Abstract
We evaluated the contribution of changes in systemic arterial pressure and local vasodilation to blood flow restoration in contracting human muscles during acute hypoperfusion. Healthy subjects (n = 10) performed rhythmic forearm exercise (10% and 20% of maximum) while a balloon in the brachial artery located above the elbow was inflated. Each trial included 3 min of rest, exercise, exercise with balloon inflation, and exercise after balloon deflation. Forearm blood flow (FBF) was measured using Doppler ultrasound. Blood pressure on both sides of the balloon was measured using a brachial artery catheter (distal pressure), and Finometer for proximal (systemic) arterial pressure. Balloon inflation during exercise reduced distal arterial pressure, and FBF fell 37–41%. There was also a surprising acute increase in forearm vascular resistance (distal pressure/FBF). This was followed by recovery of distal arterial pressure and forearm vasodilation that caused a marked (∼75%) restoration of flow that was not associated with significant changes in systemic arterial pressure. During validation trials (n = 6) at rest and with exercise both balloon and brachial artery diameters were stable when the balloon was inflated. Our findings indicate that at these exercise intensities 1) the restoration of FBF during exercise with hypoperfusion relied primarily on local dilator responses in conjunction with restoration of distal perfusion pressure likely as a result of increased collateral flow around the elbow, and 2) a loss of pulsatile flow and elastic recoil in the forearm may have contributed to the acute increase in vascular resistance seen at the onset of hypoperfusion.
Keywords: exercise hyperemia, vasodilation, vascular resistance
skeletal muscle contractions evoke an increase in blood flow that is proportional to the metabolic demand of the tissue (30). In animals, when blood flow is restricted and/or perfusion pressure is reduced, the active muscle is capable of autoregulating its blood flow (2, 10, 11, 20, 33), which appears to be controlled intrinsically (2, 11). However, the ability to restore blood flow in response to hypoperfusion in active human skeletal muscle is unclear and has undergone little testing. Studies using external pressure to reduce limb blood flow during rhythmic exercise have produced conflicting results (3, 12, 28). Studies in the forearm by Joyner (12) and also by Daley et al. (3) concluded that blood flow to rhythmically contracting muscles was not restored despite a marked rise in arterial pressure. In contrast, Rowell et al. (28) concluded that leg blood flow was partially restored over time in response to external positive pressure and that much of the restoration in flow was due to a reflex rise in arterial pressure evoked by the hypoperfusion.
In the present study, we characterized the blood flow response to acute hypoperfusion in resting and exercising human skeletal muscle. We inflated a balloon in the brachial artery above the elbow to reduce blood flow to contracting forearm muscles and test the hypothesis that local vasodilator mechanisms contribute to the restoration of flow in the human forearm during exercise with muscle hypoperfusion. This is a novel model of hypoperfusion in humans that permitted us to examine vasoregulatory response under conditions of reduced flow and perfusion pressure in contracting skeletal muscle. Additionally, information generated using this model may also provide insight in conditions associated with microvascular dysfunction like diabetes, hypertension, and vascular disease that might contribute to skeletal muscle hypoperfusion during exercise.
METHODS
Subjects
A total of 16 young healthy male subjects volunteered to participate in the study. All subjects were nonobese, nonsmokers, and were not taking any medications. Each provided written informed consent before participation, and all protocols were approved by the Institutional Review Board at Mayo Clinic and in accordance with the Declaration of Helsinki. Subjects arrived in the laboratory at least 4 h postprandial and refrained from intake of caffeine or exercise for at least 24 h. Subjects were screened to determine brachial artery diameter because an end-diastolic resting brachial artery diameter of ≥0.40 cm was needed for instrumentation, and as a result no women qualified for the study.
Heart Rate and Systemic Blood Pressure
Heart rate (HR) was measured by three-lead electrocardiography (ECG). Systemic blood pressure was assessed (beat-to-beat) with a finger plethysmograph (Finometer) on the nonexercising hand and verified with an automated blood pressure cuff on the same arm. The pressure was used as an index of pressure proximal (upstream) from the balloon described below. Cardiac output (CO) was estimated using the Modelflow technique, which has been validated against other techniques and used in recent exercise studies (24, 35).
Arterial Catheterization and Balloon Placement
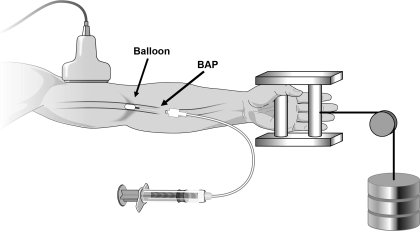
A 20-gauge, 5-cm catheter was placed in the brachial artery in the experimental (nondominant) arm under aseptic conditions after local anesthesia (2% lidocaine). A guide wire was then placed in the artery, which was then cannulated with a 4-Fr. introducer (Cook, Bloomington, IN) that permitted insertion of a 2-Fr. Fogarty balloon catheter into the brachial artery. A port and stopcock system permitted the measurement of brachial arterial pressure (BAP) distal to the balloon and drawing of arterial blood samples. The system was continuously flushed (3 ml/h) with heparinized saline. The configuration of the balloon upstream from the lumen of the introducer allowed measurement of the arterial pressure distal to the balloon that was perfusing the contracting forearm muscles (Fig. 1).
Fig. 1.
Schematic of forearm exercise model. Brachial artery blood velocity was measured (via Doppler ultrasound) proximal to the balloon. The configuration of the balloon upstream from the lumen of the introducer allowed measurement of the brachial arterial pressure (BAP) distal to the balloon that was perfusing the contracting forearm muscles. Rhythmic forearm exercise was performed with a hand grip device by lifting a weight (10% and 20% of maximal voluntary contraction) 4–5 cm over a pulley system at a duty cycle of 1-s contraction and 2-s relaxation (20 contractions/min).
Forearm Blood Flow
Brachial artery mean blood velocity (MBV) and brachial artery diameter were determined proximal (e.g., upstream) from the balloon with a 12-MHz linear-array Doppler probe (Model M12L, Vivid 7, General Electric, Milwaukee, WI, USA). Brachial artery blood velocity was measured throughout each condition with a probe insonation angle previously calibrated to 60°. Brachial artery and balloon diameter measurements were obtained at end diastole and between contractions during steady-state conditions. Diameter measurement typically results in the loss of the pulse wave signal for 15–20 s. Velocity and diameter measurements were made 2–3 cm proximal to the balloon. Forearm blood flow (FBF) was calculated as the product of MBV (cm/s) and brachial artery cross-sectional area (cm2) and multiplied by 60 to present as milliliters per minute.
Rhythmic Forearm Exercise
Rhythmic forearm exercise was performed with a hand grip device at 10% and 20% of each subject's maximal voluntary contraction (MVC, mean 49 ± 2 kg, range 39–58 kg). The weight was lifted 4–5 cm over a pulley at a duty cycle of 1 s contraction and 2-s relaxation (20 contractions/min) using a metronome to ensure correct timing. The average weight used for forearm exercise was 5.0 ± 0.2 and 10.0 ± 0.4 kg for 10 and 20% MVC, respectively.
Brachial Artery Balloon Inflation
To reduce forearm blood flow the brachial artery was partially occluded via inflation of the Fogarty balloon catheter with saline using a calibrated microsyringe for tight control of balloon volume. Balloon inflations were targeted to reduce blood velocity by 40–50%.
Catecholamine Analysis
Arterial plasma catecholamine (epinephrine and norepinephrine) levels were determined by HPLC with electrochemical detection.
Experimental Protocol
A schematic diagram of the general experimental design is illustrated in Fig. 2. Each subject completed one rest trial and two exercise trials (10% and 20% MVC). The rest trial consisted of baseline (3 min) followed by balloon inflation (3 min) and recovery following deflation (3 min). Each exercise trial consisted of 3 min of rest, exercise, exercise with balloon inflation, exercise following balloon deflation, and recovery (15 min total; 9 min of total exercise). Each trial was separated by 20 min of rest to allow FBF to return to baseline. Arterial blood samples were obtained for plasma catecholamine determination during the last 30 s of each phase of the exercise trials.
Fig. 2.
Schematic diagram of experimental protocol. Subjects completed 1 resting and 4 exercise trials. The resting trial consisted of baseline, inflation, and recovery measurements (3 min each). Each exercise trial consisted of baseline, exercise (control), exercise during inflation, exercise following deflation, and recovery measurements (3 min each). Each trial was separated by at least 20 min of rest to allow forearm blood flow (FBF) to return to baseline values. MVC, maximal voluntary contraction.
Validation Protocol
To verify that the balloon inflation in the brachial artery created a stable mechanical system throughout inflation, balloon and brachial artery diameters (proximal to the balloon and vessel around length of balloon) were measured with ultrasound at target inflation and every 30 s throughout inflation in six subjects. Each subject completed one rest trial and two exercise trials (10% and 20% MVC), as described in Experimental Protocol. Brachial artery blood velocity was measured throughout each condition to calculate changes in blood flow.
As an additional validation step in these six subjects, venous occlusion plethysmography (13) was used to determine if the downstream (distal) forearm blood flow response to balloon inflation was similar to measurements proximal to the balloon (Doppler ultrasound). During measurement of FBF, circulation to the hand was arrested by inflation of a wrist cuff to suprasystolic levels (220 mmHg) and a venous occlusion cuff was placed on the upper arm and rapidly inflated to 40 mmHg every 7.5 s, yielding one blood flow every 15 s. Flow was measured for 3 min before inflation, 3 min during inflation, and 2 min following balloon deflation. These studies were only performed at rest due to the inability to obtain quality FBF measures with venous occlusion plethysmography during exercise. FBF was expressed in milliliters per minute per 100 ml of forearm tissue (ml·min−1·100 ml−1). Forearm vascular conductance (FVC) was calculated as (FBF × 100)/BAP and expressed as milliliters per minute per 100 ml of forearm tissue per 100 mmHg (ml·min−1·100 ml−1·100 mmHg−1).
Data Analysis and Statistics
Data were collected at 200 Hz, stored on a computer, and analyzed offline with signal processing software (WinDaq, DATAQ Instruments, Akron, OH). Local mean arterial pressure (BAP) was determined from the brachial artery pressure waveform measured distal to the balloon, systemic MAP (e.g., pressure proximal to the balloon) was derived from the Finometer pressure waveform, and HR was determined from the electrocardiogram. FBF, BAP, MAP, CO, and HR were determined by averaging values during the last 30–45 s of rest, exercise, exercise with inflation, exercise following deflation, and recovery. In addition, all values were analyzed and averaged during the first 10 s of target balloon inflation (nadir) and the first 10 s immediately following balloon deflation. Forearm vascular conductance (FVC) was calculated as (FBF/BAP) × 100 and expressed as milliliters per minute per 100 mmHg.
All values are expressed as means ± SE. Within a given protocol, the FBF, FVC, BAP, systemic MAP, HR, CO, and plasma catecholamines during rest, exercise, the nadir after balloon inflation, exercise at the end of the balloon inflation, exercise following deflation, and recovery were analyzed by repeated-measures ANOVA. When significance was detected, Tukey's post hoc test was used to identify individual differences and adjust P values to account for multiple comparisons, to preserve an overall type I error rate of 0.05. To examine the magnitude of restoration during balloon inflation, the percent recovery of FBF and FVC were calculated as (steady-state inflation plus exercise value − nadir)/[steady-state exercise (i.e., control) value − nadir]. Statistical significance was set a priori at P < 0.05.
To further explore the contribution of local vasodilation to any restoration of flow, we analyzed balloon resistance (Rb) and forearm vascular resistance (Rv) and considered them individually and in series (23). Using systemic arterial pressure (SAP; Finometer), brachial artery pressure distal to the balloon (BAP; catheter) and brachial artery blood flow (Doppler), we calculated the resistance of the balloon [(SAP − BAP)/flow] and vascular resistance (BAP/flow). The total resistance (Rt) was calculated as the sum of these two resistors. Repeated-measures ANOVAs were used to compare each resistance during rest or exercise (before inflation), nadir (after balloon inflation), and at the end of the inflation period.
To investigate the stability of balloon and brachial artery diameter throughout the period of inflation, brachial artery and balloon diameters were measured every 30 s of the inflation period in the validation protocol. Repeated-measures ANOVA were used to compare diameters throughout each trial.
RESULTS
Ten young (30 ± 2 yr) and lean (height 179 ± 2 cm, weight 80 ± 3 kg, body mass index 25 ± 1 kg/m2) men completed the experimental protocol. An additional six young (26 ± 1 yr) and lean (24 ± 1 kg/m2) men completed the validation protocol.
Experimental Protocol
Forearm blood flow and vasodilation during rest with balloon inflation.
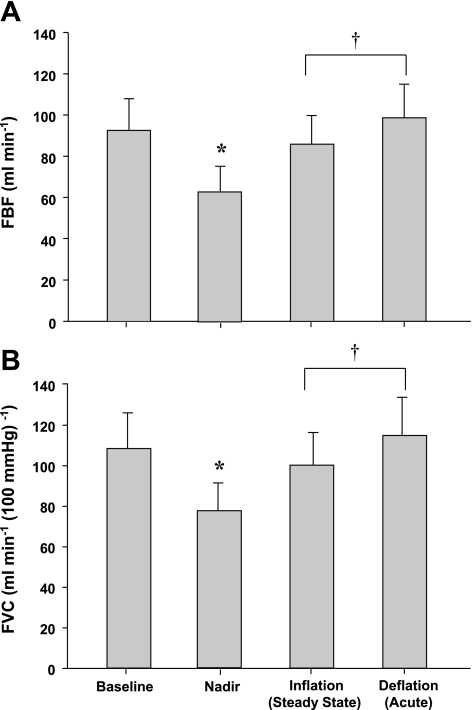
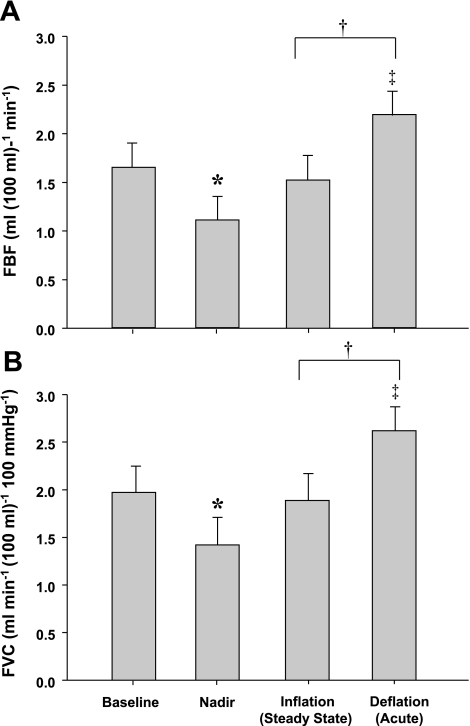
Group mean data for FBF and FVC responses are presented in Fig. 3, A and B. Balloon inflation during resting conditions resulted in an acute 32% reduction in FBF (P < 0.001) and a 28% reduction in FVC (P < 0.001). By the end of the 3-min inflation period FBF and FVC were greater than the nadir (P < 0.01) and were partially restored to preinflation values. This resulted in a 74 ± 11% recovery of FBF and 71 ± 13% recovery of FVC. FBF and FVC remained higher than the nadir following rapid deflation (P < 0.01) but did not differ from steady-state inflation or baseline values.
Fig. 3.
Effect of balloon-induced hypoperfusion on FBF (A) and forearm vascular conductance (FVC; B) at rest. Balloon inflation resulted in an acute reduction in FBF and FVC (nadir), which were partially restored. *P < 0.001 vs. baseline. †P < 0.001 vs. nadir.
Forearm blood flow and vasodilation during exercise with balloon inflation.
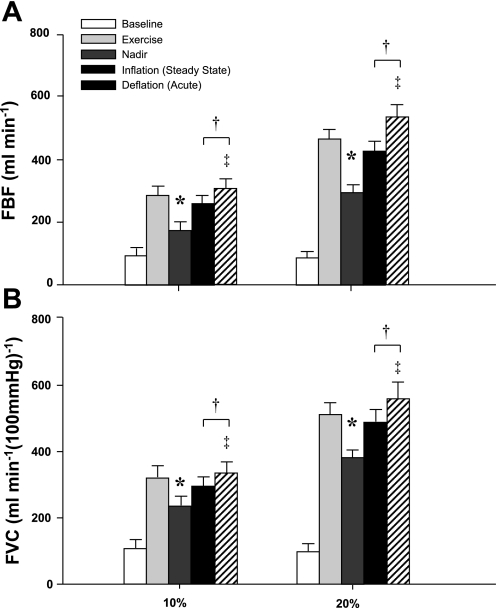
Figures 4 and 5 are a representative tracing and fully analyzed record of the FBF response to exercise with balloon-induced hypoperfusion. Group mean data for FBF and FVC responses are presented in Fig. 6, A and B. As expected, exercise increased FBF and FVC in both exercise trials (P < 0.001). Balloon inflation (nadir) during the exercise trials resulted in an acute 41% and 37% reduction in FBF and an acute 27% and 25% reduction in FVC at 10% and 20% MVC, respectively (P < 0.001). FBF and FVC at the end of inflation were partially restored to exercise (control) levels at 10% and 20% MVC, which were substantially higher than their respective nadir values (P < 0.001). The percent recovery of FBF and FVC during the 10% trial was 75 ± 8% and 76 ± 7%, respectively. Similarly, the percent recovery of FBF and FVC during the 20% trial was 76 ± 6% and 80 ± 5%, respectively. Rapid deflation of the balloon during exercise resulted in an acute elevation in FBF and FVC compared with steady-state inflation values (P < 0.05). Changes in balloon, vascular, and total resistance during the inflation period are presented in Table 1.
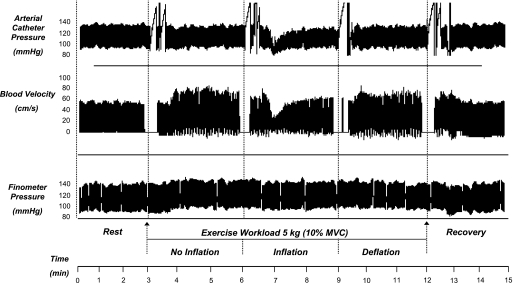
Fig. 4.
Documentation of fall in blood velocity with balloon inflation. Individual record (compressed) of arterial catheter pressure, brachial artery velocity, and Finometer blood pressure during mild rhythmic hand gripping. Exercise caused a rapid increase in blood velocity. Balloon inflation caused a fall in blood velocity that recovered with no obvious increase in systemic pressure. With balloon deflation there was a modest reactive hyperemia. Breaks in velocity signal indicate times of image acquisition for diameter measurements and breaks in arterial pressure tracing indicate times for blood sampling.
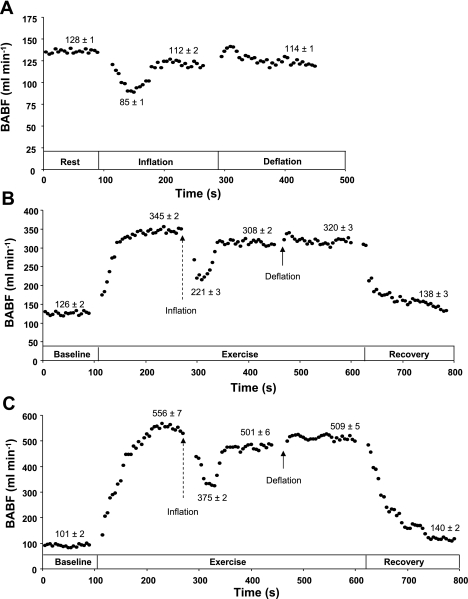
Fig. 5.
Typical blood flow response. A sample tracing of FBF [i.e., brachial artery blood flow (BABF)] in 5-s averages (•) under resting conditions (A) and during exercise at 10% (B) and 20% (C) of maximal voluntary contraction. Arrows indicate start of balloon inflation (dashed) and deflation (solid).
Fig. 6.
Effect of balloon-induced hypoperfusion on FBF (A) and (FVC; B) during exercise. Balloon inflation resulted in an acute reduction in FBF and FVC (nadir), which were partially restored in both trials. *P < 0.001 vs. exercise. †P < 0.001 vs. nadir. ‡P < 0.05 vs. inflation (steady state).
Table 1.
Resistance values during balloon inflation for all experimental trials
| Resistance, mmHg·ml−1·min |
|||
|---|---|---|---|
| Before inflation | Nadir | End of inflation | |
| Rest | |||
| Balloon | 0.00±0.00 | 0.11±0.03* | 0.06±0.01 |
| Vascular | 1.12±0.15 | 1.58±0.20* | 1.18±0.19† |
| Total | 1.14±0.15 | 1.69±0.22* | 1.26±0.18† |
| 10% MVC | |||
| Balloon | 0.00±0.00 | 0.08±0.01* | 0.05±0.01† |
| Vascular | 0.34±0.04 | 0.52±0.07* | 0.35±0.04† |
| Total | 0.35±0.04 | 0.61±0.09* | 0.38±0.04† |
| 20% MVC | |||
| Balloon | 0.00±0.00 | 0.05±0.01* | 0.03±0.01† |
| Vascular | 0.20±0.01 | 0.28±0.01* | 0.21±0.02† |
| Total | 0.21±0.02 | 0.33±0.03* | 0.23±0.02† |
Values are means ± SE; n = 10. MVC, maximum voluntary contraction.
P < 0.01 vs. before inflation.
P < 0.01 vs. nadir.
Timing of compensatory vasodilation.
The time it took to reach a steady-state FVC during balloon inflation during the rest trial (80 ± 8 s) was significantly longer than the 10% (54 ± 7 s; P < 0.05) and 20% (43 ± 6 s; P < 0.01) MVC trails. There was no difference in the timing of compensation between the 10 and 20% exercise trials (P = 0.46).
Hemodynamic changes.
Systemic hemodynamic responses at rest and during exercise are presented in Table 2. During the rest trial there was no change in MAP, HR, and CO during balloon inflation or deflation. Exercise resulted in an increase in MAP in both trials (P < 0.05). MAP remained elevated above baseline values throughout each trial (P < 0.05). HR increased with exercise only during the 20% trial (P < 0.05). Likewise, HR remained elevated above baseline values throughout the 20% trial (P < 0.05). Estimated CO did not change with exercise (control) in either trial. MAP, HR, and CO did not change with balloon inflation compared with exercise (control) values. Furthermore, balloon deflation did not alter any of these variables compared with exercise (control) values. Arterial catecholamine levels did not change throughout any of the trials (Table 3).
Table 2.
Systemic hemodynamic responses for experimental protocol
| Baseline | Exercise (Control) | Inflation (Nadir) | Inflation (Steady State) | Deflation (Acute) | Deflation (Steady State) | |
|---|---|---|---|---|---|---|
| Rest | ||||||
| Mean arterial pressure, mmHg | 86±2 | 85±3 | 87±3 | 87±3 | 87±2 | |
| Brachial artery pressure, mmHg | 86±2 | 80±2* | 84±1† | 86±2† | 88±2† | |
| Heart rate, beats/min | 65±1 | 66±1 | 66±1 | 66±1 | 65±1 | |
| Cardiac output, l/min | 5.2±0.3 | 5.4±0.3 | 5.2±0.3 | 5.3±0.3 | 5.3±0.4 | |
| 10% MVC | ||||||
| Mean arterial pressure, mmHg | 90±3 | 93±3* | 94±3* | 95±3* | 94±3* | 94±3* |
| Brachial artery pressure, mmHg | 88±2 | 90±2 | 79±2*‡ | 84±2†‡ | 90±2† | 90±2† |
| Heart rate, beats/min | 65±1 | 66±1 | 66±1 | 67±1* | 67±1 | 67±1 |
| Cardiac output. l/min | 5.3±0.3 | 5.3±0.3 | 5.5±0.3 | 5.6±0.4 | 5.5±0.3 | 5.6±0.4 |
| 20% MVC | ||||||
| Mean arterial pressure, mmHg | 93±3 | 98±3* | 99±3* | 100±3* | 101±3* | 99±4* |
| Brachial artery pressure, mmHg | 90±2 | 92±2 | 78±2*‡ | 86±3†‡ | 93±2† | 92±2† |
| Heart rate, beats/min | 66±1 | 68±1* | 68±1* | 68±1* | 68±1* | 68±2* |
| Cardiac output, l/min | 5.0±0.3 | 5.3±0.4 | 5.4±0.4* | 5.4±0.3* | 5.5±0.3* | 5.6±0.3* |
Values are means ± SE; n = 10.
P < 0.05 vs. baseline.
P < 0.05 vs. nadir.
P < 0.05 vs. exercise (control).
Table 3.
Arterial catecholamine levels during exercise trial for experimental protocol
| Baseline | Exercise (Control) | Inflation (Steady State) | Deflation (Steady State) | |
|---|---|---|---|---|
| 10% MVC | ||||
| Norepinephrine, pg/ml | 148±15 | 153±14 | 157±16 | 160±17 |
| Epinephrine, pg/ml | 58±9 | 62±10 | 59±9 | 66±10 |
| 20% MVC | ||||
| Norepinephrine, pg/ml | 164±15 | 174±16 | 178±17 | 177±16 |
| Epinephrine, pg/ml | 67±10 | 71±9 | 71±12 | 70±11 |
Values are means ± SE; n = 10.
Validation Protocol
Balloon and brachial artery diameters.
There were no changes in the brachial artery diameter (proximal to and at the site of the balloon) throughout the inflation period under resting and exercise conditions. Likewise, the diameter of the balloon did not change from target inflation to the end of the inflation period (Table 4). Similar to the experimental protocol, balloon inflation during resting conditions resulted in an acute 35 ± 3% and 28 ± 4% reduction in FBF and FVC, respectively (P < 0.01). By the end of the 3-min inflation period there was an 83 ± 10% recovery of FBF and 86 ± 14% recovery of FVC. Balloon inflation (nadir) during the 10% exercise trial resulted in an acute 38 ± 3% and 23 ± 3% reduction in FBF and FVC, respectively (P < 0.001). The percent recovery of FBF and FVC during the 10% trial was 82 ± 5% and 71 ± 11%, respectively. Balloon inflation (nadir) during the 20% exercise trial resulted in an acute 35 ± 2% and 23 ± 2% reduction in FBF and FVC, respectively (P < 0.001). Similarly, the percent recovery of FBF and FVC during the 20% trial was 84 ± 6% and 90 ± 6%, respectively.
Table 4.
Brachial artery and balloon diameter measurements for validation protocol
| Baseline | Exercise | Balloon Inflation |
Deflation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Target | 60 s | 90 s | 120 s | 150 s | 180 s | ||||
| Rest | |||||||||
| Proximal vessel, cm | 0.43±0.01 | 0.43±0.01 | 0.43±0.01 | 0.44±0.01 | 0.43±0.01 | 0.44±0.01 | 0.43±0.01 | 0.44±0.01 | |
| Vessel at site, cm | 0.43±0.01 | 0.43±0.01 | 0.43±0.01 | 0.43±0.01 | 0.44±0.01 | 0.44±0.01 | 0.43±0.01 | 0.44±0.01 | |
| Balloon, cm | 0.39±0.01 | 0.39±0.01 | 0.38±0.01 | 0.38±0.01 | 0.38±0.01 | 0.39±0.01 | |||
| 10% MVC | |||||||||
| Proximal vessel, cm | 0.44±0.01 | 0.44±0.01 | 0.44±0.01 | 0.44±0.01 | 0.44±0.01 | 0.44±0.01 | 0.44±0.01 | 0.44±0.01 | 0.44±0.01 |
| Vessel at site, cm | 0.44±0.01 | 0.44±0.01 | 0.44±0.01 | 0.44±0.01 | 0.44±0.01 | 0.44±0.01 | 0.44±0.01 | 0.44±0.01 | 0.44±0.01 |
| Balloon, cm | 0.40±0.01 | 0.39±0.01 | 0.39±0.01 | 0.39±0.01 | 0.40±0.01 | 0.39±0.01 | |||
| 20% MVC | |||||||||
| Proximal vessel, cm | 0.43±0.01 | 0.44±0.01 | 0.44±0.01 | 0.44±0.01 | 0.44±0.01 | 0.44±0.01 | 0.44±0.01 | 0.44±0.01 | 0.45±0.01 |
| Vessel at site, cm | 0.44±0.01 | 0.44±0.01 | 0.44±0.01 | 0.44±0.01 | 0.44±0.01 | 0.44±0.01 | 0.44±0.01 | 0.44±0.01 | 0.45±0.01 |
| Balloon, cm | 0.40±0.01 | 0.40±0.01 | 0.40±0.01 | 0.40±0.01 | 0.40±0.01 | 0.40±0.01 | |||
Values are means ± SE; n = 6. Proximal vessel refers to brachial artery diameter 2–3 cm proximal to balloon; Vessel at site refers to brachial artery diameter at site of the balloon. Balloon is balloon diameter.
Downstream blood flow response to balloon inflation (plethysmography).
Group mean data for FBF and FVC responses are presented in Fig. 7, A and B. Balloon inflation during resting conditions resulted in an acute 34% reduction in FBF (P < 0.01) and a 29% reduction in FVC (P < 0.01). By the end of the 3-min inflation period, FBF and FVC were greater than the nadir (P < 0.02) and partially restored to preinflation values. This resulted in an 81 ± 9% and 83 ± 4% recovery of FBF and FVC, respectively. Balloon deflation resulted in an acute increase in FBF and FVC, which were greater than their respective values at rest and at the end of the 3-min inflation period (P < 0.05). Of note, these results were qualitatively similar to those obtained with Doppler ultrasound.
Fig. 7.
Effect of balloon-induced hypoperfusion on downstream FBF (A) and FVC (B) at rest measured by plethysmography. Balloon inflation resulted in an acute reduction in FBF and FVC (nadir), which were partially restored. *P < 0.01 vs. baseline. †P < 0.05 vs. nadir. ‡P < 0.05 vs. baseline.
DISCUSSION
The primary novel findings of this study are 1) that local vasodilation plays a major role in blood flow restoration to contracting human skeletal muscles subjected to acute hypoperfusion; and 2) in contrast to most but not all findings in animals, there is an initial rise in vascular resistance in response to acute hypoperfusion, which decreases over time. Finally, we were surprised that our model of acute hypoperfusion in contracting forearm muscles did not evoke a marked pressor response. However, there was evidence that collateral flow around the elbow in some subjects contributed to a restoration of distal perfusion pressure during balloon inflation.
Vascular Responses to Acute Hypoperfusion During Exercise
An interesting and unexpected finding of the present study was the initial fall in FVC (increase in Rv) in the forearm at the onset of balloon inflation. Previous studies in animal muscle have demonstrated that acute reductions in perfusion pressure cause vasodilation over the course of a few minutes or less via autoregulatory mechanisms (2, 8, 10, 25, 33). The reason for the acute drop in FVC at the onset of balloon inflation in the present study is not clear but may be related to dampening of pulsatile flow by balloon inflation, which might limit the release of endothelium-derived vasodilators (29). Another possibility is that a sudden drop in perfusion pressure caused the resistance vessels to recoil before autoregulatory vasodilation, a response that was also noted by Koch et al. (15) during mild exercise in dogs.
Despite the initial fall in FVC with balloon inflation, there was a substantial vasodilation and a restoration of blood flow by the end of the inflation period. The vasodilation during balloon inflation was faster with exercise and reached steady-state levels in ∼40–50 s vs. ∼80 s at rest. These observations are consistent with findings in isolated contracting dog muscle where steady-state flow was achieved in 5–15 s after acute reductions in perfusion pressure vs. 1–2 min in resting muscle (33). In the present study the faster compensatory dilatation during exercise was relatively independent of exercise intensity.
Potential Mechanisms for Blood Flow Restoration
Since flow was restored by 54–78% during exercise after balloon inflation without a significant pressor response, local vasodilator mechanisms likely played a major role in this response. Evidence from animal experiments suggest that during periods of hypoperfusion or other maneuvers that reduce O2 delivery, adenosine released from the active muscles is a major compensatory vasodilator signal (15, 18). Additionally, if oxygen extraction was increased during the periods of reduced flow, oxygen-sensitive ATP release from erythrocytes may have been enhanced and contributed to a local vasodilatory response (4, 14, 27, 36). Along the lines noted above, compensatory vasodilation in resting and exercising muscle has also been observed with reduced O2 delivery via systemic hypoxia (7, 16, 37, 38). Hypoxic vasodilation appears to be mediated by a combination of nitric oxide, adenosine, and β2-adrenergic receptor activation (19, 34, 37).
It is also possible that the myogenic response, albeit delayed, contributed to the compensatory dilation. When intravascular pressure falls vessels can dilate to restore flow, and this response plays a major role in the autoregulation of flow in a variety of organs, including skeletal muscle (5, 25). Therefore, it is likely that the regulation of flow in response to changes in arterial perfusion pressure during exercise is shared between vasodilator metabolites, produced during periods of decreased oxygen supply to the muscle (balloon inflation), and a myogenic mechanism. However, at rest when the metabolic rate is low, a myogenic mechanism would likely predominate.
Absence of Pressor Response
We were surprised that our hypoperfusion model, balloon inflation, did not evoke a systemic pressor response during exercise. The classic view based on the “Seattle model” in chronically instrumented conscious dogs (39) is that a pressor response restores blood flow to underperfused exercising muscle. Using this model, reductions in blood flow to the exercising hindlimbs, via inflation of a terminal aortic occluder, elicits a reflex increase in pressure and partial restoration (up to 50%) of blood flow (9, 17, 21–23, 31, 39). The absence of a significant pressor response (above exercise alone) during hypoperfusion in our model suggests that a reflex increase in pressure is not necessary to restore blood flow to hypoperfused contracting human muscle. The differences observed in the present study and the “Seattle model” may be attributed to a species difference and the fact that the two models are not identical. We used a balloon inside the artery to induce hypoperfusion, whereas the “Seattle model” used an occluder around the terminal aorta to reduce flow to a much larger mass of active muscle. Additionally, the differences may be explained by a larger mass of active muscle, greater vascular occlusion, and higher exercise intensity in the “Seattle model”, thus resulting in a greater flow metabolism mismatch compared with the model used in the present study.
Validity of Model and Experimental Considerations
Data from the imaging studies demonstrate that the balloon diameter and brachial artery diameter around the balloon were stable throughout the inflation period (Table 4). Furthermore, the use of venous occlusion plethysmography to measure flow below the balloon yielded flow patterns similar to those measured above the balloon with Doppler. In the context of the stable mechanical system noted above, the restoration of distal pressure without a systemic pressor response was surprising. One explanation for this response is that collateral vessels around the elbow dilated and contributed to the restoration of pressure. Robinson et al. (26) demonstrated that occlusion of the brachial artery resulted in an acute fall in radial arterial pressure and forearm blood flow that recovered after ∼30 s and was attributed to a decrease in collateral resistance around the elbow. Plethysmographic studies have also shown good distal blood flow despite thrombosis of the brachial artery, supporting the existence of collateral channels to the forearm (1). Furthermore, substantial vasodilation and blood flow are observed in collateral arteries in response to occlusion of a feed artery of the rat mesentery (32).
To explore this possibility further, we calculated balloon resistance and noted variable responses between individuals; balloon resistance was maintained in some subjects but fell in others, while vascular resistance distal to the balloon fell in all subjects as flow was restored. This suggests that more extensive collateral circulation and/or more collateral vasodilation around the elbow explains the restoration of distal pressure in some of the subjects. Additionally, the magnitude of change in vascular resistance was always greater than changes in balloon resistance (Table 1). Of note, careful examination of Fig. 1 in Ref. 23 and Fig. 1 in Ref. 39 shows a fall in occluder resistance in dogs during exercise using the Seattle model.
There are potential limitations to our model and findings that should be noted. First, we were concerned that the restoration of distal perfusion pressure after balloon inflation indicated that either the balloon was losing volume over time or that the brachial artery near the balloon was dilating. However, our validation experiments demonstrate that we had a stable mechanical system, and the rise in distal pressure can be explained by dilation in collateral vessels. Second, the observation that FVC did not reach preinflation values during balloon inflation is in contrast to studies in isolated perfused animal muscles that demonstrate greater changes in conductance in response to reduced perfusion pressure via autoregulatory mechanisms (8, 25, 33). This may be explained by a loss in pulsatile flow following balloon inflation, which likely effected the contribution of flow-sensitive vasodilator mechanisms, and/or by the small muscle mass exercise used. Additionally, the contracting forearm muscles were likely “overperfused” during exercise before inflation and thus did not require total restoration of flow to meet their metabolic needs. Last, our exercise and hypoperfusion model did not evoke a pressor response so we could not test the role of this response in the restoration of flow.
Perspectives
The observation that flow did not return completely (100% recovery) to preinflation levels suggests that autoregulation may be less than perfect in humans under conditions of hypoperfusion. This is in contrast to other conditions in which oxygen delivery to contracting muscles is decreased (e.g., hypoxia and anemia). Under these conditions there is a compensatory vasodilation that mirrors the decrease in oxygen content (6, 37, 38). The partial compensation to acute hypoperfusion in young healthy subjects with apparently normal microcirculation raises the concern that the restoration of flow may be markedly less in conditions with impaired microvascular function (i.e., diabetes, hypertension, vascular disease). In this context, a reduced compensatory response in conditions associated with microvascular dysfunction might have a negative impact on exercise tolerance in the face of concurrent large-vessel disease, which would reduce perfusion pressure in the active muscles.
Conclusions
Our findings indicate that in contrast to most but not all previous work in animals, the vascular bed of human skeletal muscle demonstrates an initial vasoconstriction in response to acute hypoperfusion. Despite the initial increase in vascular resistance, there is a substantial restoration of flow to hypoperfused exercising human muscle. The restoration of flow occurs in the absence of a pressor response, thus suggesting local vasodilator mechanisms in conjunction with increased collateral flow around the elbow are likely responsible. Finally, our observations raise important new questions about the mechanisms responsible for the initial constriction and the factors responsible for the later restoration of flow during hypoperfusion in exercising human muscles.
GRANTS
This study was supported by National Institutes of Health Research Grants HL-46493 (M. J. Joyner), AR-55819 (D. P. Casey), and by CTSA RR-024150.
Acknowledgments
We thank Branton Walker, Christopher Johnson, Pam Engrav, Karen Krucker, Jean Knutson, and Shelly Roberts for technical assistance. We also thank the volunteers for their time.
REFERENCES
- 1.Bell JW Treatment of post-catheterization arterial injuries: use of survey plethysmography. Ann Surg 155: 591–598, 1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Britton SL, Metting PJ, Ronau TF, Strader JR, Weldy DL. Autoregulation of hind-limb blood flow in conscious dogs. J Physiol 368: 409–422, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daley JC, Khan MH, Hogeman CS, Sinoway LI. Autonomic and vascular responses to reduced limb perfusion. J Appl Physiol 95: 1493–1498, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am J Physiol Heart Circ Physiol 269: H2155–H2161, 1995. [DOI] [PubMed] [Google Scholar]
- 5.Faber JE, Meininger GA. Selective interaction of alpha-adrenoceptors with myogenic regulation of microvascular smooth muscle. Am J Physiol Heart Circ Physiol 259: H1126–H1133, 1990. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez-Alonso J, Mortensen SP, Dawson EA, Secher NH, Damsgaard R. Erythrocytes and the regulation of human skeletal muscle blood flow and oxygen delivery: role of erythrocyte count and oxygenation state of haemoglobin. J Physiol 572: 295–305, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez-Alonso J, Richardson RS, Saltin B. Exercising skeletal muscle blood flow in humans responds to reduction in arterial oxyhaemoglobin, but not to altered free oxygen. J Physiol 530: 331–341, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Granger HJ, Goodman AH, Granger DN. Role of resistance and exchange vessels in local microvascular control of skeletal muscle oxygenation in the dog. Circ Res 38: 379–385, 1976. [DOI] [PubMed] [Google Scholar]
- 9.Hammond RL, Augustyniak RA, Rossi NF, Churchill PC, Lapanowski K, O'Leary DS. Heart failure alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 278: H818–H828, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Jones RD, Berne RM. Intrinsic regulation of skeletal muscle blood flow. Circ Res 14: 126–138, 1964. [DOI] [PubMed] [Google Scholar]
- 11.Jones RD, Berne RM. Local regulation of blood flow in skeletal muscle. Circ Res 15, Suppl: 30–38, 1964. [PubMed] [Google Scholar]
- 12.Joyner MJ Does the pressor response to ischemic exercise improve blood flow to contracting muscles in humans? J Appl Physiol 71: 1496–1501, 1991. [DOI] [PubMed] [Google Scholar]
- 13.Joyner MJ, Dietz NM, Shepherd JT. From Belfast to Mayo and beyond: the use and future of plethysmography to study blood flow in human limbs. J Appl Physiol 91: 2431–2441, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Kirby BS, Voyles WF, Carlson RE, Dinenno FA. Graded sympatholytic effect of exogenous ATP on postjunctional alpha-adrenergic vasoconstriction in the human forearm: implications for vascular control in contracting muscle. J Physiol 586: 4305–4316, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koch LG, Strick DM, Britton SL, Metting PJ. Reflex versus autoregulatory control of hindlimb blood flow during treadmill exercise in dogs. Am J Physiol Heart Circ Physiol 260: H436–H444, 1991. [DOI] [PubMed] [Google Scholar]
- 16.Koskolou MD, Calbet JA, Radegran G, Roach RC. Hypoxia and the cardiovascular response to dynamic knee-extensor exercise. Am J Physiol Heart Circ Physiol 272: H2655–H2663, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Laprad SL, Augustyniak RA, Hammond RL, O'Leary DS. Does gender influence the strength and mechanisms of the muscle metaboreflex during dynamic exercise in dogs? Am J Physiol Regul Integr Comp Physiol 276: R1203–R1208, 1999. [DOI] [PubMed] [Google Scholar]
- 18.Laughlin MH, Klabunde RE, Delp MD, Armstrong RB. Effects of dipyridamole on muscle blood flow in exercising miniature swine. Am J Physiol Heart Circ Physiol 257: H1507–H1515, 1989. [DOI] [PubMed] [Google Scholar]
- 19.Leuenberger UA, Gray K, Herr MD. Adenosine contributes to hypoxia-induced forearm vasodilation in humans. J Appl Physiol 87: 2218–2224, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Metting PJ, Weldy DL, Ronau TF, Britton SL. Effect of aminophylline on hindlimb blood flow autoregulation during increased metabolism in dogs. J Appl Physiol 60: 1857–1864, 1986. [DOI] [PubMed] [Google Scholar]
- 21.Mittelstadt SW, Bell LB, O'Hagan KP, Clifford PS. Muscle chemoreflex alters vascular conductance in nonischemic exercising skeletal muscle. J Appl Physiol 77: 2761–2766, 1994. [DOI] [PubMed] [Google Scholar]
- 22.O'Leary DS, Augustyniak RA. Muscle metaboreflex increases ventricular performance in conscious dogs. Am J Physiol Heart Circ Physiol 275: H220–H224, 1998. [DOI] [PubMed] [Google Scholar]
- 23.O'Leary DS, Sheriff DD. Is the muscle metaboreflex important in control of blood flow to ischemic active skeletal muscle in dogs? Am J Physiol Heart Circ Physiol 268: H980–H986, 1995. [DOI] [PubMed] [Google Scholar]
- 24.Ogoh S, Fadel PJ, Nissen P, Jans O, Selmer C, Secher NH, Raven PB. Baroreflex-mediated changes in cardiac output and vascular conductance in response to alterations in carotid sinus pressure during exercise in humans. J Physiol 550: 317–324, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ping P, Johnson PC. Role of myogenic response in enhancing autoregulation of flow during sympathetic nerve stimulation. Am J Physiol Heart Circ Physiol 263: H1177–H1184, 1992. [DOI] [PubMed] [Google Scholar]
- 26.Robinson BF, Dobbs RJ, Kelsey CR, Saverymuttu S. A method for assessing responses of small arteries in man: effect of physiological and pharmacological stimuli. Clin Sci (Lond) 60: 659–666, 1981. [DOI] [PubMed] [Google Scholar]
- 27.Rosenmeier JB, Hansen J, Gonzalez-Alonso J. Circulating ATP-induced vasodilatation overrides sympathetic vasoconstrictor activity in human skeletal muscle. J Physiol 558: 351–365, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rowell LB, Savage MV, Chambers J, Blackmon JR. Cardiovascular responses to graded reductions in leg perfusion in exercising humans. Am J Physiol Heart Circ Physiol 261: H1545–H1553, 1991. [DOI] [PubMed] [Google Scholar]
- 29.Rubanyi GM, Romero JC, Vanhoutte PM. Flow-induced release of endothelium-derived relaxing factor. Am J Physiol Heart Circ Physiol 250: H1145–H1149, 1986. [DOI] [PubMed] [Google Scholar]
- 30.Shepherd JT Circulation to skeletal muscle. In: Handbook of Physiology. The Cardiovascular System. Peripheral Circulation and Organ Blood Flow. Bethesda, MD: Am. Physiol Soc, 1983, sect. 2, vol. III, pt. 1, chapt. 11, p. 319–370. [Google Scholar]
- 31.Sheriff DD, Wyss CR, Rowell LB, Scher AM. Does inadequate oxygen delivery trigger pressor response to muscle hypoperfusion during exercise? Am J Physiol Heart Circ Physiol 253: H1199–H1207, 1987. [DOI] [PubMed] [Google Scholar]
- 32.Smiesko V, Lang DJ, Johnson PC. Dilator response of rat mesenteric arcading arterioles to increased blood flow velocity. Am J Physiol Heart Circ Physiol 257: H1958–H1965, 1989. [DOI] [PubMed] [Google Scholar]
- 33.Stainsby WN Autoregulation of blood flow in skeletal muscle during increased metabolic activity. Am J Physiol 202: 273–276, 1962. [DOI] [PubMed] [Google Scholar]
- 34.Weisbrod CJ, Minson CT, Joyner MJ, Halliwill JR. Effects of regional phentolamine on hypoxic vasodilatation in healthy humans. J Physiol 537: 613–621, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wesseling KH, Jansen JR, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol 74: 2566–2573, 1993. [DOI] [PubMed] [Google Scholar]
- 36.Wihlborg AK, Malmsjo M, Eyjolfsson A, Gustafsson R, Jacobson K, Erlinge D. Extracellular nucleotides induce vasodilatation in human arteries via prostaglandins, nitric oxide and endothelium-derived hyperpolarising factor. Br J Pharmacol 138: 1451–1458, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilkins BW, Pike TL, Martin EA, Curry TB, Ceridon ML, Joyner MJ. Exercise intensity-dependent contribution of beta-adrenergic receptor-mediated vasodilatation in hypoxic humans. J Physiol 586: 1195–1205, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilkins BW, Schrage WG, Liu Z, Hancock KC, Joyner MJ. Systemic hypoxia and vasoconstrictor responsiveness in exercising human muscle. J Appl Physiol 101: 1343–1350, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wyss CR, Ardell JL, Scher AM, Rowell LB. Cardiovascular responses to graded reductions in hindlimb perfusion in exercising dogs. Am J Physiol Heart Circ Physiol 245: H481–H486, 1983. [DOI] [PubMed] [Google Scholar]