Abstract
Objectives
To determine in healthy people aged ≥75 years 1) if restricting time in bed and education in health sleep practices are superior to an attention-only control condition (i.e., education in healthy dietary practices) for maintaining or enhancing sleep continuity and depth over 2.5 years; and 2) if maintenance or enhancement of sleep continuity and depth promotes the maintenance or enhancement of health-related quality of life.
Methods
Single-blind, randomized, clinical trial in a university-based sleep center, enrolling 64 adults (n = 30 women, 34 men; mean age = 79 years) without sleep/wake complaints (e.g., insomnia or daytime sleepiness), followed by randomized assignment to either: 1) restriction of time in bed by delaying bedtime 30 minutes nightly for 18 months, together with education in healthy sleep practices (SLEEP); or 2) attention-only control condition with education in health dietary practices (NUTRITION).
Results
SLEEP did not enhance sleep continuity or depth; however, compared with NUTRITION, SLEEP was associated with decreased time spent asleep (about 30 minutes nightly over 18 months). Contrary to hypothesis, participants in SLEEP reported a decrement in physical health-related quality of life and an increase in medical burden (cardiovascular illness), relative to NUTRITION. Neither markers of inflammation, body mass index, or exercise explained treatment-related changes in medical burden.
Conclusions
Although we cannot exclude a positive effect of education in healthy nutrition, for healthy elderly >75 years of age without sleep complaints, reducing sleep time may be detrimental, whereas allowing more time to sleep (about 7.5 hours nightly) is associated with better maintenance of physical health-related quality of life and stability of medical illness burden over 30 months.
Keywords: sleep, aging, quality of life, physical health, inflammation
INTRODUCTION
Profound decreases in sleep continuity and depth of sleep are observed in later life (1). Sleep fragmentation, decreased slow-wave sleep, and changes in sleep duration associated with advancing age, together with daytime sleepiness, can compromise functioning and well-being, amplify disability associated with prevalent medical conditions, further degrade health-related quality of life, and hasten mortality (1–7). Protecting sleep may be important to maintaining and promoting good health in later life (1). This randomized, clinical trial tested the hypothesis that attenuation or reversal of age-dependent loss of sleep continuity and depth would improve health-related quality of life in older persons. For this purpose, we chose an intervention involving a modification of sleep restriction therapy (8), which entails modest delays in bedtime without changing risetime. Sleep restriction may increase homeostatic sleep drive, resulting in increased sleep efficiency and slow-wave sleep, which are measures of sleep continuity and depth, respectively. Sleep restriction therapy has been used successfully in older adults with insomnia to improve sleep efficiency and depth (8). The question addressed by this study was whether strategies derived from sleep restriction therapy would promote better sleep and health-related quality of life in older adults without primary sleep disorders (e.g., symptomatic sleep apnea, insomnia).
We recruited persons aged ≥75 years without sleep-wake complaints, but at risk for developing disturbed sleep by virtue of advanced age (1,7). In preliminary studies, we had observed that: 1) very old participants who maintain a highly entrained sleep-wake schedule and limit time in bed to about 7.0 to 7.5 hours daily report fewer sleep quality complaints and exhibit higher sleep efficiency as measured by polysomnography (9); 2) poor sleep efficiency affects immunocompetence (10) and predicts future decline in subjective sleep quality, higher chronic medical burden, and shorter life expectancy (11); and 3) sleep restriction (by delaying bedtime 30 minutes nightly) in normal elderly sleepers improves sleep quality and well-being (12). Hence, we sought to test the potential benefits to health of restricting time in bed combined with education in healthy sleep practices (e.g., the importance of stable bed- and risetimes) for maintaining and even enhancing sleep continuity and depth in elderly participants, and to examine the persistence of any such effects for 12 months beyond the end of the intervention. In addition to sleep efficiency, the experimental design also allowed us to address sleep duration. Too little (generally <5 hours) or too much sleep (generally >9 hours) may be correlated to health status and longevity (13).
To account for the nonspecific effects of time, face-to-face contact, and social support, we chose as a control condition education in health dietary practices (“NUTRITION”). We thought it unlikely that NUTRITION would affect sleep in older adults without sleep complaints and already in good health for their age cohort, with very good levels of health-related quality of life.
Our hypotheses were as follows:
Restricting time in bed (by delaying bedtime by 30 minutes) plus education in healthy sleep practices (“SLEEP”) will be superior to a control condition (i.e., education in health dietary practices: “NUTRITION”) for maintaining or enhancing sleep continuity and depth over an 18-month intervention and during a 12-month postintervention follow-up; and
SLEEP will lead to better maintenance or enhancement of health-related quality of life than NUTRITION.
METHODS
Participants were 64 healthy elderly men and women aged ≥75 years. We reported the sociodemographic and clinical characteristics of participants (1), summarized in Table 1. (The schedule of repeated assessments is shown in Table 2.) We recruited participants without sleep dissatisfaction or complaints of insomnia, daytime sleepiness, or other sleep disturbances. Participants had no current or past psychiatric disorder or sleep disorder as determined by Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (14). To be eligible, participants were required to score ≤7 on the 17-item Hamilton Depression Rating Scale (15) and ≥24 on the Folstein Mini-mental Status Examination (16). Potential participants had physical and neurologic examinations, electrocardiograms, complete blood cell counts, thyroid function tests, and metabolic chemistry analysis; those under a physician’s care for a stable medical illness (e.g., heart disease, hypertension, and arthritis) and with health conditions that posed no major limitations to activities of daily living were invited to participate. A potential participant was excluded from the study if s/he had a mean sleep latency of lt]6 minutes on a screening Multiple Sleep Latency Test, because such a finding could indicate pathological sleepiness. Of 110 who were evaluated, two potential participants were excluded from the study because of mean sleep latencies of <6 minutes. An additional seven participants were excluded because of elevated apnea-hypopnea indices (≥30). All participants with apnea-hypopnea indices ≥10 were referred to a pulmonary sleep specialist for further assessment, even in the absence of symptoms. Three of 34 participants in SLEEP and four of 30 participants in NUTRITION were prescribed continuous positive airway pressure as a result of the pulmonary evaluation. After consultation with our scientific advisory board, we decided to retain these subjects and to use their data in final analyses because they were aymptomatic. However, we examined outcomes both with and without these seven participants’ data and found no differences.
TABLE 1.
Baseline Demographic and Clinical Measures
| All Participants n = 64 | Sleep Restriction + Education n = 34 | Healthy Dietary Education n = 30 | t or χ2 | df | p | |
|---|---|---|---|---|---|---|
| Demographic | ||||||
| Age | 78.9 (3.3) | 78.9 (3.5) | 78.9 (3.0) | −0.05 | 62 | .96 |
| Men (%) | 53 | 56 | 50 | 0.22 | 1 | .64 |
| Caucasian (%) | 89 | 94 | 83 | 1.90 | 1 | .17 |
| Education in years | 15.3 (2.2) | 15.0 (2.3) | 15.6 (2.1) | 1.13 | 62 | .26 |
| Marital status, n | 6.33 | 3 | .097 | |||
| Never married | 5 | 2 | 3 | |||
| Married/living with partner | 37 | 24 | 13 | |||
| Separated/divorced | 5 | 3 | 2 | |||
| Widowed | 17 | 5 | 12 | |||
| General health | ||||||
| BMI | 26.6 (3.9) | 26.2 (3.6) | 27.0 (4.3) | 0.82 | 62 | .42 |
| Cumulative Illness Rating Scale | 7.3 (2.9) | 7.1 (2.9) | 7.5 (2.9) | 0.52 | 62 | .61 |
| Quality of life | ||||||
| MOS SF-36 (PCS) | 48.5 (5.6) | 48.9 (5.5) | 48.0 (5.9) | −0.63 | 62 | .53 |
| MOS SF-36 (MCS) | 58.5 (5.0) | 58.1 (5.3) | 58.9 (4.7) | 0.59 | 62 | .55 |
| Physical Functioning Index (0–100) | 82.5 (14.3) | 84.4 (12.8) | 80.3 (15.7) | −1.15 | 62 | .26 |
| Physical activity | ||||||
| Exercise (0–3) | 1.09 (0.63) | 1.07 (0.66) | 1.12 (0.60) | 0.28 | 62 | .78 |
| Cognitive function | ||||||
| Mini-mental State Exam (0–30) | 29.1 (1.0) | 29.0(1.0) | 29.2 (0.8) | 0.70 | 61 | .48 |
| TONI-III (63–151) | 103.2 (12.0) | 102.0 (12.2) | 104.5 (11.8) | 0.81 | 61 | .42 |
| Number letter (0–21) | 9.4 (2.3) | 9.6 (2.6) | 9.3 (1.9) | −0.57 | 60 | .57 |
| Memory immediate recall (0–25) | 23.0 (5.6) | 23.8 (5.1) | 22.1 (6.2) | −1.14 | 60 | .26 |
| Memory delayed recall (0–25) | 18.8 (6.3) | 19.4 (5.7) | 18.1 (6.9) | −0.79 | 60 | .43 |
| Mental health | ||||||
| HAM-D | 1.7 (1.5) | 1.8 (1.6) | 1.5 (1.4) | −0.95 | 62 | .34 |
| HAM-A | 2.5 (1.9) | 2.5(1.8) | 2.4 (2.0) | −0.20 | 62 | .84 |
| Social support | ||||||
| Interpersonal self-evaluation list—tangible (0–12) | 10.1 (1.7) | 10.0 (1.7) | 10.3 (1.7) | 0.86 | 61 | .39 |
| Interpersonal self-evaluation list—belonging (0–12) | 9.8 (1.7) | 9.8 (1.7) | 9.9 (1.7) | 0.09 | 61 | .93 |
| Interpersonal self-evaluation list—appraisal (0–12) | 9.8 (1.8) | 9.7(1.9) | 9.8 (1.8) | 0.27 | 61 | .79 |
| Interpersonal self-evaluation list—self-esteem (0–12) | 9.0 (1.7) | 9.4 (1.6) | 8.5 (1.6) | −2.27 | 61 | .027 |
BMI = body mass index; MOS SF-36 (PCS) = Medical Outcomes Study Short Form-36 (Physical Component Summary); MOS SF-36 (MCS) = Medical Outcomes Study Short Form-36 (Mental Component Summary); HAM-D = Hamilton Depression Rating Scale; HAM-A = Hamilton Anxiety Scale.
TABLE 2.
Schedule of Repeated Assessments
| Baseline | 6 Months (T2) | 18 Months (T3) | 30 Months (T4) | |
|---|---|---|---|---|
| Demographic | X | |||
| General health | ||||
| BMI | X | X | X | X |
| CIRS-G | X | X | ||
| Quality of life | ||||
| MOS-SF 36 | X | X | X | X |
| Physical functioning index | X | |||
| Physical Activity Exercise | X | X | X | X |
| Cognitive function | ||||
| Mini Mental State | X | |||
| TONI | X | X | X | X |
| Logical Memory | X | X | X | X |
| Mental health | ||||
| HAM-D | X | X | X | X |
| HAM-A | X | X | X | X |
| Social support | ||||
| ISEL | X | |||
| Sleep | ||||
| PSQI | X | X | X | X |
| Sleep Diary | X | X | X | X |
| Epworth sleepiness | X | X | X | X |
| Polysomnography | X | X | X | X |
| Actigraphy | X | X | X | X |
| IL-6 and TNF-α | X | X | X | X |
BMI = body mass index; MOS-SF = Medical Outcomes Study-Short Form; HAM-D = Hamilton Depression Rating Scale; HAM-A = Hamilton Anxiety Scale; ISEL = Illinois Snapshot of Early Literacy; PSQI = Pittsburgh Sleep Quality Index; IL = interleukin; TNF = tumor necrosis factor.
We obtained informed consent from all participants. Participants were recruited via Institutional Review Board approved advertising (n = 52) or other referral sources (n = 12). We imposed no restrictions based on sex, race, or ethnic group. Data collection began in July 2003 and ended in June 2008.
Procedure
We randomly assigned participants, stratified by gender, to either SLEEP or NUTRITION. The stratified blocked randomization list was computer-generated by our statistician (S.M.) and kept by the data manager (S.B.), who recorded cell assignments at the time of randomization by the project coordinator (L.S.). The clinical interventionist (S.P.) met with participants in both conditions weekly for eight consecutive weeks, followed by once-monthly meetings for the balance of 16 months. Thus, each participant had a total of 24 intervention sessions, about 25 minutes each, over 18 months, and a posttreatment follow-up 1 year later at 30 months from study entry. If participants were unable to attend a scheduled study visit for reasons, such as illness, vacation, or other special circumstances, the study clinician contacted them by telephone to review and assess practices. Sessions were audiotaped and rated blindly for fidelity to randomly assigned intervention.
SLEEP
The purpose of SLEEP was to educate participants about good sleep practices and to accumulate a modest sleep debt, to enhance sleep consolidation (continuity) and depth by increasing homeostatic sleep drive (17). During the initial 8 weeks, participants met weekly for 20 minutes to 25 minutes with the clinician. The first contact focused on education about the principles of reducing time in bed and included instructions for delaying bedtime by 30 minutes nightly from the average bedtime reported in the 2-week diary, observing regular bed- and wake-up times, and keeping written logs of daily sleep-wake schedules. Subsequent weekly meetings reviewed sleep-wake logs, assessed complaints of daytime sleepiness (if any), reinforced bed restriction and good sleep practices, completed study measures, monitored and facilitated compliance, and answered questions. Subjects in SLEEP were discouraged from napping. During weeks 9 to 78, participants met monthly for 20 minutes to 25 minutes with the study clinician to encourage and assess compliance, reinforce time-in-bed restriction and good sleep hygiene practices, trouble-shoot any incident problems with adherence to SLEEP, and complete scheduled study assessments (Table 2). Seven of 34 SLEEP participants were willing or able to restrict time in bed by only 15 minutes nightly rather than 30 minutes.
NUTRITION
This intervention was designed to control for the effects of attention associated with one-on-one visits. Total number of visits and face-to-face time in this intervention were similar to SLEEP. Visits focused on diet and healthy eating practices. The clinician reviewed general nutrition guidelines including the Food Pyramid, and helped participants prepare their weekly menus and grocery lists, cut out food coupons, and review food intake from the time of the last visit. NUTRITION did not focus on weight loss, but rather on planning and executing a meal plan that was nutritious, good-tasting, and affordable. NUTRITION also did not include any components likely to affect sleep (e.g., recommendations to avoid evening ingestions of caffeine or to increase levels of physical activity). At the end of the study, after the final assessment, participants in NUTRITION were given a copy of sleep education materials.
We repeated clinical- and laboratory-based assessments during the study. The schedule of repeated assessments is shown in Table 2. We describe those assessments here. Rater-administered assessments and sleep scoring were conducted by research staff blinded to randomized interventions assignment.
Laboratory - Based Sleep Measures
Participants underwent two consecutive overnight sleep studies at each of the four scheduled time points (T1: preintervention baseline; T2: 6 months; T3: 18 months; and T4: 30 months), including one night of monitoring of sleep-disordered breathing and periodic limb movements. Habitual bedtimes and risetimes were estimated from daily diary reports completed over 14 consecutive days before laboratory-based sleep studies. NUTRITION participants’ mean bedtime (± standard deviation [SD]) was 11:19 PM (SD = 49.3 minutes) versus 10:54 PM (SD = 46.8 minutes) in SLEEP participants. Mean risetime recorded by NUTRITION participants was 7:06 AM (SD = 61.0 minutes) versus 6:58 AM (SD = 68.7 minutes) in SLEEP participants. Thus, diary-based estimates of baseline total bedtime were similar in both groups: 7 hours 47 minutes in NUTRITION and 8 hours 4 minutes in SLEEP.
Polysomnography was conducted in the Neuroscience Clinical and Translational Research Center with sleep onset and offset scheduled to occur at the participant’s habitual sleep time. Signals collected on each study night included bilateral central referential electroencephalogram channels (C3 and C4, referenced to A1–A2), electro-oculogram, and submentalis electromyogram. Additional signals were collected on the first night of sleep studies for the assessment of sleep-disordered breathing (nasal pressure and oral-nasal thermistors, fingertip oximeter, and abdominal and thoracic excursion, as measured by inductance plethysmography to reflect ventilatory effort) and periodic leg movements (bilateral electromyogram of anterior tibialis). Visual sleep stage scoring was conducted by trained polysomnographic technologists with established reliability (intraclass correlation coefficients for wake, nonrapid eye movement, and rapid eye movement were each >0.90), who were blinded to participant characteristics and intervention group. Sleep was scored in 20-second epochs, using standard scoring criteria (18).
Summary sleep measures included those directly manipulated by the intervention (time in bed) and dimensions of sleep targeted by the intervention (sleep efficiency, slow-wave sleep). Tim e in bed was quantified as minutes from “lights out” to “morning awakening.” Sleep efficiency was defined as total sleep time/time spent in bed × 100 and slow - wave sleep was calculated as minutes of nonrapid eye movement stages 3 and 4/total sleep time × 100. Increased daytime sleepiness, as measured by the Multiple Sleep Latency Test, and decreased total sleep time were also assessed due to the possibility that they might be affected by SLEEP and that they might adversely affect health and functioning (19–22). Total sleep time (sleep duration) was calculated as total minutes of any stage of sleep from sleep onset to morning awakening. Sleep-disordered breathing (apnea-hypopnea index [AHI]) and periodic leg movements were also monitored to establish study eligibility and monitor possible sleep disorders (23). We repeated assessment of sleep-disordered breathing at T3 (18 months). T2 (month 6) and T4 (month 30) studies did not include assessments for sleep-disordered breathing.
Self-Report Sleep Measures
At each time point (T1–T4), we used the Pittsburgh Sleep Quality Index (24) to assess habitual subjective sleep quality over the previous month. In addition, participants were asked to complete the Pittsburgh Sleep Diary (25) for 14 days before the laboratory recordings. The diary assessed daily activities including physical activity, naps, subjective nocturnal sleep quality, bedtime, and risetime. Participants in SLEEP additionally completed sleep diaries daily during the 18 months of the intervention to evaluate adherence with the intervention and possible increases in daytime sleepiness. Participants in NUTRITION completed a sleep diary daily for the 2 weeks before each scheduled assessment only. Other self-report measures of sleep included the Composite Scale of Morningness (26), to determine if someone is more active and alert in the morning or evening and the Epworth Sleepiness Scale (27), to evaluate daytime sleepiness. During the 2 weeks before each polysomnographic assessment, participants wore a wrist actigraph to verify diary reports of bedtime and risetime (1). As previously reported (1), we observed good concordance between diary-based and wrist actigraphic estimates of sleep-onset and sleep-offset times.
Measures of Health and Functioning
The Medical Outcomes Survey-Short Form 36 (28) was used to measure self-reported mental and physical health-related quality of life (T1–T4). Clinician-rated medical burden was evaluated, using the Cumulative Illness Rating Scale-Geriatrics (29), which quantifies the presence and severity of medical disorders for each organ system (T1 and T3). At all four time points, the study medical evaluator contacted participants by telephone to inventory all medications being taken (over-the-counter and prescription) and evaluate any changes in medical status from the time of the last scheduled assessment. The Logical Memory Test (30,31) for episodic memory and nonverbal intelligence for reasoning (TONI-III) (32) were used to assess cognitive functioning.
Markers of Inflammation
Dysregulation of the inflammatory response is considered a biological pathway through which sleep may affect health, functioning, and longevity in older adults (33). Markers of inflammation, interleukin (IL)-6 and tumor necrosis factor (TNF)-α levels, were assayed from morning blood samples drawn during the T1 and T3 medical examination (33). Plasma levels of TNF-α and IL-6 were determined by enzyme-linked immunosorbent assay (ELISA) (R& D Systems, Minneapolis, Minnesota) according to the manufacturer’s instructions. The range for TNF-α was between 0.5 pg/mL and 32 pg/mL and for IL-6 between 0.156 pg/mL and 10 pg/mL. All samples were run in duplicate and coefficient of variation between samples was <10% (Biobehavioral Medicine Laboratory, University of Pittsburgh Cancer Institute, F. Jenkins, Director).
Measures of Stress, Coping, and Social Support
Also measured at each time point were life events, perceived stress, coping, and social support. Number and severity of stressful life events as well as perceived stress were measured with the Life Experience Survey (34) and the Perceived Stress Scale (35), respectively. The Brief COPE (36) was used to assess coping strategies for a participant-identified stressor, and the Interpersonal Support Evaluation List (37) assessed perceived social support, sense of belonging, and self-esteem. We reported data on these measures elsewhere (1).
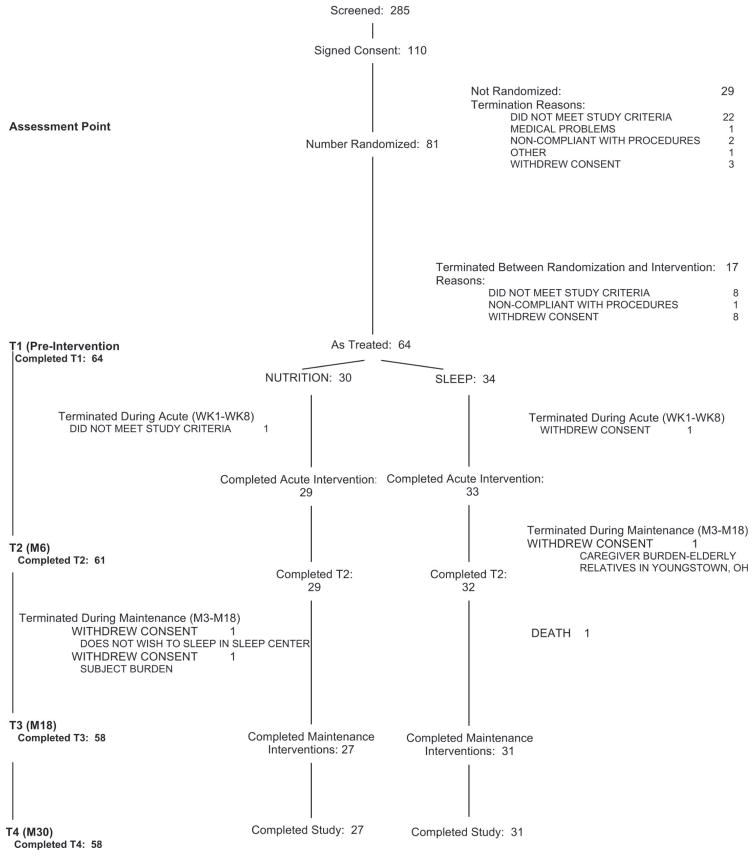
Participant Accrual, Retention, and Flow Through the Entire Protocol (Fig. 1)
Figure 1.
Subject Accrual and Flow Chart.
Our sample as treated comprised 64 participants, 34 randomly assigned to SLEEP and 30 to NUTRITION. Fifty-eight (90.6%) of 64 participants completed the assigned interventions (27 of 30 NUTRITION and 31 of 34 SLEEP). Of the six participants who did not complete their interventions, five withdrew consent (n = 3 NUTRITION, 2 SLEEP) and one participant died after a fall and head injury (SLEEP). (We do not have evidence that the fall was related to the intervention itself.) Fifty-four (84.3%) of 64 participants completed all four assessments (T1-T4): 25 (83.3%) of 30 NUTRITION and 29 (85.3%) of 34 SLEEP.
Statistical Analyses
Base line Participant Characteristics
We used group t tests to compare baseline (pre intervention) demographic, clinical, health-related quality of life, cognitive, mental health, and social support measures between SLEEP and NUTRITION. Similarly, group t tests compared baseline (pre intervention) measures of sleep quality (PSQ I, Pittsburgh Sleep Diary), daytime sleepiness (Epworth), and daytime mood and alertness (Pittsburgh Sleep Diary). As detailed in Tables 1 and 2, respectively, baseline sample characteristics did not differ for SLEEP and NUTRITION.
To examine any group differences in first-night effect at T1 and capacity for adaptation to the sleep laboratory, we performed a repeated-measures analysis of variance on selected electroencephalogram sleep measures with SLEEP or NUTRITION as the between-group factor and night (first versus second) as the within-participant factor. An interaction term (group × night) was included in the analyses to test for differential first-night effects in the two groups. As detailed in Table 3, we observed no baseline (preintervention) differences related to intervention group or group × night interactions. Expected night effects were evident in sleep onset latency, time spent asleep, sleep efficiency, rapid eye movement percent, and delta percent on night 2.
TABLE 3.
Baseline Sleep Characteristics: Self-Report, Diary, PSG
| Sleep Restriction + Education, n = 34 | Healthy Dietary Education, n= 30 | t | df | p | |
|---|---|---|---|---|---|
| Pittsburgh Sleep Quality Index (0–21) | 3.3 (1.8) | 2.8 (1.6) | 1.13 | 62 | .26 |
| Epworth Sleepiness | 6.3 (2.9) | 6.6 (2.3) | 0.51 | 62 | .61 |
| Pittsburgh Sleep Diary (0–100) | |||||
| Quality of sleep | |||||
| Mood on awakening | 78.2 (11.2) | 74.6 (11.5) | 1.50 | 58 | .14 |
| Alert on awakening | 81.4 (13.1) | 79.8 (9.7) | 0.29 | 58 | .77 |
| 80.0 (14.4) | 79.4 (11.1) | 0.07 | 58 | .94 | |
| Sleep Restriction + Education |
Healthy Dietary Education |
Effect F(df = 1.62) p |
|||||
|---|---|---|---|---|---|---|---|
| Night 1 | Night 2 | Night 1 | Night 2 | Group | Night | G × N | |
| Time in bed (minutes) | 426 (49) | 427 (47) | 429 (58) | 446 (49) | 1.04 | 2.79 | 2.32 |
| .31 | .099 | .13 | |||||
| Time spent asleep (minutes) | 298 (63) | 340 (48) | 305 (71) | 349 (44) | 0.52 | 25.36 | .01 |
| .48 | .0001 | .94 | |||||
| Sleep latencya (minutes) | 25.4 (22.1) | 18.2 (14.8) | 25.3 (23.9) | 22.4 (23.0) | 0.13 | 3.55 | .77 |
| .72 | .064 | .38 | |||||
| Sleep efficiency b (%) | 69.9 (13.1) | 79.6 (7.5) | 70.7 (12.9) | 78.5 (8.7) | 0.00 | 31.53 | .23 |
| .98 | .0001 | .63 | |||||
| REM % | 17.3 (5.8) | 20.8 (5.6) | 17.8 (6.4) | 21.9 (7.0) | 0.38 | 19.66 | .11 |
| .54 | .0001 | .74 | |||||
| Slow-wave %a | 2.6 (4.7) | 2.1 (3.2) | 3.0 (4.9) | 1.6 (2.7) | 0.06 | 3.26 | .78 |
| .81 | .076 | .38 | |||||
PSG = polysomnographic; REM = rapid eye movement.
Square root transformation before statistical comparison.
Natural log transformation before statistical comparison.
Equality of Exposure to Each Intervention
The mean number of NUTRITION sessions was 21.6 (SD = 4.2); and SLEEP, 22.1 (SD = 4.0). Of these, 13.4 (SD = 7.4) and 13.0 (SD = 9.0) were conducted in person for NUTRITION and SLEEP, respectively. (The rest were conducted on the telephone.) The mean length of NUTRITION sessions was 27 (SD = 5.8) minutes; and of SLEEP sessions, 22.9 minutes (SD = 10.0) (t = 2.32, df = 62, p = .024).
Intervention Integrity
NUTRITION and SLEEP sessions were tape-recorded; audiotapes during the first eight sessions were rated by an independent blind scorer for session content for a total of 23 participants (n = 10 NUTRITION, 13 SLEEP). In NUTRITION, an average of 84% of all items to be covered during the intervention were covered over the first eight sessions. In SLEEP, an average of 79.1% of items was covered. We found only one instance of crossover topics, when a topic from NUTRITION was discussed in SLEEP.
Data Analysis of Primary Outcomes
We used repeated-measures mixed-effects models to compare hypothesized differences in sleep and health-related outcomes, with treatment group and time as fixed effects, and participants as random effects. We report Satterwwaite df. We applied the Holm’s step-down Bonferroni procedure to the outcome measures within each domain to control for type I error inflation (38). We have found the Holm’s procedure to perform well in correlated outcome measures (38). Regression modeling was used to examine the mediation role of reduced time in bed and cytokines (IL-6 and TNF-α) on medical burden (CIRS-G) scores. Both cytokines were log transformed and assessed as continuous variables.
Our power calculations, based on pilot data, called for numbers of 30 in each cell to detect large effect sizes (Cohen’s d or ≥0.75) likely to have clinical meaning and to achieve statistical significance at a two-tailed α of 0.05 with 80% power. We report effect sizes for statistically significant outcomes; other effect size data are available on request.
RESULTS
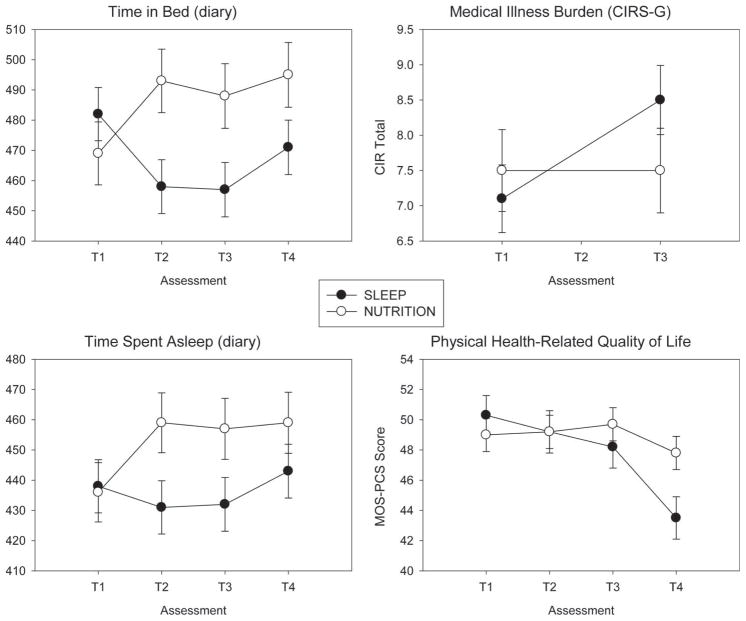
Diary-based measures of time in bed (Fig. 2) confirmed the expected group × time interactions (F(3,159) = 8.94, p = .0001): Compared with baseline values, participants in SLEEP spent 25 minutes to 30 minutes less time in bed at T2 (6 months) and T3 (18 months), whereas subjects in NUTRITION spent 15 minutes to 20 minutes longer time in bed at T2 and T3. Diary-based measures showed a similar pattern of greater time spent asleep (TSA) (20–25 minutes) in NUTRITION than in SLEEP at T2 and T3 (group × time interaction) (F(3,159) = 4.09, p = .008). Thus, for TSA, the group differences were due more to the increase in NUTRITION TSA than to SLEEP decreasing. NUTRITION TSA stayed high at T4 and increased for SLEEP from T3–T4 but was still lower for SLEEP than NUTRITION at T4. Thus, it seemed that some SLEEP subjects continued to practice restricting time in bed, even though they received no instruction to do so.
Figure 2.
Sleep and health in older adults. NUTRITION allowed more time in bed (F = 8.94, df = 3,159, p = .0001) and time asleep (F = 4.09, df = 3,159, p = .008) than SLEEP. NUTRITION was associated with greater stability of medical status (F = 7.44, df = 3,54, p = .009) and physical health-related quality of life than SLEEP (F = 2.95, df = 3,172, p = .034). Two interpretations are possible: (1) NUTRITION might have had a positive effect, rather than SLEEP having a negative effect; or (2) vice versa.
Hypothesis 1: Intervention Effects on Polysomnographic-, Questionnaire -, and Diary - Based Outcome Measures of Sleep
We hypothesized that SLEEP participants would show greater stability or even improvements in laboratory measures of sleep efficiency and slow-wave sleep percent (as a result of increased homeostatic sleep drive secondary to restriction of time in bed) relative to NUTRITION participants. However, we detected no group × time interactions in polysomnographic measures of sleep continuity and depth (data not shown but available on request). Nor did we observe group × time interactions for questionnaire-based measures of sleep quality (Pittsburgh Sleep Quality Index) (24) or daytime sleepiness (Epworth Sleepiness Scale) (27). Finally, we did not detect intervention-related effects on diary-based measures (Pittsburgh Sleep Diary) (25) of sleep quality, duration of daytime napping, daytime alertness or mood over the 30-month period of participation (data not shown; available on request).
Hypothesis 2: Intervention Effects on Health - Related Quality of Life, Inflammatory Markers, and Objectively Measured Cumulative Medical Illness Burden
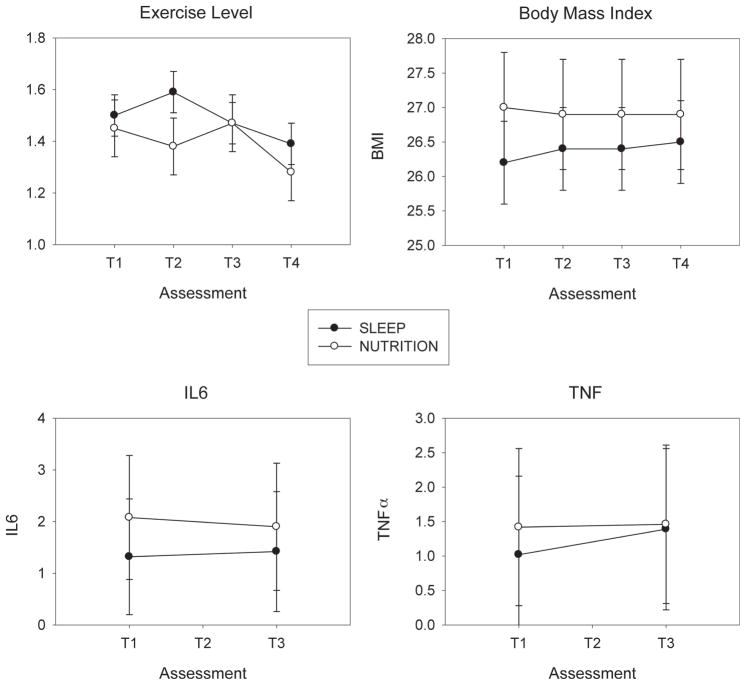
We hypothesized that SLEEP participants would show greater stability or even improvements in health-related quality of life (Medical Outcomes Survey Short Form) (39) and cumulative medical illness burden (Cumulative Illness Rating Scale for Geratrics) (29) compared with NUTRITION participants. We detected no group × time interaction in the mental component score of the Medical Outcomes Survey (F(3,177) = 0.72, p = .54). We did detect an interaction for the physical health component; however, the effect was opposite of the hypothesized direction (Fig. 2). That is, NUTRITION participants showed greater stability in physical health-related quality of life over time (T1–T4) compared with SLEEP participants (group × time interaction F(3,172) = 2.95, p = .034). SLEEP participants showed a gradual decline in Medical Outcomes Survey physical health scores from T1 to T3, followed by a steeper decline from T3 to T4, as compared with NUTRITION participants (Fig. 2) (effect size = 0.35). There were no significant group or group × time interaction effects for Il-6 or TNF-α (Fig. 3).
Figure 3.
Exercise, body mass index, and cytokines (IL-6, TNF-α) in SLEEP and NUTRITION participants. SLEEP and NUTRITION participants did not differ over time in exercise (F = 0.71, df = 3,138, p = .55), body mass index (F = 0.65, df = 3,118, p = .58), IL-6 (F = 0.41, df = 1,32, p = .53), or TNF-α (F = 2.24, df = 1,32, p = .14). T1 = baseline; T2 = 6 months; T3 = 18 months; T4 = 30 months. IL = interleukin; TNF = tumor necrosis factor.
Intervention effects on the Cumulative Illness Rating Scale for Geriatrics (29), a clinician-rated measure of medical illness severity, were similar to those observed for physical health-related quality of life. SLEEP participants showed a greater increase in medical burden scores from baseline (T1) to 18 months (T3), compared with NUTRITION participants, whose trajectories remained stable (group × time interaction F(1,56) = 7.76, p = .008) (Fig. 3) (effect size = 0.34). (Note that CIRS-G measures were not collected at T4.) Group differences in CIRS scores were accounted for by changes in cardiovascular disease, which increased in SLEEP and decreased in NUTRITION over 18 months (nonparametric Wilcoxon statistic χ2 = 5.90, p = .015). There was no significant difference in neurological disease burden change over 18 months.
We next examined several possible mechanisms by which SLEEP might have been associated with decreased physical health-related quality of life and increased medical burden. As noted above, the SLEEP intervention was associated with significant decreases in diary-based indices of sleep duration including time in bed and time spent asleep (Fig. 2). Markers of inflammation, body mass index, and exercise did not contribute to the effects of SLEEP on decreased physical health-related quality of life or increased medical burden, as they did not change as a function of group (Fig. 3).
Finally, we detected no differences in any outcome measures when data from the seven subjects on continuous positive airway pressure were omitted from the analyses.
DISCUSSION
We did not confirm the primary study hypotheses that 30 minutes restriction of time in bed, combined with education in health sleep practices, would slow age-dependent declines in sleep continuity/depth and lead to better preservation of health and functioning. In other words, because SLEEP was not associated with an increase in sleep pressure, we cannot say whether increased homeostatic drive is beneficial to health. Rather, unexpectedly, we observed that NUTRITION (which allowed participants to spend more time in bed compared with SLEEP) was associated with greater time spent asleep (about 30 minutes more: 7 hours 30 minutes nightly versus 7 hours in SLEEP), more stable health-related quality of life, and more stable medical burden over the observation period (Fig. 2). Markers of inflammation, body mass index, and exercise did not account for the apparent decrease in health-related quality of life or increased medical burden in participants randomly assigned to SLEEP.
We cannot exclude the possibility that NUTRITION had a positive effect on health, rather than SLEEP having a negative effect. A limitation of the study is the absence of a noninterventional (or observational) control group to confirm one or another of these two possibilities. We observed stability of health measures in NUTRITION versus deterioration in SLEEP, suggesting that, for healthy elderly without sleep complaints, reducing sleep time may be detrimental. NUTRITION could have led to preservation of cardiovascular health through better food choices, associated with improved lipid profiles even in the absence of weight loss, particularly as these healthy, noncomplaining older adults were only mildly overweight (per body mass indices) at study entry. A second limitation of the study is that we did not measure lipid profiles.
In a recent report of cross-sectional data from this study (1), we observed that better physical health-related quality of life at baseline (before intervention) was associated with better sleep quality on the Pittsburgh Sleep Quality Index (Pearson r = −.26, df = 62, p < .05). Possibly, a modest increase in time spent asleep from 7 hours to about 7.5 hours nightly does lead to better preservation of physical health-related quality of life and could protect from faster accretion of concurrent medical (e.g., cardiovascular) burden. Recent studies have supported this concept with respect to coronary heart disease, where short and long self-reported sleep durations are independently associated with increased risk of coronary events (19); and with respect to the development of Type 2 diabetes, where short (≤5 hours of sleep duration) and long (>8 hours) self-reported sleep increase the risk of developing diabetes (40). We have also reported that sleep duration is a significant correlate of the metabolic syndrome, which is a risk factor for cardiovascular disease and diabetes (41). This hypothesis is consistent with observations from the epidemiologic literature that too little, or too much, sleep is associated with decrements in health and longevity (13). We have also reported that longer sleep latency predicts greater mortality (7). Thus, the current data suggest that steps taken to protect sleep and sleep duration may yield benefits to health-related quality of life in very old age, whereas even modest curtailment of time spent asleep to ≤7 hours may not benefit physical health and quality of life. The decrease in sleep duration observed in SLEEP did not significantly affect circulating levels of inflammatory markers. Although there is some evidence that short sleep duration is associated with increased levels of inflammatory markers (42), the modest sleep restriction imposed by SLEEP (e.g., 15–30 minutes a night) seems to have been insufficient to increase circulating markers of inflammation. It remains possible that other indices of inflammation or cellular processes more sensitive to sleep restriction and not measured in the present study may have contributed to the increased medical burden associated with the SLEEP condition.
In conclusion, modest sleep restriction in healthy elders without sleep complaints or disorders did not improve sleep continuity or depth and was associated with increases in medical burden over an 18-month period. In contrast, a NUTRITION intervention focused on education in healthy dietary practices in the elderly was associated with modest increases in sleep duration and stable indices of health over the same period of time. Although the mechanisms by which SLEEP and NUTRITION affected physical health-related quality of life and medical burden are unknown, modest treatment-related changes in sleep duration may have affected lipid and glucose metabolism, blood pressure, or other cellular mechanisms integral to the maintenance of health including cardiovascular health in older adults. That the intervention was associated with a worse outcome suggests the importance of time spent asleep in old age.
Acknowledgments
This work was supported, in part, by the following grants: P01 AG20677 (Timothy Monk), P30 MH071944 (C.F.R.), R01 MH37869 (C.F.R.), R01 MH43832 (C.F.R.), RR024153 (Daniel Buysse), the John A. Hartford Foundation Center of Excellence in Geriatric Psychiatry (C.F.R.), and the University of Pittsburgh Medical Center Endowment in Geriatric Psychiatry (C.F.R.).
We thank the following individuals for their helpful reviews of earlier drafts: Daniel Buysse, MD, Anne Germain, PhD, David J. Kupfer, MD, Timothy Monk, PhD, and Ruth O’Hara, PhD.
- IL-6
interleukin-6
- TNF-α
tumor necrosis factor-α
References
- 1.Driscoll HC, Serody L, Patrick S, Maurer J, Bensasi S, Houck PR, Mazumdar S, Nofzinger EA, Bell B, Nebes RD, Miller MD, Reynolds CF. Sleeping well, aging well: a descriptive and cross-sectional study of sleep in “successful agers” 75 and older. Am J Geriatr Psychiatry. 2008;16:74–82. doi: 10.1097/JGP.0b013e3181557b69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16:40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- 3.Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18:425–32. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 4.Foley DJ, Monjan AA, Izmirlian G, Hays JC, Blazer DG. Incidence and remission of insomnia among elderly adults in a biracial cohort. Sleep. 1999;22:S373–8. [PubMed] [Google Scholar]
- 5.Morin CM, Gramling SE. Sleep patterns and aging: comparison of older adults with and without insomnia complaints. Psychol Aging. 1989;4:290–4. doi: 10.1037//0882-7974.4.3.290. [DOI] [PubMed] [Google Scholar]
- 6.Prinz PN, Vitiello MV, Raskind MA, Thorpy MJ. Geriatrics: sleep disorders and aging. N Engl J Med. 1990;323:520–6. doi: 10.1056/NEJM199008233230805. [DOI] [PubMed] [Google Scholar]
- 7.Dew MA, Hoch CC, Buysse DJ, Monk TH, Begley AE, Houck PR, Hall M, Kupfer DJ, Reynolds CF. Healthy older adults’ sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom Med. 2003;65:63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- 8.Friedman L, Benson K, Noda A, Zarcone V, Wicks DA, O’Connell K, Brooks JO, Bliwise DL, Yesavage JA. An actigraphic comparison of sleep restriction and sleep hygiene treatments for insomnia in older adults. J Geriatr Psychiatry Neurol. 2000;13:17–27. doi: 10.1177/089198870001300103. [DOI] [PubMed] [Google Scholar]
- 9.Hoch CC, Reynolds CF, Kupfer DJ, Houck PR, Berman SR, Stack JA. The superior sleep of healthy elderly nuns. Int J Aging Hum Dev. 1987;25:1–9. doi: 10.2190/P1A2-K0X5-K27M-TJ49. [DOI] [PubMed] [Google Scholar]
- 10.Hall M, Baum A, Buysse DJ, Prigerson HG, Kupfer DJ, Reynolds CF. Sleep as a mediator of the stress-immune relationship. Psychosom Med. 1998;60:48–51. doi: 10.1097/00006842-199801000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Dew MA, Reynolds CF, Monk TH, Buysse DJ, Hoch CC, Jennings JR, Kupfer DJ. Psychosocial correlates and sequelae of electroencephalographic sleep in healthy elders. J Gerontol. 1994;49:8–18. doi: 10.1093/geronj/49.1.p8. [DOI] [PubMed] [Google Scholar]
- 12.Hoch CC, Reynolds CF, 3rd, Buysse DJ, Monk TH, Nowell P, Begley AE, Hall F, Dew MA. Protecting sleep quality in later life: a pilot study of bed restriction and sleep hygiene. J Gerontol B Psychol Sci Soc Sci. 2001;56:52–9. doi: 10.1093/geronb/56.1.p52. [DOI] [PubMed] [Google Scholar]
- 13.Kripke DF, Simons RN, Garfinkel L, Hammond EC. Short and long sleep and sleeping pills. Is increased mortality associated? Arch Gen Psychiatry. 1979;36:103–16. doi: 10.1001/archpsyc.1979.01780010109014. [DOI] [PubMed] [Google Scholar]
- 14.First MB, Spitzer RL, Gibbon M, Williams JBW. Administration Booklet. Washington, DC: American Psychiatric Publishing; 1997. Structured Clinical Interview for DSM-IV Axis I Disorders. Clinician Version. SCID-I. [Google Scholar]
- 15.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–96. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 16.Folstein MF, Folstein SW, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 17.Friedman L, Bliwise DL, Yesavage JA, Salom SR. A preliminary study comparing sleep restriction and relaxation treatments for insomnia in older adults. J Gerontol. 1991;46:1–8. doi: 10.1093/geronj/46.1.p1. [DOI] [PubMed] [Google Scholar]
- 18.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 19.Ayas NT, White DP, Manson JE, Stampfer MJ, Speizer FE, Malhotra A, Hu FB. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–9. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 20.Cappuccio FP, Stranges S, Kandala NB, Miller MA, Taggart FM, Kumari M, Ferrie JE, Shipley MJ, Brunner EJ, Marmot MG. Gender-specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II Study. Hypertension. 2007;50:693–700. doi: 10.1161/HYPERTENSIONAHA.107.095471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, Rundle AG, Zammit GK, Malaspina D. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–9. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 22.Ferrie JE, Shipley MJ, Cappuccio FP, Brunner D, Miller MA, Kumari M, Marmot MG. A prospective study of change in sleep duration: associations with mortality in the Whitehall II cohort. Sleep. 2007;30:1659–66. doi: 10.1093/sleep/30.12.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rechtschaffen A, Kales A. NIH Publication 204. Washington, DC: U.S. Government Printing Office, Department of Health Education and Welfare; 1968. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. [Google Scholar]
- 24.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Q uality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 25.Monk TH, Reynolds CF, 3rd, Kupfer DJ, Buysse DJ, Coble PA, Hayes AJ, Machen MA, Petrie SR, Ritenour AM. The Pittsburgh Sleep Diary. J Sleep Res. 1994;3:111–20. [PubMed] [Google Scholar]
- 26.Monk TH, Buysse DJ, Potts JM, DeGrazia JM, Kupfer DJ. Morningness-eveningness and lifestyle regularity. Chronobiol Int. 2004;21:435–43. doi: 10.1081/cbi-120038614. [DOI] [PubMed] [Google Scholar]
- 27.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 28.Ware JE, Sherbourne CD. The MOS-36-Item short form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 29.Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, Mulsant B, Reynolds CF. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41:237–48. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 30.Wechsler D. Wechsler Memory Scale-Revised. San Antonio: The Psychological Corporation; 1987. [Google Scholar]
- 31.Wechsler D. Wechsler Adult Intelligence Scale-III. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- 32.Brown L, Sherbenou RJ, Johnsen SK. Test of Nonverbal Intelligence: A Language-Free Measure of Cognitive Ability, Third Edition (TONI-3) Austin, TX: Pro-Ed; 1998. [Google Scholar]
- 33.von Kanel R, Dimsdale JE, Ancoli-Israel S, Mills PJ, Patterson TL, McKibbin CL, Archuleta C, Grant I. Poor sleep is associated with higher plasma proinflammatory cytokine interleukin-6 and procoagulant marker fibrin D-dimer in older caregivers of people with Alzheimer’s disease. J Am Geriatr Soc. 2006;54:431–7. doi: 10.1111/j.1532-5415.2005.00642.x. [DOI] [PubMed] [Google Scholar]
- 34.Russo J, Vitaliano PP. Life events as correlates of burden in spouse caregivers of persons with Alzheimer’s disease. Exp Aging Res. 1995;21:273–94. doi: 10.1080/03610739508253985. [DOI] [PubMed] [Google Scholar]
- 35.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 36.Carver CS. You want to measure coping but your protocol’s too long: consider the Brief COPE. Int J Behav Med. 1997;4:92–100. doi: 10.1207/s15327558ijbm0401_6. [DOI] [PubMed] [Google Scholar]
- 37.Williamson G, Schulz R. Physical illness and symptoms of depression among elderly outpatients. Psychol Aging. 1992;7:343–51. doi: 10.1037//0882-7974.7.3.343. [DOI] [PubMed] [Google Scholar]
- 38.Blakesley RE, Mazumdar S, Dew MA, Houck PR, Tang G, Reynolds CF, Butters MA. Comparisons of methods for multiple hypothesis testing in neuropsychological research. Neuropsychology. 2009;23:255–64. doi: 10.1037/a0012850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stewart AL, Hays RD, Ware JE. The MOS short-form general health survey. Reliability and validity in a patient population. Med Care. 1988;26:724–35. doi: 10.1097/00005650-198807000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care. 2006;29:657–61. doi: 10.2337/diacare.29.03.06.dc05-0879. [DOI] [PubMed] [Google Scholar]
- 41.Hall MH, Muldoon MF, Jennings JR, Buysse DJ, Flory J, Manuck SB. Self-reported sleep duration is associated with the metabolic syndrome in midlife adults. Sleep. 2008;31:635–43. doi: 10.1093/sleep/31.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mattis S. Dementia Rating Scale-2™ (DRS-2™) Odessa, FL: Psychological Assessment Resources; 2004. [Google Scholar]