Abstract
Cardiovascular disease remains the number one killer in western nations in spite of declines in death rates following improvements in clinical care. It has been 20 years since David Barker and colleagues showed that slow rates of prenatal growth predict mortality from ischemic heart disease. Thus, fetal undergrowth and its associated cardiovascular diseases must be due, in part, to placental inadequacies. This conclusion is supported by a number of studies linking placental characteristics with various adult diseases. A “U” shaped relationship between placental-to-fetal weight ratio and heart disease provides powerful evidence that placental growth-regulating processes initiate vulnerabilities for later heart disease in offspring. Recent evidence from Finland indicates that placental morphological characteristics predict risks for coronary artery disease, heart failure, hypertension and several cancers. The level of risk imparted by placental shape is sex dependent. Further, maternal diet and body composition strongly influence placental growth, levels of inflammation, nutrient transport capacity and oxidative stress, with subsequent effects on offspring health. Several animal models have demonstrated the placental roots of vulnerability for heart disease. These include findings that abnormal endothelial development in the placenta is associated with undergrown myocardial walls in the embryo, and that placental insufficiency leads to depressed maturation and proliferation of working cadiomyocytes in the fetal heart. Together these models suggest that the ultimate fitness of the heart is determined by hemodynamic, growth factor, and oxygen/nutrient cues before birth, all of which are influenced, if not regulated by the placenta.
1. Introduction
Across the globe, far more people die of cardiovascular diseases than any other disease in spite of the fact that deaths due to coronary artery disease have declined since the early 1970s and continue to do so in developed countries. Nevertheless, the annual cost of cardiovascular disease continues to increase in the US and is expected to reach the half trillion dollar mark next year. This continual rise in cost is due in part to the increase in the numbers of people with myocardial damage who now progress to heart failure. Heart failure is the fastest growing reason for hospitalization in the US, and the most expensive. An additional dark cloud sits on the horizon. An increasing number of children and adolescents are gaining excessive body weight and crossing the health threshold into the realm of chronic disease. This portends a further increase in health care costs in the coming decades as increasing numbers of middle aged adults with type 2 diabetes and coronary heart disease require medical attention [1]. Medical scientists are wondering how this epidemic of metabolic disease evolved and how it might be stopped. There is reason to believe that a better understanding of early human development might shed light on the problem.
2. Programming of cardiovascular disease
Twenty years ago David Barker and colleagues showed that mortality due to ischemic heart disease increased with decreasing birthweights over the 5 to 9 pound (2.3 to 4.1 kg) range; in addition, they found that babies at the largest end of the birthweight scale, above 9.5 pounds (4.3 kg), also had high rates of mortality [2]. Thus it appears that babies at both ends of the birthweight spectrum are at high risk for cardiovascular disease in adulthood. Over the intervening 20 years, a number of human and animal studies have strengthened the original findings. It is now known that in addition to ischemic heart disease, obesity, hypertension and type 2 diabetes are also associated with low birthweight. Thus, the primary chronic diseases that affect western societies have their origins in prenatal life.
A developing mammal can respond to detrimental environmental conditions in the womb only within the constraints of its gene expression options. Thus, stressful responses are necessarily limited by biological options and have become rather similar among individuals within, and even across, species. The term “fetal programming” has been applied to the structural, physiological and gene-activated responses to intrauterine stresses that lead to increased disease risk in later life. While the term “programming” has its detractors, the underlying concept that gene expression patterns in the womb, conditioned by adverse surroundings, predict future disease outcomes has withstood two decades of human and animal investigations and the terminology has taken root. Programming is merely a reflection of developmental plasticity that has been recognized for many decades. However in the case of programming the plasticity leads to susceptibility for adult-onset disease. Alterations in maternal-fetal nutrition, hormone levels, cardiac load and placental function have all been shown to lead to adaptations in fetal cardiovascular development. The placenta is in a unique position to influence all of these intrauterine challenges.
The term “heart disease” is in reality a catchall phrase that represents many diverse heart and blood pathologies and conditions. For a few, like hypertension and coronary heart disease, links to prenatal stress are well established. However, for most cardiovascular conditions, associations with placental growth and function have not even been considered, let alone investigated. Thus, the roles of the placenta in the etiology of adult onset arrhythmias, valvular disease, congenital structural defects, coagulopathies, coronary endothelial dysfunction and dyslipidemias remain unstudied.
There are now many lines of animal evidence showing that the placenta is important in regulating the development of the cardiovascular system. Thus it is not surprising to find that epidemiological evidence supporting an association of placental growth and cardiovascular disease has been accumulating over the past two decades. Barker and colleagues showed a U-shaped relationship between the placenta-to-birthweight ratio and subsequent death from coronary heart disease in 2571 men in Sheffield, UK [as reviewed by Godfrey, 3]. The risk of cardiac death was least when the placenta weighed just under 20% of fetal body weight but the risk increased when the placental-to-fetal weight ratio deviated (increased or decreased) from 20%. Risnes et al., [4] also found that a high placental ratio (placental weight-to-birthweight) predicted death from coronary artery disease among 31,000 men and women in Norway.
3. The placenta and cardiovascular disease: Data from the Helsinki Birth Cohorts
The Helsinki Birth Cohorts have been especially helpful in uncovering the relationships between placental size and shape, and cardiovascular diseases in later life. Two primary Helsinki Birth Cohorts have been studied: men and women born between 1924–1933, and a younger cohort born between 1934–1944. Data from these cohorts are highly valuable because they contain information regarding birth size, placental size, childhood growth and health records. The team of Johann Eriksson, Eero Kajantie, Clive Osmond, and David Barker has investigated these cohorts and discovered many new relationships between placental size and shape and chronic diseases in later life. For example, the team analyzed data from 279 women hospitalized for coronary heart disease from among 3447 women in the 1924–1934 Birth Cohort [5]. They were compared to a group of men with coronary disease from the same cohort [6]. For the women, the greatest risk for coronary disease was associated with the lowest birthweights (≤2.5 kg) and shortest birth lengths (≤48 cm). In men, disease was associated with low birthweight and thinness at birth. Among women, hazard ratios rose with an increasing ratio of placental weight to birth length. These data indicate that altered patterns of prenatal growth (both of the fetus and the placenta) can impart sex-dependent risks for later disease.
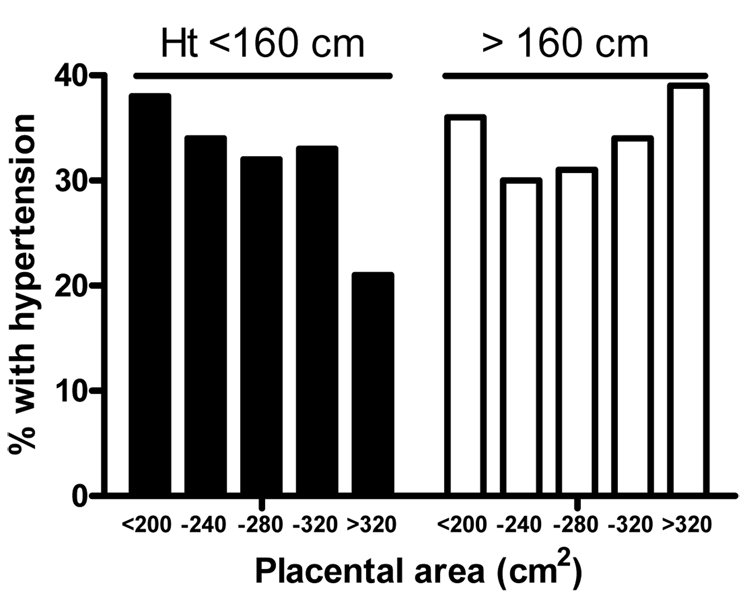
Hypertension is a major underlying determinant of heart disease. The number of people with uncontrolled hypertension is increasing in the USA despite the increase in the number of drug regimens available for antihypertensive therapy [7]. Recent data from Helsinki have shown that specific features of placental development are related to hypertension [8]. In the younger Helsinki cohort, placental weights and measurements were taken by nurse midwives at the time of delivery. The major axis of the placenta was measured as the longest dimension of the placenta and the minor axis the widest point at right angles to it. From these axial dimensions and the placental weight, placental area was estimated as the length of the major axis × minor axis dimension × π/4. From the area and the weight, placental thickness could also be estimated. Among 644 people being treated for hypertension, the disease was generally associated with a small placenta (as measured by weight, area and diameters). However, upon close inspection, hypertension was found to be primarily associated with small placentas in mothers whose heights were below the median of 160cm. In women who were taller than 160cm, there was no association between placental area, weight or diameters, and offspring’s hypertension. Indeed, a significant interaction was found between effects of maternal height and placental area (p for interaction=0.02) and height and length of the minor axis (p for interaction=0.002) on hypertension. Thus, indices of maternal nutrition (such as height) are important variables in the relationship between placental growth and the fetal programming of cardiovascular disease (see Figure 1).
Figure 1.
Hypertension in offspring of short and tall mothers as a function of placental area. Incidence of hypertension according to placental area is divided based upon maternal height (black bars: <160 cm; white bars: >160 cm). Individual bars represent placental area categories. There was a significant relationship between hypertension and placental area in offspring of short mothers (p<0.002) but not tall mothers (p=0.72). Data from Barker et al. [8].
It is known that women who have had a pregnancy complicated by preeclampsia are more likely to have heart disease in later life, and studies from the Helsinki Birth Cohort have shown that the offspring from a preeclamptic pregnancy have an elevated risk for stroke [9]. Recent data show that placentas from mothers with preeclampsia are of a predictable size and shape. In a subset of 6410 mothers in the 1934–44 Helsinki birth cohort, the relationship between placental measurements and preeclampsia was determined [10]. Maternal hypertension without proteinuria (hypertension without preeclampsia) was associated with a reduction in placental area (p<0.009) but not thickness (p=0.7) when compared to placentas from normotensive pregnancies. Severe preeclampsia was associated with a reduction in the weight, area and length of both axes but also an increase in placental thickness when compared to normotensive pregnancies (p<0.001 for all measurements). When mild and severe cases of preeclampsia were combined, there was a strong trend for increasing risk of preeclampsia as the absolute length of the minor axis decreased (p<0.0001) whereas there was no trend for the major axis (p=0.9) [10]. This suggests that growth of the major and minor axes of the placenta are regulated differently and that length of the minor axis may be closely related to processes underlying preeclampsia. Taken together, the Helsinki Birth Cohort data demonstrate that placental size and shape are highly powerful predictors of future risk for coronary heart disease and hypertension. Further investigation may reveal the mechanisms underlying the placental programming of cardiovascular disease.
4. Fetal cardiac development is vulnerable to placental vascular development
The heart must grow in capacity throughout gestation to provide sufficient blood flow for growing fetal organs. Thus, the fractional cardiac output that supplies the placenta remains constant, at least over the last half of gestation [11]. Increases in placental flow impedance, as found in placental insufficiency, lead to adjustments in growth by the embryonic and fetal heart. This is understandable because the heart must eject against the instantaneous resistance of the placental bed, the largest fetal vascular bed, with each beat. Thus, the mechanical force required to eject against a high resistance bed is sensed by the contracting muscle with every beat. The developing heart is primed to alter its growth patterns in order to maintain its stroke volume to accommodate changing vascular conditions. In humans, severe placental insufficiency, indicated by absent or reversed diastolic flow in the umbilical artery, can lead to increased loading of the right ventricle [12] and because of central shunts, the left ventricle also.
The importance of appropriate vascular development in the placenta has been further highlighted in a recent study of HOXA13 knockout embryonic mice [13]. HOXA13 is not expressed in the heart, but in the placenta it regulates Tie-2 and is critical for normal vascular branching in the placenta. In HOXA13 −/− mice the placental endothelium is abnormal in appearance but the structure of the underlying trophoblast layers is not affected by the gene deficiency. The interesting feature of this finding is that ventricular wall thickness of the embryonic heart is reduced by some 43% in HOXA13−/− mice [13]; they die at embryonic day 14. Thus, placental endothelial defects appear to underlie hemodynamic alterations, such changes in impedance, wall stretch and/or endothelial shear stresses which result in lethal abnormalities of the embryonic heart. Thus, even at an early stage in development, the placenta plays an important role in the development of the heart.
5. Placental insufficiency
Most of our understanding of the biological mechanisms that underpin placental insufficiency has come from animal models. The functional surface area of the sheep placenta can be reduced by various means including umbilical arterial embolization with microspheres or by carunclectomy. The first method produces placental insufficiency by infusion of 50 micrometer mucopolysaccharide microspheres into the umbilico-placental circulation; embolization causes umbilical blood flow to decrease as placental resistance is temporarily increased [14;15]. The second method requires the surgical removal of most placentation sites before pregnancy and reduces the surface area over which maternal and fetal tissues can interact to form a placenta; this leads to reduced placental mass. These two models alter fetal growth in similar ways and have several features in common. They can both lead to fetal hypoxemia, hypercapnia and mild acidemia, i.e. fetal conditions like those seen in human cases of intrauterine growth restriction. Interestingly, not all carunclectomy fetuses are hypoxemic [16], and development and stress responses of these fetuses may differ from those that are hypoxemic; variability in oxygenation is also present in human intrauterine growth restriction (IUGR) whereby many but not all IUGR fetuses are hypoxic [17].
One would assume that a reduction in placental area or a reduction in placental mass increases placental vascular impedance and would load and stimulate the heart to grow in a way that mimicked a systolic pressure load. It is known that an increase in systolic load will stimulate the heart to grow by hyperplasia and hypertrophy and will stimulate maturation and binucleation of the cardiomyocyte [18]. However, the effect of reductions in placental exchange area on heart growth in these sheep models was unexpected and not predicted by systolic load models. Instead, cell cycle activity and proliferation were depressed in the placental insufficiency models. In addition, cardiomyocyte binucleation, a reliable indicator of cardiomyocyte maturation and terminal differentiation, did not undergo its normal maturational increase with age. Thus, the heart of the fetus suffering from placental insufficiency is characterized by a smaller heart for age and a lower number of cardiomyocytes that remain immature [19;20]. What is not known is whether hearts which have developed under the conditions of placental insufficiency are able to recover from their disadvantaged state and return their cell numbers to their expected number and level of maturity after birth, or if this remains a lifelong impairment.
Animal models have shown that the placenta is sensitive to the nutritional status of the mother. The stimulation of placental growth by early gestational undernutrition in sheep is associated with increased expression of placental IGF binding proteins [21] and the placental glucose transporter, GLUT-1 [22]. Although placental insufficiency is generally associated with lower circulating fetal IGF levels [23], in the hyperthermia model of placental insufficiency in sheep, the expression of placental IGF binding proteins is increased [24]. Cotyledonary expression levels of VEGF, angiopoietins (ang-1, ang-2) and the angiopoietin receptor, Tie-2, are transiently increased early in gestation in this model but are not maintained at high levels throughout gestation [25;26]. It is possible that placental compromise initially stimulates vascular growth but failure to maintain this growth leads to placental insufficiency, with related consequences on the heart. Thus, these studies highlight the critical importance of adequate placental vascular growth throughout gestation to maintain normal fetal cardiovascular development.
6. The placenta is the link between maternal nutrition, fetal growth and cardiovascular disease risk
Human and animal studies have consistently shown that maternal diet during pregnancy has a profound impact on fetal growth and future risk of chronic disease. Children born to poorly nourished (high protein and fat intake) mothers have a significantly higher incidence of coronary heart disease and hypertension in adulthood [27;28]. As mentioned above, recent studies from the Helsinki Birth Cohort demonstrate that placental size and shape, conditioned by maternal height, are also important risk factors for offspring hypertension [8]. Analyses of maternal diets in relation to fetal outcomes in the Southampton Women’s Survey cohort have demonstrated that placental weight is inversely related to energy intake in early pregnancy, a condition also related to an infant’s thinness at birth [29;30]. Farmers have long recognized that different nutrition regimens can be used to manipulate placental growth and lamb size. Farmers will often graze well-fed ewes on poor pastures at mid-pregnancy in order to increase placental weight and birth size of the lamb [31]. This reduction in nutritional supply leads to a potentially adaptive increase in placental growth and nutrient transport capacity. Despite the importance of diet during pregnancy, a mother’s nutritional history has a profound influence on pregnancy outcomes. Undernutrition in ewes stimulates placental growth only in those ewes that were well nourished before pregnancy [22]. Interestingly, women with low pre-pregnancy body weight give birth to smaller babies compared to women of average weight, even if their weight gains during pregnancy are similar [32]. This suggests that pre-pregnancy nutritional status is as important to placental function and fetal growth as is maternal diet during pregnancy.
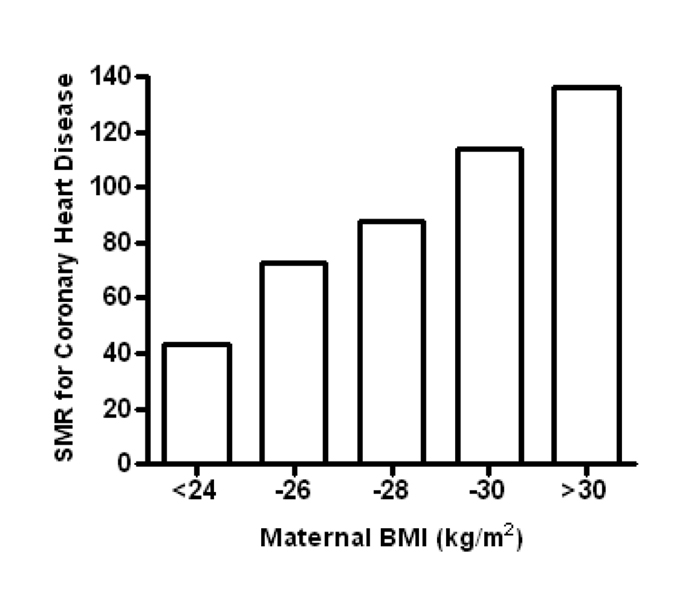
A woman’s periconceptional height and body mass index (BMI) are markers of her nutritional history [32]; maternal BMI predicts birth weight [32;33] and childhood fat mass of her offspring [34]. Maternal BMI also has a significant impact on a child’s risk of developing type 2 diabetes and is a major risk factor for coronary artery disease (see Figure 2) [6;35–37]. Obese women (BMI >30 kg/m2) who are pregnant have an increased risk of gestational diabetes, venous thromboembolism, preeclampsia and fetal loss among other poor outcomes [38]. Markers of poor placental function, such as the plasminogen activator inhibitor (PAI)-1/PAI-2 ratio, are elevated in obese women [39]. Furthermore, reports of inflammation [40] and nitrative stress [41] in obese placentas (similar to preeclamptic placentas) may explain the increased risk for fetal morbidity. Conversely, low maternal pre-pregnancy weight (BMI<20) is associated with fetal growth restriction and preterm delivery [42]. The degree to which the placenta is responsible for the slowed fetal growth in these pregnancies has not been determined, but is likely to play a key role.
Figure 2.
Standardized mortality ratios (SMR) for coronary heart disease for adult offspring of short women (<158 cm) according to maternal BMI at term. Data from Forsen et al. [6].
Given that the placenta regulates nutrient flow from the mother to the fetus, one would presume that the placenta plays a central role in the programming of cardiovascular disease. If true, taking into account the above studies, the placenta must be sensitive to maternal nutritional markers (i.e. diet and body composition). Consistent with this notion, intrauterine growth restriction reported in offspring of rats fed a low protein diet during pregnancy was preceded by a decrease in placental amino acid transporter activity and gene expression [43] indicating that placental function is sensitive to maternal nutrition, leading to changes in fetal growth. Intrauterine growth restriction in humans (below 10th percentile) is associated with increases in placental inflammatory cytokines, alterations in angiogenesis, nutrient metabolism-related genes and decreases in placental growth factor gene expression [44–46]. In addition to nutritional deficits as associated with growth restriction, the placenta is sensitive to the ‘over-nourished’ state. Obese women have elevated placental inflammatory cytokine expression [40] and nitrative stress [41] which can impact placental nutrient transport. Pro-inflammatory molecules such as interleukin (IL)-6 and tumor necrosis factor (TNF)-α stimulate system A amino acid transport by cytotrophoblast cells in vitro [47]; conversely, a previous study found that the pro-inflammatory IL-1β inhibited this system in cytotrophoblasts [48]. Thus, although evidence suggests that inflammatory cytokines alter placental nutrient transport, further studies are required to determine the effect of a general pro-inflammatory state on placental function.
Little is known about how the placenta “senses” maternal nutritional status. Jansson et al. have proposed the concept of a “nutrient sensor” within the placenta which is linked to nutrient transport and cell growth pathways and may be “dialed” up or down in response to placental nutrient supply [49]. This idea is very attractive. These authors suggested that the mammalian target of rapamycin (mTOR) plays this role. Indeed, placental mTOR stimulates amino acid transporters, cell growth and differentiation, is responsive to amino acid levels and is associated with fetal growth. Maternal nutrition is also likely to have an important effect on epigenetic mechanisms within the placenta [50]; epigenetic control of placental gene expression and function is an additional potential ‘nutrient sensor’ mechanism. Furthermore, nutrient transporters have been shown to be responsive to inflammatory cytokines in several tissues, including the placenta [47;48] and thus a pro-inflammatory state, as associated with maternal obesity is a potential contributing factor to modified nutrient transport in the placenta.
Recent evidence suggests that maternal body composition during the periconceptional period affects placental development from the blastocyst stage onward. Early nutritional conditions may affect placental nutrient transport, metabolism, inflammation, oxidative stress and blood flow. A number of animal models using either “undernutrition” or “excessive nutrition” support this view [43;51]. In humans, placental oxidative stress is associated with poor placental vascular function and preeclampsia. IUGR fetuses also have elevated oxidative stress but at present, the contribution made by maternal body composition and diet to feto-placental oxidative stress is not known. Another understudied area is placental function in low risk obese pregnancies. Much attention has been paid to the effects of diabetes on placental function and fetal growth. While it is well known that gestational diabetes often accompanies obesity, the separate effects of obesity and diabetes on placental function require further investigation. Wijendran et al. suggest that maternal BMI explains the association between gestational diabetes and fetal omega-3 fatty acid deficiency [52]. This could be due to the effects on placental lipid transport, given that circulating maternal omega-3 levels were increased. Both maternal obesity and neonatal omega-3 fatty acid deficiencies are known risk factors for development of future cardiovascular disease; the placenta potentially plays a critical role in this pathway.
7. Future Directions
Despite several decades of materno-feto-placental studies, many fundamental questions remain unanswered. We know that as the interface between mother and fetus, the placenta is critical for normal fetal growth and development but the mechanisms underlying the placental origins of adult cardiovascular disease in offspring remain unclear. A thorough investigation of maternal influences on placental development (both at the time of implantation and throughout pregnancy) in addition to the specific macro- and micronutrient transport capabilities under different nutritional (including oxygen), inflammatory and oxidative stress states will be key to our understanding of how intrauterine stress predisposes for disease in offspring many decades later. The field is now ripe to find placental markers of future cardiovascular illness which could lead to interventions and prevention of cardiovascular disease for future generations.
Acknowledgements
This research at Oregon Health and Science University was supported by the National Institute of Child Health and Human Development Program Project Grant (P01HD34430), Fellowships by the American Heart Association (Louey and O’Tierney) and the M. Lowell Edwards Endowment (Thornburg).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Bibbins-Domingo K, Coxson P, Pletcher MJ, Lightwood J, Goldman L. Adolescent overweight and future adult coronary heart disease. N.Engl.J.Med. 2007;357:2371–2379. doi: 10.1056/NEJMsa073166. [DOI] [PubMed] [Google Scholar]
- 2.Martyn CN, Barker DJ, Osmond C. Mothers' pelvic size, fetal growth, and death from stroke and coronary heart disease in men in the UK. Lancet. 1996;348:1264–1268. doi: 10.1016/s0140-6736(96)04257-2. [DOI] [PubMed] [Google Scholar]
- 3.Godfrey KM. The role of the placenta in fetal programming-a review. Placenta. 2002;23 Suppl A:S20–S27. doi: 10.1053/plac.2002.0773. [DOI] [PubMed] [Google Scholar]
- 4.Risnes KR, Romundstad PR, Nilsen TI, Eskild A, Vatten LJ. Placental weight relative to birth weight and long-term cardiovascular mortality: findings from a cohort of 31,307 men and women. Am.J.Epidemiol. 2009;170:622–631. doi: 10.1093/aje/kwp182. [DOI] [PubMed] [Google Scholar]
- 5.Forsen T, Eriksson JG, Tuomilehto J, Osmond C, Barker DJ. Growth in utero and during childhood among women who develop coronary heart disease: longitudinal study. BMJ. 1999;319:1403–1407. doi: 10.1136/bmj.319.7222.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forsen T, Eriksson JG, Tuomilehto J, Teramo K, Osmond C, Barker DJ. Mother's weight in pregnancy and coronary heart disease in a cohort of Finnish men: follow up study. BMJ. 1997;315:837–840. doi: 10.1136/bmj.315.7112.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chobanian AV. Shattuck Lecture. The hypertension paradox--more uncontrolled disease despite improved therapy. N.Engl.J.Med. 2009;361:878–887. doi: 10.1056/NEJMsa0903829. [DOI] [PubMed] [Google Scholar]
- 8.Barker DJ, Thornburg KL, Osmond C, Kajantie E, Eriksson JG. The surface area of the placenta and hypertension in the offspring in later life. Int.J.Dev.Biol. 2009 doi: 10.1387/ijdb.082760db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kajantie E, Eriksson JG, Osmond C, Thornburg K, Barker DJ. Pre-eclampsia is associated with increased risk of stroke in the adult offspring: the Helsinki birth cohort study. Stroke. 2009;40:1176–1180. doi: 10.1161/STROKEAHA.108.538025. [DOI] [PubMed] [Google Scholar]
- 10.Kajantie E, Thornburg K, Eriksson JG, Osmond L, Barker DJ. In preeclampsia, the placenta grows slowly along its minor axis. Int.J.Dev.Biol. 2009 doi: 10.1387/ijdb.082833ek. [DOI] [PubMed] [Google Scholar]
- 11.Sutton MG, Plappert T, Doubilet P. Relationship between placental blood flow and combined ventricular output with gestational age in normal human fetus. Cardiovasc.Res. 1991;25:603–608. doi: 10.1093/cvr/25.7.603. [DOI] [PubMed] [Google Scholar]
- 12.Kiserud T, Ebbing C, Kessler J, Rasmussen S. Fetal cardiac output, distribution to the placenta and impact of placental compromise. Ultrasound Obstet.Gynecol. 2006;28:126–136. doi: 10.1002/uog.2832. [DOI] [PubMed] [Google Scholar]
- 13.Shaut CA, Keene DR, Sorensen LK, Li DY, Stadler HS. HOXA13 Is essential for placental vascular patterning and labyrinth endothelial specification. PLoS.Genet. 2008;4:e1000073. doi: 10.1371/journal.pgen.1000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trudinger BJ, Stevens D, Connelly A, Hales JR, Alexander G, Bradley L, et al. Umbilical artery flow velocity waveforms and placental resistance: the effects of embolization of the umbilical circulation. Am.J.Obstet.Gynecol. 1987;157:1443–1448. doi: 10.1016/s0002-9378(87)80241-7. [DOI] [PubMed] [Google Scholar]
- 15.Morrow RJ, Adamson SL, Bull SB, Ritchie JW. Effect of placental embolization on the umbilical arterial velocity waveform in fetal sheep. Am.J.Obstet.Gynecol. 1989;161:1055–1060. doi: 10.1016/0002-9378(89)90783-7. [DOI] [PubMed] [Google Scholar]
- 16.Robinson JS, Kingston EJ, Jones CT, Thorburn GD. Studies on experimental growth retardation in sheep. The effect of removal of a endometrial caruncles on fetal size and metabolism. J.Dev.Physiol. 1979;1:379–398. [PubMed] [Google Scholar]
- 17.Pardi G, Cetin I, Marconi AM, Lanfranchi A, Bozzetti P, Ferrazzi E, et al. Diagnostic value of blood sampling in fetuses with growth retardation. N.Engl.J.Med. 1993;328:692–696. doi: 10.1056/NEJM199303113281004. [DOI] [PubMed] [Google Scholar]
- 18.Barbera A, Giraud GD, Reller MD, Maylie J, Morton MJ, Thornburg KL. Right ventricular systolic pressure load alters myocyte maturation in fetal sheep. Am.J.Physiol Regul.Integr.Comp Physiol. 2000;279:R1157–R1164. doi: 10.1152/ajpregu.2000.279.4.R1157. [DOI] [PubMed] [Google Scholar]
- 19.Louey S, Jonker SS, Giraud GD, Thornburg KL. Placental insufficiency decreases cell cycle activity and terminal maturation in fetal sheep cardiomyocytes. J.Physiol. 2007;580:639–648. doi: 10.1113/jphysiol.2006.122200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morrison JL, Botting KJ, Dyer JL, Williams SJ, Thornburg KL, McMillen IC. Restriction of placental function alters heart development in the sheep fetus. Am.J.Physiol Regul.Integr.Comp Physiol. 2007;293:R306–R313. doi: 10.1152/ajpregu.00798.2006. [DOI] [PubMed] [Google Scholar]
- 21.Osgerby JC, Wathes DC, Howard D, Gadd TS. The effect of maternal undernutrition on the placental growth trajectory and the uterine insulin-like growth factor axis in the pregnant ewe. J.Endocrinol. 2004;182:89–103. doi: 10.1677/joe.0.1820089. [DOI] [PubMed] [Google Scholar]
- 22.Dandrea J, Wilson V, Gopalakrishnan G, Heasman L, Budge H, Stephenson T, et al. Maternal nutritional manipulation of placental growth and glucose transporter 1 (GLUT-1) abundance in sheep. Reproduction. 2001;122:793–800. [PubMed] [Google Scholar]
- 23.Langford K, Blum W, Nicolaides K, Jones J, McGregor A, Miell J. The pathophysiology of the insulin-like growth factor axis in fetal growth failure: a basis for programming by undernutrition? Eur.J.Clin.Invest. 1994;24:851–856. doi: 10.1111/j.1365-2362.1994.tb02030.x. [DOI] [PubMed] [Google Scholar]
- 24.de Vrijer B, Davidsen ML, Wilkening RB, Anthony RV, Regnault TR. Altered placental and fetal expression of IGFs and IGF-binding proteins associated with intrauterine growth restriction in fetal sheep during early and mid-pregnancy. Pediatr.Res. 2006;60:507–512. doi: 10.1203/01.PDR.0000242364.78002.71. [DOI] [PubMed] [Google Scholar]
- 25.Hagen AS, Orbus RJ, Wilkening RB, Regnault TR, Anthony RV. Placental expression of angiopoietin-1, angiopoietin-2 and tie-2 during placental development in an ovine model of placental insufficiency-fetal growth restriction. Pediatr.Res. 2005;58:1228–1232. doi: 10.1203/01.pdr.0000185266.23265.87. [DOI] [PubMed] [Google Scholar]
- 26.Regnault TR, Orbus RJ, de Vrijer B, Davidsen ML, Galan HL, Wilkening RB, et al. Placental expression of VEGF, PlGF and their receptors in a model of placental insufficiency-intrauterine growth restriction (PI-IUGR) Placenta. 2002;23:132–144. doi: 10.1053/plac.2001.0757. [DOI] [PubMed] [Google Scholar]
- 27.Painter RC, de Rooij SR, Bossuyt PM, Simmers TA, Osmond C, Barker DJ, et al. Early onset of coronary artery disease after prenatal exposure to the Dutch famine. Am.J.Clin.Nutr. 2006;84:322–327. doi: 10.1093/ajcn/84.1.322. [DOI] [PubMed] [Google Scholar]
- 28.Shiell AW, Campbell-Brown M, Haselden S, Robinson S, Godfrey KM, Barker DJ. High-meat, low-carbohydrate diet in pregnancy: relation to adult blood pressure in the offspring. Hypertension. 2001;38:1282–1288. doi: 10.1161/hy1101.095332. [DOI] [PubMed] [Google Scholar]
- 29.Godfrey K, Robinson S, Barker DJ, Osmond C, Cox V. Maternal nutrition in early and late pregnancy in relation to placental and fetal growth. BMJ. 1996;312:410–414. doi: 10.1136/bmj.312.7028.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Godfrey KM, Barker DJ, Robinson S, Osmond C. Maternal birthweight and diet in pregnancy in relation to the infant's thinness at birth. Br.J.Obstet.Gynaecol. 1997;104:663–667. doi: 10.1111/j.1471-0528.1997.tb11975.x. [DOI] [PubMed] [Google Scholar]
- 31.Thomas WJK. Lowland sheep: production policies and practices. Exeter: University of Exeter. 1970 [Google Scholar]
- 32.Neggers Y, Goldenberg RL. Some thoughts on body mass index, micronutrient intakes and pregnancy outcome. J.Nutr. 2003;133:1737S–1740S. doi: 10.1093/jn/133.5.1737S. [DOI] [PubMed] [Google Scholar]
- 33.Sanin Aguirre LH, Reza-Lopez S, Levario-Carrillo M. Relation between maternal body composition and birth weight. Biol.Neonate. 2004;86:55–62. doi: 10.1159/000077586. [DOI] [PubMed] [Google Scholar]
- 34.Gale CR, Javaid MK, Robinson SM, Law CM, Godfrey KM, Cooper C. Maternal size in pregnancy and body composition in children. J.Clin.Endocrinol.Metab. 2007;92:3904–3911. doi: 10.1210/jc.2007-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115:e290–e296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 36.Loos RJ, Phillips DI, Fagard R, Beunen G, Derom C, Mathieu C, et al. The influence of maternal BMI and age in twin pregnancies on insulin resistance in the offspring. Diabetes Care. 2002;25:2191–2196. doi: 10.2337/diacare.25.12.2191. [DOI] [PubMed] [Google Scholar]
- 37.Mi J, Law C, Zhang KL, Osmond C, Stein C, Barker D. Effects of infant birthweight and maternal body mass index in pregnancy on components of the insulin resistance syndrome in China. Ann.Intern.Med. 2000;132:253–260. doi: 10.7326/0003-4819-132-4-200002150-00002. [DOI] [PubMed] [Google Scholar]
- 38.Nohr EA, Bech BH, Davies MJ, Frydenberg M, Henriksen TB, Olsen J. Prepregnancy obesity and fetal death: a study within the Danish National Birth Cohort. Obstet.Gynecol. 2005;106:250–259. doi: 10.1097/01.AOG.0000172422.81496.57. [DOI] [PubMed] [Google Scholar]
- 39.Stewart FM, Freeman DJ, Ramsay JE, Greer IA, Caslake M, Ferrell WR. Longitudinal assessment of maternal endothelial function and markers of inflammation and placental function throughout pregnancy in lean and obese mothers. J.Clin.Endocrinol.Metab. 2007;92:969–975. doi: 10.1210/jc.2006-2083. [DOI] [PubMed] [Google Scholar]
- 40.Challier JC, Basu S, Bintein T, Minium J, Hotmire K, Catalano PM, et al. Obesity in Pregnancy Stimulates Macrophage Accumulation and Inflammation in the Placenta. Placenta. 2008 doi: 10.1016/j.placenta.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roberts VH, Smith J, McLea SA, Heizer AB, Richardson JL, Myatt L. Effect of increasing maternal body mass index on oxidative and nitrative stress in the human placenta. Placenta. 2009;30:169–175. doi: 10.1016/j.placenta.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ehrenberg HM, Dierker L, Milluzzi C, Mercer BM. Low maternal weight, failure to thrive in pregnancy, and adverse pregnancy outcomes. Am.J.Obstet.Gynecol. 2003;189:1726–1730. doi: 10.1016/s0002-9378(03)00860-3. [DOI] [PubMed] [Google Scholar]
- 43.Jansson N, Pettersson J, Haafiz A, Ericsson A, Palmberg I, Tranberg M, et al. Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J.Physiol. 2006;576:935–946. doi: 10.1113/jphysiol.2006.116509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Street ME, Seghini P, Fieni S, Ziveri MA, Volta C, Martorana D, et al. Changes in interleukin-6 and IGF system and their relationships in placenta and cord blood in newborns with fetal growth restriction compared with controls. Eur.J.Endocrinol. 2006;155:567–574. doi: 10.1530/eje.1.02251. [DOI] [PubMed] [Google Scholar]
- 45.Gauster M, Hiden U, Blaschitz A, Frank S, Lang U, Alvino G, et al. Dysregulation of placental endothelial lipase and lipoprotein lipase in intrauterine growth-restricted pregnancies. J.Clin.Endocrinol.Metab. 2007;92:2256–2263. doi: 10.1210/jc.2006-2403. [DOI] [PubMed] [Google Scholar]
- 46.Jarvenpaa J, Vuoristo JT, Savolainen ER, Ukkola O, Vaskivuo T, Ryynanen M. Altered expression of angiogenesis-related placental genes in pre-eclampsia associated with intrauterine growth restriction. Gynecol.Endocrinol. 2007;23:351–355. doi: 10.1080/09513590701350291. [DOI] [PubMed] [Google Scholar]
- 47.Jones HN, Jansson T, Powell TL. IL-6 stimulates system A amino acid transporter activity in trophoblast cells through STAT3 and increased expression of SNAT2. Am.J.Physiol Cell Physiol. 2009;297:C1228–C1235. doi: 10.1152/ajpcell.00195.2009. [DOI] [PubMed] [Google Scholar]
- 48.Thongsong B, Subramanian RK, Ganapathy V, Prasad PD. Inhibition of amino acid transport system a by interleukin-1beta in trophoblasts. J.Soc.Gynecol.Investig. 2005;12:495–503. doi: 10.1016/j.jsgi.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 49.Roos S, Powell TL, Jansson T. Placental mTOR links maternal nutrient availability to fetal growth. Biochem.Soc.Trans. 2009;37:295–298. doi: 10.1042/BST0370295. [DOI] [PubMed] [Google Scholar]
- 50.Gheorghe CP, Goyal R, Holweger JD, Longo LD. Placental gene expression responses to maternal protein restriction in the mouse. Placenta. 2009;30:411–417. doi: 10.1016/j.placenta.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones HN, Woollett LA, Barbour N, Prasad PD, Powell TL, Jansson T. High-fat diet before and during pregnancy causes marked up-regulation of placental nutrient transport and fetal overgrowth in C57/BL6 mice. FASEB J. 2009;23:271–278. doi: 10.1096/fj.08-116889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wijendran V, Bendel RB, Couch SC, Philipson EH, Cheruku S, Lammi-Keefe CJ. Fetal erythrocyte phospholipid polyunsaturated fatty acids are altered in pregnancy complicated with gestational diabetes mellitus. Lipids. 2000;35:927–931. doi: 10.1007/s11745-000-0602-2. [DOI] [PubMed] [Google Scholar]