Abstract
Multipotent cardiovascular progenitor cells derived from ES cells or induced pluripotent stem (iPS) cells are an intriguing source for stem cell–based therapies for congenital and acquired heart diseases. From a clinical perspective, the ideal cardiac progenitor cells are those that can proliferate, survive, and differentiate into multiple mature cardiac cell types when transplanted into normal or diseased heart. In this issue of JCI, Blin et al. report the isolation and characterization of a group of early mesodermal cardiovascular progenitor cells, induced by BMP2 and marked by the cell surface protein, stage-specific embryonic antigen 1 (SSEA-1). BMP2-induced SSEA-1+ cells were purified from ES and iPS cells and could be directed to differentiate into cardiomyocytes, endothelial cells, and smooth muscle cells by treatment with defined cytokines and signaling molecules. Most importantly, purified SSEA+ progenitor cells from Rhesus monkey ES cells engrafted into nonhuman primate hearts, in which they differentiated into cardiac cells without forming teratomas. These findings move the field another step closer to clinical use of ES or iPS cell–derived cardiovascular progenitors in cardiac repair.
Heart failure is a progressive disease that affects over 5 million individuals in the United States. In addition, many of the over 1 million survivors of congenital heart disease are destined to develop heart failure as they age. Current pharmacologic therapies have limited efficacy and only slow the progression of cardiac dysfunction. Heart transplantation is often the last resort of treatment, but the limited donor pool makes this option unrealistic for the vast majority of patients. Given the disease burden and the unsatisfying therapeutic modalities for heart failure, the potential of cardiac regenerative medicine has generated tremendous interest worldwide.
Approaches to regenerate functional myocardium in damaged hearts have made substantial strides in recent years. Of major importance was the identification of multiple cardiovascular progenitor cells (CPCs) that have embryonic origin and can be isolated from heart tissue, or that can be differentiated from ES or induced pluripotent stem (iPS) cells (1–4). The previously characterized CPCs retain intrinsic competence to differentiate into various cardiac lineages. Among them, Flk1+ (KDR+ in human) precursors retain the capacity to differentiate into blood cells and three of the major cell types of the heart cells, namely cardiomyocytes, smooth muscle cells, and endothelial cells (5, 6). CPCs expressing the transcription factor Isl1 are also multipotent and give rise to cardiomyocytes, smooth muscle cells, and endothelial cells (7–9). Finally, CPCs marked by the transcription factor Nkx2.5 are more lineage restricted but can differentiate into cardiomyocytes and smooth muscle cells (10). Despite these advances, practical isolation of cardiac lineage-committed progenitor cells using a surface marker and introduction of such cells into a damaged heart has remained problematic.
Isolation of cardiovascular progenitors using a cell-surface marker
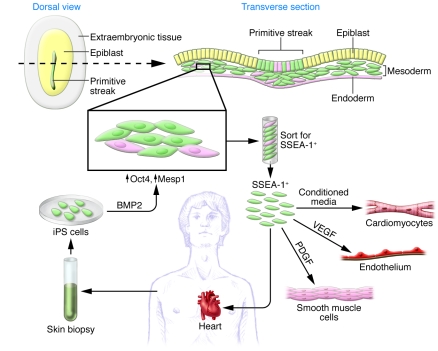
In this issue of the JCI, Blin and colleagues identified a very early cardiac progenitor population derived from primate (human and monkey) ES and iPS cells. They used knowledge from embryonic development and mimicked the conditions that may be present in the early primitive streak, just as epiblasts give rise to newly formed mesodermal cells that are destined to acquire a cardiac fate (Figure 1). Such cells express transient but high levels of the transcription factors Oct4 and Mesp1 (11). Blin et al. treated ES and iPS cells with BMP2, which activates Wnt3a, to simulate early epiblast conditions, and then used magnetic beads to isolate cells that expressed stage-specific embryonic antigen 1 (SSEA-1), an early surface marker of general differentiation. Remarkably, the isolated SSEA-1+ cells expressed early mesodermal and cardiac lineage markers and appeared by gene expression and epigenetic marks to be very early CPCs.
Figure 1. Schematic of primate BMP2-induced SSEA-1+ cells.
Epiblast cells in the embryo express the surface marker SSEA-1 as they differentiate and transiently express Oct4 and Mesp1 during the commitment to CPCs. Blin et al. show that using BMP2 to mimic the environment of epiblast cells, primate ES and iPS cells can be induced to differentiate into SSEA-1+ CPCs. Isolated BMP2-induced SSEA-1+ cells were multipotent CPCs that could be differentiated into endothelial cells, smooth muscle cells, and cardiomyocytes under different growth conditions. The SSEA-1+ cells were also multipotent in vivo upon transplantation into primate hearts.
Only a small portion of SSEA-1+ CPCs were Flk1+ (~5%–7%), reflecting a difference in developmental stage between the two CPC populations and suggesting the SSEA-1+ CPCs might appear earlier than Flk1+ and other previously isolated CPCs. Of particular interest, BMP2-induced SSEA-1+ CPCs exhibited an interesting pattern of microRNA expression. Specifically, the miR-17-92 cluster (e.g., miR-106, miR-513, and miR-302a) was upregulated, while miR-302b,c,d and miR-125b were downregulated. Future studies of SSEA-1+ CPC-specific microRNAs (e.g., miR-513) will help to elucidate the biological mechanisms underlying the ability to differentiate toward a cardiogenic fate.
To demonstrate the multipotency and clonality of the SSEA-1+ CPCs quantitatively and qualitatively, Blin et al. elegantly used high-content cell imaging and flow cytometry (11). The cells could be induced to differentiate into any one of the three major cardiac lineages, simply by changing the conditions in which they were maintained (Figure 1). For example, when treated with PDGF or VEGF, the CPCs developed into smooth muscle or endothelial cells, respectively. In the presence of conditioned medium of both fibroblasts and cardiomyocytes, they differentiated mostly into ventricular-like cardiomyocytes. The ability to manipulate the fate of SSEA-1+ CPCs will be important for generating large numbers of relatively pure cells for clinically relevant testing or basic biology studies.
Implications for regenerative medicine
Powered in part by the discovery of iPS cells (12–15), CPCs or cardiomyocytes derived from pluripotent cells have provided a potential avenue for cardiac regenerative approaches. In particular, the plasticity and cardiogenic differentiation potential of SSEA-1+ CPCs make them attractive sources for cells to replace damaged myocytes in an infarcted heart.
A true test of the value of these cells is to determine whether they functionally incorporate themselves into heart tissue. Before moving to animal graft experiments, Blin and colleagues examined the maturation of cardiomyocytes derived from BMP2-induced SSEA-1+ CPCs ex vivo (11). A large proportion of differentiated cells (60%–80%) displayed organized sarcomeres and expressed cardiac α-actinin, ventricular myosin light chain, and the adult β-myosin heavy chain isoform. Importantly, the gap junction protein connexin 43 (Cx43) was phosphorylated in these cells, indicating the potential for cell-cell coupling. However, whether these cardiomyocytes are in fact electrophysiologically functional will have to be established by standard patch-clamp single-cell recordings.
As a proof of concept, Blin and colleagues transplanted Rhesus ES cell–derived SSEA-1+ CPCs into a Rhesus monkey model of myocardial infarction (11). Previous studies used human ESC-derived cardiomyocytes or CPCs in a rodent myocardial infarction model (16, 17), but the human cardiomyocytes failed to couple to rodent host myocardium, possibly due to differences in their intrinsic beating frequency. The unsuccessful coupling might explain the absence of long-term (i.e., up to 12 weeks) functional benefits (16). The Rhesus SSEA-1+ CPCs engrafted in the infarcted monkey hearts, without forming teratomas, and differentiated into morphologically matured cardiomyocytes that were positive for myosin light chain 2 and myosin light chain kinase.
However, a major goal of cardiac repair is to restore long-term function in an effort to prevent or treat heart failure. Newly formed muscle must provide passive mechanical support and, more importantly, couple and contract in synchrony with the host myocardium. Using immunohistochemistry, Blin et al. reported activation of Cx43 expression within and surrounding the graft. Although this indicates electrical coupling between the graft and the surrounding myocardium, additional electrophysiological studies (e.g., intracellular calcium imaging) will be needed to unequivocally show successful coupling. Since incomplete coupling can cause ectopic electrical activity and increase the risk of arrhythmia, more functional analyses are needed to prove the procedure is safe and effective. Moreover, the functional benefit of cells engrafted into nonhuman primate hearts remains to be determined.
Despite these issues, the pioneering studies of Blin et al. are very encouraging. Their imaginative work using a nonhuman primate model of myocardial infarction to test the capacity of SSEA-1+ CPCs to engraft and differentiate into matured cardiomyocytes represents an important milestone. Future studies on the long-term survival, functional integration, physiological compatibility of engrafted cells, and beneficial effects on cardiac function will provide new insights into the potential use of SSEA-1+ CPCs for cardiovascular regenerative medicine. Most importantly, the ability to isolate nonhuman primate CPCs using a cell surface marker brings us one step closer to the ultimate dream of cell-based therapies for some of the most devastating forms of heart disease.
Footnotes
Conflict of interest: Deepak Srivastava serves on the Scientific Advisory Board of iPierian Inc. and RegeneRx Pharmaceuticals.
Citation for this article: J Clin Invest. 2010;120(4):1034–1036. doi:10.1172/JCI42643.
See the related article beginning on page 1125.
References
- 1.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132(4):661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Srivastava D, Ivey KN. Potential of stem-cell-based therapies for heart disease. Nature. 2006;441(7097):1097–1099. doi: 10.1038/nature04961. [DOI] [PubMed] [Google Scholar]
- 3.Passier R, van Laake LW, Mummery CL. Stem-cell-based therapy and lessons from the heart. Nature. 2008;453(7193):322–329. doi: 10.1038/nature07040. [DOI] [PubMed] [Google Scholar]
- 4.Yi BA, Wernet O, Chien KR. Pregenerative medicine: developmental paradigms in the biology of cardiovascular regeneration. J Clin Invest. 2010;120(1):20–28. doi: 10.1172/JCI40820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kattman SJ, Huber TL, Keller GM. Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell. 2006;11(5):723–732. doi: 10.1016/j.devcel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Yang L, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453(7194):524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 7.Bu L, et al. Human ISL1 heart progenitors generate diverse multipotent cardiovascular cell lineages. Nature. 2009;460(7251):113–117. doi: 10.1038/nature08191. [DOI] [PubMed] [Google Scholar]
- 8.Laugwitz KL, et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433(7026):647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moretti A, et al. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127(6):1151–1165. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 10.Wu SM, et al. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell. 2006;127(6):1137–1150. doi: 10.1016/j.cell.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 11.Blin G, et al. A purified population of multipotent cardiovascular progenitors derived from primate pluripotent stem cells engrafts in postmyocardial infarcted nonhuman primates. J Clin Invest. 2010;120(4):1125–1139. doi: 10.1172/JCI40120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park IH, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451(7175):141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 15.Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 16.van Laake LW, et al. Human embryonic stem cell-derived cardiomyocytes survive and mature in the mouse heart and transiently improve function after myocardial infarction. Stem Cell Res. 2007;1(1):9–24. doi: 10.1016/j.scr.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Laflamme MA, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25(9):1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]