. (2001) Cytotoxicity of halothane on human gingival fibroblast cultures in vitro. J Endod 27:82–84 [DOI] [PubMed] [Google Scholar]
- Cheung KH, Shineman D, Muller M, Cardenas C, Mei LJ, Yang J, Tomita T, Iwatsubo T, Lee VMY, Foskett JK. (2008) Mechanism of Ca2+ disruption in Alzheimer's disease by presenilin regulation of InsP3 receptor channel gating. Neuron 58:871–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culley DJ, Baxter MG, Yukhananov R, Crosby G. (2004) Long-term impairment of acquisition of a spatial memory task following isoflurane-nitrous oxide anesthesia in rats. Anesthesiology 100:309–314 [DOI] [PubMed] [Google Scholar]
- Denborough M. (1998) Malignant hyperthermia. Lancet 352:1131–1136 [DOI] [PubMed] [Google Scholar]
- Dong Y, Zhang G, Zhang B, Moir RD, Xia W, Marcantonio ER, Culley DJ, Crosby G, Tanzi RE, Xie Z. (2009) The common inhalational anesthetic sevoflurane induces apoptosis and increases β-amyloid protein levels. Arch Neurol 66:620–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckenhoff RG, Johansson JS, Wei H, Carnini A, Kang B, Wei W, Pidikiti R, Keller JM, Eckenhoff MF. (2004) Inhaled anesthetic enhancement of amyloid-β oligomerization and cytotoxicity. Anesthesiology 101:703–709 [DOI] [PubMed] [Google Scholar]
- Galeotti N, Bartolini A, Ghelardini C. (2006) Blockade of intracellular calcium release induces an antidepressant-like effect in the mouse forced swimming test. Neuropharmacology 50:309–316 [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260:3440–3450 [PubMed] [Google Scholar]
- Hanson CJ, Bootman MD, Roderick HL. (2004) Cell signaling: IP3 receptors channel calcium into cell death. Curr Biol 14:R933–R935 [DOI] [PubMed] [Google Scholar]
- Hewavitharana T, Deng XX, Soboloff J, Gill DL. (2007) Role of STIM and orai proteins in the store-operated calcium signaling pathway. Cell Calcium 42:173–182 [DOI] [PubMed] [Google Scholar]
- Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. (2003) Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci 23:876–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkman CJ, Peelen L, Moons KG, Veenhuizen M, Bruens M, Sinnema G, de Jong TP. (2009) Behavior and development in children and age at the time of first anesthetic exposure. Anesthesiology 110:805–812 [DOI] [PubMed] [Google Scholar]
- Li Y, Liang G, Wang S, Meng Q, Wang Q, Wei H. (2007) Effect of fetal exposure to isoflurane on postnatal memory and learning in rats. Neuropharmacology 53:942–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang G, Wang QJ, Li Y, Kang B, Eckenhoff MF, Eckenhoff RG, Wei HF. (2008) A presenilin-1 mutation renders neurons vulnerable to isoflurane toxicity. Anesth Analg 106:492–500 [DOI] [PubMed] [Google Scholar]
- Loepke AW, Mccann JC, Kurth CD, McAuliffe JJ. (2006) The physiologic effects of isoflurane anesthesia in neonatal mice. Anesth Analg 102:75–80 [DOI] [PubMed] [Google Scholar]
- Ma D, Williamson P, Januszewski A, Nogaro MC, Hossain M, Ong LP, Shu Y, Franks NP, Maze M. (2007) Xenon mitigates isoflurane-induced neuronal apoptosis in the developing rodent brain. Anesthesiology 106:746–753 [DOI] [PubMed] [Google Scholar]
- Malledant Y, Siproudhis L, Tanguy M, Clerc C, Chesne C, Saint-Marc C, Guillouzo A. (1990) Effects of halothane on human and rat hepatocyte cultures. Anesthesiology 72:526–534 [DOI] [PubMed] [Google Scholar]
- Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD, et al. (1998) Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Postoperative Cognitive Dysfunction. Lancet 351:857–861 [DOI] [PubMed] [Google Scholar]
- Paschen W, Frandsen A. (2001) Endoplasmic reticulum dysfunction-a common denominator for cell injury in acute and degenerative diseases of the brain? J Neurochem 79:719–725 [DOI] [PubMed] [Google Scholar]
- Sedlak TW, Snyder SH. (2006) Messenger molecules and cell death: therapeutic implications. J Am Med Assoc 295:81–89 [DOI] [PubMed] [Google Scholar]
- Stratmann G, May LDV, Sall JW, Alvi RS, Bell JS, Ormerod BK, Rau V, Hilton JF, Dai R, Lee MT, et al. (2009) Effect of hypercarbia and isoflurane on brain cell death and neurocognitive dysfunction in 7-day-old rats. Anesthesiology 110:849–861 [DOI] [PubMed] [Google Scholar]
- Tang TS, Tu H, Chan EY, Maximov A, Wang Z, Wellington CL, Hayden MR, Bezprozvanny I. (2003) Huntingtin and huntingtin-associated protein 1 influence neuronal calcium signaling mediated by inositol-(1,4,5) triphosphate receptor type 1. Neuron 39:227–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Peretich K, Zhao Y, Liang G, Meng Q, Wei H. (2009) Anesthesia-induced neurodegeneration in fetal rat brains. Pediatr Res 66:435–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Kang B, Wei W, Liang G, Meng QC, Li Y, Eckenhoff RG. (2005) Isoflurane and sevoflurane affect cell survival and BCL-2/BAX ratio differently. Brain Res 1037:139–147 [DOI] [PubMed] [Google Scholar]
- Wei H, Leeds P, Chen RW, Wei W, Leng Y, Bredesen DE, Chuang DM. (2000) Neuronal apoptosis induced by pharmacological concentrations of 3-hydroxykynurenine: characterization and protection by dantrolene and Bcl-2 overexpression. J Neurochem 75:81–90 [DOI] [PubMed] [Google Scholar]
- Wei H, Liang G, Yang H. (2007) Isoflurane preconditioning inhibited isoflurane-induced neurotoxicity. Neurosci Lett 425:59–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei HF, Liang G, Yang H, Wang QJ, Hawkins B, Madesh M, Wang SP, Eckenhoff RG. (2008) The common inhalational anesthetic isoflurane induces apoptosis via activation of inositol 1,4,5-trisphosphate receptors. Anesthesiology 108:251–260 [DOI] [PubMed] [Google Scholar]
- Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, Gleich SJ, Schroeder DR, Weaver AL, Warner DO. (2009) Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology 110:796–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise-Faberowski L, Zhang H, Ing R, Pearlstein RD, Warner DS. (2005) Isoflurane-induced neuronal degeneration: an evaluation in organotypic hippocampal slice cultures. Anesth Analg 101:651–657 [DOI] [PubMed] [Google Scholar]
- Xie ZC, Dong YL, Maeda U, Moir RD, Xia WM, Culley DJ, Crosby G, Tanzi RE. (2007) The inhalation anesthetic isoflurane induces a vicious cycle of apoptosis and amyloid β-protein accumulation. J Neurosci 27:1247–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Liang G, Hawkins BJ, Madesh M, Pierwola A, Wei HF. (2008) Inhalational anesthetics induce cell damage by disruption of intracellular calcium homeostasis with different potencies. Anesthesiology 109:243–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GH, Dong YL, Zhang B, Ichinose F, Wu X, Culley DJ, Crosby G, Tanzi RE, Xie ZC. (2008) Isoflurane-induced caspase-3 activation is dependent on cytosolic calcium and can be attenuated by memantine. J Neurosci 28:4551–4560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen Y, Dong YL, Wu X, Xu ZP, Lu Y, Zhang YY, Norton D, Tian M, Li SR, Xie ZC. (2009) Nitrous oxide plus isoflurane induces apoptosis and increases β-amyloid protein levels. Anesthesiology 111:741–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.