Abstract
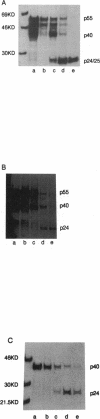
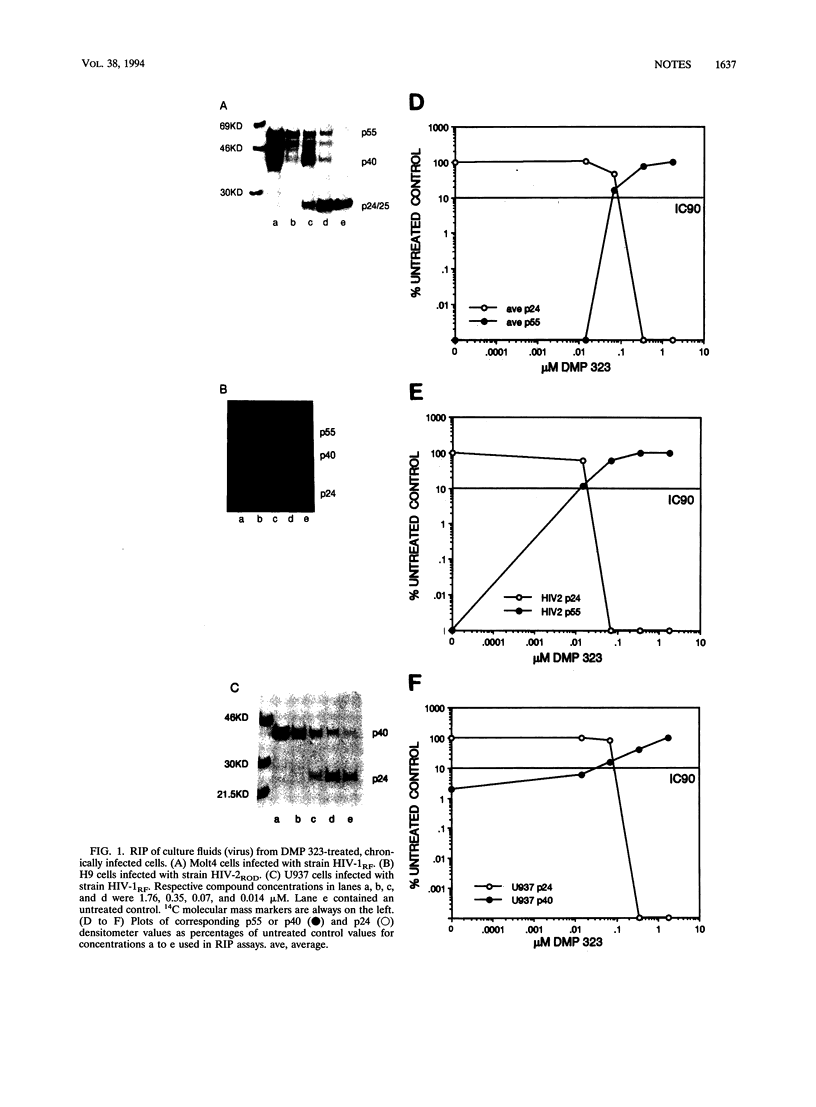
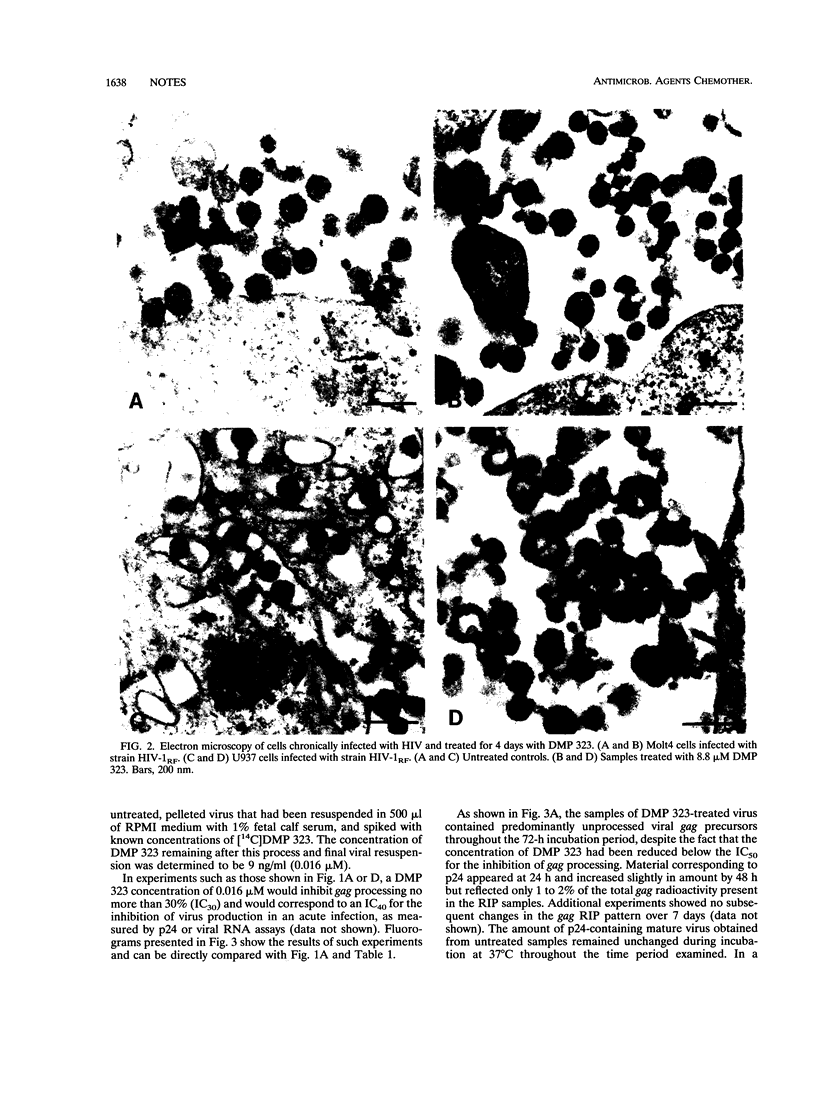
DMP 323, a C-2-symmetrical cyclic urea, is representative of a new class of inhibitors of human immunodeficiency virus protease. In this study, we correlate the potent antiviral activity of DMP 323 in acute infections with antiprotease activity assessed by monitoring the inhibition of the processing of viral gag precursor polyprotein from chronically infected lymphoid and monocytoid cell lines. Electron microscopic examination confirmed that the inhibition of gag processing was associated with the production of immature viral particles. Reduction of DMP 323 in the environment of unprocessed gag viral particles did not result in the resumption of gag processing for at least 72 h.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asjö B., Ivhed I., Gidlund M., Fuerstenberg S., Fenyö E. M., Nilsson K., Wigzell H. Susceptibility to infection by the human immunodeficiency virus (HIV) correlates with T4 expression in a parental monocytoid cell line and its subclones. Virology. 1987 Apr;157(2):359–365. doi: 10.1016/0042-6822(87)90278-9. [DOI] [PubMed] [Google Scholar]
- Bagasra O., Pomerantz R. J. Human immunodeficiency virus type I provirus is demonstrated in peripheral blood monocytes in vivo: a study utilizing an in situ polymerase chain reaction. AIDS Res Hum Retroviruses. 1993 Jan;9(1):69–76. doi: 10.1089/aid.1993.9.69. [DOI] [PubMed] [Google Scholar]
- Bugelski P. J., Kaplan J. M., Hart T. K., Miller J., Laydon J. T., Lee J. C., Dreyer G. B., Kirsh R. Effect of a human immunodeficiency virus protease inhibitor on human monocyte function. AIDS Res Hum Retroviruses. 1992 Dec;8(12):1951–1958. doi: 10.1089/aid.1992.8.1951. [DOI] [PubMed] [Google Scholar]
- Embretson J., Zupancic M., Ribas J. L., Burke A., Racz P., Tenner-Racz K., Haase A. T. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993 Mar 25;362(6418):359–362. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- Erickson-Viitanen S., Klabe R. M., Cawood P. G., O'Neal P. L., Meek J. L. Potency and selectivity of inhibition of human immunodeficiency virus protease by a small nonpeptide cyclic urea, DMP 323. Antimicrob Agents Chemother. 1994 Jul;38(7):1628–1634. doi: 10.1128/aac.38.7.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom H. R., Ozel M., Pauli G. Morphogenesis and morphology of HIV. Structure-function relations. Arch Virol. 1989;106(1-2):1–13. doi: 10.1007/BF01311033. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Orenstein J. M., Baca L. M., Weiser B., Burger H., Kalter D. C., Meltzer M. S. The macrophage in the persistence and pathogenesis of HIV infection. AIDS. 1989 Aug;3(8):475–495. doi: 10.1097/00002030-198908000-00001. [DOI] [PubMed] [Google Scholar]
- Holloway M. Lethal cascade. A model for the neurologic damage found in AIDS. Sci Am. 1993 Mar;268(3):28–30. [PubMed] [Google Scholar]
- Ikuta K., Luftig R. B. Inhibition of cleavage of Moloney murine leukemia virus gag and env coded precursor polyproteins by cerulenin. Virology. 1986 Oct 15;154(1):195–206. doi: 10.1016/0042-6822(86)90441-1. [DOI] [PubMed] [Google Scholar]
- Jacobsen H., Ahlborn-Laake L., Gugel R., Mous J. Progression of early steps of human immunodeficiency virus type 1 replication in the presence of an inhibitor of viral protease. J Virol. 1992 Aug;66(8):5087–5091. doi: 10.1128/jvi.66.8.5087-5091.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A. H., Swanstrom R. Human immunodeficiency virus type 1 Gag proteins are processed in two cellular compartments. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4528–4532. doi: 10.1073/pnas.88.10.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A. H., Zack J. A., Knigge M., Paul D. A., Kempf D. J., Norbeck D. W., Swanstrom R. Partial inhibition of the human immunodeficiency virus type 1 protease results in aberrant virus assembly and the formation of noninfectious particles. J Virol. 1993 Jul;67(7):4050–4055. doi: 10.1128/jvi.67.7.4050-4055.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh I., Yoshinaka Y., Luftig R. B. The effect of cerulenin on Moloney murine leukemia virus morphogenesis. Virus Res. 1986 Aug;5(2-3):265–276. doi: 10.1016/0168-1702(86)90023-7. [DOI] [PubMed] [Google Scholar]
- Kenealy W., Reed D., Cybulski R., Tribe D., Taylor P., Stevens C., Matthews T., Petteway S. Analysis of human serum antibodies to human immunodeficiency virus (HIV) using recombinant ENV and GAG antigens. AIDS Res Hum Retroviruses. 1987 Spring;3(1):95–105. doi: 10.1089/aid.1987.3.95. [DOI] [PubMed] [Google Scholar]
- Koenig S., Gendelman H. E., Orenstein J. M., Dal Canto M. C., Pezeshkpour G. H., Yungbluth M., Janotta F., Aksamit A., Martin M. A., Fauci A. S. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986 Sep 5;233(4768):1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- Kort J. J., Bilello J. A., Bauer G., Drusano G. L. Preclinical evaluation of antiviral activity and toxicity of Abbott A77003, an inhibitor of the human immunodeficiency virus type 1 protease. Antimicrob Agents Chemother. 1993 Jan;37(1):115–119. doi: 10.1128/aac.37.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lam P. Y., Jadhav P. K., Eyermann C. J., Hodge C. N., Ru Y., Bacheler L. T., Meek J. L., Otto M. J., Rayner M. M., Wong Y. N. Rational design of potent, bioavailable, nonpeptide cyclic ureas as HIV protease inhibitors. Science. 1994 Jan 21;263(5145):380–384. doi: 10.1126/science.8278812. [DOI] [PubMed] [Google Scholar]
- McDougal J. S., Cort S. P., Kennedy M. S., Cabridilla C. D., Feorino P. M., Francis D. P., Hicks D., Kalyanaraman V. S., Martin L. S. Immunoassay for the detection and quantitation of infectious human retrovirus, lymphadenopathy-associated virus (LAV). J Immunol Methods. 1985 Jan 21;76(1):171–183. doi: 10.1016/0022-1759(85)90489-2. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Otto M. J., Reid C. D., Garber S., Lam P. Y., Scarnati H., Bacheler L. T., Rayner M. M., Winslow D. L. In vitro anti-human immunodeficiency virus (HIV) activity of XM323, a novel HIV protease inhibitor. Antimicrob Agents Chemother. 1993 Dec;37(12):2606–2611. doi: 10.1128/aac.37.12.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo G., Graziosi C., Demarest J. F., Butini L., Montroni M., Fox C. H., Orenstein J. M., Kotler D. P., Fauci A. S. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993 Mar 25;362(6418):355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- Pauwels R., Balzarini J., Baba M., Snoeck R., Schols D., Herdewijn P., Desmyter J., De Clercq E. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J Virol Methods. 1988 Aug;20(4):309–321. doi: 10.1016/0166-0934(88)90134-6. [DOI] [PubMed] [Google Scholar]
- Piatak M., Jr, Saag M. S., Yang L. C., Clark S. J., Kappes J. C., Luk K. C., Hahn B. H., Shaw G. M., Lifson J. D. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science. 1993 Mar 19;259(5102):1749–1754. doi: 10.1126/science.8096089. [DOI] [PubMed] [Google Scholar]
- Potts B. J., Maury W., Martin M. A. Replication of HIV-1 in primary monocyte cultures. Virology. 1990 Apr;175(2):465–476. doi: 10.1016/0042-6822(90)90431-p. [DOI] [PubMed] [Google Scholar]
- Price R. W., Brew B., Sidtis J., Rosenblum M., Scheck A. C., Cleary P. The brain in AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science. 1988 Feb 5;239(4840):586–592. doi: 10.1126/science.3277272. [DOI] [PubMed] [Google Scholar]
- Roberts N. A., Martin J. A., Kinchington D., Broadhurst A. V., Craig J. C., Duncan I. B., Galpin S. A., Handa B. K., Kay J., Kröhn A. Rational design of peptide-based HIV proteinase inhibitors. Science. 1990 Apr 20;248(4953):358–361. doi: 10.1126/science.2183354. [DOI] [PubMed] [Google Scholar]
- Schmidtmayerova H., Bolmont C., Baghdiguian S., Hirsch I., Chermann J. C. Distinctive pattern of infection and replication of HIV1 strains in blood-derived macrophages. Virology. 1992 Sep;190(1):124–133. doi: 10.1016/0042-6822(92)91198-4. [DOI] [PubMed] [Google Scholar]
- Schätzl H., Gelderblom H. R., Nitschko H., von der Helm K. Analysis of non-infectious HIV particles produced in presence of HIV proteinase inhibitor. Arch Virol. 1991;120(1-2):71–81. doi: 10.1007/BF01310950. [DOI] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin A., Albert J., Fenyö E. M., Asjö B. HIV-1 infection of normal human macrophage cultures: implication for silent infection. Virology. 1990 Aug;177(2):790–794. doi: 10.1016/0042-6822(90)90551-2. [DOI] [PubMed] [Google Scholar]