Abstract
The authors examined internalizing behavior problems at middle childhood, adolescence, and young adulthood and brain-based measures of stress vulnerability in 154 right-handed, non-impaired young adults (M age = 23 years): 71 (30 males, 41 females) born at extremely low birth weight (ELBW; < 1000 grams), and 83 (35 males, 48 females) controls born at normal birth weight (NBW). Internalizing behavior problems increased from adolescence to young adulthood among ELBW individuals. ELBW adults exhibited greater relative right frontal electroencephalogram (EEG) activity at rest and more concurrent internalizing behavior problems than NBW controls. Being born at ELBW may have subtle influences on brain-behavior relations even in survivors without major impairments and evidence of these influences may not emerge until young adulthood.
Extremely low birth weight (ELBW) is a phenomenon that provides developmental researchers with a model for understanding how adverse events early in life may impact brain development and developmental outcomes across a variety of domains. Infants born at ELBW (< 1000 grams) are the smallest and most at-risk infants. Because the survival rate of infants born at ELBW has markedly improved over the last few decades due to advances in neonatal care (see Lorenz, 2001, for a review), there is an increased need to track development in ELBW survivors.
Previous research from our group and others has established that children born at ELBW are at risk for a variety of neurodevelopmental and psychiatric disorders and stress-related problems. Saigal and her colleagues (Saigal, Rosenbaum, Szatmari, & Campbell, 1991) reported that the prevalence rate for a number of neurological and neurosensory impairments (e.g., cerebral palsy, hydrocephaly, microcephaly, blindness, and deafness) is significantly higher among children born at ELBW than in normal birth weight (NBW) matched controls. ELBW survivors are also at higher risk for a range of academic, behavioral, social, and psychiatric problems during preschool (Szatmari, Saigal, Rosenbaum, Campbell, & King, 1990; Taylor, Hack, & Klein, 1998), middle childhood (Miller, Bowen, Gibson, Hand, & Ungerer, 2001; Szatmari, Saigal, Rosenbaum, & Campbell, 1993; Taylor, Klein, & Hack, 2000), adolescence (Grunau, Whitfield, & Fay, 2004; Saigal, Hoult, Streiner, Stoskopf, & Rosenbaum, 2000; Saigal, Lambert, Russ, & Hoult, 2002; Saigal, Pinelli, Hoult, Kim, & Boyle, 2003), and young adulthood (Schmidt, Miskovic, Boyle, & Saigal, 2008) compared with their NBW counterparts.
A similar pattern of stress-related difficulties such as psychiatric, behavioral, and socioemotional problems noted in children, adolescents, and young adults born at ELBW has been documented in children, adolescents, and young adults who were born at very low birth weight (VLBW < 1500 grams; see Chapieski & Evankovich, 1997; Hack, Klein, & Taylor, 1995; Schothorst & Van Engeland, 1996; Zwicker & Harris, 2008, for reviews). These behaviors include: lower levels of social competence (Ross, Lipper, & Auld, 1990), more psychiatric and behavioral problems (Botting, Powls, Cooke, & Marlow, 1997; Cooke, 2004; Dahl et al., 2006; Hack et al., 2002, 2004, 2005; Hack, Cartar, Schluchter, Klein, & Forrest, 2007; Indredavik, Vik, Heyerdahl, Kulseng, & Brubakk, 2005; Levy-Schiff et al., 1994; Reijneveld et al., 2006; Richards, Kelly, Doyle, & Callanan, 2001; Sykes et al., 1997), and lower self-concept (Richards et al., 2001) in children, adolescents, and young adults born at VLBW. Hack and colleagues (2007) also found that, although VLBW participants reported similar health, well-being, and functioning compared with NBW controls, they exhibited greater risk avoidance. Hack et al. suggested that lesser resilience in VLBW individuals may prove detrimental to their future adult health. In other high risk birth groups, Allin and colleagues (Allin et al., 2006) recently reported significantly lower extraversion and higher neuroticism scores in adults born very preterm (< 33 weeks gestation age) compared with full-term control adults.
Although pre- and peri-natal risk factors such as low birth weight and prematurity are presumed to shape the developing brain and predict the development of stress-related problems (Als, 1995), we know little about their impact on brain-based and behavioral measures of stress vulnerability in adult survivors. The reason for this lack of attention is largely that there have been relatively few opportunities to study ELBW survivors as young adults. Because medical advances have improved survival rates further for infants born at extremely low birth weight only in the last few decades (Lorenz, 2001), the long-term impact of pre- and peri-natal risk factors on stress vulnerability can now be examined in adult survivors.
Frontal Electroencephalogram Asymmetry and Stress Vulnerability
One approach to the study of stress vulnerability has been to use measures of electroencephalogram (EEG) activity to examine patterns of regional brain asymmetry in infants, children, adults, and clinical populations (Davidson, 1993, 2000; Fox, 1991, 1994). Davidson and Fox have argued that emotions are organized around approach-withdrawal motivational tendencies. The experience of positive emotions (e.g., joy, happiness, interest) facilitates approach-related responses whereas the experience of negative emotions (e.g., fear, sadness, disgust) facilitates withdrawal-related responses. Moreover, the anterior cerebral hemispheres are differentially lateralized for approach-withdrawal responses and the regulation of emotion. The left frontal region subserves approach-related behaviors and emotions, whereas the right frontal region subserves withdrawal-related emotions.
Over the past three decades, researchers have used the frontal EEG asymmetry-emotion model as a theoretical platform to derive predictions about emotional regulation and socioemotional development (see Coan & Allen, 2004, for a review). Overall, studies have shown that the pattern of resting frontal EEG alpha asymmetry (1) reflects a bias to experience either positive or negative emotion, (2) is related to individual differences in affective style, and (3) predicts psychopathology across development. Individuals who exhibit greater relative left frontal EEG activity at rest are social, extraverted, and outgoing, whereas those who exhibit greater relative right frontal EEG activity at rest are shy, anxious, and depressed.
For example, infants who are fearful and easily distressed (Buss et al., 2003; Fox, Bell, & Jones, 1992), toddlers who are behaviorally inhibited (Calkins, Fox, & Marshall, 1996; Fox, Bell, & Calkins, 1994), and children who are socially withdrawn (Fox et al., 1995, 2001), shy (Theall-Honey & Schmidt, 2006) and score high on internalizing behaviors (Fox, Schmidt, Calkins, Rubin, & Coplan, 1996) all exhibit greater relative right frontal EEG activity at rest. Adults who are shy (Schmidt, 1999) and socially anxious (Beaton, Schmidt, Ashbaugh, Santesso, Antony, & McCabe, 2008) are known to display greater relative right frontal EEG activity at rest. In addition, clinically depressed adults are characterized by greater relative right frontal EEG activity at rest even when their symptoms of depression are in remission (Henriques & Davidson, 1990). Individuals who exhibit stable resting right, versus left, frontal EEG activity across time are also known to score high on measures of internalizing behaviors such as behavioral inhibition and withdrawal (Sutton & Davidson, 1997).
Studies have noted short- and long-term stability over time and context in resting frontal EEG alpha asymmetry and power measures in healthy individuals as well as some clinical populations. Schmidt and his colleagues reported stability in frontal EEG asymmetry measured second-by-second in healthy infants (Schmidt, 2008) and across different stages of sleep in adults (Schmidt, Cote, Santesso, & Milner, 2003). Tomarken and his colleagues found stability in frontal EEG asymmetry and power across three weeks in healthy adults (Tomarken, Davidson, Wheeler, & Kinney, 1992). Still other studies have noted stability across a longer period of time (i.e., 8- and 16-week intervals) in frontal alpha asymmetry in non-medicated clinically depressed individuals, where changes in asymmetry were not related to changes in clinical state (Allen, Urry, Hitt, & Coan, 2004). More recent studies by Fox’s group have reported long-term stability in the resting frontal asymmetry metric over a 6-month to 3-year interval in preschool- (3 to 5 years) and school-aged (6 to 9 years) children (Vuga, Fox, Cohn, Kovacs, & George, 2008), and over a 1- to 3-year interval in healthy adults and in adults with a history of major depression (Vuga, Fox, Cohn, George, Levenstein, & Kovacs, 2006). More recently, Schmidt and colleagues have noted long-term stability in the pattern of frontal EEG alpha asymmetry over a 3 year period in a sample of stable community outpatients with schizophrenia before and after controlling for positive and negative symptoms of the disorder (Jetha, Schmidt, & Goldberg, 2008). Because the pattern of frontal EEG asymmetry at rest is stable across time (Tomarken et al., 1992) and context (Schmidt et al., 2003), and its appearance early in life is predictive of socioemotional and stress-related problems (see Coan & Allen, 2004; Fox et al., 1995, 1996, 2001), some have argued that this metric indexes stress vulnerability (Coan & Allen, 2004; Davidson, 1993, 2000; Fox, 1991, 1994).
There have been, however, no studies that have examined the impact of pre- and peri-natal risk factors on resting frontal EEG asymmetry measures in adult survivors. The EEG studies that do exist are concerned primarily with premature infants at birth in neonatal intensive care units (Victor, Appleton, Beirne, Marson, & Weindling, 2005) or those early in the post-natal period (Beckwith & Parmelee, 1986; Duffy, Als, & McNulty, 1990; Hayakawa et al., 2001; Vecchierini, Andre, & d’Allest, 2007) in relation to behavioral and cognitive outcomes. Some researchers also have examined the EEGs of premature infants in relation to later manifestations of congenital brain disorders (Ellingson, Dutch, & McIntire, 1974). A primary goal of the present study was to examine the patterns of resting frontal EEG alpha asymmetry in young adults born at extremely low birth weight.
Salivary Cortisol and Stress Vulnerability
A second approach to the study of stress vulnerability has been to use neuroendocrine measures such as salivary cortisol (see Gunnar, 2000; Gunnar & Tagle, 2008, for reviews). The endocrine system has long been implicated in the regulation of emotion (see Gunnar, 1994). Cortisol is one of the critical molecules of the endocrine system. It is produced in response to stress and is easily measured in saliva. A number of studies have shown that cortisol increases in response to stress and the experience of fear in human infants, children, and adults, and baseline salivary cortisol predicts individual differences in stress vulnerability (see Gunnar & Tagle, 2008, for a review).
Human infants with high basal cortisol levels exhibit more freezing behavior in response to novel stimuli and greater relative right frontal EEG activity (Buss et al., 2003). Infants who remained temperamentally distressed from 9 to 13 months of age exhibited increases in adrenocortical activity during the presentation of laboratory vignettes (Gunnar, Mangelsdorf, Larson, & Hertsgaard, 1989). Kagan and his colleagues reported that temperamentally shy and behaviorally inhibited children exhibited higher morning basal cortisol levels compared with uninhibited children (Kagan, Reznick, & Snidman, 1987, 1988). Schmidt and his colleagues replicated the morning basal cortisol finding in temperamentally shy preschoolers (Schmidt et al., 1997), and noted increased cortisol reactivity in shy children at age seven in response to self-presentation (Schmidt et al., 1999). Adults with agitated depression (i.e., depression with comorbid anxiety) are also known to be characterized by high basal cortisol levels (Gold, Goodwin, & Chrousos, 1988a, 1988b; Gold, Drevets, & Charney, 2002).
Gunnar and her colleagues found that stressful events early in life shaped neuroendocrine responses. Gunnar’s group noted high cortisol levels among 6- to 12-year-old children who spent more than 8 months in Romanian orphanages in their first years of post-natal life compared with early adopted children and non-adopted North American children (Gunnar, Morison, Chisholm, & Schuder, 2001). Gunnar and her colleagues also reported that behaviorally inhibited children who were in insecure attachment relationships displayed greater increases in salivary cortisol during fear-eliciting conditions compared with uninhibited and securely attached children (Nachmias, Gunnar, Mangelsdorf, Parritz, & Buss, 1996).
There have been relatively few studies that have examined the impact of pre- and peri-natal risk factors on adult salivary cortisol levels. One exception is the work of Wust and colleagues (Wust et al., 2005) who recently reported that birth weight was associated with salivary cortisol responses to psychosocial stress in adult life. Lower birth weight due to twin births predicted higher cortisol reactivity in young adulthood. A second goal of the present study was to examine patterns of resting baseline salivary cortisol levels in young adults born at extremely low birth weight.
The Present Study
We examined whether being born at extremely low birth weight would have any long-term negative impact on brain-behavior relations and stress vulnerability. More specifically, we were interested in testing whether ELBW survivors without major impairments would exhibit more internalizing behavior problems in middle childhood, adolescence, and young adulthood and more stress vulnerability as indexed by two brain-based measures than their normal birth weight peers. To our knowledge, there appear to be no studies examining multiple brain-based and behavioral measures of stress vulnerability in young adult survivors who were born at extremely low birth weight. It is possible that there may be subtle influences on brain development due to pre- and peri-natal risk factors that may only manifest later in life. For example, a recent study reported reduced fertility in adult survivors of extreme prematurity (Swamy, Østbye, Skjaerven, 2008). Although causality could not be inferred from this study, the results do raise the possibility that pre- and peri-natal risk factors may negatively impact development across different domains in subtle ways that do not manifest (or are not observed) until young adulthood.
By design, we selected relatively homogenous groups from the ELBW and NBW cohorts composed of participants who were free from neurosensory or psychiatric impairment, right-handed, and tested at approximately the same time of day, as these variables are known to influence the study measures. The two groups did not differ significantly on key maternal and familial risk factors such as parental social class, education, family functioning, maternal psychopathology, and social support measures collected in middle childhood, adolescence, and young adulthood. The two groups also did not differ on any demographic, individual or familial risk factors other than birth weight from infancy.
We collected regional resting brain electroencephalogram (EEG) activity, baseline salivary cortisol, and measures of internalizing behavior problems in ELBW young adults and NBW controls. These measures were examined in relation to measures of child, adolescent, and young adult internalizing behavior problems. The ELBW cohort has been followed longitudinally since birth and assessed at key developmental milestones (see Saigal et al., 1990, 1991, 1996, 2003, 2006a; Szatmari, et al. 1991, 1993).
Hypotheses
We made the following predictions: 1) young adults born at ELBW would exhibit significantly more internalizing behavior problems, greater relative right frontal EEG activity at rest, and higher baseline salivary cortisol levels compared with their NBW controls; 2) internalizing behavior problems would increase from adolescence to young adulthood in ELBW adults; and 3) greater relative right frontal EEG activity would be related to concurrent internalizing behavior problems and higher salivary cortisol levels.
Method
Participants and Study Overview
ELBW
The extremely low birth weight (ELBW) cohort comprised 397 predominantly Caucasian infants (birth weight: 501 to 1000 grams) born between 1977 and 1982 to residents of a geographically-defined region in central-west Ontario, Canada, and recruited at birth. Of these 397 infants, 179 (45%) survived to hospital discharge from the Neonatal Intensive Care Unit. There were 13 late deaths; therefore, 166 survived to young adulthood. Adults who were born at ELBW were followed longitudinally from birth, and neurosensory impairments (e.g., cerebral palsy, blindness, deafness, mental retardation, microcephaly) were diagnosed by a neonatologist and a developmental pediatrician. By design, we chose to constitute a fairly homogeneous group of participants and therefore excluded individuals with neurosensory impairments (n = 46), psychiatric problems (n = 2), and those who were non-right handed (cf. Oldfield, 1971; n = 25). We selected only right-handed individuals because left-handers are known to differ in the lateralization of emotion (Heller & Levy, 1981). Of the 93 ELBW adult survivors eligible to participate, 7 were lost to follow-up, 4 refused the entire young adulthood study, and 11 either refused or were unable to be recalled to the laboratory. A total of 71 (76%) eligible ELBW adult survivors participated in the present study.
NBW
The normal birth weight (NBW) control group comprised 145 individuals who were selected from a random sample of children born at term and obtained from class lists of 8-year-old children from several local public school boards, and were of similar race, sex, and socioeconomic status distribution as the ELBW cohort (Szatmari et al., 1993). The controls were not followed prospectively from birth. Of the 145 NBW controls originally enrolled, we excluded those with neurosensory impairments (n = 3), non-right handed individuals (n = 27), and those who had epilepsy and multiple sclerosis at young adulthood (n = 2). Of the 113 eligible NBW control participants, 4 were lost to follow-up, 7 refused the entire young adulthood study, and 19 either refused or were unable to be recalled to the laboratory. A total of 83 (74%) NBW adults participated in the present study (see Table 1 for an overview).
Table 1.
ELBW and NBW Sample Overview
| ELBW | NBW |
|---|---|
| 179 survivors of Neonatal Intensive Care Unit |
|
| ⇓ | |
| 13 late deaths | |
| ⇓ | |
| 166 survivors to young adulthood |
145 enrolled at age 8 (no deaths) |
| exclusions | exclusions |
| 46 neurosensory impaired | 3 neurosensory impaired |
| 25 non-right handed | 27 non-right handed |
| 2 mental illness at young adulthood |
2 epilepsy and Multiple Sclerosis at young adulthood |
| eligible to participate | eligible to participate |
| ⇓ | ⇓ |
| 93 | 113 |
| 7 lost to follow-up | 4 lost to follow-up |
| 4 refused entire young adult study |
7 refused entire young adult study |
| 11 refused/unable to recall to lab |
19 refused/unable to recall to lab |
| ⇓ | ⇓ |
| 71 participated (76%) | 83 participated (73%) |
The 154 (ELBW, n = 71; NBW, n = 83) participants in the present study were part of a larger study examining transition to young adulthood (Saigal et al., 2006a, 2006b), health status (Saigal et al., 2007), and quality of life (Saigal et al., 2006c) of individuals born at ELBW. The analyses below focus on the measures of internalizing behavior problems, resting regional EEG activity and salivary cortisol collected during their young adulthood visit to our laboratory as well as measures of internalizing behavior problems collected during their middle childhood and adolescent follow-up visits.
Instruments and Measures
Internalizing behavior problems measured at middle childhood
Both cohorts of participants and their parents (ELBW: n = 64; NBW: n = 80) were asked to complete the Ontario Child Health Study (OCHS; see Boyle et al., 1987) at the middle childhood visit (see Szatmari et al., 1993). The OCHS Scales are comprised of a psychiatric checklist which measures conduct disorder, ODD, ADHD, overanxious disorder, separation anxiety, and depression based on criteria of the Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised (DSM-III-R; American Psychiatric Association, 1987). The externalizing problems scale is formed by summing the first three scales and the internalizing problems by summing the last three scales. The CBCL (Achenbach & Edelbrock, 1983) and the original OCHS scale (Boyle et al., 1987; Offord et al., 1987) provided the basic pools of items and additional items were added by consensus when necessary, to describe a particular disorder. Each item has 3 response categories “never or not true,” or “somewhat or sometimes true,” and “very or often true,” with scores of 0, 1 or 2, respectively. Respondents were asked to base their answer on the preceding 6 months. Of particular interest to the present study was in the internalizing behavior problems scale (i.e., overanxious disorder, separation anxiety, and depression). All three subscales were inter-related within age (p’s < .05). Accordingly, the three individual subscales were first z-scored and then summed to form a separate composite measure of middle childhood internalizing behavior problems. The composite measure of middle childhood internalizing behavior problems was then z-scored.
Internalizing behavior problems measured at adolescence
The participants in both cohorts (ELBW: n = 65; NBW: n = 74) were asked to complete the Ontario Child Health Study-Revised (OCHS-R; see Boyle et al., 1993) scales, which were self-administered at the adolescent visit (see Saigal et al., 2003). Of particular interest from the OCHS-R was the internalizing behavior problems scale (i.e., overanxious disorder + separation anxiety + depression). All three subscales were inter-related within age (p’s < .05). Accordingly, the three individual subscales were first z-scored and then summed to form a separate composite measure of adolescent internalizing behavior problems. The composite measure of middle childhood internalizing behavior problems was then z-scored.
Internalizing behavior problems measured at young adulthood
At the young adult visit, the participants in both cohorts (ELBW: n = 71; NBW: n = 82) completed 1) the CBCL Youth Self-Report (YSR; Achenbach, 1991) measure; of particular interest from the YSR was the internalizing behavior problems scale (social withdrawal + somatic complaints + anxiety/depression scales); 2) the Spielberg Trait Anxiety Inventory (Spielberger et al., 1983), which is a 20-item Likert-format measure that indexes trait anxiety (e.g., ‘I am a steady person’, ‘I lack self-confidence’); and 3) items related to social phobia from the interviewer administered World Organization Composite International Diagnostic Interview Short-Form (CIDI-SF; Kessler et al., 1998). Sample items include: Have you ever had such a strong, unreasonable fear of: (i) giving a speech or speaking in public, (ii) eating or drinking where someone could watch you, (iii) talking to people because you might have nothing to say or might sound foolish?, (iv) writing while someone watches, (v) taking part or speaking in a meeting or class, (vi) going to a party or other social outing? Response format is yes or no. The CBCL YSR Internalizing Scale, Spielberger Trait Anxiety, and CIDI-SF Social Phobia scale were all highly inter-related (p’s < .05). Accordingly, each was first z-scored and then added together to form a composite measure of young adulthood internalizing behavior problems. This composite measure was then z-scored.
Demographic risk
Information on demographic factors was obtained from the parents through parent report questionnaires at the middle childhood (see Szatmari et al., 1993), adolescent (see Saigal et al., 2003), and young adult visits. Parental socioeconomic status was measured using the Hollingshead index of social position (Hollingshead, 1969). Maternal education was assessed by responses to the OCHS questionnaire (Boyle et al., 1987).
Maternal and familial risk
Information on family factors was obtained from parent report questionnaires at the middle childhood (see Szatmari et al., 1993), adolescent (see Saigal et al., 2003), and young adulthood visits. Maternal mood was measured by a 10-item scale derived from the Bradburn Scale of positive and negative affect (Bradburn, 1969). This scale is comprised of 5 items which measure positive affect and five items which measure negative affect. Maternal depression was indexed using the Center for Epidemiologic Studies-Depression Scale (CES-D; Carpenter et al., 1998; Radloff, 1977). Maternal anxiety was measured by the Speilberger State-Trait Anxiety Inventory (Speilberger et al., 1983). Maternal social support was assessed using Social Support Index (SSI; McCubbin, Patterson, Glynn, 1987). The SSI measures degree of social networks and connectedness. Family functioning was measured by the 12-item general functioning scale derived from the McMaster Family Assessment Device (FAD; Byles, Byrne, Boyle, & Offord, 1998). This instrument assesses family functioning on six dimensions: problem solving, communication, roles, affective responsiveness, affective involvement, and behavior control.
Individual risk
Young adults from both cohorts completed the Social Support Index (SSI; McCubbin et al., 1987), the CES-D (Carpenter et al., 1998; Radloff, 1977), and the Rosenberg Self-esteem Short Form (Rosenberg, 1965). Body-mass index was measured by dividing participant weight by height, squared. Level of respiratory support (0-none to 4-high) at birth also was examined within the ELBW group.
Procedures
Upon arrival to the laboratory at the young adult visit, we explained the procedures to the participants and obtained written informed consent. After allowing the participants 30 min to adjust to the laboratory, three saliva samples were collected approximately 15 min apart. Then the participant was led to a room used for EEG testing. Regional EEG was recorded during a 2 min resting baseline [1 min eyes-open (EO), 1 min eyes-closed (EC)] condition. Studies have shown that EEG power estimates as short as 2 min have proven reliable (Allen et al., 2004; Schmidt, 2008).
After the EEG recording, the EEG cap was removed, and the participant was debriefed and provided with remuneration for participating. All procedures were conducted under the supervision of trained research staff and approved by the McMaster University Health Sciences Research Ethics Board. The study was conducted from 2001 to 2004.
Electroencephalogram (EEG) Procedures and Measures
EEG recording
Regional EEG was collected using a lycra stretch cap. The cap electrodes are positioned according to the 10/20 system of the International Federation (Jasper, 1958). The experimenter used the blunt end of a Q-tip in combination with an abrasive gel (Omni-prep) and gently abraded each electrode surface. Each electrode site was then filled with a small amount of electrolyte gel which served as a conductor. Electrode impedances below 10 K ohms per site and within 500 ohms between homologous sites were considered acceptable.
EEG was recorded from the left and right anterior and posterior regions of the scalp (i.e., mid-frontal: F3, F4; central: C3, C4). EEG activity was collected in posterior regions in order to examine if asymmetry differences were specific to the frontal region. All active EEG sites were referenced to Cz during acquisition.
A calibration signal (10 Hz/.47 V rms sine wave) was input through each amplifier prior to each data collection. The output of this signal was 50 uV, with a gain of 10,000. The five channels were amplified by individual SA Instrumentation Bioamplifiers, with filter settings for all channels set at 1 Hz (high pass) and 100 Hz (low pass). The data from all five channels were digitized on-line at a sampling rate of 512 Hz.
EEG data reduction and quantification
The EEG data were visually scanned for artifact due to movement (e.g., eye blinks, body movements) and edited out using software developed by James Long Company (EEG Analysis Program, Caroga Lake, NY). If artifact was present in one channel, then data in all channels were excluded. All artifact free EEG data were analyzed using a discrete Fourier transform (DFT), with a Hanning window of one sec width and 50% overlap. Regional EEG power (in uV2) was derived in the alpha (8 to 13 Hz) frequency band separately for the EO and EC conditions. It is well-documented that this EEG frequency band is linked to individual differences in stress vulnerability (see Coan & Allen, 2004; Davidson, 2000). Because EEG power in the EO and EC conditions was highly related for each of the sites (r’s > .80, p’s < .01), a composite measure of resting EEG alpha power was computed separately for each EEG site by averaging power in the EO and EC conditions. This aggregate measure is known to produce a more reliable estimate of EEG power and asymmetry than separate EO and EC conditions (see Tomarken et al., 1992). A separate EEG asymmetry measure was then computed for each region (e.g., F4 alpha power minus F3 alpha power). Because EEG power is inversely related to activity, negative values on the frontal asymmetry metric reflect greater relative right EEG activity (Davidson & Tomarken, 1989). The two study groups did not differ in the amount of EEG artifact data removed, or in the amount of artifact free EEG data used in the analyses.
Salivary Cortisol Procedures and Measures
Data collection
Each participant was asked to refrain from eating or drinking 1 hr prior to coming to the laboratory and to report any atypical recent life events, as these factors are known to influence cortisol levels. Each participant was asked to donate three individual saliva samples during the laboratory visit. Passive saliva was collected by having each participant expectorate into a 50 ul cryogenic tube. Approximately 50 ul of saliva were collected at each sample. The average time of day for saliva collection was approximately 1300 hr. The time of day that the three saliva samples were collected did not vary between groups or systematically with any of the other study measures.
Salivary cortisol assay determinations
All saliva samples were transported on ice and stored at −20 °C prior to assays. Saliva was centrifuged at 3000×g for 15 min, and only the supernatant was assayed in the Neuroendocriniology Laboratory at Brock University (St. Catharines, Ontario). All enzyme immunoassays were carried out on NUNC Maxisorb plates. Cortisol antibodies (R4866) and corresponding horseradish peroxidase conjugate were obtained from C. Munro of the Clinical Endocrinology Laboratory, University of California, Davis. Steroid standards were obtained from Steraloids, Inc. (Newport, Rhode Island). Each sample was assayed in duplicate and averages were used. Interplate variation (CV) was 6.45%, while intraplate variation was 6.51%. Because all three salivary cortisol samples were correlated (p’s < .05), they were aggregated and an average measure of baseline salivary cortisol (expressed in ng/ml) was computed. The natural log (ln) of this composite measure was then computed to reduce skewness.
Missing Data
Of the 154 participants, EEG data were missing from 21 participants (6 ELBW, 15 NBW), owing to several reasons: 6 (3 ELBW, 3 NBW) refused the EEG procedure; 10 (2 ELBW, 8 NBW) were excluded either due to equipment failure (n = 4) or excessive movement and motor artifacts rendered their EEG unusable (n = 6); and 5 (2 ELBW, 3 NBW) were excluded because they had extreme EEG power values +3SD above the mean. The ELBW and NBW groups did not differ, nor did males and females disproportionately differ in the number of participants excluded or without useable data.
Results
Preliminary Analyses
Sociodemographic risk factors
Socio-demographic data for the ELBW and NBW young adult groups are provided in Table 2. Separate chi-square analyses revealed that there were no significant differences in group membership for gender, parental social class, and educational status (p’s > .05). A between-groups t-test also revealed that the two groups did not differ on age at assessment at the young adult visit (p > .05). In addition, there were no significant between-group differences on any of these sociodemographic factors at their middle childhood (see Szatmari et al., 1993) and adolescent (see Saigal et al., 2003) visits.
Table 2.
Socio-Demographic Variables for the ELBW and NBW Groups
| Group | |||
|---|---|---|---|
| Measure | ELBW | NBW | |
| (n = 71) | (n = 83) | ||
| Birth Weight (in grams) | |||
| Mean | 874 | 3,395 | t = −44.04; p < .0001 |
| SD | 11 | 470 | |
| Gestational Age (in weeks) | |||
| Maximum | 34 | term | |
| Minimum | 23 | ||
| Mean | 27.59 | ||
| SD | 2.18 | ||
| Sex (F/M) | 41/30 | 48/35 | X2 = .001; p > .05 |
| Parental Social Classa | X2 = 2.39; p > .05 | ||
| I–II | 28 | 48 | |
| III | 23 | 15 | |
| IV–V | 17 | 18 | |
| Current highest education (%)b | X2 = 2.97; p > .05 | ||
| < High school | 7 | 5 | |
| Completed high school | 36 | 39 | |
| Partial postsecondary | 23 | 22 | |
| Completed university/college | 5 | 16 | |
| Age at adult assessment (in years) | |||
| Mean | 23.3 | 23.6 | t = −1.44; p > .05 |
| SD | 1.4 | 1.1 | |
Based on Hollingshead (1969) classification. Data were missing for 3 ELBW participants and 2 NBW participants.
Data were missing for 1 NBW participant.
Individual, maternal, and familial risk factors
A separate analysis of variance (ANOVA) with group (ELBW, NBW) as the between-groups factor was performed on the separate individual, maternal, and familial risk factors. These analyses revealed that the two groups did not significantly differ on individual social support, depression, self-esteem, and body-mass index, or on maternal social support, mood, depression, anxiety, social support, and family functioning measured at the young adult visit (p’s > .05). In addition, the two groups did not differ on these individual, maternal, and familial risk factors at their middle childhood (see Szatmari et al., 1993) or adolescent (see Saigal et al., 2003) visits.
Between-Group Analyses
Internalizing behavior problems at middle childhood, adolescence, and young adulthood
We predicted that individuals born at ELBW would exhibit significantly more internalizing behavior problems than those born at NBW. In order to test this prediction, we performed a repeated measures analysis of variance (ANOVA) with Group (ELBW, NBW) and Sex (male, female) as the between-groups factors and Age (Middle Childhood, Adolescence, Young Adulthood) as a within-groups factor. The dependent measure was the composite z-score measure of internalizing behavior problems.
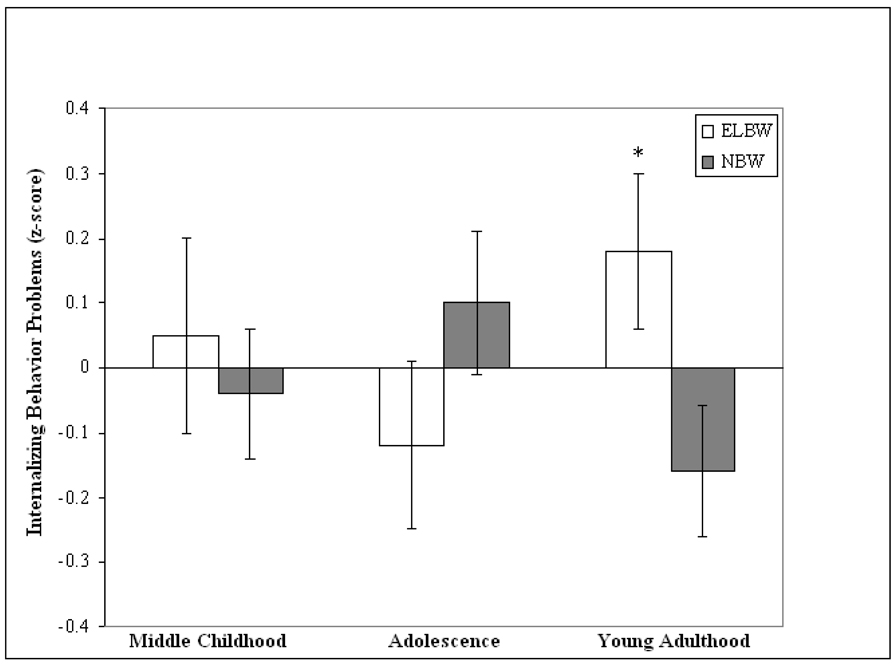
The analyses revealed a significant main effect for Sex, F(1, 129) = 4.81, p < .05. Overall, females (M = .114, SE = .08) exhibited significantly more internalizing behavior problems than males (M = −.153, SE = .09). The analyses also revealed a significant Group × Age interaction F(2, 128) = 4.74, p < .01 (see Figure 1).
Figure 1.
Standardized means (standard error bars) of internalizing problems in individuals born at extremely low birth weight (ELBW) and at normal birth weight (NBW) as children (ELBW: n = 64; NBW: n = 80) adolescents (ELBW: n = 65; NBW: n = 74), and young adults (ELBW: n = 71; NBW: n = 82; *between group difference, p < .05)
In order to decompose this interaction, we performed a separate ANOVA at each age, with Group (ELBW, NBW) as the between-groups factor. The dependent measure was the composite z-score measure of internalizing behavior problems at each age. As predicted, the analyses revealed a significant main effect for Group on internalizing behavior problems only at young adulthood, F(1, 131) = 4.97, p < .05, Cohen’s d = 0.35 (see Figure 1). ELBW adults (M = .21, SE = .14) exhibited significantly more internalizing behavior problems as young adults than their NWB peers (M = −.19, SE = .11). Pairwise t-tests also revealed that, as predicted, ELBW adults showed a significant increase in internalizing behavior problems from adolescence (M = −.11, SE = .13) to young adulthood (M = .21, SE = .14), t(60) = 2.19, p < .05, Cohen’s d = 0.28, while NBW adults showed a significant decrease in internalizing behavior problems from adolescence (M = .11, SE = .11) to young adulthood (M = −.19, SE = .11), t(71) = 2.25, p < .05, Cohen’s d = 0.29. There were no significant between-group differences on internalizing behavior problems at middle childhood (p > .05) or adolescence (p > .05).
Resting frontal brain activity (EEG) at young adulthood
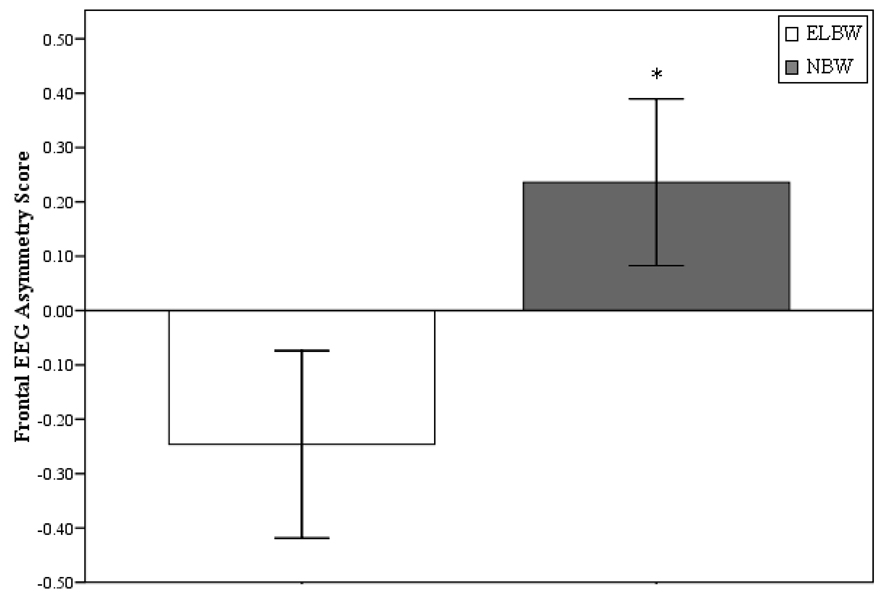
We predicted that adults born at ELBW would exhibit significantly greater relative right frontal EEG activity at rest compared with adults born at NBW. In order to test this prediction, we computed a frontal EEG asymmetry (right frontal power – left frontal power) metric. An ANCOVA with Group (ELBW, NBW) as the between-groups factor and concurrent depression ratings (CES-D measure) as a covariate was then performed on the frontal EEG asymmetry metric. Concurrent depression was covaried because concurrent mood is known to influence frontal asymmetry measures (see Beaton et al., 2008; Henriques & Davidson, 1990). This analysis revealed a significant main effect for Group on resting frontal EEG asymmetry measure before F(1, 131) = 4.39, p < .05; Cohen’s d = 0.37 and after F(1, 129) = 3.95, p = .05; Cohen’s d = 0.36, controlling for concurrent depression ratings (see Figure 2). As predicted, adults born at ELBW (M = −.25, SE = .17) exhibited significantly greater relative right frontal EEG activity at rest compared with adults born at NBW (M = .24, SE = .17).
Figure 2.
Mean difference (standard error bars) between individuals born at extremely low birth weight (ELBW; n = 65) and at normal birth weight (NBW; n = 68) on the pattern of resting frontal EEG alpha (8 to 13 Hz) asymmetry score (i.e., right frontal power minus left frontal power). Note: EEG power is inversely related to activity so negative scores reflect greater relative right frontal EEG activity; *between group difference, p < .05)
In order to understand the source of the asymmetry difference, a repeated measures ANOVA was computed with Group (ELBW, NBW) and Sex (male, female) as the between-groups factors and Region (Frontal, Central) and Hemisphere (left, right) as the within-groups factors. The dependent measure was EEG alpha (8 to 13 Hz) power (uV2). There were no significant main or interaction effects for Sex, so this variable was collapsed in all subsequent analyses. The analyses revealed a significant Group × Region × Hemisphere interaction, F(1, 131) = 4.59, p < .05.
To decompose this interaction, we performed a separate ANOVA for each region with Group (ELBW, NBW) as the between-subjects factor and Hemisphere (left, right) as the within-subjects factor. The dependent measure was EEG alpha (8 to 13 Hz) power (uV2). The analyses revealed a significant Group × Hemisphere interaction on alpha power only in the frontal region, F(1, 131) = 4.39, p < .05, confirming the frontal asymmetry difference above. The ANOVA with Group and Hemisphere as between-subjects factors on EEG power for the central region was not significant, (p > .05). Follow-up between-group and pairwise t-tests computed on left and right frontal and central EEG power failed to yield any significant differences (p’s > .05).
Baseline salivary cortisol at young adulthood
Table 3 presents the descriptive statistics on the salivary cortisol measures collected for the two groups. We predicted that ELBW adults would exhibit significantly higher salivary cortisol at baseline compared with adults born at NBW.
Table 3.
Descriptive statistics for the ELBW and NBW Young Adults on the Salivary Cortisol Measures
| Group | |||
|---|---|---|---|
| Measure | ELBW | NBW | |
| ln Baseline Salivary Cortisol | |||
| Mean (in ng/ml) | .962 | 1.047 | t = −0.37; p > .05 |
| SE | .150 | .171 | |
| Average Time of Day of Saliva Collection | |||
| Mean (in hr) | 13:25 | 13:42 | t = −0.40; p > .05 |
| SE (in min) | 0:29 | 0:17 | |
An ANCOVA with Group (ELBW, NBW) and Sex (male, female) as the between-groups factors was computed on the average baseline salivary cortisol measure. Time of day was included as a co-variate in the salivary cortisol analysis, given that it is known to be related to cortisol levels (Daly & Evans, 1974). The analysis failed to reveal any significant main or interaction effects on the average natural log baseline salivary cortisol measure (p > .05).
Within-Group Analyses
Full sample analyses
Table 4 presents the Pearson correlations among the study measures for the full sample. As can be seen in Table 4, internalizing behavior problems at middle childhood and young adulthood (r = .21, p < .01) and at adolescence and young adulthood (r = .33, p < .001) were significantly correlated. As expected, children and adolescents who exhibited internalizing behavior problems were likely to display internalizing behavior problems as young adults.
Table 4.
Pearson Correlations Among Study Measures Across Participants
| Measure | 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|---|
| 1. Child Internalizing Problems | --- | |||||
| 2. Adolescent Internalizing Problems | .06 | --- | ||||
| 3. Young Adult Internalizing Problems | .21*** | .33*** | --- | |||
| 4. Resting Frontal EEG Alpha Asymmetry | .11 | −.11 | −.14* | --- | ||
| 5. ln Baseline Salivary Cortisol | −.01 | .02 | .04 | −.20** | --- |
Note:
p < .01;
p < .05;
p < .06;
time of day was partialled out of all correlations involving the ln baseline salivary cortisol measure; EEG power is inversely related to activity so negative scores reflect greater relative right frontal EEG activity.
Frontal EEG asymmetry at rest was related to concurrent internalizing behavior problems (r = −14, p < .06) and baseline salivary cortisol (r = −.20, p < .05) at the young adult visit, although the former relation only approached statistical significance. As predicted, adults who exhibited greater relative right frontal EEG activity also tended to exhibit more internalizing behavior problems and higher baseline salivary cortisol levels contemporaneously.
Within-ELBW group analyses
We computed a series of within-group Pearson correlations within the ELBW group in order to examine if the two measures of stress vulnerability (i.e., resting frontal EEG asymmetry and baseline salivary cortisol) covaried in any systematic way with adverse early life events (e.g., birth weight, gestational age and respiratory support). Table 5 presents the within-group Pearson correlations among the study measures for the ELBW group.
Table 5.
Pearson Correlations Among Study Measures Within Participants in the ELBW Group
| Measure | 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|---|
| 1. Birth weight | --- | |||||
| 2. Gestation Age | .34* | --- | ||||
| 3. Respiratory Support Level at Birth | −.39* −.23 | --- | ||||
| 4. Resting Frontal EEG Alpha Asymmetry | .22* | .03 | −.08 | --- | ||
| 5. ln Baseline Salivary Cortisol | .01 | .01 | −.08 | −.23* | --- |
Note:
p < .05;
time of day was partialled out of all correlations involving the log baseline salivary cortisol measure; EEG power is inversely related to activity so negative scores reflect greater relative right frontal EEG activity.
As can be seen in Table 5, resting frontal EEG asymmetry was related to baseline salivary cortisol (r = −.23, p < .05), and birth weight (r = .22, p < .05), but not gestation age (r = .03, p > .05) or level of respiratory support at birth (r = −.08, p > .05). As predicted, ELBW adults who exhibited greater relative right frontal EEG activity at rest were likely to exhibit higher salivary cortisol levels and lower birth weights.
Discussion
Pre- and peri-natal risk factors such as being born at extremely low birth weight are presumed to influence brain-behavioral relations. We examined internalizing behavior problems at middle childhood, adolescence, and young adulthood and patterns of resting frontal brain activity and baseline salivary cortisol in a sample of adult survivors of ELBW without major impairments. As predicted, we found that internalizing behavior problems significantly increased from adolescence to young adulthood among ELBW adults. Also as predicted, we found that ELBW young adults exhibited significantly greater relative right frontal EEG activity and more internalizing behavior problems than NBW controls. These findings were based, overall, on medium effect sizes and are consistent with a large corpus of literature suggesting a relation between right frontal brain asymmetry at rest and individual differences in stress vulnerability (see Coan & Allen, 2004; Davidson, 2000; Fox, 1991, for substantive reviews). Here we extend these earlier findings on resting frontal EEG asymmetry as a stress vulnerability marker to a sample of adults born at ELBW without major impairments.
Why did differences in internalizing behaviors emerge in young adulthood and not earlier in development in ELBW adults? It is possible that there may be subtle influences on brain development due to adverse events early in life that may only manifest later in life. A recent study reported reduced fertility in adult survivors of extreme prematurity (Swamy et al., 2008). It is possible that adverse life events such as being born at extremely low birth weight may negatively impact brain-behavior relations among ELBW survivors only later in life at developmental transition points. One such developmental transition is young adulthood, which is characterized by psychosocial stressors such as the development of personal autonomy, striving for financial independence, career development, romantic relationships, and starting a family. This speculation needs to be tempered, however, in that a recent study noted pervasive behavior problems at 6 years of age in a sample of children born extremely premature (i.e., ≤ 25; Samara, Marlow, & Wolke, 2008), so behavioral problems may exist in extremely premature populations before adulthood. However, it is also important to note that the study by Samara et al. included a heterogenous sample of premature children likely including children with impairments. Such impairments may have contributed to their behavioral problems at younger ages. In the present study, we used a non-impaired sample of adult survivors by design to mitigate some potential confounds due to impairment.
We also found that right frontal EEG asymmetry predicted concurrent internalizing behavior problems and cortisol levels across the full sample, and was significantly related to higher cortisol levels and lower birth weights among ELBW adults. These results are consistent with findings from studies of stress responses in humans (Buss et al., 2003) and nonhuman primates (Kalin et al., 1998). Buss and her colleagues (2003) found that human infants with high basal cortisol levels exhibited more freezing behavior in response to novel stimuli and greater relative right frontal EEG activity at age 6 months than infants with relatively lower levels of basal cortisol. Kalin and his group reported that nonhuman primate Rhesus monkeys who exhibited right frontal EEG activity also displayed high basal cortisol levels and fearful behavior (Kalin et al., 1998). Taken together, these two studies suggest that (1) the pattern of resting right frontal EEG asymmetry is predictive of high baseline cortisol and stress responses, (2) this pattern emerges early in life, and (3) it plays a role in stress dysregulation that is conserved across mammals. The results of the present study extend these findings to adult survivors of extremely low birth weight.
What does resting right frontal brain asymmetry and its relation to high cortisol reflect in extremely low birth weight? Over the last three decades, there have been more than 100 studies published that have examined patterns of frontal EEG activity during the processing of emotion and its role in explaining individual differences in affective style (see Coan & Allen, 2004, for a review). Overall, these studies suggest that individuals exhibit greater relative left frontal EEG during the processing of positive emotion (e.g., happy, joy), and greater relative right frontal EEG activity during the processing of negative emotion (e.g., fear, sadness). These patterns are evidenced across a variety of sensory modalities used to induce emotion, including visual, auditory, imagery, and olfactory senses. The findings from these studies also suggest that the pattern of resting frontal EEG activity emerges in the first months of post-natal life, remains moderately stable during development from childhood through adolescence and young adulthood, and predicts individual differences in affective style and risk for psychopathology. Individuals who present with tonic left frontal EEG activity tend to be social, extraverted and outgoing, whereas individuals who exhibit tonic right frontal EEG activity are shy, anxious and depressed.
It is possible that the pattern of greater relative right frontal EEG activity may constitute a mechanism predisposing individuals to difficulties in regulating stress, such as physical and mental problems. The pattern of right frontal EEG asymmetry observed in adult survivors of ELBW without major impairments might be due to adverse events early in life that shaped brain development in a relatively subtle way. Adverse early experiences are known to alter the development of the prefrontal brain regions, resulting in differences in right, but not left, adult prefrontal volumes in nonhuman primates (Lyons, Afarian, Schatzberg, Sawyer-Glover, & Moseley, 2002). The observed relation between right frontal EEG asymmetry and high cortisol in young adults born at ELBW may reflect a less obvious impairment due to this adverse event early in life, placing some young adults born at ELBW at risk for stress-related problems.
What may account for the lack of significant group differences on the salivary cortisol measure used in the present study? Because the HPA axis is a dynamic system, it is possible that individuals despite having similar basal salivary cortisol, may have different adrenocorticotrophic hormone (ACTH) levels. That is, although ELBW and NBW adults have similar cortisol levels (possibly for down-regulation of adrenal ACTH receptors); it is possible that ELBW participants have greater ACTH levels than controls. This hypothesis is in line with what others have reported in nondepressed adult survivors of childhood trauma (see Gunnar & Quevedo, 2007; Heim, et al, 2000).
It also may be that adverse events early in life such as low birth weight only perturb the neuroendocrine system early in post-natal life on reactivity measures (see Wust et al., 2005) and not on basal measures. Or, by young adulthood, development and maturational control of this system may have been possibly repaired. The lack of group differences also might be related to issues of differential risk and resiliency and specificity of different stress vulnerability systems. The impact of extremely low birth weight may affect some, but not all, brain-based aspects of stress vulnerability in the same way. Some brain systems may be more vulnerable to the impact of adverse early life events than others.
Theoretical Implications
The findings from the present study may be interpreted by, and have theoretical implications for, at least two models of development. One model of development is a brain “insult” hypothesis. It is possible that the events producing reduced birth weight also have perturbed select brain systems involved in the regulation of stress. Right frontal brain asymmetry and internalizing behavior problems may develop as a result of early brain insult in survivors without major impairments. We found that lower birth weight predicted right frontal brain asymmetry at young adulthood in ELBW survivors without impairments.
It is important to note, however, that there is an emerging literature suggesting another variation of a brain insult model. That is, the brain develops quite differently at extreme prematurity if the infant is raised extra-uterine (e.g., cortical folding), resulting in the building of a different brain (e.g., Kapellou et al., 2006). This idea would predict that the impact is not specific to any one function, but influences general domains of functioning (e.g., language difficulties and school achievement) as has been shown for both very and extremely premature children (e.g., Wolke, Samara, Bracewell, & Marlow, 2008).
A second model that the present results may inform is a fetal programming hypothesis (Barker, 1995). Although controversial, Barker posited that stressful events during fetal development may “program” the developing brain for how it handles subsequent stress. A proposed mechanism is perturbations in the hypothalamic-pituitary-adrenal axis (HPA-axis) due to early life events, which contribute to chronically elevated cortisol levels (Fall et al., 2002) and autonomic responses (Phillips & Jones, 2006). This early programming places individuals born extremely premature at risk for disease (Reynolds et al., 2001) and may influence individual differences in temperamental style (Pesonen et al., 2006). However, other studies have not found support for the fetal programming hypothesis. For example, Hanna and her colleagues failed to find chronically elevated cortisol levels in infant survivors of ELBW (Hanna et al., 1993). Hanna et al. argued that the relatively low levels of cortisol noted in infants born at ELBW may be due to the extremely premature brain’s inability to recognize the stress of the illness or because of inadequate hypothalamic secretion of corticotropin-releasing hormone (CRH). If the fetal programming hypothesis were correct, we would have expected to find between group differences on salivary cortisol levels. As noted above, we did not find between-group differences on the cortisol measure. It might be that although there were no differences between the groups on cortisol, the differences might exist at the level of ACTH. However, we did not index ACTH, but future studies should consider doing so to test this hypothesis. The results of the present study also suggest that stress early in life may alter fetal programming of specific stress vulnerability measures (e.g., resting frontal brain asymmetry), but not others (e.g., baseline salivary cortisol).
Limitations
There are at least four limitations in the present study. First, we recorded resting frontal EEG asymmetry and baseline salivary cortisol levels on one occasion only. Accordingly, we do not have information on the stability of these measures or information on how frontal EEG asymmetry may or may not covary with internalizing behavior problems at earlier ages. Although empirical evidence suggests that the pattern of resting frontal brain activity is stable across time (Tomarken et al., 1992; Vuga et al., 2006, 2008), it would be advisable to have repeated measures over time and earlier in post-natal development. Second, EEG is measured at the scalp and thought to reflect cortical electrical activity. However, we do not know the exact sources of the EEG signal so caution needs to be exercised in terms of interpretation of EEG findings as being specific to the cerebral cortex and a particular brain region. Future studies should consider the use of more dense arrays of EEG electrodes than used in the present study so that EEG source analyses can be performed. As well, measures of functional magnetic imaging might prove useful in combination with electrocortical measures to provide accurate spatial and temporal resolution of brain-based measures of stress vulnerability. Third, issues of causation cannot be ascertained. Although we presume that the adverse early life event of being born extremely low birth weight preceded the development of right frontal EEG asymmetry, we need to caution against making any kind of causal interpretation. It might be that right frontal EEG asymmetry is a consequence of extremely low birth weight, or a co-existing factor. Tracking brain development at pre- and early post-natal stages may hold some of the answers to questions of causality. Fourth, although we studied adult survivors of extremely low birth weight, there are other populations characterized by early adversity that are of interest. For example, survivors of very low birth weight (Hack et al., 2002), babies born small for their gestational age (Strauss, 2000), and babies born prematurely (Schothorst, Swaab-Barneveld, & van Engeland, 2007) are also known to be at risk for stress-related problems. In future studies examining multiple brain-based measures of stress vulnerability, these populations would further inform our understanding of the relation between early adversity and stress vulnerability.
Conclusions
Pre- and peri-natal risk factors such as being born at extremely low birth weight are known to have a profound impact on a range of developmental processes. We found that internalizing behavior problems significantly increased from adolescence to young adulthood among ELBW individuals without major impairments. We also found that ELBW adults exhibited significantly more internalizing behavior problems and greater relative right frontal EEG activity at rest than NBW controls. Greater relative right frontal EEG activity was related to higher cortisol levels and lower birth weights among ELBW adults. Findings suggest that extremely low birth weight may negatively influence brain-behavior relations in subtle ways even among survivors without major impairments and that evidence of these influences may not emerge until young adulthood. Extremely low birth weight may influence the development of right frontal brain asymmetry, a putative mechanism for difficulties regulating stress responses, which can manifest as internalizing behavior problems in young adulthood.
Acknowledgments
This research was supported by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Social Science and Humanities Research Council of Canada (SSHRC) awarded to Louis Schmidt; a pre-doctoral fellowship from NSERC awarded to Vladimir Miskovic under the direction of Louis Schmidt; and grants from Canadian Institutes of Health and Research (CIHR; Grant #MOP42536) and National Institute of Child Health and Human Development (NICHD; Grant #RO1HD40219) awarded to Saroj Saigal and her colleagues. We wish to thank the participants for their continued cooperation; Lindsay Bennett, Sylvia Nowakowski, Caroline Parkin, and Diane Santesso for their help with data collection; Lorraine Hoult and Barbara Stoskopf for arranging the assessments; and Alison Niccols for helpful comments on earlier drafts.
References
- Achenbach TM. Manual for the Child Behavior Checklist. Burlington: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- Achenbach TM, Edelbrock C. Manual for the Child Behavior Checklist and Revised Behavioral Profile. Burlington: University of Vermont, Department of Psychiatry; 1983. [Google Scholar]
- Allen JJ, Urry HL, Hitt SK, Coan JA. The stability of resting frontal electroencephalographic asymmetry in depression. Psychophysiology. 2004;41:269–280. doi: 10.1111/j.1469-8986.2003.00149.x. [DOI] [PubMed] [Google Scholar]
- Allin M, Rooney M, Cuddy M, Wyatt J, Walshe M, Rifkin L, Murray R. Personality in young adults who are born preterm. Pediatrics. 2006;117:309–316. doi: 10.1542/peds.2005-0539. [DOI] [PubMed] [Google Scholar]
- Als H. The preterm infant: A model for the study of fetal brain expectation. In: Lecanuet JP, Fifer WP, Krasnegor NA, Smotherman WP, editors. Fetal development: A psychobiological perspective. Hillsdale, NJ: Erlbaum; 1995. pp. 439–472. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd Edition-Revised. Washington, DC: Author; 1987. [Google Scholar]
- Barker DJP. Fetal origins of coronary heart disease. British Medical Journal. 1995;311:171–174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaton EA, Schmidt LA, Ashbaugh AR, Santesso DL, Antony MM, McCabe RE. Resting and reactive frontal brain electrical activity (EEG) among a non-clinical sample of socially anxious adults: Does concurrent depressive mood matter? Neuropsychiatric Disease and Treatment. 2008;4:187–192. doi: 10.2147/ndt.s1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckwith L, Parmelee AH., Jr EEG patterns of preterm infants, home environment, and later IQ. Child Development. 1986;57:777–789. [PubMed] [Google Scholar]
- Botting N, Powls A, Cooke RWI, Marlow N. Attention deficit hyperactivity disorders and other psychiatric outcomes in very low birth weight children at 12 years. Journal of Child Psychology & Psychiatry. 1997;38:931–941. doi: 10.1111/j.1469-7610.1997.tb01612.x. [DOI] [PubMed] [Google Scholar]
- Boyle MH, Offord DR, Hofmann HG, Catlin GP, Byles JA, Cadman DT, Crawford JW, Links PS, Rae-Grant NI, Szatmari P. Ontario Child Health Study: Methodology. Archives of General Psychiatry. 1987;44:826–831. doi: 10.1001/archpsyc.1987.01800210078012. [DOI] [PubMed] [Google Scholar]
- Boyle MH, Offord DR, Racine Y, Fleming JE, Szatmari P, Sanford M. Evaluation of the revised Ontario Child Health Study Scales. Journal of Child Psychology and Psychiatry. 1993;34:189–213. doi: 10.1111/j.1469-7610.1993.tb00979.x. [DOI] [PubMed] [Google Scholar]
- Bradburn NM. The structure of psychological well-being. Chicago, IL: Aldine; 1969. [Google Scholar]
- Buss KA, Schumacher JR, Dolski I, Kalin NH, Goldsmith HH, Davidson RJ. Right frontal brain activity, cortisol, and withdrawal behavior in 6-month-old infants. Behavioral Neuroscience. 2003;117:11–20. doi: 10.1037//0735-7044.117.1.11. [DOI] [PubMed] [Google Scholar]
- Byles J, Byrne C, Boyle MH, Offord DR. Ontario Child Health Study: Reliability and validity of the general functioning subscale of the McMaster Family Assessment Device. Family Process. 1988;27:97–104. doi: 10.1111/j.1545-5300.1988.00097.x. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Fox NA, Marshall TR. Behavioral and physiological antecedents of inhibited and uninhibited behavior. Child Development. 1996;67:523–540. [PubMed] [Google Scholar]
- Carpenter JS, Andykowski MA, Wilson J, Hall LA, Rayens MK, Sachs B, Cunningham LLC. Psychometrics for two short forms of the Center for Epidemiologic Studies-Depression Scale. Issues Mental Health Nursing. 1998;19:481–494. doi: 10.1080/016128498248917. [DOI] [PubMed] [Google Scholar]
- Chapieski ML, Evankovich KD. Behavioral effects of prematurity. Seminar in Perinatology. 1997;21:221–239. doi: 10.1016/s0146-0005(97)80065-1. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJB. Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology. 2004;67:7–49. doi: 10.1016/j.biopsycho.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Cooke RW. Health, lifestyle, and quality of life in young adults born very preterm. Archives of Disease in Childhood. 2004;89:201–206. doi: 10.1136/adc.2003.030197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl LB, Kaaresen PI, Tunby J, Handegard BH, Kvernmo S, Ronning JA. Emotional, behavioral, social, and academic outcomes in adolescents born with very low birth weight. Pediatrics. 2006;118:e449–e459. doi: 10.1542/peds.2005-3024. [DOI] [PubMed] [Google Scholar]
- Daly JR, Evans JI. Daily rhythms of steroid and associated pituitary hormones in man and their relationship to sleep. In: Biggs MH, Christie GA, editors. Advances in steroid biochemistry and pharmacology. New York: Academic Press; 1974. pp. 61–109. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. The neuropsychology of emotion and affective style. In: Lewis M, Haviland JM, editors. Handbook of emotion. New York: Guilford; 1993. pp. 143–154. [Google Scholar]
- Davidson RJ. Affective style, psychopathology, and resilience: Brain mechanisms and plasticity. American Psychologist. 2000;55:1196–1214. doi: 10.1037//0003-066x.55.11.1196. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Tomarken AJ. Laterality and emotion: An electrophysiological approach. In: Boller F, Grafman J, editors. Handbook of neuropsychology. New York: Elsevier; 1989. pp. 419–441. [Google Scholar]
- Duffy FH, Als H, McNulty GB. Behavioral and electrophysiological evidence for gestation age effects in healthy preterm and fullterm infants studied two weeks after expected due date. Child Development. 1990;61:1271–1286. [PubMed] [Google Scholar]
- Ellingson RJ, Dutch SJ, McIntire MS. EEG’s of prematures: 3–8 year follow-up study. Developmental Psychobiology. 1974;7:529–538. doi: 10.1002/dev.420070605. [DOI] [PubMed] [Google Scholar]
- Fall CHD, Dennison E, Cooper C, Pringle J, Kellinggray SD, Hindmarsh P. Does birth weight predict adult serum cortisol concentrations? Twenty-four-hour profiles in the United Kingdom 1920–1930 Hertfordshire Birth Cohort. The Journal of Clinical Endocrinology & Metabolism. 2002;87:2001–2007. doi: 10.1210/jcem.87.5.8469. [DOI] [PubMed] [Google Scholar]
- Fox NA. If it’s not left, it’s right: Electroencephalograph asymmetry and the development of emotion. American Psychologist. 1991;46:863–872. doi: 10.1037//0003-066x.46.8.863. [DOI] [PubMed] [Google Scholar]
- Fox NA. Dynamic cerebral processes underlying emotion regulation. The development of emotion regulation: Behavioral and biological considerations. In: Fox NA, editor. Monographs of the Society for Research in Child Development. Vol. 59. 1994. pp. 152–166. (2–3 Serial No. 240) [PubMed] [Google Scholar]
- Fox NA, Bell MA, Calkins SD. Neural plasticity and development in the first two years of life: Evidence from cognitive and socioemotional domains of research. Development and Psychopathology. 1994;6:677–696. [Google Scholar]
- Fox NA, Bell MA, Jones NA. Individual differences in response to stress and cerebral asymmetry. Developmental Neuropsychology. 1992;8:161–184. [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Development. 2001;72:1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Fox NA, Rubin KH, Calkins SD, Marshall TR, Coplan RJ, Porges SW, Long JM, Stewart S. Frontal activation asymmetry and social competence at four years of age. Child Development. 1995;66:1770–1784. [PubMed] [Google Scholar]
- Fox NA, Schmidt LA, Calkins SD, Rubin KH, Coplan RJ. The role of frontal activation in the regulation and dysregulation of social behavior during the preschool years. Development and Psychopathology. 1996;8:89–102. [Google Scholar]
- Gold PW, Drevets WC, Charney DS. New insights into the role of cortisol and the glucocorticoid receptor in severe depression. Biological Psychiatry. 2002;52:381–385. doi: 10.1016/s0006-3223(02)01480-4. [DOI] [PubMed] [Google Scholar]
- Gold PW, Goodwin FK, Chrousos GP. Clinical and biochemical manifestations of depression (Part I) New England Journal of Medicine. 1988a;319:348–353. doi: 10.1056/NEJM198808113190606. [DOI] [PubMed] [Google Scholar]
- Gold PW, Goodwin FK, Chrousos GP. Clinical and biochemical manifestations of depression (Part II) New England Journal of Medicine. 1988b;319:413–420. doi: 10.1056/NEJM198808183190706. [DOI] [PubMed] [Google Scholar]
- Grunau RE, Whitfield MF, Fay TB. Psychosocial and academic characteristics of extremely low birth weight (≤ 800 g) adolescents who are free of major impairment compared with term-born control subjects. Pediatrics. 2004;114:e725–e732. doi: 10.1542/peds.2004-0932. [DOI] [PubMed] [Google Scholar]
- Gunnar MR. Psychoendocrine studies of temperament and stress in early childhood: Expanding current models. In: Bates JE, Wachs TD, editors. Temperament: Individual differences at the interface of biology and behavior. Washington, DC: American Psychological Association; 1994. pp. 175–198. [Google Scholar]
- Gunnar MR. Early adversity and the development of stress reactivity and regulation. In: Nelson CA, editor. The effects of adversity on neurobehavioral development: Minnesota symposium on child psychology. Vol. 31. Mahwah, NJ: Lawrence Erlbaum; 2000. pp. 163–200. [Google Scholar]
- Gunnar MR, Mangelsdorf S, Larson M, Hertsgaard L. Attachment, temperament, and adrenocortical activity in infancy: A study of psychoendocrine regulation. Developmental Psychology. 1989;25:355–363. [Google Scholar]
- Gunnar MR, Morison SJ, Chisholm K, Schuder M. Salivary cortisol levels in children adopted from Romanian orphanages. Development and Psychopathology. 2001;13:611–628. doi: 10.1017/s095457940100311x. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annual Review of Psychology. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Tagle NM. Neuroendocrine measures in developmental research. In: Schmidt LA, Segalowitz SJ, editors. Developmental psychophysiology: Theory, systems, and methods. New York: Cambridge University Press; 2008. pp. 343–364. [Google Scholar]
- Hack M, Cartar L, Schluchter M, Klein N, Forrest CB. Self-perceived health, functioning and well-being of very low birth weight infants at age 20 years. Journal of Pediatrics. 2007;151:635–641. doi: 10.1016/j.jpeds.2007.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack M, Flannery D, Schluchter M, Cartar L, Borawski E, Klein N. Outcomes in young adulthood for very-low-birth-weight infants. New England Journal of Medicine. 2002;346:149–157. doi: 10.1056/NEJMoa010856. [DOI] [PubMed] [Google Scholar]
- Hack M, Klein NK, Taylor HG. Long-term developmental outcomes of low birth weight infants. The Future of Children. 1995;5:176–196. [PubMed] [Google Scholar]
- Hack M, Youngstrom EA, Cartar L, Schluchter M, Taylor HG, Flannery D, Klein N, Borawski E. Behavioral outcomes and evidence of psychopathology among very low birth weight infants at age 20 years. Pediatrics. 2004;114:932–940. doi: 10.1542/peds.2003-1017-L. [DOI] [PubMed] [Google Scholar]
- Hack M, Youngstrom EA, Cartar L, Schluchter M, Taylor GH, Flannery DJ, Klein N, Borawski E. Predictors of internalizing symptoms among very low birth weight young women. Journal of Developmental & Behavioral Pediatrics. 2005;26:93–104. doi: 10.1097/00004703-200504000-00004. [DOI] [PubMed] [Google Scholar]
- Hanna CE, Keith LD, Colasurdo MA, Buffkin DC, Laird MR, Mandel SH, Cook DM, LaFranchi SH, Reynolds JW. Hypothalamic pituitary adrenal function in the extreme low birth weight infant. Journal of Clinical Endocrinology and Metabolism. 1993;76:384–387. doi: 10.1210/jcem.76.2.8381799. [DOI] [PubMed] [Google Scholar]
- Hayakawa M, Okumura A, Hayakawa F, Watanabe K, Ohshiro M, Kato Y, Takahaski R, Tauchi N. Background electroencephalographic (EEG) activities of very preterm infants born at less than 27 weeks gestation: A study on the degree of continuity. Archives of Disease in Childhood, Fetal Neonatology Edition. 2001;84:F163–F167. doi: 10.1136/fn.84.3.F163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Newport J, Heit S, Graham YP, Wilcox M, Bonsall R, Miller A, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. Journal of the American Medical Association. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Heller W, Levy J. Perception and expression of emotion in right-handers and left-handers. Neuropsychologia. 1981;19:263–272. doi: 10.1016/0028-3932(81)90110-x. [DOI] [PubMed] [Google Scholar]
- Henriques JB, Davidson RJ. Regional brain electrical asymmetries discriminate between previously depressed and healthy controls. Journal of Abnormal Psychology. 1990;99:22–31. doi: 10.1037//0021-843x.99.1.22. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Two-factor index of social position (mimeograph) New Haven CT: Yale University Press; 1969. [Google Scholar]
- Indredavik MS, Vik T, Heyerdahl S, Kulseng S, Brubakk AM. Psychiatric symptoms in low birth weight adolescents, assessed by screening questionnaires. European Child & Adolescent Psychiatry. 2005;14:226–236. doi: 10.1007/s00787-005-0459-6. [DOI] [PubMed] [Google Scholar]
- Jasper HH. The ten-twenty electrode system of the International Federation. Electroencephalography and Clinical Neurophysiology. 1958;10:371–375. [PubMed] [Google Scholar]
- Jetha MK, Schmidt LA, Goldberg JO. Long-term stability of resting frontal EEG alpha asymmetry and power in a sample of stable community outpatients with schizophrenia. International Journal of Psychophysiology. 2008 doi: 10.1016/j.ijpsycho.2008.12.011. In press. [DOI] [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Snidman N. The physiology and psychology of behavioral inhibition in children. Child Development. 1987;58:1459–1473. [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Snidman N. Biological basis of childhood shyness. Science. 1988;240:167–171. doi: 10.1126/science.3353713. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Larson C, Shelton SE, Davidson RJ. Asymmetric frontal brain activity, cortisol, and behavior associated with fearful temperament in Rhesus monkeys. Behavioral Neuroscience. 1998;112:286–292. doi: 10.1037//0735-7044.112.2.286. [DOI] [PubMed] [Google Scholar]
- Kapellou O, Counsell SJ, Kennea N, Dyet L, Saeed N, Stark J, et al. Abnormal cortical development after premature birth shown by altered allometric scaling of brain growth. PLoS Med. 2006;3:e265. doi: 10.1371/journal.pmed.0030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Andrews G, Mroczek D, Ustun B, Wittchen HU. The World Health Organization Composite International Diagnostic Interview Short-Form (CIDI-SF) International Journal of Methods Psychiatric Research. 1998;7:171–185. doi: 10.1002/mpr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy-Schiff R, Einat G, Har-Even D, Mogilner M, Mogilner S, Lerman M, Krikler R. Emotional and behavioral adjustment in children born prematurely. Journal of Clinical Child Psychology. 1994;23:323–333. [Google Scholar]
- Lorenz JM. The outcome of extreme prematurity. Seminars in Perinatology. 2001;25:348–359. doi: 10.1053/sper.2001.27164. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Afarian H, Schatzberg AF, Sawyer-Glover A, Moseley ME. Experience-dependent asymmetric variation in primate prefrontal morphology. Behavioural Brain Research. 2002;136:51–59. doi: 10.1016/s0166-4328(02)00100-6. [DOI] [PubMed] [Google Scholar]
- McCubbin H, Patterson J, Glynn T. Social support index (SSI) In: McCubbin H, Thompson A, editors. Family assessment inventories for research and practice. Madison, WI: University of Wisconsin–Madison; 1987. pp. 283–302. [Google Scholar]
- Miller M, Bowen JR, Gibson FL, Hand PJ, Ungerer JA. Behaviour problems in extremely low birthweight children at 5 and 8 years of age. Child: Care, Health and Development. 2001;27:569–581. doi: 10.1046/j.1365-2214.2001.00223.x. [DOI] [PubMed] [Google Scholar]
- Nachmias M, Gunnar M, Mangelsdorf S, Parritz R, Buss KA. Behavioral inhibition and stress reactivity: The moderating role of attachment security. Child Development. 1996;67:508–522. [PubMed] [Google Scholar]
- Offord DR, Boyle MH, Szatmari P, Rae-Grant NI, Links PS, Cadman DT, Byles JA, Crawford JW, Munroe-Blum H, Byrne C, Thomas H, Woodward CA. Ontario Child Health Study II: Six-month prevalence of disorder and rates of service utilization. Archives of General Psychiatry. 1987;44:832–836. doi: 10.1001/archpsyc.1987.01800210084013. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Phillips DI, Jones A. Fetal programming of autonomic and HPA function: Do people who were small babies have enhanced stress responses? The Journal of Physiology. 2006;572:45–50. doi: 10.1113/jphysiol.2005.104695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesonen AK, Raikkonen K, Kajantie E, Heinonen K, Strandberg TE, Jarvenpaa AL. Fetal programming of temperamental negative affectivity among children born healthy at term. Developmental Psychobiology. 2006;48:633–643. doi: 10.1002/dev.20153. [DOI] [PubMed] [Google Scholar]
- Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;3:385–401. [Google Scholar]
- Reijneveld SA, de Kleine MJK, van Baar AL, Kollee LAA, Verhaak CM, Verhulst FC, Verloove-Vanhorick P. Behavioural and emotional problems in very preterm and very low birthweight infants at age 5 years. Archives of Disease in Childhood, Fetal and Neonatal Edition. 2006;91:F423–F428. doi: 10.1136/adc.2006.093674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds RM, Walker BR, Syddall HE, Andrew R, Wood PJ, Whorwood CB, Phillips DIW. Altered control of cortisol secretion in adult men with low birth weight and cardiovascular risk factors. The Journal of Clinical Endocrinology & Metabolism. 2001;86:245–250. doi: 10.1210/jcem.86.1.7145. [DOI] [PubMed] [Google Scholar]
- Richards AL, Kelly EA, Doyle LW, Callanan C. Cognition, academic progress, behavior and self-concept at 14 years of very low birth weight children. Journal of Developmental and Behavioral Pediatrics. 2001;22:11–18. doi: 10.1097/00004703-200102000-00002. [DOI] [PubMed] [Google Scholar]
- Rosenberg M. Society and the adolescent self-image. Princeton, NJ: Princeton University Press; 1965. [Google Scholar]
- Ross G, Lipper EG, Auld PAM. Social competence and behavior problems in premature children at school age. Pediatrics. 1990;86:391–397. [PubMed] [Google Scholar]
- Saigal S, Feeny D, Rosenbaum P, Furlong W, Burrows E, Stoskopf B. Self-perceived health status and health related quality of life in extremely low-birth-weight infants at adolescence. Journal of the American Medical Association. 1996;276:453–459. [PubMed] [Google Scholar]
- Saigal S, Hoult LA, Streiner DL, Stoskopf BL, Rosenbaum PL. School difficulties at adolescence in a regional cohort of children who were extremely low birth weight. Pediatrics. 2000;105:325–331. doi: 10.1542/peds.105.2.325. [DOI] [PubMed] [Google Scholar]
- Saigal S, Lambert M, Russ C, Hoult L. Self-esteem of adolescents who were born prematurely. Pediatrics. 2002;109:429–433. doi: 10.1542/peds.109.3.429. [DOI] [PubMed] [Google Scholar]
- Saigal S, Pinelli J, Hoult L, Kim MM, Boyle M. Psychopathology and social competencies of adolescents who were extremely low birth weight. Pediatrics. 2003;111:969–975. doi: 10.1542/peds.111.5.969. [DOI] [PubMed] [Google Scholar]
- Saigal S, Rosenbaum P, Szatmari P, Campbell D. Learning disabilities and school problems in a regional cohort of extremely low birthweight (<1000 g) children: A comparison with matched term controls. Journal of Developmental and Behavioral Pediatrics. 1991;12:294–300. [PubMed] [Google Scholar]
- Saigal S, Stoskopf B, Boyle M, Paneth N, Pinelli J, Streiner D, Goddeeris J. Comparison of current health, functional limitations and health care utilization of young adults born with extremely low birthweight and normal birthweight. Pediatrics. 2007;119:e562–e573. doi: 10.1542/peds.2006-2328. [DOI] [PubMed] [Google Scholar]
- Saigal S, Stoskopf B, Pinelli J, Streiner D, Hoult L, Paneth N, Goddeeris J. Self-perceived health-related quality of life of former extremely low birthweight infants at young adulthood. Pediatrics. 2006b;118:1140–1148. doi: 10.1542/peds.2006-0119. (Erratum: Dr. Michael Boyle) [DOI] [PubMed] [Google Scholar]
- Saigal S, Stoskopf B, Streiner D, Boyle M, Pinelli J, Paneth N, Goddeeris J. Transition of extremely low-birth-weight infants from adolescence to young adulthood: Comparison with normal birth-weight controls. The Journal of the American Medical Association. 2006a;295:667–675. doi: 10.1001/jama.295.6.667. [DOI] [PubMed] [Google Scholar]
- Saigal S, Stoskopf B, Streiner D, Paneth N, Pinelli J, Boyle M. Growth trajectories of extremely low birthweight infants from birth to young adulthood: A longitudinal population-based study. Pediatric Research. 2006c;60:751–758. doi: 10.1203/01.pdr.0000246201.93662.8e. [DOI] [PubMed] [Google Scholar]
- Saigal S, Szatmari P, Rosenbaum P, Campbell D, King S. Intellectual and functional status at school entry of children who weighed 1000 grams or less at birth: A regional perspective of births in the 1980’s. Journal of Pediatrics. 1990;116:409–416. doi: 10.1016/s0022-3476(05)82835-5. [DOI] [PubMed] [Google Scholar]
- Samara M, Marlow N, Wolke D. Pervasive behavior problems at 6 years of age in a total-population sample of children born at ≤ 25 weeks gestation. Pediatrics. 2008;122:562–573. doi: 10.1542/peds.2007-3231. [DOI] [PubMed] [Google Scholar]
- Schmidt LA. Frontal brain electrical activity in shyness and sociability. Psychological Science. 1999;10:316–320. [Google Scholar]
- Schmidt LA. Patterns of second-by-second resting frontal brain (EEG) asymmetry and their relation to heart rate and temperament in 9-month-old human infants. Personality and Individual Differences. 2008;44:216–225. [Google Scholar]
- Schmidt LA, Cote KA, Santesso DL, Milner CE. Frontal electroencephalogram alpha asymmetry during sleep: Stability and its relation to affective style. Emotion. 2003;3:401–407. doi: 10.1037/1528-3542.3.4.401. [DOI] [PubMed] [Google Scholar]
- Schmidt LA, Fox NA, Schulkin J, Gold PW. Behavioral and psychophysiological correlates of self-presentation in temperamentally shy children. Developmental Psychobiology. 1999;35:119–135. [PubMed] [Google Scholar]
- Schmidt LA, Fox NA, Rubin KH, Sternberg EM, Gold PW, Smith CC, Schulkin J. Behavioral and neuroendocrine responses in shy children. Developmental Psychobiology. 1997;30:127–140. doi: 10.1002/(sici)1098-2302(199703)30:2<127::aid-dev4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Schmidt LA, Miskovic V, Boyle M, Saigal S. Shyness and timidity in young adults who were born at extremely low birth weight. Pediatrics. 2008;122:e181–e187. doi: 10.1542/peds.2007-3747. [DOI] [PubMed] [Google Scholar]
- Schothorst PF, Swaab-Barneveld H, van Engeland H. Psychiatric disorders and MND in non-handicapped preterm children: Prevalence and stability from school age into adolescence. European Child & Adolescent Psychiatry. 2007;16:439–448. doi: 10.1007/s00787-007-0617-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schothorst PF, van Engeland H. Long-term behavioral sequelae of prematurity. Journal of the American Academy of Child & Adolescent Psychiatry. 1996;35:175–183. doi: 10.1097/00004583-199602000-00011. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene PR, Vagg PR, Jacobs AG. Manual for the State-Trait Anxiety Inventory (Form Y) Palo Alto: Consulting Psychologists Press, Inc.; 1983. [Google Scholar]
- Strauss RS. Adult functional outcome of those born small for gestation age. Journal of the American Medical Association. 2000;283:625–632. doi: 10.1001/jama.283.5.625. [DOI] [PubMed] [Google Scholar]
- Sutton SK, Davidson RJ. Prefrontal brain asymmetry: A biological substrate of the behavioral approach and inhibition systems. Psychological Science. 1997;8:204–210. [Google Scholar]
- Swamy GK, Østbye T, Skjaerven R. Association of preterm birth with long-term survival, reproduction, and next-generation preterm birth. Journal of the American Medical Association. 2008;299:1429–1436. doi: 10.1001/jama.299.12.1429. [DOI] [PubMed] [Google Scholar]
- Sykes DH, Hoy EA, Bill JM, McClure BG, Halliday HL, Reid M. Behavioral adjustment in school of very low birthweight children. Journal of Child Psychology and Psychiatry. 1997;38:315–325. doi: 10.1111/j.1469-7610.1997.tb01516.x. [DOI] [PubMed] [Google Scholar]
- Szatmari P, Saigal S, Rosenbaum P, Campbell D. Psychopathology and adaptive functioning among extremely low birthweight children at eight years of age. Development and Psychopathology. 1993;5:345–357. [Google Scholar]
- Szatmari P, Saigal S, Rosenbaum P, Campbell D, King S. Prevalence of psychiatric disorders at five years of age among children born under 1000 g birthweight: A regional perspective. Developmental Medicine of Child Neurology. 1990;32:954–962. [PubMed] [Google Scholar]
- Taylor HG, Hack M, Klein N. Attention deficits in children with < 750 gram birthweight. Child Neuropsychology. 1998;4:21–34. [Google Scholar]
- Taylor HG, Klein N, Hack M. School-age consequences of birth weight less than 750 g: A review and update. Developmental Neuropsychology. 2000;17:289–321. doi: 10.1207/S15326942DN1703_2. [DOI] [PubMed] [Google Scholar]
- Theall-Honey LA, Schmidt LA. Do temperamentally shy children process emotion differently than non-shy children? Behavioral, psychophysiological, and gender differences in reticent preschoolers. Developmental Psychobiology. 2006;48:187–196. doi: 10.1002/dev.20133. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Davidson RJ, Wheeler RE, Kinney L. Psychometric properties of resting anterior EEG asymmetry: Temporal stability and internal consistency. Psychophysiology. 1992;29:576–592. doi: 10.1111/j.1469-8986.1992.tb02034.x. [DOI] [PubMed] [Google Scholar]
- Vecchierini MF, Andre M, d’Allest AM. Normal EEG of premature infants born between 24 and 30 weeks gestation age: Terminology, definitions and maturation aspects. Clinical Neurophysiology. 2007;37:311–323. doi: 10.1016/j.neucli.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Victor S, Appleton RE, Beirne M, Marson AG, Weindling AM. Spectral analysis of electroencephalography in premature newborn infants: Normal ranges. Pediatric Research. 2005;57:336–341. doi: 10.1203/01.PDR.0000153868.77623.43. [DOI] [PubMed] [Google Scholar]
- Vuga M, Fox NA, Cohn JF, George CJ, Levenstein RM, Kovacs M. Long-term stability of frontal electroencephalographic asymmetry in adults with a history of depression and controls. International Journal of Psychophysiology. 2006;59:107–115. doi: 10.1016/j.ijpsycho.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Vuga M, Fox NA, Cohn JF, Kovacs M, George CJ. Long-term stability of electroencephaplographic asymmetry and power in 3 to 9 year old children. International Journal of Psychophsyiology. 2008;67:70–77. doi: 10.1016/j.ijpsycho.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolke D, Samara M, Bracewell M, Marlow N. Specific language difficulties and school achievement in children born at 25 weeks of gestation or less. Journal of Pediatrics. 2008;152:256–262. doi: 10.1016/j.jpeds.2007.06.043. [DOI] [PubMed] [Google Scholar]
- Wust S, Entringer S, Federenko IS, Schlotz W, Hellhammer DH. Birth weight is associated with salivary cortisol responses to psychosocial stress in adult life. Psychoneuroendocrinology. 2005;30:591–598. doi: 10.1016/j.psyneuen.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Zwicker JG, Harris SR. Quality of life of formerly preterm and very low birth weight infants from preschool age to adulthood: A systematic review. Pediatrics. 2008;121:e366–e376. doi: 10.1542/peds.2007-0169. [DOI] [PubMed] [Google Scholar]