Abstract
Our aim was to test the hypothesis that the vinca alkaloid vincristine could prevent doxorubicin-induced cardiomyocyte death and to identify the mechanisms involved. Adult mouse cardiac myocytes were incubated for 24 h with doxorubicin, with and without concurrent vincristine. Trypan blue exclusion showed that 50–60% of myocytes treated with doxorubicin alone survived. Concurrent vincristine treatment increased survival to ≥85%. Treatment with doxorubicin + vincristine activated the prosurvival signal Akt and diminished cytochrome C release. The PI3K/Akt inhibitor LY294002 and the MEK/ERK inhibitor PD98059 augmented doxorubicin cardiotoxicity and attenuated salvage during concurrent vincristine treatment, indicating that the mechanism of vincristine cardioprotection involves activation of specific survival signals. Vincristine retarded the onset of apoptosis in association with a delay in poly(ADP) ribose polymerase activation. Vincristine also exhibited greater protection than the antioxidant MPG. These novel findings may have clinical implications for the prevention of doxorubicin cardiomyopathy.
Keywords: Cardiac myocytes, Doxorubicin, Vincristine, Signal transduction, Cell culture
The anthracycline anticancer drug doxorubicin is an effective chemotherapeutic agent that is extensively used for the treatment of many malignancies [1,2]. Its major limitation is cardiotoxicity. Doxorubicin cardiomyopathy accompanied by congestive heart failure ranges from 0.1% to 18% [3], and this complication is associated with a poor prognosis [2,4]. Thus it is not surprising that considerable effort has been expended to understand the mechanisms of doxorubicin cardiotoxicity and to identify therapies that reduce this adverse response [2].
Several pharmacologic agents have been used to reduce doxorubicin cardiotoxicity. Antioxidants [3], iron-chelating agents [4] and hematopoetic cytokines such as erythropoietin [5] are effective in animal models. We have previously reported that the vinca alkaloid vincristine exerts cardioprotective effects on cultured adult mouse cardiac myocytes exposed to chemical and hypoxic oxidative stress [6]. As vincristine is often administered to patients together with doxorubicin, we used a cell culture model to test the hypothesis that vincristine would also attenuate doxorubicin-induced cardiac myocyte toxicity. We also wished to identify potential mechanisms of such vincristine-induced cardioprotection.
Methods
Materials
Doxorubicin (DOX), vincristine (VCR), and N-(2-mercaptoproprionyl)-glycine) (MPG) were purchased from Sigma–Aldrich (St. Louis, MO). Antibodies against poly ADP-ribose (PAR) and cytochrome C were purchased from BD Biosciences (Franklin Lakes, NJ) and antibodies against GAPDH were obtained from Abcam (Cambridge, MA). An AKT antibody and LY294002 and PD98059 were obtained from Biomol (Plymouth Meeting, PA). Mouse laminin was purchased from Invitrogen (Carlsbad, CA).
Cell culture
The study was approved by the Institutional Animal Care and Use Committee of the San Francisco Veterans Affairs Medical Center. The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). Male C57Bl/6 mice weighing 19–25 gm were obtained from Charles River Laboratories (Hollister, CA). Mice received standard rodent chow and water ad libitum. Methods for isolation and culture of adult mouse myocytes were as previously reported from our laboratory [6,7]. Following anticoagulation with heparin (50 U i.p.), mice were euthanized with sodium pentobarbital (200 mg/kg i.p.). After excision hearts were cannulated via the aorta for retrograde perfusion for 2 min with Ca2+ free isolation buffer containing (mmol/L) NaCl 120, KCl 5.4, MgSO4 1.2, NaH2PO4 1.2, glucose 5.6, NaHCO3 4.6, HEPES 10, taurine 5, 2, 3 butanedione monoxine (BDM) 10. The hearts were then perfused for approximately 7 min with the same isolation buffer which in addition contained 50 µmol/L CaCl2 and 2 mg/mL collagenase II (Worthington, Lakewood, NJ). Ventricles were then separated by trimming the other cardiac and major vascular structures. Ventricular fibers were teased apart with forceps, pipetted in collagenase II buffer, filtered through a cell dissociation sieve, and centrifuged at 40g for 1 min. Cardiac myocyte pellets were resuspended in isolation buffer serially supplemented with 100, 250, 500, and 1200 µmol/L CaCl2. The final myocyte pellets were resuspended in minimal essential medium (MEM) containing Hanks Buffered Salt Solution (HBSS), 10 µg/mL penicillin, 1.5 µmol/L vitamin B12, 2.5% bovine calf serum (BCS), and 10 mmol/L BDM. Isolated myocytes were then plated on dishes coated with 10 µg/L laminin and in media containing 2.5% serum and 10 mM BDM at a density of 50 rod-shaped cells/mm2 for 2 h. Culture media were changed to 1 mM BDM without serum for overnight incubation at 37 °C in a humidified atmosphere of 1% CO2 and air. Cells were viable at pH 7.2 for 72 h. Experimental protocols as described under Results were performed the day following myocyte isolation and plating. For short-term signaling experiments, medium was changed to remove BDM, while for long-term survival experiments 1 mM BDM remained in the medium. However, in the absence of BDM, long-term survival did not differ.
Assessment of cell survival
Myocyte survival using trypan blue exclusion was quantified as previously reported in our laboratory [6,7]. For calculation of viability and morphologic changes myocytes were visualized at 100× magnification by microscopy. Cells that excluded trypan blue (TBE) were considered viable. Healthy rod-shaped myocytes (rods) were identified when the length/width ratio was >3:1 as previously described [6,7]. Contracted cells were defined when the length/width ratio was <3:1. Trypan blue positive cells were identified when the trypan blue was present intracellularly irrespective of whether the cells were rod shaped or contracted [6,7]. Morphologic changes were measured by determining the number of rods relative to all TBEs (rods and contracted cells) in 10 fields/dish.
A second technique employed to assess cell survival was the Live/Dead Viability/Cytotoxicity assay (Molecular Probes, Carlsbad, CA) following the manufacturer’s instruction as previously reported [6]. It is based on determination of intracellular esterase activity and plasma membrane integrity. The polyanionic dye calcein, which fluoresces green, is retained exclusively by live cells. Ethidium homodimer enters the cells only when the plasma membrane is damaged, and after binding to nucleic acids, it exhibits red fluorescence. For these experiments myocytes were grown on chamber slides. The culture medium was replaced with 2 µmol/L calcein acetoxymethyl ester and 4 µmol/L ethidium homodimer-1. Live cells were recognized by the intense uniform green fluorescence of calcein and the dead cells by red fluorescence of the ethidium homodimer.
Assessment of apoptosis
A direct TUNEL labeling assay (Roche Applied Science, Indianapolis, IN) was used to determine apoptotic death of the myocytes. Cardiomyocytes were cultured overnight on 25 mm circular glass cover slips (Fisher Scientific, Pittsburgh, PA) and treated with doxorubicin (15 µg/ml) with or without vincristine (10 µmol/L) for 2–8 h. At each time point glass cover slips were submerged in 4% paraformaldehyde in PBS for 10 min at −20 °C. Following fixation, cells were air dried and PBS was added to the cover slip and incubated for 10 min at room temperature. The TUNEL reaction was performed according to the manufacturer’s instructions and the incorporated fluorescein was visualized with a Zeiss Axiophoto fluorescence microscope and recorded by an LEI-750 digital imaging system. Apoptotic cells were identified by fragmented nuclear structure in cells stained with 0.5 µg/ml of Hoechst 33258 (Molecular Probes).
In addition, immunofluorescence microscopy was used to detect poly (ADP) ribose (PAR) as an index of poly(ADP) ribose polymerase activity. Cardiomyocytes cultured on 25 mm glass cover slips in 35 mm dishes were treated with the following: doxorubicin (15 µg/ml) with or without vincristine (10 µmol/L) at different time points. The cardiomyocytes were fixed with MEOH/acetone (70:30 v/v) for 10 min at −20 °C. Following fixation, cells were dried and PBS was added to the glass cover slips and incubated for 10 min at room temperature. After cells were permeablized in blocking buffer (PBS, 5% non-fat dry milk (w/v), 0.1% Tween 20) for 30 min at room temperature, cells were then incubated with purified anti-poly (ADP-ribose) (PAR) antibody at a dilution of 1:2000 for 2 h at room temperature. Cells were washed 5 times in PBS and reacted with Alexa Fluor 488 conjugated goat antimouse IgG (H + L) and visualized with a Zeiss Axiophoto fluorescence microscope and recorded by a LEI-750 digital imaging system.
Western blot analysis
Following appropriate treatments cultured myocytes were lysed and the lysates were subjected to SDS–PAGE as previously described [6,7]. Selected primary antibodies, appropriate secondary antibodies conjugated with horseradish peroxidase, and an enhanced chemiluminescence system (ECL, Amersham Biosciences, Piscataway, NJ) were used for detection of proteins of interest. Densitometry was used for quantitative assessment of the signals with NIH Image 1.61 software.
Mitochondrial isolation and cytochrome C release
Mitochondria were isolated from cultured cardiomyocytes using a commercially available kit (BD Biosciences Clontech, Palo Alto, CA) and cytochrome C release was measured by western blot analysis with a cytochrome C specific antibody.
Statistics
All results are reported as means ± SEM. Comparisons were made by one-way analysis of variance (ANOVA). Post hoc analysis was performed using the Student-Newman-Keuls test. P < 0.05 was considered significant.
Results
Doxorubicin-induced decrease in cardiomyocyte survival is prevented by vincristine
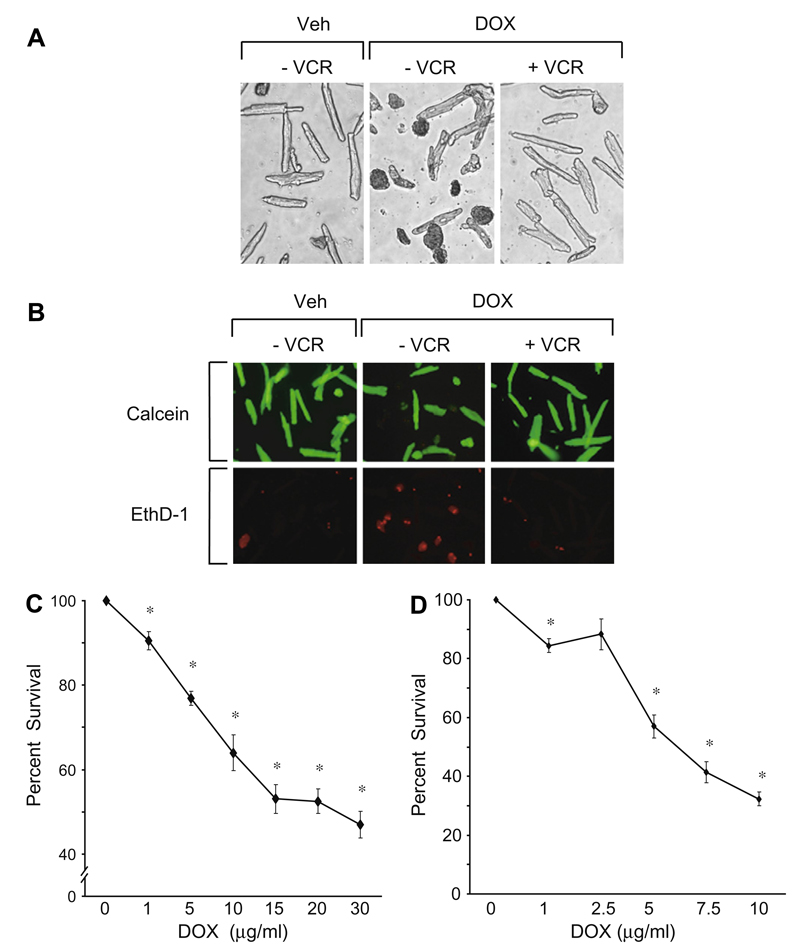
A trypan blue exclusion assay during doxorubicin incubation with and without co-treatment with vincristine in a representative experiment is illustrated in Fig. 1A. Compared to control, doxorubicin was associated with a large number of trypan blue positive cells indicating cell death. With vincristine co-treatment the majority of cells were trypan blue negative suggesting persistent viability. Results of the qualitative “Live/Dead” assay for the assessment of death and survival of the cultured adult myocytes treated with doxorubicin alone and during co-treatment with vincristine are illustrated in Fig. 1B.
Fig. 1.
(A) Typical results of trypan blue exclusion assay in a representative experiment. Doxorubicin (15 µg/ml) treatment for 20–24 h was associated with an increased number of trypan blue positive cells and a decrease in rod-shaped cells compared to the vehicle control panel. Concurrent treatment with 10 µmol/L of vincristine was associated with a fewer trypan blue positive cells and persistence of rod-shaped cells indicating increased survival. These results were replicated multiple times. (see text and C and D and Fig. 2). VCR, vincristine; Veh, vehicle; Dox, doxorubicin. (B) Results of a Live/Dead assay. With doxorubicin (15 µg/ml) treatment alone for 24 h, there was an increased proportion of dead cells (ethidium positive/red fluorescence) and a decreased proportion of live cells (calcein ester positive/green fluorescence) compared to vehicle treatment. With concurrent vincristine treatment (10 µmol/L) there were many more live than dead cells. This experiment was replicated 4 times. VCR, vincristine; Veh, vehicle; Dox, doxorubicin; EthD-1, ethidium homodimer-1. (C, D) Time and dose dependent effects of doxorubicin on the survival of cultured adult mouse myocytes as measured by the trypan blue exclusion assay. Cell counts are expressed as the percentage relative to control values which are set at 100%. (C) Cells were treated with doxorubicin (1–30 µg/ml) for approximately 20 h. At 15–30 µg/ml, cell survival decreased by more than 50%. (D) Cells were treated with doxorubicin (1–10 µg/ml) for approximately 48 h. After longer exposure cell survival decreased significantly despite a lower concentration of doxorubicin. *p < 0.05, n = 7.
Treatment with increasing concentrations of doxorubicin for approximately 24 h is illustrated in Fig. 1C. Cell counts are expressed as the percentage relative to control values which are set at 100%. Even at 1 µg/ml of doxorubicin adult mouse cardiac myocyte survival began to decrease and at 15–30 µg/ml survival decreased dramatically. When the exposure time was increased to 48 h, myocyte survival decreased by a similar amount at a lower concentration of doxorubicin (Fig. 1D). These concentrations are in the range of those achieved in human patients undergoing cancer chemotherapy [8]. For the remainder of the experiments, unless specified, 15 µg/ml doxorubicin and 20–24 h of incubation were employed.
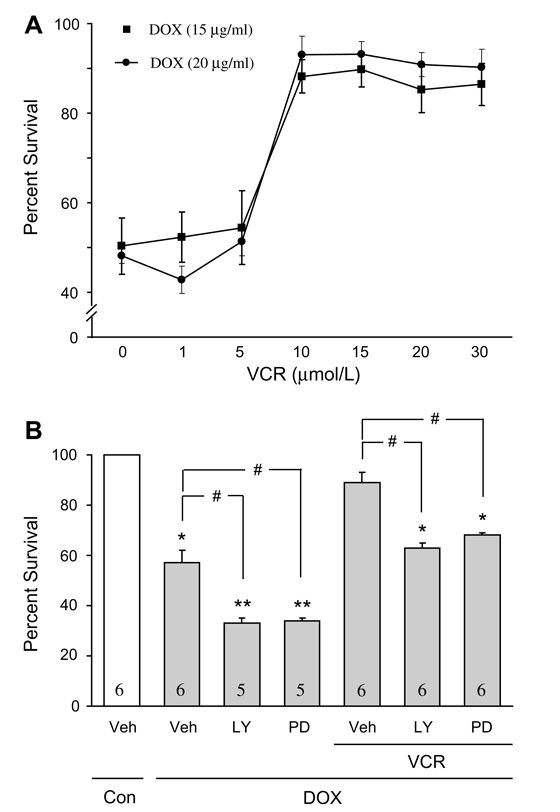
In Fig. 2A, the effects of increasing concentrations of vincristine on the survival of cultured adult mouse myocytes exposed to doxorubicin are illustrated. Similar to doxorubicin, these concentrations of vincristine are within the range of levels reported in human patients receiving chemotherapy [9,10]. Co-treatment with vincristine dramatically increased cardiomyocyte survival (>85%) compared to untreated control cells.
Fig. 2.
(A) Effect of increasing concentration of vincristine co-treatment on the proportion of surviving myocytes (trypan blue assay), in the presence of 15 µg/ml and 20 µg/ml of doxorubicin. With vincristine (10–30 µmol/L) there was about 90% salvage of myocytes. *p < 0.05, n = 5. (B) The effects pharmacological inhibitors of PI3-K/AKT (LY294002) and MEK/ERK (PD98059) on cell survival (trypan blue exclusion assay), with or without doxorubicin (15 µg/ml) and with vincristine co-treatment (10 µmol/L). Myocytes were pretreated with 10 µmol/L of LY294002 (LY) or 30 µmol/L of PD98059 (PD) for 30 min. Compared to control, doxorubicin treatment alone was associated with a significant reduction in cell survival; with addition of the inhibitors there was a further reduction in survival. After addition of vincristine alone to doxorubicin, myocyte survival was about 90%. Following the addition of the inhibitors the protective effect of vincristine was substantially reduced. Numbers in bars indicate the number of experiments for each condition. *p < 0.05 vs. all other conditions except LY or PD + VCR and DOX; **p < 0.05 vs. all other conditions except for DOX vehicle; #p < 0.05 for indicated comparisons. Veh, vehicle; VCR, vincristine; Dox, doxorubicin.
Potential protective mechanisms activated by vincristine
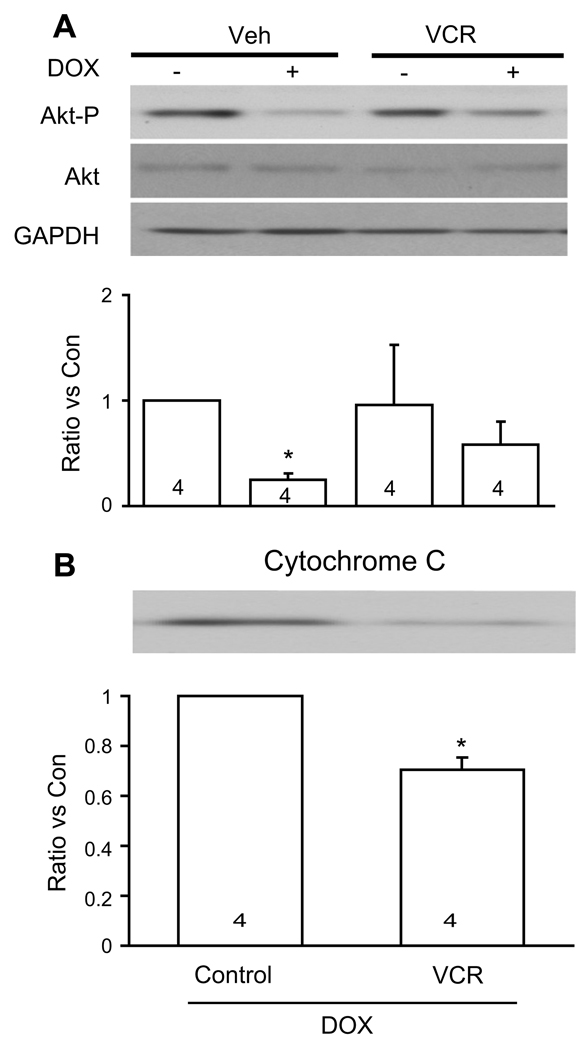
Previously we reported that vincristine activates several prosurvival signaling pathways which contribute to its protective effect on cardiomyocytes exposed to chemical oxidative stress [6]. In the current study we asked whether doxorubicin inhibits these pathways. PI3-K/Akt and MEK/ERK inhibitors (LY294002 and PD98059, respectively) further reduced myocyte survival compared to doxorubicin alone (Fig. 2B). However, during vincristine co-treatment without the inhibitors, myocyte salvage was markedly enhanced. With the addition of the inhibitors the protective effect of vincristine was substantially reduced (Fig. 2B). Fig. 3A illustrates the effects of doxorubicin with and without vincristine co-treatment on phosphorylation of the prosurvival factor Akt (S473). Although doxorubicin significantly decreased Akt phosphorylation, vincristine co-treatment maintained Akt phosphorylation at control levels. Compared to doxorubicin treatment alone, co-treatment with vincristine was associated with a significant decrease in cytochrome C release (Fig. 3B), suggesting reduction of oxidative stress and inhibition of mitochondrial permeability transition.
Fig. 3.
(A) Illustrated are changes in Akt phosphorylation in cultured adult mouse myocytes after 24 h of doxorubicin treatment (15 µg/ml) with or without vincristine (10 µmol/L). The upper panel shows representative western blots and the bottom panel shows the fold- increase in signal intensity. Numbers in bars show the number of experiments for each of signals depicted. Vincristine maintained phosphorylated Akt at control levels during doxorubicin treatment. *p < 0.05 vs all other conditions. Veh, vehicle; VCR, vincristine; DOX, Doxorubicin; Akt-P, phosphorylated Akt. (B) Western blot analysis of cytochrome C release in response to 15 µg/ml doxorubicin for 2 h. Co-treatment with 10 µmol/L vincristine decreased cytochrome C release into the cytosol. Equal loading was verified with anti- GAPDH antibody (not shown). Numbers in bars show the number of experiments for each of signals depicted. *p < 0.05 vs control. Con, control; DOX, doxorubicin; VCR, vincristine.
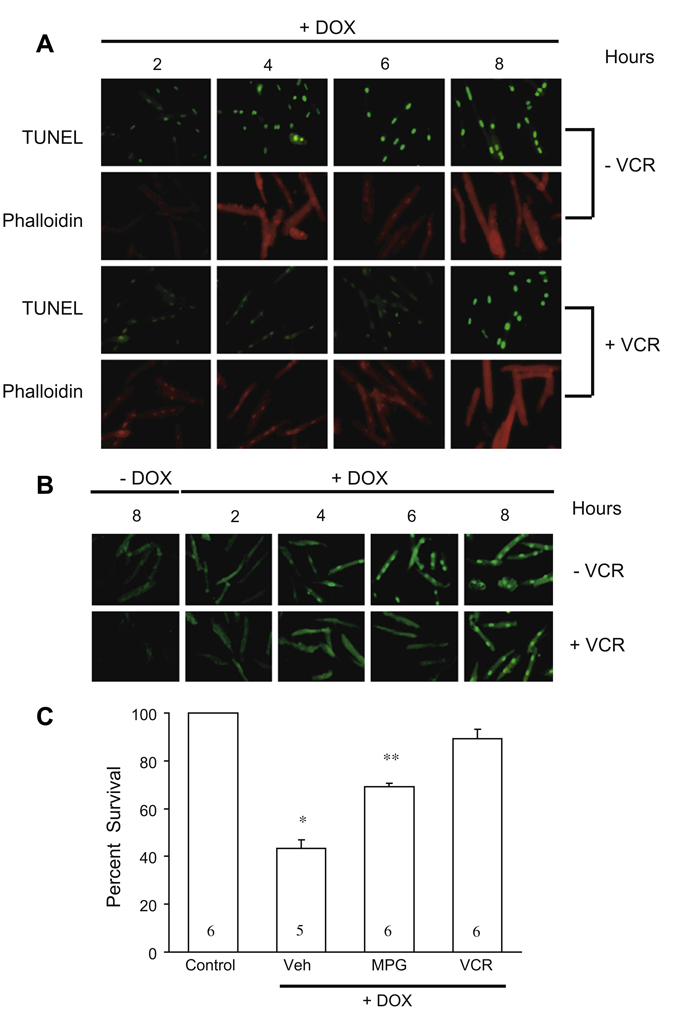
In addition to examining cell necrosis as described above, wealso studied the effects of vincristine on doxorubicin-induced apoptosis as measured by TUNEL staining and PARP activation. The results of TUNEL staining are illustrated in Fig. 4A. With doxorubicin treatment, apoptosis was obvious within 4 h of incubation, which is much earlier than evidence of widespread trypan blue uptake described above. During co-treatment with vincristine apoptosis of cultured adult myocytes was delayed by several hours. We also monitored the DNA repair activity of poly (ADP-ribose) polymerase-1 (PARP-1) by measuring its product poly (ADP-ribose) (PAR). During treatment with doxorubicin PAR was detected as early as 4 h; with vincristine co-treatment PAR was not detected until 8 h (Fig. 4B).
Fig. 4.
(A) Results of TUNEL staining to assess changes in the magnitude of apoptosis with doxorubicin treatment alone and co-treatment with vincristine. Co-treatment with vincristine delayed the onset of apoptosis. Phalloidin, which stains actin fibers, was used to confirm that the cells studied were rod-shaped myocytes. DOX, doxorubicin; VCR, vincristine. (B) Effects of doxorubicin treatment alone and with vincristine co-treatment on PARP activity monitored by assessment of poly (ADP)ribose (PAR) generation. The appearance of PAR was delayed during vincristine co-treatment. DOX, doxorubicin; VCR, vincristine. (C) Vincristine compared to the antioxidant N-(2-mercaptoproprionyl)-glycine) (MPG). Compared to 0.1 mmol/L MPG, the magnitude of cell survival (trypan blue exclusion assay) with vincristine (VCR, 15 µg/ml) was greater. Numbers in bars represent the number of experiments performed for each of the treatments depicted. Veh, vehicle; *p < 0.01 vs. all other bars; **p < 0.01 vs. Veh and VCR + DOX.
One mechanism of doxorubicin toxicity is the induction of free radicals [2]. As vincristine has been shown to prevent cardiac myocyte death due to oxidative stress, we next asked whether vincristine is more or less effective than an agent that has been reported to decrease doxorubicin-induced cardiotoxicity [11]. Accordingly we compared N-(2-mercaptopropionyl glycine) (MPG), a synthetic aminothiol that exhibits antioxidant properties with vincristine in our cardiac myocyte cell culture model. As shown in Fig. 4C and noted above, doxorubicin alone substantially reduced myocyte survival. MPG co-treatment increased survival significantly. However, during co-treatment with vincristine cardiac myocyte survival was significantly higher than with MPG (Fig. 4C).
Discussion
The major finding of the present study was that concurrent vincristine treatment provided dramatic salvage of cultured adult mouse myocytes exposed to doxorubicin. Previously we reported that vincristine is cardioprotective in adult mouse cardiac myocytes exposed either to hypoxia or chemical oxidative stress [6]. As doxorubicin and vincristine are often given together, we wondered whether vincristine could prevent or delay the toxic effects of doxorubicin. Our results indicate that this indeed appears to be the case. Despite the presence of doxorubicin, vincristine retains the ability to activate MAPK pathways. Stimulation of these pathways has been found to confer cardioprotection, especially after preconditioning [12].
Doxorubicin-induced apoptotic death of cardiomyocytes has been reported previously [13]. Within 4 h of doxorubicin treatment substantial apoptotic demise of cultured adult mouse cardiac myocytes was evident as reflected by TUNEL staining. However during co-treatment with vincristine apoptosis was considerably delayed and was not evident until 8 h (Fig. 4A). It has been proposed that cardiomyocyte apoptosis is related to hydrogen peroxide production and activation of the p53-induced apoptosis pathway [13]. Although it is possible that the anti-apoptotic effect of vincristine we observed is due to its effect on hydrogen peroxide and/or free radicals generated by doxorubicin, using 1H NMR spectroscopy we previously observed no alteration in the spectra of vincristine when it was incubated with hydrogen peroxide in the presence or absence of cardiomyocytes [6]. These data did not support a direct interaction of vincristine and hydrogen peroxide. Moreover, vincristine was even more potent than the antioxidant MPG, suggesting that the beneficial effect of vincristine results from activation of alternative prosurvival pathways reported in the present study, including elements of the MAPK pathway.
In this connection, when reactive oxygen species are produced, DNA strand breaks are induced which may cause excessive activation of the nuclear enzyme poly (ADP-ribose) polymerase-1 (PARP-1) which leads to depletion of intracellular NAD+ and cell death [14]. When PARP-1 is activated, there is increased synthesis of the poly (ADP) ribose polymer (PAR) [15]. Thus by monitoring PAR production the activity of PARP-1 can be assessed. In the present study, with doxorubicin alone, PAR was detected after 4 h of treatment; with vincristine co-treatment, PAR was not detected until 8 h (Fig. 4B). These findings suggest that inhibition of doxorubicin-induced PARP-1 activity by vincristine at least partly contributed to its protective effect.
In summary, this study, in which the concentrations of doxorubicin and vincristine used are in the range of those achieved clinically in human patients [8–10], demonstrates for the first time that in the experimental model used vincristine provides substantial protection of adult cardiomyocytes exposed to doxorubicin via multiple pathways.
Acknowledgments
Funded by the Foundation for Cardiac Research, UCSF and a National Institutes of Health Grant (PO1 HL 68738 to JSK).
References
- 1.Weiss RB. The anthracyclines: will we ever find a better doxorubicin? Semin Oncol. 1992;19:670–686. [PubMed] [Google Scholar]
- 2.Takemura G, Fujiwara H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog. Cardiovasc. Dis. 2007;49:330–352. doi: 10.1016/j.pcad.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Oliveira PJ, Bjork JA, Santos MS, Leino RL, Froberg MK, Moreno AJ, Wallace KB. Carvedilol mediated antioxidant protection against doxorubicin-induced cardiac mitochondrial toxicity. Toxicol. Appl. Pharmacol. 2004;200:159–168. doi: 10.1016/j.taap.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Della Torre P, Imondi AR, Bernardi C, Podestà A, Moneta D, Riflettuto M, Mazué G. Cardioprotection by dexrazoxane in rats treated with doxorubicin and paclitaxel. Cancer Chemother. Pharmacol. 1999;44:138–142. doi: 10.1007/s002800050958. [DOI] [PubMed] [Google Scholar]
- 5.Li L, Takemura G, Li Y, Miyata S, Esaki M, Okada H, Kanamori H, Khai NC, Maruyama R, Ogino A, Minatoguchi S, Fujiwara T, Fujiwara H. Preventive effect of erythropoietin on cardiac dysfunction in doxorubicin-induced cardiomyopathy. Circulation. 2006;113:535–543. doi: 10.1161/CIRCULATIONAHA.105.568402. [DOI] [PubMed] [Google Scholar]
- 6.Chatterjee K, Zhang J, Honbo N, Simonis U, Shaw R, Karliner JS. Acute vincristine pretreatment protects adult mouse cardiac myocytes from oxidative stress. J. Mol. Cell. Cardiol. 2007;43:327–336. doi: 10.1016/j.yjmcc.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Honbo N, Goetzl EJ, Chatterjee K, Karliner JS, Gray MO. Signals from type 1 sphingosine 1-phosphate receptors enhance adult mouse cardiac myocyte survival during hypoxia. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H3150–H3158. doi: 10.1152/ajpheart.00587.2006. [DOI] [PubMed] [Google Scholar]
- 8.Bronchud MH, Margison JM, Howell A, Lind M, Lucas SB, Wilkinson PM. Comparative pharmacokinetics of escalating doses of doxorubicin in patients with metastatic breast cancer. Cancer Chemother. Pharmacol. 1990;25:435–439. doi: 10.1007/BF00686055. [DOI] [PubMed] [Google Scholar]
- 9.Dorr RT, Von Hoff DD. Cancer Chemotherapy Handbook. 2nd ed. East Norwalk, Conn: Appleton &Lange; 1994. Vincristine Sulfate; pp. 951–957. [Google Scholar]
- 10.Gronninger E, Koopmans P, Kamps W, de Graaf S, Uges D. An automated HPLC method to determine intracellular vincristine concentrations in mononuclear cells of children with acute lymphoblastic leukemia. Ther. Drug Monit. 2003;25:441–446. doi: 10.1097/00007691-200308000-00004. [DOI] [PubMed] [Google Scholar]
- 11.el-Missiry MA, Othman AI, Amer MA, Abd el-Aziz MA. Attenuation of the acute adriamycin-induced cardiac and hepatic oxidative toxicity by N-(2-mercaptopropionyl) glycine in rats. Free Radic. Res. 2001;35:575–581. doi: 10.1080/10715760100301581. [DOI] [PubMed] [Google Scholar]
- 12.Hausenloy DJ, Mocanu MM, Yellon DM. Cross-talk between the survival kinases during early reperfusion: its contribution to ischemic preconditioning. Cardiovasc. Res. 2004;63:305–312. doi: 10.1016/j.cardiores.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Sawyer DB, Fukazawa R, Arsttall MA, Kelly RA. Daunorubicin-induced apoptosis in rat cardiac myocytes is inhibited by dexrazoxane. Circ. Res. 1999;84:257–265. doi: 10.1161/01.res.84.3.257. [DOI] [PubMed] [Google Scholar]
- 14.Chua CC, Liu X, Gao J, Hamdy RC, Chua BL. Multiple actions of pifithrin-α on doxorubicin-induced apoptosis in rat myoblastic H9c2 cells. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H2606–H2613. doi: 10.1152/ajpheart.01138.2005. [DOI] [PubMed] [Google Scholar]
- 15.van Wijk SJ, Hageman GJ. Poly (ADP-ribose) polymerase-1 mediated caspase-independent cell death after ischemia/reperfusion. Free Radic. Biol. Med. 2005;39:81–90. doi: 10.1016/j.freeradbiomed.2005.03.021. [DOI] [PubMed] [Google Scholar]