Abstract
Background
Up to 50% of depressed older adults either do not adequately respond to or are unable to tolerate treatment with a serotonin-specific reuptake inhibitor. On the basis of previous experience with serotonin-norepinephrine reuptake inhibitors, we predicted at least a 50% response rate to open-label treatment with duloxetine in subjects who were resistant to treatment with the selective serotonin reuptake inhibitor (SSRI) escitalopram.
Method
Community-dwelling subjects aged 65 years or older with current nonpsychotic major depressive disorder as established by the Structured Clinical Interview for DSM-IV received escitalopram under protocolized conditions between April 2004 and September 2006. Subjects who failed to meet response criteria or relapsed after achieving an initial response were subsequently switched to open treatment with duloxetine up to 120 mg/day. Side effects were assessed at every visit.
Results
Subjects (N = 40) switched to duloxetine had a mean (SD) age of 74.4 (7.0) years and a baseline (before escitalopram) 17-item Hamilton Rating Scale for Depression (HAM-D-17) score of 20.0 (3.5) and were predominantly female (65.0%) and white (82.5%). The mean (SD) maximum dose of duloxetine was 93.0 (27.8) mg/day. Subjects received this maximum dose for a median duration of 6.9 weeks. Fifty percent of subjects (N = 20) met criteria for full response, 17.5% (N = 7) were partial responders, and 32.5% (N = 13) did not respond. The median time to response was 12.0 weeks (95% CI = 8.4 to 14.6). Five of the subjects (12.5%) discontinued duloxetine because of intolerable side effects.
Discussion
These open-label data suggest that duloxetine at doses up to 120 mg/day is a well-tolerated and potentially effective treatment for older adults who fail to respond to an adequate trial of an SSRI. These results are preliminary, and future controlled studies are required to test the efficacy of rescue pharmacotherapy with duloxetine.
Trial Registration
clinicaltrials.gov Identifier: NCT00177671
When older adults with major depressive disorder (MDD) are treated with an antidepressant at adequate dosage, 50% to 70% either fail to adequately respond or respond but then relapse within the first 6 to 12 weeks of treatment.1–3 While up to 67% of older depressed patients will eventually respond to vigorous antidepressant pharmacotherapy,4,5 there is little published evidence regarding the success of treating older adults with depression who have failed to respond to pharmacotherapy with a selective serotonin reuptake inhibitor (SSRI).5 Our group previously demonstrated an equivalent rate of response to an “augmentation strategy” (adding bupropion sustained release, nortriptyline, or lithium to paroxetine) or a “switch strategy” (venlafaxine extended release [XR]) in a cohort of paroxetine nonresponders.4 In that report, we observed that venlafaxine XR was generally better tolerated than the augmentation strategies.4 This observation led us to hypothesize that, in older depressed adults who do not respond to an SSRI, switching to a dual reuptake inhibitor may be an effective and well-tolerated pharmacotherapy strategy.
Duloxetine has been shown to be safe and effective in the treatment of MDD among adults aged 55 years or older.6 Specifically, in this analysis that pooled older subjects from 2 randomized controlled trials in which subjects received duloxetine 60 mg/day for up to 9 weeks,6 the estimated probability of remission for duloxetine-treated subjects was 44.1%, and the discontinuation rate due to adverse events was 21.0%. In an open-label study of subjects aged 65 years or older treated with duloxetine at doses of 80 to 120 mg/day,7 the rate of remission was 69.8% at week 28 with a discontinuation rate due to adverse events of 26.7% by week 52. In that open-label study, the adverse events leading to discontinuation in greater than 1.0% of enrolled patients were somnolence (4.0%), dizziness (3.0%), diarrhea (2.0%), hypertension (2.0%), and vomiting (2.0%). At these doses of 80 to 120 mg/day, Wohlreich et al.7 also did not report any significant changes in cardiac intervals detected by electrocardiogram. These studies provide preliminary evidence that duloxetine is effective and tolerated in older adults. While reflecting both efficacy and effectiveness, these studies may not reflect real-world stepped-care practice in which serotonin-norepinephrine reuptake inhibitors (SNRIs) are prescribed after nonresponse to an SSRI.8 In order to improve the evidence base of treatment options for older adults who do not respond to optimized treatment with an SSRI, it is useful to describe the response rates for these nonresponders after being switched to an SNRI.
In this report, we present open-label data examining how older subjects fare when they are treated with duloxetine as a second-line agent after failing SSRI monotherapy with escitalopram. Our goal is to describe the antidepressant response achieved during treatment in an uncontrolled study and the reported side effects among older adults treated with open-label duloxetine delivered under protocolized conditions after failure of protocolized treatment with escitalopram. On the basis of our experience with venlafaxine XR rescue pharmacotherapy,4 we predicted that approximately half of escitalopram nonresponders would respond to duloxetine.
METHOD
Subjects
Between April 2004 and September 2006, 274 subjects aged 65 years or older were recruited from primary care practices and from our specialty mental health clinic for older adults with mood and anxiety disorders. Recruitment occurred by word of mouth, referrals from clinicians, advertisements, and presentations to lay groups of older adults and their families. All subjects were currently experiencing a nonpsychotic, unipolar, major depressive episode as established by the Structured Clinical Interview for DSM-IV (SCID),9 had a baseline rating of 15 or higher on the 17~item Hamilton Rating Scale for Depression10 (HAM-D-17), and scored at least 18 on the Mini-Mental State Examination (MMSE).11 We chose to accept subjects with MMSE scores within the impaired range in order to increase the generalizability of our findings. However, we excluded subjects if they received a diagnosis of dementia after undergoing protocolized neuropsychological testing. These tests were administered by dedicated neuropsychological technicians. Determination of the final cognitive diagnosis was made through a multi-disciplinary consensus process at the University of Pittsburgh School of Medicine Alzheimer’s Disease Research Center. Subjects diagnosed with mild cognitive impairment12 remained in the current analysis. Participants were excluded if they had a SCID lifetime diagnosis of bipolar disorder, schizophrenia, schizoaffective or other psychotic disorders, or dementia; a history of alcohol/drug abuse within the past 12 months; or a history of nonresponse or nontolerance to escitalopram. The study was approved by the University of Pittsburgh Institutional Review Board, and all subjects provided written informed consent.
Assessments
Subjects’ demographic information and history of depression were assessed, and a physical examination was performed before study entry. Baseline assessments included depression severity (HAM-D-17), anxiety severity (Hamilton Rating Scale for Anxiety13), cognition (MMSE11), side effects (Udvalg for Kliniske Undersogelser-Committee of Clinical Investigations Side Effect Rating Scale14 [UKU]), chronic medical illness burden (Cumulative Illness Rating Scale for Geriatrics15 [CIRS-G]), and health-related quality of life (Medical Outcomes Study [MOS] Short Form Health Survey16 physical component and mental component scores).
Intervention
All ineffective psychotropic medications (e.g., antide-pressants) and over-the-counter psychoactive medications (e.g., diphenhydramine for insomnia) were discontinued in a tapered fashion over 1 to 2 weeks concurrent with the start of escitalopram pharmacotherapy. Participants unable to discontinue benzodiazepine therapy were converted to an equivalent dose of lorazepam (generally 0.5–2.0 mg/day). Clinical management conducted by study clinicians consisted of education about geriatric depression and interventions, side effects of treatment, risks for suicide, and sleep hygiene, as well as a careful review of symptoms of depression and side effects using the HAM-D-17 and UKU, respectively.
All subjects were openly treated with escitalopram and supportive clinical management before being switched to open-label treatment with duloxetine due to escitalopram nonresponse, partial response, relapse after initial response, or intolerable side effects. Subjects received escitalopram 10 mg/day for 6 weeks. If no response, defined as a HAM-D-17 score less than or equal to 10, was achieved by week 6, the dose was increased to 20 mg/day. If a subject experienced intolerance of adverse effects during treatment with escitalopram, the dosage could be adjusted to no less than 10 mg/day.
Subjects were switched to open-label treatment with duloxetine for the following reasons: (1) nonresponse to escitalopram after 8 weeks of treatment (i.e., defined as HAM-D-17 scores ≥ 15 or change in the HAM-D-17 score from baseline of 0%–30%), (2) partial response to escitalopram after 16 weeks of treatment (defined as HAM-D-17 scores of 11–14), (3) relapse of a major depressive episode within the context of the index episode, or (4) intolerance of escitalopram.
Before switching to duloxetine, escitalopram was tapered to 10 mg for 3 to 4 days and then discontinued. Duloxetine was initiated at 30 mg/day for 1 week and then increased to 60 mg/day. After 6 weeks of treatment with 60 mg/day, the dose could be subsequently increased up to a maximum of 120 mg/day based on clinical ratings (e.g., HAM-D-17 score ≥ 11) and reported side effects. If a subject experienced significant adverse effects, the dose was reduced to a tolerable but potentially still efficacious dose (e.g., ≥ 60 mg/day). The criterion for response during treatment with duloxetine was more stringent, requiring a HAM-D-17 score less than or equal to 10 for 3 consecutive weeks. The criterion for partial response was 3 consecutive HAM-D-17 scores of 11 to 14 and for nonresponse was HAM-D-17 scores greater than or equal to 15.
The different response criteria for the escitalopram and duloxetine trials reflect the 2 separate but linked National Institute of Health–funded protocols from which the subjects in this report were participating. All subjects were initially participating in 1 study (Geriatric Depression: Getting Better, Getting Well [MH037869]; principal investigator: C.F.R.); those who did not respond received duloxetine in the context of a second study (Maintenance Therapies in Late-Life Depression-III [MH043832]; principal investigator: C.F.R.).
Analyses
We used descriptive statistics to characterize treatment response and a Kaplan-Meier survival analysis to estimate median time to response. When describing subjects’ experience with open-label duloxetine, we truncated the data to 16.5 weeks and treated subjects who responded after that point as censored observations. This timepoint was chosen so that (1) the focus would be on acute treatment with duloxetine (e.g., within 4 months) and (2) all subjects would have had the opportunity to have the dose of duloxetine increased to 120 mg/day if needed. A Wilcoxon signed rank test was used to examine somatic changes assessed with the UKU. Nonparametric tests were used due to the distribution of the measures. A repeated-measures analysis of variance was used to examine changes in anxiety over time.
RESULTS
Two hundred sixteen subjects were initially treated with escitalopram. Forty subjects discontinued escitalopram after receiving a mean (SD) maximum dose of 18.3 (3.8) mg/day (median = 20.0). The mean (SD) time subjects received this maximum dose of escitalopram was 14.5 weeks (19.3) (median = 8.6 weeks). The reasons for escitalopram discontinuation included nonresponse (N = 23), partial response (N = 9), relapse (N = 5), and intolerable side effects (N = 3). Side effects leading to discontinuation included diarrhea (N = 2), nausea (N = 1), headache (N = 1), and sweating (N = 1). Table 1 presents the clinical and demographic characteristics of these 40 subjects.
Table 1.
Demographic and Clinical Data at the Start of Escitalopram Treatment for the 40 Subjects With Late-Life Depression Who Were Subsequently Switched to Duloxetine
| Variable | Value |
|---|---|
| Age, mean (SD), y | 74.4(7.0) |
| Female, N (%) | 26 (65.0) |
| White, N (%) | 33 (82.5) |
| Duration of index episode, mean (SD) (median), wk | 251.8 (587.0) (69.0) |
| Age at illness onset, mean (SD), y | 51.2 (22.6) |
| Recurrent depression, N (%) | 24 (60.0) |
| HAM-D-17 score, mean (SD) (range) | 20.0 (3.5) (0 [better]–52 [worse]) |
| HAM-A score, mean (SD) (range)a | 18.4 (5.1) (0 [better]–56 [worse]) |
| MMSE score, mean (SD) | 27.7 (3.1) |
| CIRS-G total score, mean (SD) (range) | 9.7 (3.0) (0 [better]–52 [worse]) |
| MOS physical component subscale score, mean (SD) (range)b | 42.0 (11.5) (0 [worse]–100 [better]) |
| MOS mental component subscale score, mean (SD) (range)b | 30.5 (7.2) (0 [worse]–100 [better]) |
| UKU Side Effect Rating Scale score, mean (SD) (range) | |
| Total | 15.0 (4.2) (0 [better]–138 [worse]) |
| Psychological subscalec | 8.7 (2.8) (0 [better]–30 [worse]) |
| Neurological subscaled | 1.1 (0.9) (0 [better]–24 [worse]) |
| Autonomic subscalee | 3.5 (2.0) (0 [better]–33 [worse]) |
| Other systems subscalef | 1.8 (1.1) (0 [better]–51 [worse]) |
N = 33; 7 subjects had missing data.
N = 35; 2 subjects refused to complete the scale and 3 subjects had missing data.
Change in concentration and in sleep or dream activity, fatigue, and emotional indifference.
Dystonia, rigidity, tremor, akathisia, paresthesia, and headache.
Accommodation disturbances, change in salivation, gastrointestinal distress, orthostasis, sweating, and palpitations.
Rash, pruritis, photosensitivity, and change in sexual functioning and in weight.
Abbreviations: CIRS-G = Cumulative Illness Rating Scale for Geriatrics, HAM-A = Hamilton Rating Scale for Anxiety, HAM-D-17 = 17-item Hamilton Rating Scale for Depression, MMSE = Mini-Mental State Examination, MOS = Medical Outcomes Study, UKU = Udvalg for Kliniske Undersogelser-Committee of Clinical Investigations.
Response to Duloxetine
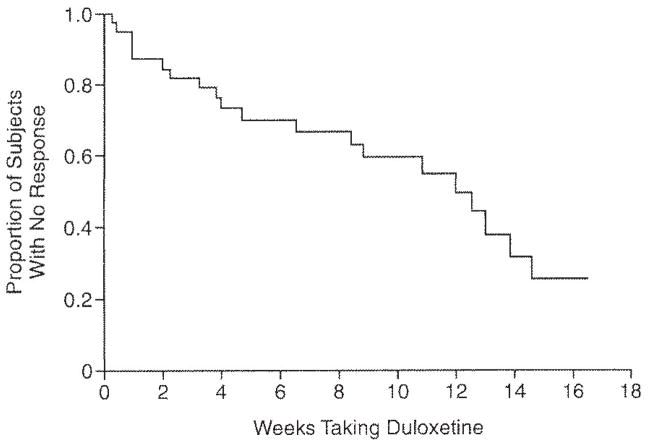
Before starting open-label treatment with duloxetine, the mean (SD) HAM-D-17 score was 17.2 (3.7). The 40 subjects received a mean (SD) maximum dose of duloxetine of 93.0 (27.8) mg/day (median = 90.0 mg/day, range, 30.0–120.0 mg/day). Subjects received this maximum dose for a median duration of 6.9 weeks. Fifty percent of duloxetine-treated subjects (N = 20) achieved a response (defined as a HAM-D-17 score ≤ 10 for 3 consecutive weeks) by 16.5 weeks. The median time to response was 12.0 weeks (95% CI = 8.4 to 14.6 weeks; Figure 1). Because the duloxetine-treated subjects were a subset of escitalopram nonresponders, it was not feasible to compare time to response between the escitalopram-treated and duloxetine-treated subjects.
Figure 1. Rescue Pharmacotherapy With Duloxetine Among Escitalopram Nonresponders With Late-Life Depressiona,b.
aFigure 1 shows the Kaplan-Meier survival curve truncated at 16.5 weeks. Response was defined as a 17-item Hamilton Rating Scale for Depression score ≤ 10 for 3 consecutive weeks.
bMedian time to response = 12.0 weeks (95% CI = 8.4 to 14.6).
Of the 20 subjects who did not respond to open-label treatment with duloxetine, 13 were classified as nonresponders (2 of whom discontinued duloxetine due to side effects) (comprising 32.5% of the sample), and 7 of the subjects were classified as partial responders (17.5% of the sample). A comparison between the 20 responders and 20 nonresponders found that significantly more responders were female (85% [N = 17]; Fisher exact p = .02). The responders and nonresponders did not differ in terms of age, marital status, education, proportion with recurrent depression, or age at onset of first episode of depression. The MMSE scores at baseline were not different between responders and nonresponders (Wilcoxon exact p = .12).
There was a significant change on the mental component of the MOS from the beginning of treatment with escitalopram to the end of treatment with duloxetine (median change = 6.7, N = 27, Wilcoxon exact p = .03). However, there was no similar statistically significant change on the MMSE or the physical component of the MOS (median change = −1.0, N = 23, Wilcoxon exact p = .20 and median change = −1.1, N = 27, Wilcoxon exact p = .95, respectively).
Effect of Duloxetine on Anxiety
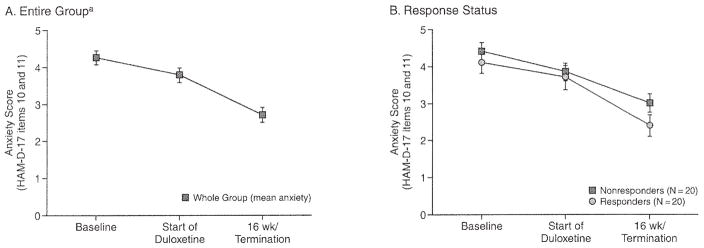
The Hamilton Rating Scale for Anxiety was collected only during treatment with escitalopram. To describe the effect of duloxetine on anxiety, we created an anxiety subscale from the 2 anxiety-specific items from the HAM-D-17.17 These 2 items (10 and 11) assess psychological and somatic anxiety. As illustrated in Figures 2A and 2B, scores on this anxiety subscale decreased over time in subjects treated with duloxetine. There was a significant effect of time on decrease in anxiety scores for the entire group of subjects treated with duloxetine (F = 19.76; df = 2,39; p < .0001).
Figure 2. Effect of Duloxetine on Anxiety Scores for the Entire Group of Subjects With Late-Life Depression and by Response Status.
aTime effect: F = 19.76; df = 2,39; p < .0001.
Abbreviation: HAM-D-17 = 17-item. Hamilton Rating Scale for Depression.
At the beginning of open-label treatment with duloxetine, 15 subjects were receiving lorazepam. The mean (SD) dose of lorazepam was 0.8 (0.5) mg/day with a range of 0.5 to 2.0 mg/day. At the end of treatment with duloxetine, 11 participants were receiving lorazepam. The mean (SD) dose of lorazepam at the end of treatment with duloxetine was 1.0 (0.5) mg/day with a range of 0.5 to 2.0 mg/day.
Tolerability
Five of the 40 subjects (12.5%) discontinued duloxetine because of side effects or adverse events. Three of the subjects who discontinued duloxetine had responded to the medication, whereas 2 had not responded. Reasons for discontinuation included dry mouth and bloating (N = 1), sedation (N= 1), elevated transaminase levels (N= 1), sweating and diarrhea (N = 1), and substance-induced mania (N = 1). It was unclear whether this substance-induced mania was secondary to duloxetine therapy or to concomitant initiation of opioid analgesics.
Table 2 lists the UKU scores (total and subscale) at the beginning of treatment with duloxetine and at the end of treatment (e.g., week 16 or withdrawal from treatment). Table 2 also lists the percent change for the UKU total and subscale scores during treatment with duloxetine. There were small but statistically significant decreases in somatic complaints as reflected in decreases in UKU total, psychological subscale, and other systems subscale scores (a mean [SD] change of −0.6 [1.5] points, Wilcoxon exact p = .02). The specific items that comprise the subscales are listed in Table 1.
Table 2.
UKU Side Effect Rating Scale Score Change During Treatment With Duloxetine Among Subjects With Late-Life Depression
| UKU Side Effect Rating Scale (N = 34) | Prior to Starting Duloxetine (< 14 days), Mean (SD) | 16 Weeks or End of Duloxetine, Mean (SD) | Baseline-to-End Change Between Initiating and Stopping Duloxetine, Mean (SD) | % Change, Median | Wilcoxon Exact p Value |
|---|---|---|---|---|---|
| Total score | 12.8 (3.7) | 10.9 (3.1) | −1.9 (4.3)a | −12.9 | .01 |
| Psychological subscale score | 7.1 (2.1) | 5.6 (2.1) | −1.4 (2.5)b | −16.7 | .001 |
| Neurological subscale score | 0.8 (0.8) | 0.8 (1.0) | 0.1 (0.9)c | 0 | .86 |
| Autonomic subscale score | 3.4 (2.3) | 3.5 (1.8) | 0.0 (2.5)d | 0 | .49 |
| Other systems subscale score | 1.5 (1.3) | 1.0 (1.2) | −0.6 (1.5)c | −16.7 | .02 |
Median = −2; range, −14 to 6.
Median = −1; range, −8 to 3.
Median = 0; range, −1 to 3.
Median = 0; range, −9 to 4.
Median = −0.5; range, −5 to 3.
Abbreviation: UKU = Udvalg for Kliniske Undersogelser-Commitlee of Clinical Investigations.
DISCUSSION
In this sample of nonresponders to escitalopram monotherapy, 50% of the depressed older adults responded fully to open-label duloxetine “rescue” pharmacotherapy by 16.5 weeks, and another 17.5% responded partially. Reported side effects as measured by the UKU decreased over time. Five of the 40 subjects (12.5%) discontinued duloxetine because of intolerable side effects or adverse events.
Our finding that 50% of the subjects responded to the rescue pharmacotherapy with duloxetine is consistent with reports that the majority of older depressed patients will eventually respond to stepped care with antidepressant pharmacotherapy.3,5,18,19 Indeed, our group has reported that 42% of older adults (N = 5) who were nonresponders to open-label treatment with paroxetine responded to open-label venlafaxine XR.4 It is notable that the median dose of duloxetine was 90 mg/day. This dose is in contrast to the package insert for duloxetine that states, “There is no evidence that doses greater than 60 mg/day confer any additional benefits.”20 The higher median dose in our study reflects the operationalized titration schedule used. However, our study suggests that higher doses and/or longer exposure may be necessary to achieve a response to duloxetine in older adults who do not respond to treatment with an SSRI and require rescue pharmacotherapy with a dual-mechanism agent. Our observations that higher doses may result in improved effectiveness and still be tolerated are consistent with work reported by Wohlreich et al.7
The observed decrease in anxiety scores in our duloxetine-treated cohort is consistent with the report by Nelson et al.6 that, among older adults with MDD, the difference on the anxiety subscale of the HAM-D-17 between the duloxetine-treated and placebo-treated groups neared statistical significance after 9 weeks of treatment (t = −2.00, p = .051). It is notable that anxiety symptoms predict diminished acute treatment response and increased recurrence in elderly patients with MDD. We previously reported, in this cohort using open-label escitalopram, that greater severity of anxiety symptoms (both psychological and somatic) and lower self-esteem predicted worse response status after 6 weeks of treatment even after controlling for other variables.21 Our group has also examined the effects of comorbid anxiety on both acute treatment: response and recurrence of MDD during 2 years of maintenance treatment with paroxetine or placebo.22 We found that patients with greater pretreatment anxiety took on average 4.3 weeks longer to respond to depression treatment and had higher rates of recurrence of depression during maintenance treatment.22
Within this context, 1 possible explanation for the success of duloxetine as a rescue agent may be that it provides greater anxiolysis than escitalopram, even though both agents have recently received approval by the U.S. Food and Drug Administration for the treatment of generalized anxiety disorder.20,23 We were unable to test the differential decrease in anxiety scores between the duloxetine responders and nonresponders without an additional available anxiety assessment instrument. Future work to assess anxiety over time using more sensitive and validated anxiety assessment instruments such as the Hamilton Rating Scale for Anxiety or the anxiety sub-scale from the Hospital Anxiety and Depression Scale24 is warranted.
In our study, the median time to response to duloxetine rescue therapy was 12 weeks. While 12 weeks may seem like an extended period of time to achieve response for an older patient suffering from depression, this delayed but robust response is consistent with an earlier report from our group, which found that older patients with MDD treated with nortriptyline appear to benefit as much as, but perhaps more slowly than, midlife patients treated with imipramine.19 It should also be noted that subjects in this report had been depressed for a mean duration of 251.8 weeks prior to starting treatment with escitalopram. Greater duration of depressive episode in nongeriatric adults is associated with longer time to response.25,26 Taken into context, for this sample, the median time to achieve antidepressant response after being switched to duloxetine was 5% of the mean duration of the index episode prior to seeking treatment.
Five participants (12.5%) discontinued duloxetine because of intolerable side effects or adverse events. This rate of discontinuation is consistent with that reported in a study using duloxetine at recommended doses (40–60 mg/day)27 and is less than what has been reported in a study utilizing higher doses (80–120 mg/day).7,28 Of note, there was a statistically significant decrease in reported somatic symptoms during treatment as measured with the UKU.
In a recently published article by Raskin et al. comparing duloxetine 60 mg/day with placebo in older adults with MDD,29 the rates of discontinuation due to adverse events were 9.7% versus 8.7% for placebo. It is possible that since all of our subjects received escitalopram immediately prior to treatment with duloxetine, they were less susceptible to the troubling side effects frequently encountered when starting an antidepressant de novo. This hypothesis is supported by a report that the rate of discontinuation caused by adverse events among patients switched to duloxetine from an SSRI or venlafaxine was significantly lower than that in patients initiating duloxetine therapy (6.3% vs. 16.1 %, p = .018).30
Despite the 12.5% discontinuation rate due to side effects and adverse events, neither the total nor any of the subscales of the UKU indicated more somatic problems during treatment. In fact, there was a decrease in severity between initiation and end of duloxetine therapy for the total score, psychological subscale, and other systems subscale, suggesting that the level of reported somatic preoccupation decreased during the course of treatment. To put these findings into context, in another sample of older adults treated with paroxetine, the mean UKU total score (minus the psychological subscale) at baseline was 8.8.3 Thus, the patients in this report may be a more somatically preoccupied sample. Our group has previously found similar improvements in the UKU scales in elderly subjects with or without comorbid anxiety receiving paroxetine or nortriptyline.17 Thus, it is unclear whether this decrease in UKU scores represents improvement in depression or a somatic benefit of duloxetine itself. This finding is important because older adults frequently present with depression comorbid with unpleasant bodily sensations and somatic preoccupation.31
There are several limitations to this analysis. We cannot rule out the possibility that subjects’ expectations may have inflated the rate of response in this open-label trial. In addition, because this report is of uncontrolled open-label data, we cannot rule out the possibility that response was due to spontaneous improvement of their depression unrelated to the medication. Descriptions of spontaneous response rates in older depressed patients are needed to put open-label findings into perspective. To our knowledge, such data have not been reported. Finally, the relatively small size of the sample may have resulted in unstable estimates of response rates.
In summary, our data suggest that duloxetine may be a good “rescue” pharmacotherapy for older adults who either do not respond to or do not tolerate treatment with escitalopram. Furthermore, duloxetine appears to be well tolerated even at the higher doses used in this study. Achieving response by switching antidepressant medications instead of augmenting3 or combining antidepressants, especially in older adults, may have several practical benefits. Among these are expense (the patient only has to buy 1 medication), safety (polypharmacy and the increased risks of side effects and adverse events are minimized), and increased adherence (fewer pills, fewer missed doses). The next step in evaluating the efficacy of duloxetine as a potent rescue pharmacotherapy is a randomized, double-blind, placebo-controlled trial to minimize the risk of expectation bias on the part of both subjects and investigators.
Acknowledgments
Supported in part by grants numbered P30 MH071944, R01 MH37869, K23MH067710, R01 MH43832, and T32 MH19986 from the National Institute of Mental Health and KL2 RR024154 from the National Center for Research Resources, a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research; the John A. Hartford Center of Excellence in Geriatric Psychiatry; and the University of Pittsburgh Medical Center Endowment in Geriatric Psychiatry. Medications were supplied by Forest and Eli Lilly.
Dr. Karp has received grant/research support in the form of medication supplies for an investigator-initiated trial from and served on an advisory board for Eli Lilly. Dr. Whyte has received grant/research support from Pfizer. Dr. Lenze has received grant/research support from Forest and OrthoMcNeil. Dr. Miller has served on the speakers or advisory boards of Forest, Wyeth, and Eli Lilly. Dr. Reynolds has received grant/research support from GlaxoSmithKline, Forest, Bristol-Myers Squibb, Eli Lilly, and Forest. Dr. Dew and Ms. Begley have no additional financial affiliations to report relevant to the subject of this article.
Footnotes
Drug names: bupropion (Wellbutrin and others), diphenhydramine (Benadryl and others), duloxetine (Cymbalta), escitalopram (Lexapro and others), imipramine (Tofranil and others), lithium (Eskalith, Lithobid, and others), lorazepam (Ativan and others), norepinephrine (Levophed and others), nortriptyline (Pamelor and others), paroxetine (Paxil, Pexeva, and others), venlafaxine (Effexor and others).
References
- 1.Whyte EM, Dew MA, Gildengers A, et al. Time course of response to antidepressants in late-life major depression: therapeutic implications. Drugs Aging. 2004;21:531–554. doi: 10.2165/00002512-200421080-00004. [DOI] [PubMed] [Google Scholar]
- 2.Gildengers AG, Houck PR, Mulsant BH, et al. Course and rate of antidepressant response in the very old. J Affect Disord. 2002;69:177–184. doi: 10.1016/s0165-0327(01)00334-2. [DOI] [PubMed] [Google Scholar]
- 3.Dew MA, Whyte EM, Lenze EJ, et al. Recovery from major depression in older adults receiving augmentation of antidepressant pharmacotherapy. Am J Psychiatry. 2007;164:892–899. doi: 10.1176/ajp.2007.164.6.892. [DOI] [PubMed] [Google Scholar]
- 4.Whyte EM, Basinski J, Farhi P, et al. Geriatric depression treatment in nonresponders to selective serotonin reuptake inhibitors. J Clin Psychiatry. 2004;65:1634–1641. doi: 10.4088/jcp.v65n1208. [DOI] [PubMed] [Google Scholar]
- 5.Dew MA, Whyte EM, Lenze EJ, et al. Recovery from major depression in older adults receiving augmentation of antidepressant pharmacotherapy. Am J Psychiatry. 2007;164:892–899. doi: 10.1176/ajp.2007.164.6.892. [DOI] [PubMed] [Google Scholar]
- 6.Nelson JC, Wohlreich MM, Mallinekrodt CH, et al. Duloxetine for the treatment of major depressive disorder in older patients. Am J Geriatr Psychiatry. 2005;13:227–235. doi: 10.1176/appi.ajgp.13.3.227. [DOI] [PubMed] [Google Scholar]
- 7.Wohlreich MM, Mallinekrodt CH, Watkin JG, et al. Duloxetine for the long-term treatment of major depressive disorder in patients aged 65 and older: an open-label study. BMC Geriatr. 2004;4:11. doi: 10.1186/1471-2318-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexopoulos GS, Katz IR, Reynolds CF, et al. The expert consensus guideline series. Pharmacotherapy of depressive disorders in older patients. Postgrad Med. 2001:1–86. [PubMed] [Google Scholar]
- 9.Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for Axis I DSM-IV Disorders (SCID) Washington, DC: American Psychiatric Association Press; 1995. [Google Scholar]
- 10.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Folstein M, Folstein S, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiat Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 12.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 14.Lingjaerde O, Ahlfors UG, Bech P, et al. The UKU Side Effect Rating Scale: a new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl. 1987;334:1–100. doi: 10.1111/j.1600-0447.1987.tb10566.x. [DOI] [PubMed] [Google Scholar]
- 15.Miller MD, Paradis CF, Houck PR, et al. Rating chronic medical illness burden in genopsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41:237–248. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 16.Ware J, Kosinski M, Keller S. SF-36 Health Survey Manual and Interpretation Guide. Boston, Mass: The Health Institute, New England Medical Center; 1997. [Google Scholar]
- 17.Lenze EJ, Mulsant BH, Dew MA, et al. Good treatment outcomes in late-life depression with comorbid anxiety. J Affect Disord. 2003;77:247–254. doi: 10.1016/s0165-0327(02)00177-5. [DOI] [PubMed] [Google Scholar]
- 18.Flint AJ, Rifat SL. The effect of sequential antidepressant treatment on geriatric depression. J Affect Disord. 1996;36:95–105. doi: 10.1016/0165-0327(95)00063-1. [DOI] [PubMed] [Google Scholar]
- 19.Reynolds CF, III, Frank E, Kupfer DJ, et al. Treatment outcome in recurrent major depression: a post hoc comparison of elderly (“young old”) and midlife patients. Am J Psychiatry. 1996;153:1288–1292. doi: 10.1176/ajp.153.10.1288. [DOI] [PubMed] [Google Scholar]
- 20.Cymbalta [package insert] Indianapolis, Ind: Eli Lilly; 2006. [Google Scholar]
- 21.Saghafi R, Brown C, Butters MA, et al. Predicting 6-week treatment response to escitalopram pharmacotherapy in late-life major depressive disorder. Int J Geriatr Psychiatry. 2007 May 8; doi: 10.1002/gps.1804. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andreescu C, Lenze E, Dew M, et al. Effect of comorbid anxiety on treatment response and relapse risk in late-life depression: controlled study. Br J Psychiatry. 2007;190:344–349. doi: 10.1192/bjp.bp.106.027169. [DOI] [PubMed] [Google Scholar]
- 23.Lexapro [package insert] St. Louis, Mo: Forest Laboratories; 2007. [Google Scholar]
- 24.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 25.Keller MB, Lavori PW, Mueller TI, et al. Time to recovery, chronicity, and levels of psychopathology in major depression: a 5-year prospective follow-up of 431 subjects. Arch Gen Psychiatry. 1992;49:809–816. doi: 10.1001/archpsyc.1992.01820100053010. [DOI] [PubMed] [Google Scholar]
- 26.Mueller TI, Keller MB, Leon AC, et al. Recovery after 5 years of unremitting major depressive disorder. Arch Gen Psychiatry. 1996;53:794–799. doi: 10.1001/archpsyc.1996.01830090040006. [DOI] [PubMed] [Google Scholar]
- 27.Mallinekrodt CH, Prakash A, Andorn AC, et al. Duloxetine for the treatment of major depressive disorder: a closer look at efficacy and safety data across the approved dose range. J Psychiatr Res. 2006;40:337–348. doi: 10.1016/j.jpsychires.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 28.Raskin J, Goldstein DJ, Mallinekrodt CH, et al. Duloxetine in the long-term treatment of major depressive disorder. J Clin Psychiatry. 2003;64:1237–1244. doi: 10.4088/jcp.v64n1015. [DOI] [PubMed] [Google Scholar]
- 29.Raskin J, Wiltse CG, Siegal A, et al. Efficacy of duloxetine on cognition, depression, and pain in elderly patients with major depressive disorder: an 8-week, double-blind, placebo-controlled trial. Am J Psychiatry. 2007;164:900–909. doi: 10.1176/ajp.2007.164.6.900. [DOI] [PubMed] [Google Scholar]
- 30.Wohlreich M, Martinez J, Mallinekrodt C, et al. An open-label study of duloxetine for the treatment of major depressive disorder: comparison of switching versus initiating treatment approaches. J Clin Psychopharmacol. 2005;25:552–560. doi: 10.1097/01.jcp.0000185429.10053.c8. [DOI] [PubMed] [Google Scholar]
- 31.Gallo JJ, Rabins PV. Depression without sadness: alternative presentations of depression in late life. Am Fam Physician. 1999;60:820–826. [PubMed] [Google Scholar]