Abstract
We tested the hypothesis that the basal release of nitric oxide (NO) from endothelial cells modulates contractile activity in the corpus cavernosum (CC) via inhibition of the RhoA/Rho-kinase signaling pathway. Cavernosal strips from wild-type (WT), endothelial nitric-oxide synthase knockout [eNOS(−/−)], and neuronal nitric-oxide synthase knockout [nNOS(−/−)] mice were mounted in myographs, and isometric force was recorded. mRNA and protein expression of key molecules in the RhoA/Rho-kinase pathway were analyzed by real-time polymerase chain reaction and Western blot, respectively. The cGMP levels were determined. The Rho-kinase inhibitors (R)-(+)-trans-N-(4-pyridyl)-4-(1-aminoethyl)-cyclohexanecarboxamide (Y-27632) and (S)-(+)-2-methyl-1-[(4-methyl-5-isoquinolinyl)sulfonyl] homopiperazine (H-1152) reduced cavernosal contractions evoked by phenylephrine or electrical field stimulation (EFS) in a concentration-dependent manner, although this inhibition was less effective in tissues from eNOS(−/−) mice. Y-27632 enhanced relaxations induced by sodium nitroprusside, EFS, and NO (administered as acidified NaNO2) without affecting the cGMP content of the cavernosal strips. This enhancement was less prominent in CC from eNOS(−/−). The protein expression of RhoA, Rho-guanine dissociation inhibitor, and Rho-kinase β did not differ among the strains. However, in eNOS(−/−) CC, the protein expression of Rho-kinase α and both mRNA and protein expression of p115-Rho-associated guanine exchange factor (RhoGEF), PDZ-RhoGEF, and leukemia-associated RhoGEF were up-regulated. Phosphorylation of MYPT1 at Thr696 was higher in tissues from eNOS(−/−) mice. A high concentration of Y-27632 significantly enhanced NO release in CC stimulated by EFS. These results suggest a basal release of NO from endothelial cells, which inhibits contractions mediated by the RhoA/Rho-kinase pathway and modulates the expression of proteins related to this pathway in mouse CC. It indicates that endothelial integrity is essential to the maintenance of erectile function.
Cavernosal smooth muscle relaxation is essential for normal erectile function, and strong evidence exists to implicate nitric oxide (NO) as the principal mediator of penile erection (Andersson, 2001). NO released from nitrergic nerves that supply the cavernosal smooth muscle or from sinusoidal endothelial cells causes corpus cavernosum (CC) relaxation through stimulation of soluble guanylyl cyclase (sGC) and generation of cGMP, which plays a dominant role in smooth muscle relaxation (Andersson, 2001; Dean and Lue, 2005; Priviero et al., 2007). Penile vessels and cavernosal tissue receive a rich adrenergic innervation that maintains the penis in the flaccid state mainly via a tonic activity of these nerves. Hence in the absence of an active NO/cGMP pathway, the cavernosal smooth muscle remains in the contracted state, possibly mediated by the effects of noradrenaline released from sympathetic nerves (Cellek, 2000; Andersson, 2001).
Besides the well established noradrenergic contraction mechanisms in the penis, the important role of increased calcium sensitivity has been established (Chitaley et al., 2001; Wang et al., 2002; Jin et al., 2006). Calcium sensitization is brought about by agonist activation of heterotrimeric G protein-coupled receptors, leading to the exchange of GDP for GTP on the small monomeric GTPase, RhoA. This event elicits activation of RhoA and is catalyzed by the guanine nucleotide exchange factors (RhoGEFs), which cause dissociation of RhoA from its binding partner, Rho-guanine dissociation inhibitor (RhoGDI). As a result, RhoA translocates from the cytosol to the membrane, enabling the downstream activation of various effectors such as Rho-kinase (Webb, 2003). Phosphorylation of the regulatory subunit (MYPT1) of myosin light chain (MLC) phosphatase by Rho-kinase causes inhibition of phosphatase activity, which enhances the contractile response at a constant intracellular calcium concentration (Wang et al., 2002; Webb, 2003; Bivalacqua et al., 2004).
Several studies from our laboratory and others showed that the RhoA/Rho-kinase pathway is important to maintain penile flaccidity. Rho-kinase is expressed in human, rabbit, and rat cavernosal smooth muscle (Mills et al., 2001; Rees et al., 2002; Wang et al., 2002), and antagonism of RhoA/Rho-kinase signaling causes CC relaxation and penile erection (Chitaley et al., 2001; Mills et al., 2001; Rees et al., 2001; Wang et al., 2002; Jin et al., 2006). In vascular tissues, protein kinase G (PKG), which is activated by the NO/cGMP cascade, phosphorylates RhoA on Ser188 and inhibits its translocation to the membrane (Sawada et al., 2001; Ellerbroek et al., 2003). This phenomenon indicates that NO can regulate calcium sensitivity to cause smooth muscle relaxation, in part through PKG inhibition of the RhoA/Rho-kinase/MLC phosphatase pathway (Etter et al., 2001; Sawada et al., 2001). Conversely, inhibition of the NO/cGMP pathway seems to be involved with amplification of RhoA/Rho-kinase pathway. Several pathological conditions such as diabetes, arterial and pulmonary hypertension, low urinary tract symptoms, and erectile dysfunction are associated with endothelial dysfunction and decreased NO bioavailability, as well as with increased Rho-kinase activity or expression of its related proteins (Bivalacqua et al., 2004; Jin and Burnett, 2006; Guven et al., 2007; Hilgers et al., 2007; Do et al., 2009; Guilluy et al., 2009). However, the role of the endothelial- and neuronal-derived NO in the modulation of the RhoA/Rho-kinase pathway at basal condition is still unclear. As shear stress causes the basal release of NO from endothelium (Govers and Rabelink, 2001), we hypothesized that the basal release of NO from endothelial cells modulates the contractile activity in CC via inhibition of the RhoA/Rho-kinase signaling pathway.
Materials and Methods
Animals.
All the experimental procedures were conducted in accordance with institutional guidelines and approved by the Medical College of Georgia Institutional Animal Care and Use Committee. Male C57BL/6 mice [wild-type (WT) strain] and homozygous mutant mice lacking the gene for endothelial nitric-oxide synthase [eNOS(−/−)] or neuronal nitric-oxide synthase [nNOS(−/−)], 10 to 12 weeks of age, were obtained from The Jackson Laboratory (Bar Harbor, ME). Animals were housed in temperature-controlled facilities on a 12-h light/dark cycle with ad libitum food and water access.
Functional Studies.
The animals were stunned by inhalation of CO2, sacrificed by decapitation, and exsanguinated. The penises were surgically removed and placed in chilled Krebs-Henseleit buffer of the following composition: NaCl, 130 mM; NaHCO3, 14.9 mM; dextrose, 5.5 mM; KCl, 4.7 mM; KH2PO4, 1.18 mM; MgSO47H2O, 1.17 mM; and CaCl22H2O, 1.6 mM. After removal of the glans penis and urethra, the penile tissue was cleaned from connective and adventitial tissues, and the fibrous septum separating the CCs was opened from its proximal extremity toward the penile shaft. A slit was made in the tunica albuginea along the shaft to obtain two strips (11 × 1 × 1 mm) of CC from each animal. Each strip was mounted in a myograph for isometric force recording (Danish Myograph Technology, Aarhus, Denmark) coupled to a PowerLab 8/SP data acquisition system (Chart 5.0 software; ADInstruments, Colorado Springs, CO). The bathing solution was maintained at 37°C and continuously aerated with 95% O2 and 5% CO2. Tissues were allowed to equilibrate for 45 min under a resting tension of 2 mN. Repetitive supramaximal transmural electrical field stimulation (EFS) of autonomic nerves was delivered via platinum pin electrodes placed on either side of the cavernosal strips. Electrodes were attached to a stimulus splitter unit (Stimu-Splitter II; Med-Lab Instruments, Loveland, CO), which was connected to a Grass S88 stimulator (Astro-Med, West Warwick, RI).
After equilibration, the ability of the preparations to develop contraction was assessed in 80 mM K+-substituted Krebs-Henseleit solution. Next, endothelial function was assessed by applying acetylcholine (1 μM) in strips contracted with phenylephrine (PE, 10 μM). In the first series of experiments, cumulative concentration-response curves to H-1152 (0.001–3 μM) or Y-27632 (0.01–30 μM) were obtained from PE-precontracted CC in the absence or presence of Nω-nitro-l-arginine methyl ester (l-NAME, 100 μM). One concentration-response curve was obtained in each CC. In the second series of experiments, contractile responses to EFS (16 Hz) were obtained in the absence and presence of increasing concentrations (as indicated previously) of H-1152 and Y-27632. In the third series of experiments, relaxant responses to the NO donor sodium nitroprusside (SNP, 0.01–10 μM), EFS (1–16 Hz), and NO (10–300 μM; added as acidified NaNO2 solution) were obtained in the absence and presence of H-1152 (0.1 μM) or Y-27632 (1 μM).
Determination of cGMP Levels.
Cavernosal strips were equilibrated for 20 min in warmed and oxygenated Krebs' solution and then stimulated for 10 min with SNP (1 μM), Y-27632 (1 μM), or both. Preparations were collected immediately by freezing the segments in liquid nitrogen. cGMP was extracted and quantified using a cGMP enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI) as described previously (Teixeira et al., 2005a).
Measurement of NO Release.
NO released from mouse cavernosal strips was estimated from cGMP levels in the extracellular fluid after the addition of GTP, 3-isobutyl-1-methylxanthine (IBMX), and a soluble fraction of crude extract from the rat cerebellum, as described by Noda et al. (2001). Briefly, the soluble fraction was prepared immediately before use as follows: the rat cerebellum was dissected and homogenized with 5 volumes of ice-cold buffer: 50 mM Tris-HCl containing 1 mM EDTA, 1 mM dithiothreitol, and 1 mM phenylmethylsulfonyl fluoride. The homogenate was centrifuged at 10,000g for 30 min, and the resultant supernatant fraction was used as a source of sGC in the extracellular medium. Strips of CC were placed in a 24-well plastic plate containing 1 ml of Krebs' solution and preincubated at 37°C for 1 h. The incubation medium was then replaced by freshly prepared modified Krebs' solution to which 0.5 mM GTP, 10 mM IBMX, and 80 μg of supernatant fraction were added, and the strips were incubated for 10 min in the absence or presence of EFS and test drugs. The cGMP produced in the incubation medium was measured using an enzyme immunoassay kit. A similar procedure was performed in the presence of 100 μM l-NAME, and the difference in the extracellular cGMP content determined in the absence and presence of l-NAME was regarded as the amount of NO released. After these experiments the tissues were homogenized, and the protein content was determined using a bovine serum albumin protein assay kit (Pierce Chemical, Rockford, IL).
Semiquantitative Reverse Transcription-Polymerase Chain Reaction.
Mouse homologs of PDZ-RhoGEF, leukemia-associated RhoGEF (LARG), and p115RhoGEF were identified by comparative genome analysis using publicly available rat, mouse, and human data. Primers were designed with the Primer3 program (http://www.broadinstitute.org/cmap/) based on the known mRNA sequences for each gene. To exclude the possible contamination of genomic DNA, care was taken to ensure that the two primers for one gene were located at different exons. The PDZ-RhoGEF primers were as follows: sense, 5′-GGGACCCTCTTCGAGAACGCCAAA-3′; antisense, 5′-GGGCAGCCAC-TTGTCCTTGTCAGG-3′. LARG primers were sense, 5′-AGCCATG-CGCGCTGGAGTACAAAC-3′; antisense, 5′-GCTCCAGGGGAATGAGGGGATGTC-3′. p115RhoGEF primers were sense, 5′-TCCGGACCAAGAGTGGGGACAAGA-3′; antisense, 5′-TACCCAGGCTTCCCTTCCGGTCTG-3′. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers were sense, TGCATCCTGCACCACCAACTGCTT; antisense, ACAGCCTTGGCAGCACC-AGTGGAT. Total RNA (4 μg/reaction) extracted from cavernosal strips with TRIzol reagent (Invitrogen, Carlsbad, CA) was used for the first-strand cDNA synthesis with the SuperScript II kit (Invitrogen) according to manufacturer's specifications. cDNA equal to 0.04 μg of total RNA was used for each polymerase chain reaction (PCR) reaction under the following conditions: 94°C for 2 min and 22 (for GAPDH) or 30 (for RhoGEFs) cycles at 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s, followed by 72°C for 7 min. The reaction products were analyzed by electrophoresis on agarose gel, and the expected product was extracted and verified by direct DNA sequencing. The PCR products were quantified by densitometric scanning of gel images using RFL Print software (BDI, Dublin, Ireland). Results were expressed as the densitometric ratio of RhoGEF/GAPDH.
Western Blot Analysis.
The CC muscle strips were homogenized in a lysis buffer containing 40 mM HEPES, 1% Triton X-100, 10% glycerol, 1 mM Na3VO4, and 1 mM phenylmethylsulfonyl fluoride. The tissue lysate was centrifuged at 10,000g, and the supernatant was collected. The protein concentration was determined using a bicinchoninic acid protein assay kit. An aliquot of 40 μg of protein from each sample was loaded per lane and resolved by SDS-polyacrylamide gel electrophoresis (PAGE) under reducing conditions. Proteins were subsequently transferred onto nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA). Membranes were blocked by treatment with 5% milk in Tris-buffered saline containing 0.05% Tween 20, probed with antibodies against RhoA (1:200), MYPT1 (1:1000), phosphorylated MYPT1 (p-MYPT1, 1:1000), Rho-kinase α (1:500), Rho-kinase β (1:200), RhoGDI (1:2000), PDZ-RhoGEF (1:500), p115RhoGEF (1:200), or LARG (1:200) and then incubated with a horseradish peroxidase-conjugated second antibody. Immunoreactivity was detected by enhanced chemiluminescence autoradiography, and the protein expression was normalized to the β-actin content.
Drugs and Solutions.
Acetylcholine, atropine, l-NAME, ODQ, PE, sodium nitrite, SNP, and tetrodotoxin were purchased from Sigma-Aldrich (St. Louis, MO). The compounds H-1152 and Y-27632 were acquired from Calbiochem (San Diego, CA). The antibodies against RhoA, Rho-kinase α and β isoforms, MYPT1, and p-MYPT1 were obtained from BD Biosciences Pharmingen (San Diego, CA). The antibodies against RhoGDI and RhoGEFs were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). All the other reagents used were of analytical grade. Stock solutions were prepared in deionized water and stored in aliquots at −20°C; dilutions were made up immediately before use.
Data Analysis.
Relaxation responses are expressed as percentage of PE-induced maximum contraction. Curves were fitted to all the data using nonlinear regression, and half-maximum response (pEC50) of each drug expressed as –log molar (M) was used to compare potency. Agonist-induced contraction was expressed as percentage of KCl-induced maximum contraction. Shifts are calculated as the antilog of the value given by the difference between potencies (pEC50) and expressed as fold displacement. All the data were expressed as mean ± S.E.M. of n experiments. The statistical significance of all the differences between mean values was calculated using two-way analysis of variance followed by Bonferroni post hoc test. A level of p < 0.05 was considered to be statistically significant. Statistical analysis was undertaken using Prism, version 3.00 (GraphPad Software Inc., San Diego, CA).
Results
Relaxation Responses Evoked by Rho-Kinase Inhibitors.
KCl (80 mM)-induced contractions were not significantly different in CC from any strain [WT, 1.9 ± 0.3 mN; eNOS(−/−), 2.0 ± 0.2 mN; nNOS(−/−), 1.9 ± 0.3 mN; n = 8 per group]. Isolated cavernosal segments were contracted with PE (10 μM), which achieved 70 to 80% of KCl-induced maximum contraction. There was no significant difference in PE-induced concentration (data not shown).
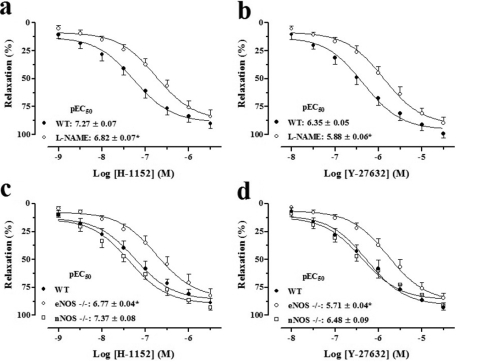
The cumulative addition of the Rho-kinase inhibitors H-1152 (0.001–3 μM; n = 6) and Y-27632 (0.01–30 μM; n = 6) caused concentration-dependent and long-lasting relaxations in precontracted CC from WT mice. In the presence of the NOS inhibitor (l-NAME, 100 μM; n = 6), the potencies of the Rho-kinase inhibitors H-1152 and Y-27632 were significantly decreased, causing a rightward shift of 2.8- and 3.0-fold, respectively (Fig. 1, a and b). When applied to CC from nNOS(−/−) animals, the Rho-kinase inhibitors produced relaxation responses similar to those evoked in tissues from WT. However, the relaxations to H-1152 and Y-27632 were significantly less potent in CC from eNOS(−/−) mice compared with the WT strain, causing a rightward shift of 3.2- and 4.4-fold for H-1152 and Y-27632, respectively (Fig. 1, c and d). Treatment with l-NAME caused similar rightward displacement of the curves to Rho-kinase inhibitors in CC from nNOS(−/−) mice, whereas no significant shifts were observed in tissues from eNOS(−/−) animals (n = 4; data not shown).
Fig. 1.
Concentration-response curves to Rho-kinase inhibitors H-1152 and Y-27632 in the WT CC in the absence or presence of l-NAME (a and b) and in the eNOS(−/−) and nNOS(−/−) CC (c and d). Data represent the mean ± S.E.M. of n experiments. ∗, p < 0.05 compared with WT and nNOS(−/−).
Inhibitory Effect of Rho-Kinase Inhibitors on EFS-Induced Contractions.
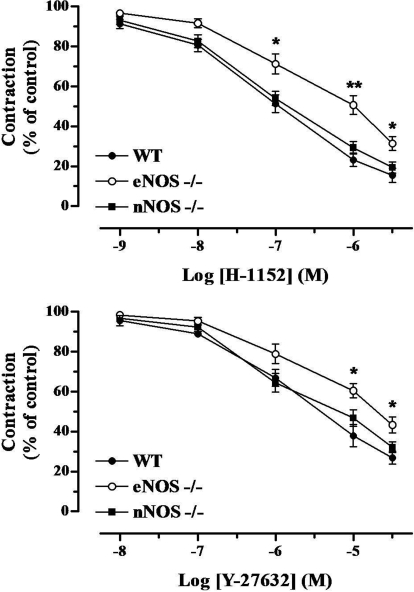
Intrinsic nerve stimulation at 16 Hz produced similar phasic contractions of CC among the three mouse strains in the presence of l-NAME (100 μM) and atropine (1 μM) to block the effects of nitrergic and cholinergic transmission, respectively. These contractions were neuronal in origin and adrenergic in nature because tetrodotoxin (1 μM), a nerve action potential blocker, and prazosin (0.1 μM), an α1 adrenergic receptor inhibitor, blocked the contractile responses. Cumulative application of either H-1152 (0.001–3 μM; n = 6) and Y-27632 (0.01–30 μM; n = 5) progressively reduced contractile responses to EFS (Fig. 2). Interestingly, Fig. 2 shows that the contractions evoked in CC strips from the eNOS(−/−) mice were significantly more resistant (p < 0.05) to Rho-kinase inhibition compared with responses obtained in segments from WT and nNOS(−/−) mice.
Fig. 2.
Effect of the Rho-kinase inhibitors H-1152 (0.001–3 μM; n = 6, a) and Y-27632 (0.01–30 μM; n = 5, b) on EFS-induced noradrenergic contractions (16 Hz) in CC strips from WT, eNOS(−/−), and nNOS(−/−) mice. Data represent the mean ± S.E.M. of n experiments. ∗, p < 0.05 and ∗∗, p < 0.01 compared with WT and nNOS(−/−).
Rho-Kinase Inhibition Enhances NO-Mediated Relaxations.
We first investigated the effects of Y-27632 (1 μM; n = 6) on concentration-response curves to SNP (0.01–10 μM) in CC strips. The potency (pEC50) of the relaxation induced by SNP was significantly enhanced after exposure to Y-27632 in WT, eNOS(−/−), or nNOS(−/−) CC preparations without significant effects on maximal responses. However, Y-27632 was approximately 16 to 20% less effective in enhancing SNP-induced tissue relaxation from eNOS(−/−) compared with the relaxation observed in CC from WT and nNOS(−/−) (Table 1; p < 0.05).
TABLE 1.
Relaxant potency (pEC50) of sodium nitroprusside (SNP) in the CC of WT, eNOS(−/−) and nNOS(−/−) in the absence and in the presence of Y-27632 (1 μM)
Addition of Y-27632 potentiated the relaxation induced by SNP.
| WT | eNOS(−/−) | nNOS(−/−) | |
|---|---|---|---|
| SNP | 6.64 ± 0.04 | 6.51 ± 0.06 | 6.59 ± 0.06 |
| SNP + Y-27632 (1 μM) | 7.21 ± 0.05* | 6.88 ± 0.04* | 7.14 ± 0.06* |
| Shift | 3.7 | 2.3 | 3.5 |
P < 0.05, compared with control condition, in the absence of Y-27632.
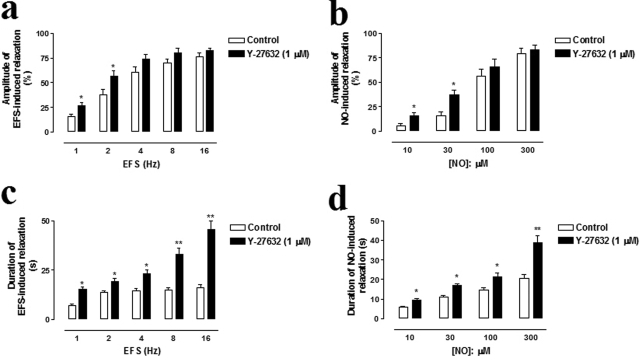
CC strips from WT mice were pretreated with bretylium tosylate (30 μM) and atropine (1 μM) to inhibit the adrenergic and cholinergic transmission. EFS-elicited (1–16 Hz) frequency-related relaxations were abolished by tetrodotoxin (1 μM; n = 4), l-NAME (100 μM; n = 4), or ODQ (10 μM; n = 4), an sGC inhibitor, confirming the nitrergic nature of these responses (data not shown). As shown in Fig. 3a, Y-27632 (1 μM; n = 6) enhanced the magnitude of relaxation evoked by lower frequencies of stimulation (1–2 Hz; p < 0.05). Most importantly, the duration of the nitrergic responses (time elapsed from 50% relaxation to 50% recovery) was significantly potentiated by treatment with Y-27632 over the full range of the frequency-response curve (Fig. 3c). To further confirm that inhibition of Rho-kinase enhances NO-mediated relaxation, we used exogenous NO (as acidified NaNO2). Addition of noncumulative concentrations of NO caused transient relaxations sensitive to ODQ (n = 6). Similarly, Y-27632 enhanced the magnitude and duration of the NO-dependent relaxations (Fig. 3, b and d; n = 6). Furthermore, Y-27632 at the same concentration failed to enhance the CC relaxations evoked by the K+ channel opener cromakalim (pEC50 = 6.18 ± 0.06 versus 6.05 ± 0.07 in the presence of Y-27632; n = 4, data not shown), suggesting the specific effect of Y-27632 on NO-induced relaxations. Similar results were obtained in tissues from eNOS(−/−) mice, although Y-27632 was approximately 19 to 25% less effective in enhancing EFS- and NO-mediated relaxations (p < 0.05; data not shown). Under the same conditions, CC from nNOS(−/−) failed to relax in response to EFS, as expected (data not shown).
Fig. 3.
Effect of Y-27632 (1 μM) on the amplitude (a and b) and duration (c and d) of the relaxation evoked by EFS (1–16 Hz; a and c) and NO (10–300 μM; b and d) in CC strips from WT mice precontracted with PE (10 μM). Y-27632 was added to the bathing medium 30 min before the second application of EFS and NO. Data represent the mean ± S.E.M. of n experiments. ∗, p < 0.05 compared with control condition in the absence of Y-27632.
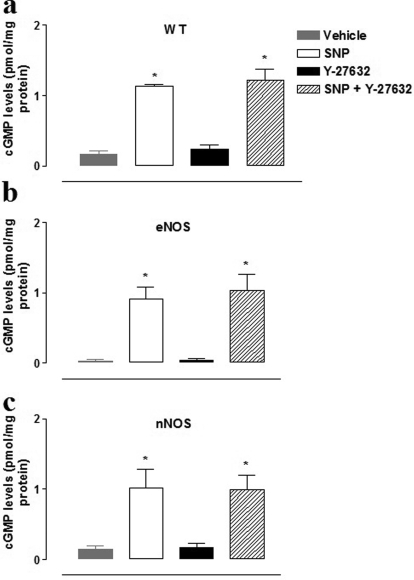
Lack of Effect of Y-27632 on cGMP Levels.
Figure 4 shows that the basal cGMP content (pmol/mg protein) of CC strips in eNOS(−/−) (0.03 ± 0.02) was significantly lower (p < 0.05) than that in WT (0.16 ± 0.05) or nNOS(−/−) (0.15 ± 0.04) tissues. SNP (1 μM) markedly increased cGMP levels (p < 0.01), whereas Y-27632 (1 μM) did not change cGMP levels either alone or in combination with SNP (Fig. 4). Incubation of strips from WT mice with l-NAME (100 μM) resulted in a basal cGMP concentration similar to that found in tissues from eNOS(−/−) animals (0.04 ± 0.01; data not shown).
Fig. 4.
cGMP content of CC strips from WT (a), eNOS(−/−) (b), and nNOS(−/−) (c) exposed to vehicle, SNP (1 μM), Y-27632 (1 μM), or their combination (n = 4). cGMP levels represent the mean ± S.E.M. of n experiments performed in duplicate.
Analysis of Endogenous RhoA, RhoGDI, and Rho-Kinase Protein Expression.
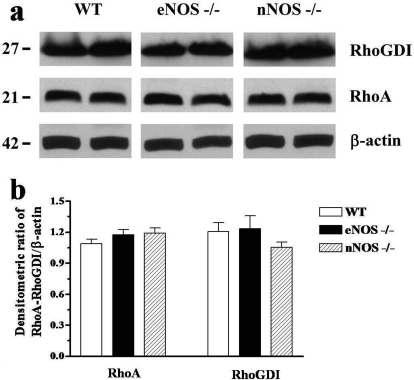
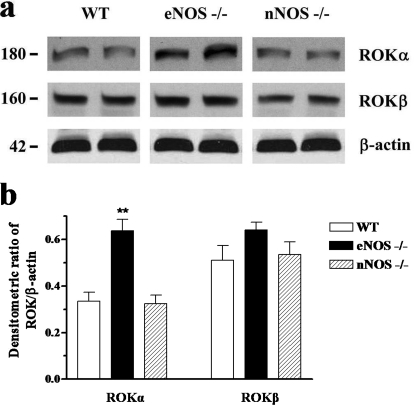
Western blot analyses were carried out using specific antibodies. Bands corresponding to RhoA and RhoGDI at approximately 21 and 27 kDa, respectively, were observed in the mouse penile tissue. The expression levels of endogenous RhoA and RhoGDI were not significantly different in CC from WT, eNOS(−/−), and nNOS(−/−) mice (Fig. 5; n = 5). The protein expression was normalized by β-actin. Antibodies against Rho-kinase α and β isoforms revealed the expression of these kinases with approximate molecular masses of 180 and 160 kDa, respectively (Fig. 6; n = 5). There were no apparent changes in the expression level of Rho-kinase β in the penile tissue from the three mouse strains (Fig. 6). However, a significant increase in protein expression of Rho-kinase α was observed in the CC from eNOS(−/−) mice compared with WT and nNOS(−/−) mice (p < 0.01).
Fig. 5.
Western blot analysis of RhoA and RhoGDI in CC isolated from WT, eNOS (−/−), and nNOS (−/−) mice. Tissue homogenates were subjected to SDS-PAGE and immunoblotted with specific antibodies. a, shows representative immunoblots. b, shows a summarized bar graph of the densitometric analysis. Data represent the mean ± S.E.M. of 5 mice/group.
Fig. 6.
Western blot analysis of Rho-kinase isoforms (α and β) in CC isolated from WT, eNOS(−/−), and nNOS(−/−) mice. Tissue homogenates were subjected to SDS-PAGE and immunoblotted with specific antibodies. a, shows representative immunoblots. b, shows a summarized bar graph of the densitometric analysis. Data represent the mean ± S.E.M. of 5 mice/group. ∗∗, p < 0.01 compared with WT and nNOS(−/−).
Expression of RhoGEFs in CC.
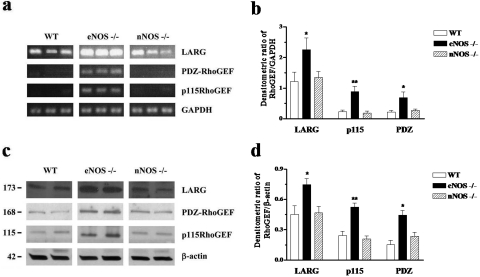
A reverse transcription-polymerase chain reaction (RT-PCR) assay was used to measure the mRNA expression of the regulator of G protein signaling-containing RhoGEFs in the mouse CC, according to methodology developed in our laboratory (Teixeira et al., 2005b). cDNAs prepared by reverse transcription of total RNA extracted from mouse CC coding for PDZ-RhoGEF, p115RhoGEF, and LARG were detectable. Figure 7 shows the electrophoretic visualization of the PCR products obtained from the amplification of cDNAs. In all the samples, the fragments exhibiting the expected sizes were amplified without any contamination from nonspecific products [210 base pairs (bp) for PDZ-RhoGEF, 226 bp for p115RhoGEF, and 237 bp for LARG]. When normalized to the GAPDH content of the samples, the expression level of each RhoGEF was markedly higher in CC isolated from eNOS(−/−) compared with those obtained from WT and nNOS(−/−) (n = 6; Fig. 7, a and b).
Fig. 7.
mRNA and protein expression of p115RhoGEF, PDZ-RhoGEF, and LARG in CC isolated from WT, eNOS(−/−), and nNOS(−/−) mice. a, total RNA was isolated from crude tissue homogenates, and expression of regulator of G protein signaling-containing RhoGEFs mRNA was analyzed by semiquantitative RT-PCR. The electrophoretic visualization of the amplicons represents six sets of separate experiments. b, bar graph summarizes the mRNA expression of RhoGEFs that was normalized by β-actin (n = 6). c, Western blot analysis of LARG, p115RhoGEF, and PDZ-RhoGEF (n = 6). Representative bands of protein expression of RhoGEFs were shown. d, bar graph summarizes the protein expression of RhoGEFs that was normalized by β-actin levels (n = 6). Each set of determinations was performed in triplicate. ∗, p < 0.05 and ∗∗, p < 0.01 compared with WT and nNOS(−/−).
Furthermore, to determine whether protein expression of RhoGEF was altered, we performed Western blot analysis with specific antibodies on the same samples. Anti-p115RhoGEF, anti-PDZ-RhoGEF, and anti-LARG antibodies reacted with proteins of approximately 115, 168, and 173 kDa, respectively. The protein expression was normalized by β-actin. Consistent with the RT-PCR results, Fig. 7, c and d, shows an increase in protein expression of the three RhoGEFs in CC samples obtained from eNOS(−/−) mice compared with WT and nNOS(−/−) mice (n = 6).
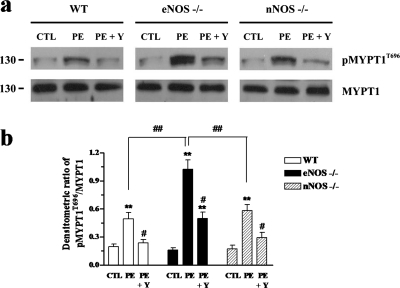
Phosphorylation of MYPT1 at Thr696 in CC.
Levels of MYPT1 phosphorylation at the inhibitory site (Thr696) were examined in CC samples. As shown in Fig. 8, stimulation with PE (1 μM) significantly increased the levels of MYPT1 phosphorylation in cavernosal tissue. This concentration was used because it represents the approximate EC50 of PE in WT CC preparations. Pretreatment with Y-27632 (10 μM) significantly reduced PE-induced MYPT1 phosphorylation. However, the level of p-MYPT1 was significantly higher in CC samples from eNOS(−/−) mice compared with WT and nNOS(−/−) mice (p < 0.01; n = 6) after normalized to the MYPT1 content of the respective samples. In addition, Y-27632 was not able to fully prevent phosphorylation of MYPT1 in eNOS(−/−) cavernosal tissue.
Fig. 8.
Western blot analysis of MYPT1 phosphorylation at Thr696 (p-MYPT1T696). The CC tissue isolated from WT, eNOS(−/−), and nNOS(−/−) mice were treated with vehicle (control, CTL), PE (1 μM), or PE + Y-27632 (10 μM). Tissue homogenates were subjected to SDS-PAGE and immunoblotted with specific antibodies. a, representative immunoblots of p-MYPT1T696 and MYPT1. b, summarized bar graph shows that increased pMYPT1T696 was detected in tissues from eNOS(−/−) mice when normalized to total MYPT1 (n = 6 per group). ∗∗, p < 0.01 compared with the respective controls; #, p < 0.05 compared with the respective PE groups; ##, p < 0.01 compared with WT and nNOS(−/−) PE groups.
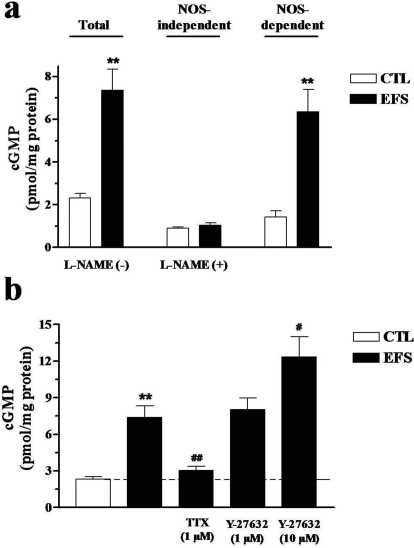
Effect of Y-27632 on NO Release in CC.
Using CC strips from WT mice, the amount of cGMP accumulated in the external medium reached 2.32 ± 0.18 pmol/mg protein (n = 7) and was decreased to 0.90 ± 0.09 pmol/mg protein by treatment with l-NAME, 100 μM (p < 0.01). Approximately 60% cGMP could be attributable to NO released from nitrergic nerves under the assay conditions. As shown in Fig. 9, EFS (50 V, 1 ms, 16 Hz for 2 min) stimulated NO release, which was fully inhibited by l-NAME (n = 7). Most importantly, pretreatment with tetrodotoxin (1 μM) also virtually abolished EFS-evoked increases in cGMP (n = 7; p < 0.01), thus indicating the neuronal origin of the NO-mediated cGMP formation in the external medium. Similar results were obtained in eNOS(−/−) CC, whereas no significant increases in cGMP were observed in the external medium when nNOS(−/−) cavernosal tissue was stimulated by EFS (data not shown). Therefore, under these established conditions, we investigated the effects of Y-27632 (1–10 μM; n = 7) on NO release. At the highest concentration, Y-27632 significantly potentiated cGMP production evoked by EFS in the external medium (p < 0.05).
Fig. 9.
EFS-induced cGMP production. a, NO synthase-dependent cGMP production in CC during EFS (16 Hz for 2 min) measured in incubation medium containing guanylyl cyclase from rat cerebellum, GTP (0.5 mM), and IBMX (10 μM), n = 7. b, shows the effects of tetrodotoxin (1 μM) and Y-27632 (1–10 μM) on EFS-induced cGMP elevation (n = 7). Data represent the mean ± S.E.M. of n experiments. ∗∗, p < 0.01 compared with control values; #, p < 0.05 and ##, p < 0.01 compared with EFS values.
Discussion
In this article we provide pharmacological and molecular evidence that there is a physiological antagonism between endothelial NO and the RhoA/Rho-kinase signaling pathway in mouse penis. Several studies showed the contribution of Rho-kinase to the penis flaccid state and inhibition of this pathway caused penile erection (Mills et al., 2002; Teixeira et al., 2005b; Lin et al., 2008). In this study, we showed that the Rho-kinase inhibitors, H-1152 and Y-27632, produced concentration-dependent and long-lasting relaxations of CC contracted with PE. However, the relaxation was significantly reduced in the CC of eNOS(−/−) mice but not that of nNOS(−/−) mice. Moreover, the inhibition of NOS by incubation with l-NAME caused a decrease in the relaxation produced by Rho-kinase inhibitors in the CC of WT and nNOS(−/−) mice, whereas no further displacement was observed in the CC of eNOS(−/−) mice. This suggests that Rho-kinase inhibition is facilitated by NO derived from endothelial cells. The interaction between NO and Rho-kinase has been extensively described, and the balance between these effectors seems to be crucial for the regulation of many physiological functions. Previous studies showed that, in pathological conditions, there is an imbalance in favor of increasing the RhoA/Rho-kinase pathway, which suppresses the eNOS in diabetic rats (Bivalacqua et al., 2004) and contributes to the hypertensive state and vascular dysfunction of eNOS(−/−) mice (Williams et al., 2006). Aging-related erectile dysfunction is also associated with the amplification of this pathway (Jin et al., 2006). Herein, we showed that nNOS deletion did not affect the relaxation induced by Rho-kinase inhibitors. On the other hand, Rho-kinase inhibitors showed resistance in relaxing the CC of eNOS(−/−) mice. This indicates a basal release of NO from endothelial cells, probably stimulated by shear stress, and the chronic suppression of NO basal release might lead to the amplification of the RhoA/Rho-kinase pathway. This suggests that endothelium-derived NO is essential to the physiological antagonism of Rho-kinase, keeping the contractile and relaxing mechanisms in balance under basal conditions.
We further evaluated the effects of Rho-kinase inhibition on the relaxation induced by the NO donors SNP, EFS, and NO in mouse CC. Inhibition of Rho-kinase significantly increased the potency of SNP and the magnitude and duration of EFS- and NO-induced relaxation in all the strains. However, these effects were smaller in the CC of eNOS(−/−) mice, corroborating that eNOS suppression enhances the RhoA/Rho-kinase pathway. The interaction between NO and the RhoA/Rho-kinase pathway was shown earlier in vascular tissue. A previous study showed that NO causes vasodilation by inhibiting Rho-kinase activity (Chitaley and Webb, 2002). On the other hand, Rho-kinase inhibition by hydroxyfasudil activates eNOS and increases NO production in human endothelial cells (Wolfrum et al., 2004). Besides the evidence showing an NO-Rho-kinase interaction, the lack of effect of Y-27632 on the relaxation induced by the K+ channel opener, cromakalim, suggests that inhibition of Rho-kinase is synergic specifically to NO-induced relaxation.
In addition, we observed that Rho-kinase inhibitors reduced EFS-induced contractions in a concentration-dependent manner, and that this effect was smaller in the CC of eNOS(−/−) mice. This is consistent with a previous study from our laboratory, showing that the combination between SNP and Rho-kinase inhibitors markedly reduced the EFS- and PE-induced contractions in rat CC (Teixeira et al., 2005b).
To investigate the nature of this synergism, we measured tissue levels of cGMP after stimulation with SNP and/or Y-27632. First, our data showed decreased cGMP levels in the CC of eNOS(−/−) mice compared with those in WT and nNOS(−/−) mice. This suggests that there is a basal release of NO from endothelial cells, which activates sGC to generate cGMP. This is confirmed by the decreased levels of cGMP in the CC of WT mice incubated with l-NAME. After stimulation with SNP, an increase in cGMP levels was observed and that was similar among the strains. This is probably because of similar sGC expression in the CC of nNOS(−/−) and eNOS(−/−) mice (Teixeira et al., 2007). In contrast, stimulation with Y-27632 did not change cGMP levels. In addition, the combination of SNP and Y-27632 did not cause a further increase in cGMP levels compared with those levels generated by SNP stimulation alone. This observation is consistent with our previous study that Y-27632 does not elevate cGMP levels in rat celiac artery (Teixeira et al., 2005a).
Because we observed resistance to inhibition of the Rho-kinase pathway in eNOS(−/−) mice, we measured expression of the proteins involved in this pathway. RhoA is a GDP-bound protein in its inactive state. RhoGEFs are responsible for catalyzing the exchange of GDP for GTP, leading to RhoA activation, which causes dissociation of RhoA from its binding partner, RhoGDI. Rho-kinase, a downstream target of RhoA, phosphorylates the myosin-binding subunit of MLC phosphatase, MYPT1, increasing the smooth muscle tone (Webb, 2003; Jin et al., 2006; Lin et al., 2008). In this study, no changes were observed in the protein expression of RhoA, RhoGDI, and Rho-kinase α in either strain. However, we saw increased expression of Rho-kinase β in the CC of eNOS(−/−) mice. Several previous studies have described increased expression of Rho-kinase in different pathological conditions associated with decreased NO bioavailability. Increased expression of Rho-kinase β was found in the CC of diabetic rabbits (Chang et al., 2003). Expression of both Rho-kinase isoforms was increased in the CC from both diabetic rats (Bivalacqua et al., 2004) and rabbits with partial bladder outlet obstruction (Chang et al., 2005). We also measured protein expression of three RhoGEFs described in the smooth muscle (PDZ-RhoGEF, p115RhoGEF, and LARG; Webb, 2003) and showed that increased expression of these proteins was observed in the CC of eNOS(−/−) mice. This indicates that, although RhoA protein expression was not increased in eNOS(−/−) mice, its activity might be increased by RhoGEFs at the post-translational level, leading to Rho-kinase activation. This activation may be responsible for the increase in the resistance to Rho-kinase inhibitors observed in eNOS(−/−) mice.
Because MYPT1 is the major effector of Rho-kinase-mediated Ca2+ sensitization of the contraction in smooth muscle (Wang et al., 2002; Webb, 2003), we measured the expression of MYPT1 and its phosphorylation after stimulation of the CC, with PE as an indicator of Rho-kinase activity. No differences were observed in the expression of MYPT1 among the strains. After stimulation with PE, phosphorylation levels of MYPT1 were significantly increased in the CC of all the strains. However, the level of p-MYPT1 was higher in eNOS(−/−) mice, which implicates a higher activity of Rho-kinase in this strain. Moreover, in the presence of Y-27632, phosphorylation of MYPT1 was virtually abolished in WT and nNOS(−/−) mice, whereas in eNOS(−/−) mice, Y-27632 only partially reduced the phosphorylation of MYPT1. Indeed, increased phosphorylation of MYPT1 was shown in several conditions known to be associated with decreased NO bioavailability, such as diabetes, aging, and hypertension (Bivalacqua et al., 2004; Jin et al., 2006; Hilgers et al., 2007).
Last, we measured EFS-induced cGMP formation as an index for NO release from the neuronal fibers in WT mice and observed that NO released from neuronal fibers also interacts with the RhoA/Rho-kinase pathway. Although 1 μM Y-27632 was not able to elevate the cGMP formation caused by SNP and EFS, this interaction can be inferred because EFS-induced cGMP formation was increased by a higher concentration of Y-27632 (10 μM). Similar results were observed in eNOS(−/−) mice, whereas there was no cGMP formation in nNOS(−/−) mice. Together with the low level of cGMP in eNOS(−/−) mice under basal conditions, these data suggest that there is no basal release of neuronal NO. Conversely, it indicates that basal release of NO from endothelial cells, probably stimulated by shear stress, is important to counteract Rho-kinase activity and might explain why eNOS deletion (but not nNOS) leads to augmented Rho-kinase activity and expression of related proteins.
In summary, our data show that eNOS suppression caused resistance to the relaxation induced by Rho-kinase inhibitors, as well as a decrease in the contribution of Rho-kinase inhibition in synergized NO-induced relaxation. eNOS suppression possibly leads to amplification of the RhoA/Rho-kinase pathway, which favors the contraction of the cavernosal smooth muscle. Taken together, these results suggest that endothelium-derived NO, released in basal physiological conditions, inhibits contractions mediated by the Rho-kinase pathway and modulates the expression of proteins involved in this pathway in mouse CC.
Supplementary Material
Acknowledgments
This work is dedicated to the memory of Dr. Cleber Evandro Teixeira, an excellent friend and scientist, who carefully designed the experimental protocols. We thank Dr. Erica Chedin for editing this manuscript.
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [Grants HL71138, HL74167].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.160606.
- NO
- nitric oxide
- CC
- corpus cavernosum
- sGC
- soluble guanylyl cyclase
- RhoGEF
- Rho-associated guanine exchange factor
- RhoGDI
- Rho-guanine dissociation inhibitor
- MLC
- myosin light chain
- PKG
- protein kinase G
- eNOS(−/−)
- endothelial nitric-oxide synthase knockout
- nNOS(−/−)
- neuronal nitric-oxide synthase knockout
- WT
- wild-type
- EFS
- electrical field stimulation
- PE
- phenylephrine
- H-1152
- (S)-(+)-2-methyl-1-[(4-methyl-5-isoquinolinyl)sulfonyl]homopiperazine
- Y-27632
- (R)-(+)-trans-N-(4-pyridyl)-4-(1-aminoethyl)-cyclohexanecarboxamide
- l-NAME
- Nω-nitro-l-arginine methyl ester
- SNP
- sodium nitroprusside
- IBMX
- 3-isobutyl-1-methylxanthine
- LARG
- leukemia-associated RhoGEF
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- PCR
- polymerase chain reaction
- PAGE
- polyacrylamide gel electrophoresis
- p-MYPT1
- phosphorylated MYPT1
- ODQ
- 1H-[1,2,4]-oxadiazolo-[4,3,-a]quinoxalin-1-one
- RT-PCR
- reverse transcription-polymerase chain reaction
- bp
- base pair.
References
- Andersson KE. (2001) Pharmacology of penile erection. Pharmacol Rev 53:417–450 [PubMed] [Google Scholar]
- Bivalacqua TJ, Champion HC, Usta MF, Cellek S, Chitaley K, Webb RC, Lewis RL, Mills TM, Hellstrom WJ, Kadowitz PJ. (2004) RhoA/Rho-kinase suppresses endothelial nitric oxide synthase in the penis: a mechanism for diabetes-associated erectile dysfunction. Proc Natl Acad Sci U S A 101:9121–9126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellek S. (2000) Nitrergic-noradrenergic interaction in penile erection: a new insight into erectile dysfunction. Drugs Today 36:135–146 [DOI] [PubMed] [Google Scholar]
- Chang S, Hypolite JA, Changolkar A, Wein AJ, Chacko S, DiSanto ME. (2003) Increased contractility of diabetic rabbit corpora smooth muscle in response to endothelin is mediated via Rho-kinase beta. Int J Impot Res 15:53–62 [DOI] [PubMed] [Google Scholar]
- Chang S, Hypolite JA, Zderic SA, Wein AJ, Chacko S, Disanto ME. (2005) Increased corpus cavernosum smooth muscle tone associated with partial bladder outlet obstruction is mediated via Rho-kinase. Am J Physiol Regul Integr Comp Physiol 289:R1124–R1130 [DOI] [PubMed] [Google Scholar]
- Chitaley K, Webb RC. (2002) Nitric oxide induces dilation of rat aorta via inhibition of rho-kinase signaling. Hypertension 39:438–442 [DOI] [PubMed] [Google Scholar]
- Chitaley K, Wingard CJ, Clinton Webb R, Branam H, Stopper VS, Lewis RW, Mills TM. (2001) Antagonism of Rho-kinase stimulates rat penile erection via a nitric oxide-independent pathway. Nat Med 7:119–122 [DOI] [PubMed] [Google Scholar]
- Dean RC, Lue TF. (2005) Physiology of penile erection and pathophysiology of erectile dysfunction. Urol Clin North Am 32:379–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do e Z, Fukumoto Y, Takaki A, Tawara S, Ohashi J, Nakano M, Tada T, Saji K, Sugimura K, Fujita H, et al. (2009) Evidence for Rho-kinase activation in patients with pulmonary arterial hypertension. Circ J 73:1731–1739 [DOI] [PubMed] [Google Scholar]
- Ellerbroek SM, Wennerberg K, Burridge K. (2003) Serine phosphorylation negatively regulates RhoA in vivo. J Biol Chem 278:19023–19031 [DOI] [PubMed] [Google Scholar]
- Etter EF, Eto M, Wardle RL, Brautigan DL, Murphy RA. (2001) Activation of myosin light chain phosphatase in intact arterial smooth muscle during nitric oxide-induced relaxation. J Biol Chem 276:34681–34685 [DOI] [PubMed] [Google Scholar]
- Govers R, Rabelink TJ. (2001) Cellular regulation of endothelial nitric oxide synthase. Am J Physiol Renal Physiol 280:F193–F206 [DOI] [PubMed] [Google Scholar]
- Guilluy C, Eddahibi S, Agard C, Guignabert C, Izikki M, Tu L, Savale L, Humbert M, Fadel E, Adnot S, et al. (2009) RhoA and Rho kinase activation in human pulmonary hypertension: role of 5-HT signaling. Am J Respir Crit Care Med 179:1151–1158 [DOI] [PubMed] [Google Scholar]
- Guven A, Onal B, Kalorin C, Whitbeck C, Chichester P, Kogan B, Levin R, Mannikarottu A. (2007) Long term partial bladder outlet obstruction induced contractile dysfunction in male rabbits: a role for Rho-kinase. Neurourol Urodyn 26:1043–1049 [DOI] [PubMed] [Google Scholar]
- Hilgers RH, Todd J, Jr, Webb RC. (2007) Increased PDZ-RhoGEF/RhoA/Rho kinase signaling in small mesenteric arteries of angiotensin II-induced hypertensive rats. J Hypertens 25:1687–1697 [DOI] [PubMed] [Google Scholar]
- Jin L, Burnett AL. (2006) RhoA/Rho-kinase in erectile tissue: mechanisms of disease and therapeutic insights. Clin Sci 110:153–165 [DOI] [PubMed] [Google Scholar]
- Jin L, Liu T, Lagoda GA, Champion HC, Bivalacqua TJ, Burnett AL. (2006) Elevated RhoA/Rho-kinase activity in the aged rat penis: mechanism for age-associated erectile dysfunction. FASEB J 20:536–538 [DOI] [PubMed] [Google Scholar]
- Lin CS, Xin ZC, Wang Z, Lin G, Lue TF. (2008) Molecular Yin and Yang of erectile function and dysfunction. Asian J Androl 10:433–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills TM, Chitaley K, Wingard CJ, Lewis RW, Webb RC. (2001) Effect of Rho-kinase inhibition on vasoconstriction in the penile circulation. J Appl Physiol 91:1269–1273 [DOI] [PubMed] [Google Scholar]
- Mills TM, Lewis RW, Wingard CJ, Chitaley K, Webb RC. (2002) Inhibition of tonic contraction—a novel way to approach erectile dysfunction. J Androl 23: S5–S9 [PubMed] [Google Scholar]
- Noda K, Oka M, Ma FH, Kitazawa S, Ukai Y, Toda N. (2001) Release of endothelial nitric oxide in coronary arteries by celiprolol, a beta(1)-adrenoceptor antagonist: possible clinical relevance. Eur J Pharmacol 415:209–216 [DOI] [PubMed] [Google Scholar]
- Priviero FB, Leite R, Webb RC, Teixeira CE. (2007) Neurophysiological basis of penile erection. Acta Pharmacol Sin 28:751–755 [DOI] [PubMed] [Google Scholar]
- Rees RW, Ralph DJ, Royle M, Moncada S, Cellek S. (2001) Y-27632, an inhibitor of Rho-kinase, antagonizes noradrenergic contractions in the rabbit and human penile corpus cavernosum. Br J Pharmacol 133:455–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees RW, Ziessen T, Ralph DJ, Kell P, Moncada S, Cellek S. (2002) Human and rabbit cavernosal smooth muscle cells express Rho-kinase. Int J Impot Res 14:1–7 [DOI] [PubMed] [Google Scholar]
- Sawada N, Itoh H, Yamashita J, Doi K, Inoue M, Masatsugu K, Fukunaga Y, Sakaguchi S, Sone M, Yamahara K, et al. (2001) cGMP-dependent protein kinase phosphorylates and inactivates RhoA. Biochem Biophys Res Commun 280:798–805 [DOI] [PubMed] [Google Scholar]
- Teixeira CE, Jin L, Ying Z, Palmer T, Priviero FB, Webb RC. (2005a) Expression and functional role of the RhoA/Rho-kinase pathway in rat coeliac artery. Clin Exp Pharmacol Physiol 32:817–824 [DOI] [PubMed] [Google Scholar]
- Teixeira CE, Priviero FB, Webb RC. (2007) Effects of 5-cyclopropyl-2-[1-(2-fluoro-benzyl)-1H-pyrazolo[3,4-b]pyridine-3-yl]pyrimidin-4-ylamine (BAY 41–2272) on smooth muscle tone, soluble guanylyl cyclase activity, and NADPH oxidase activity/expression in corpus cavernosum from wild-type, neuronal, and endothelial nitric-oxide synthase null mice. J Pharmacol Exp Ther 322:1093–1102 [DOI] [PubMed] [Google Scholar]
- Teixeira CE, Ying Z, Webb RC. (2005b) Proerectile effects of the Rho-kinase inhibitor (S)-(+)-2-methyl-1-[(4-methyl-5-isoquinolinyl)sulfonyl]homopiperazine (H-1152) in the rat penis. J Pharmacol Exp Ther 315:155–162 [DOI] [PubMed] [Google Scholar]
- Wang H, Eto M, Steers WD, Somlyo AP, Somlyo AV. (2002) RhoA-mediated Ca2+ sensitization in erectile function. J Biol Chem 277:30614–30621 [DOI] [PubMed] [Google Scholar]
- Webb RC. (2003) Smooth muscle contraction and relaxation. Adv Physiol Educ 27:201–206 [DOI] [PubMed] [Google Scholar]
- Williams J, Bogwu J, Oyekan A. (2006) The role of the RhoA/Rho-kinase signaling pathway in renal vascular reactivity in endothelial nitric oxide synthase null mice. J Hypertens 24:1429–1436 [DOI] [PubMed] [Google Scholar]
- Wolfrum S, Dendorfer A, Rikitake Y, Stalker TJ, Gong Y, Scalia R, Dominiak P, Liao JK. (2004) Inhibition of Rho-kinase leads to rapid activation of phosphatidylinositol 3-kinase/protein kinase Akt and cardiovascular protection. Arterioscler Thromb Vasc Biol 24:1842–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.