Abstract
Fetal neurobehavioral development was modeled longitudinally using data collected at weekly intervals from 24- to -38 weeks gestation in a sample of 112 healthy pregnancies. Predictive associations between 3 measures of fetal neurobehavioral functioning and their developmental trajectories to neurological maturation in the 1st weeks after birth were examined. Prenatal measures included fetal heart rate variability, fetal movement, and coupling between fetal motor activity and heart rate patterning; neonatal outcomes include a standard neurologic examination (n = 97) and brainstem auditory evoked potential (BAEP; n = 47). Optimality in newborn motor activity and reflexes was predicted by fetal motor activity; fetal heart rate variability and somatic-cardiac coupling predicted BAEP parameters. Maternal pregnancy-specific psychological stress was associated with accelerated neurologic maturation.
“If we pursue our quest beyond the newborn period, we find ourselves suddenly in an entirely new situation, where our organism is not seen, nor scarcely felt nor heard. Our environmental situation has become, if not at once more complex, at least to be likened to the postnatal environment only with great difficulty”
(Sontag & Richards, 1938, p. 1).
While the last decade has been marked by increasing appreciation of the role of the prenatal environment in providing the substrate for postnatal health and development, recognition of the continuous nature of human development from conception was evident in the earliest publications of the Fels Research Institute, established in 1929. Since that time, many of the core interests of developmental psychology concerning development and expression of individual differences and the moderating role of early environmental influences have begun to converge with epidemiologic methodology. The construct of “fetal programming” has been applied broadly to represent discoveries of prenatal influences on postnatal functioning, typically in adulthood (Barker, 2006; O’Brien, Wheeler, & Barker, 1999; Young, 2002). While this approach has generated an enormous body of data, thereby sparking great interest in the prenatal period, most studies rely on readily available data sources, such as birth weight, that provide only vague proxy for the gestational environment and can offer little information about mechanisms mediating observed associations.
A second, more direct, approach is to measure function during the prenatal period to evaluate its role as the foundation for postnatal function. This permits measurement of a single construct and allows determination of how early experiences or exposures might affect the latter via their influence on the former. Technological advances available only after the dissolution of the Fels Institute have made clear that by the end of gestation developmental parameters that are measured extensively in the neonate and infant, and are integral to theories of development, originate at neither term nor with birth (Als, 1982; Prechtl, 1984). The view that fetal neurobehaviors reflect neurological development has been supported by studies conducted in healthy populations (Amiel-Tison, Gosselin, & Kurjak, 2006; DiPietro, Irizarry, Hawkins, Costigan, & Pressman, 2001; Hepper, 1995; Krasnegor et al., 1998; Nijhuis & ten Hof, 1999; Sandman, Wadhwa, Hetrick, Porto, & Peeke, 1997). Further support is provided by observations of differences in neurobehavioral functioning in fetuses afflicted by congenital anomalies related to the nervous system (Hepper & Shahidullah, 1992; Horimoto et al., 1993; Maeda et al., 2006; Romanini & Rizzo, 1995); exposure to deleterious antenatal conditions that affect development, including growth restriction (Nijhuis et al., 2000) and maternal diabetes (Kainer, Prechtl, Engele, & Einspieler, 1997) and exposure to potentially neurotoxic substences (Gingras & O’Donnell, 1998; Mulder, Morssink, van der Schee, & Visser, 1998). The first aim of the current study is to evaluate the association between central nervous system maturation in the fetus, as indicated by fetal neurobehavioral development, and neurologic functioning of the newborn infant in an effort to identify early indicators of optimal function.
While we have examined a number of fetal neurobehavioral parameters in past cohorts, three were selected for this analysis based on conceptual considerations regarding relevance to postnatal neurological functioning. The first parameter is spontaneous variability in fetal heart rate. Measurement of phasic or non-phasic cardiac variability has had a distinguished history in developmental science as a marker of the physiological regulation that corresponds to infant and child performance and behavior. Variability in fetal heart rate is among the most prominently accessible features of the fetus, and is central to clinical antepartum assessment as an indicator of the developing balance between parasympathetic and sympathetic innervation (Freeman, Garite, & Nageotte, 1991). Recently, fetal heart rate variability at or after 28 weeks gestation and steeper developmental trajectories were shown to be significantly associated with mental, psychomotor, and language development in the third year of life (DiPietro, Bornstein, Hahn, Costigan, & Achy-Brou, 2007). Significant stability within individual fetuses during gestation was also demonstrated (DiPietro et al., 2007).
Motor activity is the second prenatal parameter, selected for its conspicuous nature as an individual difference in both the fetus and child (Eaton, McKeen, & Campbell, 2001). A few studies on small samples have suggested conservation of very specific attributes of fetal motor patterning between the fetus and infant (Almli, Ball, & Wheeler, 2001; Groome et al., 1999). Fetal motor activity is significantly associated with activity levels in early childhood for boys, and predictive of a range of regulatory temperament characteristics (DiPietro et al., 2002). The putative assumption is that fetal motor activity prepares the neurologic circuitry and musculature for postnatal function (Prechtl, 1984). Data generated from animal models further reveal that variation in fetal motor behavior both reflects ontogenic adaptation to the intrauterine environment which, in turn, fosters subsequent maturation (Smotherman & Robinson, 1987).
The third fetal parameter combines fetal heart rate and motor activity into a single measure reflecting the degree of covariation between the two. Observation of synchrony between acceleration in heart rate and motor activity in fetuses is long-standing (Sontag & Richards, 1938). Since then, cardiac-somatic coupling has become implicated as a function of parasympathetic control which becomes the increasingly prominent influence as gestation advances. The association between movement and heart rate in the fetus has been most often attributed to centrally mediated coactivation of cardiac and somatomotor processes (Johnson, Besinger, Thomas, Strobino, & Niebyl, 1992; Timor-Tritsch, Dierker, Zador, Hertz, & Rosen, 1978; Vintzileos, Campbell, & Nochinson, 1986). Documentation of normative development of this association in several longitudinal samples reveals a predictable progression during gestation marked by increased levels of correspondence and diminished latency between parameters (DiPietro et al., 2004a; DiPietro, Hodgson, Costigan, Hilton, & Johnson, 1996; DiPietro et al., 2001).
One of the most frequently investigated sources of maternal environmental variation on the developing fetus has been maternal psychological distress. Most studies examine effects on pregnancy outcomes (i.e., birth weight, gestational age). Although a number have reported significant associations between maternal stress and/or anxiety during pregnancy and shortened gestation or restricted growth, results are not uniform (Alder, Fink, Bitzer, Hosli, & Holzgreve, 2007; Littleton, Breitkopf, & Berenson, 2007). Fewer studies link maternal distress to observed child or infant outcomes; of those that do, most examine consequences on attentional or behavioral regulation (Davis et al., 2004; Gutteling et al., 2005; Huizink, Robles de Medina, Mulder, Visser, & Buitelaar, 2002; Van den Bergh et al., 2005). Examination of child performance on standardized assessments has yielded conflicting results. For example, two studies report deleterious effects between maternal psychological stress and/or anxiety on Bayley Scales of Infant Development (Buitelaar, Huizink, Mulder, Robles de Medina, & Visser, 2003; Laplante et al., 2004) while another reports that maternal psychological distress accelerates development (DiPietro, Novak, Costigan, Atella, & Reusing, 2006). The handful of studies examining the most proximal associations, those between maternal psychological distress and fetal functional activity, are neither consistent nor conclusive. The second aim of this study is to systematically examine the contribution of maternal non-specific anxiety levels and pregnancy-specific stress on both fetal and neonatal maturation. Boys have typically been observed to be more vulnerable to prenatal exposures in human studies, and a potential mechanism specific to prenatal stress has recently been uncovered in rodent models (Mueller & Bale, 2008). Thus, potential sex differences in any observed associations will be examined.
Growth and development during gestation both reflect maturative processes. Growth is generally defined as an increase in cell mass or number, while development refers to differentiation of function. While growth is relatively straightforward to measure at birth in terms of size, there are a variety of ways that functional maturation may be expressed and measured. Since our focus is on neurologic maturation, we selected two different indicators of neurologic function. The first is a traditional neurological examination of the newborn. Assessments of this type originated in the 1960s and contain a fairly standard repertoire of neonatal characteristics, including tone, posture, primitive reflexes, and behavior. The Dubowitz Neurological Exam (Dubowitz, Mercuri, & Dubowitz, 1998), used in this study, was selected because scoring has been validated around the construct of “optimality” such that the exam distinguishes the optimal point along dimensions in which suboptimal performance occupies each end (e.g., hyporesponsivity through hyperresponsivity). However, despite their widespread use, assessments of this type have a number of limitations, including restriction to features of neonatal functioning that can be observed and/or manipulated and fairly subjective scoring based on “stick figure” drawings of response ranges.
As a result, a second approach was included on a subset of infants using measurement of brainstem auditory evoked potential (BAEP) as a more objective and precise indicator of neurological maturation. BAEP has standard clinical application in the newborn period in evaluation of audition. A growing literature has indicated that it is effective in serving as a marker for more general neural maturation. Variation in BAEP parameters has been linked to prenatal lead exposure (Rothenberg, Poblano, & Schnaas, 2000), prenatal exposure to potentially neurotoxic therapeutic drugs (Poblano et al., 2003), transient depression of Apgar scores (Jiang, Xu, Brosi, Shao, & Wilkinson, 2007), iron deficiency anemia (Roncagliolo, Garrido, Walter, Peirano, & Lozoff, 1998), and breast-feeding and formula fortification (Khedr, Farghaly, El-DinAmry, & Osman, 2004; Unay et al., 2004). These results suggest that BAEP may serve as a marker for functional integrity of the nervous system beyond the specific pathway of conduction.
The BAEP consists of a series of three major peaks or waves (i.e., I, III and V) which are generated in the eighth cranial nerve and brainstem. The neural generators for waves I and III in humans are the auditory nerve and cochlear nucleus, respectively. The generator for the most positive peak of wave V is the termination of the fiber tract of the lateral lemniscus, whereas the following negative trough is produced by slow dendritic potentials in the inferior colliculus (Moller & Jannetta, 1982). BAEP responsivity can be detected in some preterm infants as early as 26 weeks gestation but is not typically measurable until 30 to 32 weeks postconceptional age (Rotteveel, de Graaf, Colon, Stegeman, & Visco, 1987). The absolute latencies of waves I, III and V as well as the interpeak latency intervals progressively decrease over the course of development (Amin, Orlando, Dalzell, Merle, & Guillet, 1999). In full-term infants, the BAEP undergoes rapid changes in the first 4 days of life with the most rapid changes occurring in the first 24 hours (Yamasaki et al., 1991). The later components (i.e., waves III and V) undergo more marked changes in latency than the most peripheral component due to the earlier maturation of the peripheral nervous system relative to the later maturing central auditory pathways (Montandon, Cao, Engel, & Grajew, 1979). The prolonged latencies in the BAEP in neonates reflect incomplete nerve myelination of the fibers in the auditory pathway, reduced axon diameter, and immaturity in synaptic function (Eggermont & Salamy, 1988; Folsom & Wynne, 1987).
Based on the constructs underlying each dependent and independent measure, we expect the following: 1) fetal motor activity will be most predictive of newborn performance on the standard neurological examination, given the focus on postnatal tone, posture, and motor behavior; 2) fetal measures more closely reflective of neural functioning (i.e., cardiac variability and somatic-cardiac coupling) will be most predictive of event related potentials (BAEP); and 3) maternal psychological distress will accelerate fetal neurobehavioral development and neonatal neurological functioning.
Method
Participants
Participants were 112 self-referred normotensive, non-smoking women with normally progressing pregnancies carrying singleton fetuses. Accurate dating of the pregnancy, based on early first trimester pregnancy testing or examination and generally confirmed by early ultrasound was required (M gestational age at pregnancy detection = 4.8 weeks; sd = 1.2). The sample represents a relatively stable population of well-educated (M years education = 17.2 years, sd = 2.1), mature (M age = 31.2, sd = 4.6), married (91%) women. Most were non-Hispanic white (79.5%); the remainder was African-American (13.4%), Hispanic or Asian (7.1%). Fifty-seven (51%) of the fetuses were female.
Procedure
Prenatal
In order to fully represent the gestational age span from 24 to 38 weeks gestation, participants were stratified into 3 cohorts with staggered entry into the protocol between 24 and 26 weeks gestation and tested in 3-week intervals. That is, data collection for the first cohort proceeded at 24, 27, 30, 33, and 36 weeks; the second at 25, 28, 31, 34, and 37 weeks; and the third at 26, 29, 32, 35, and 38 weeks. Prenatal visits were scheduled at 13:00 or 15:00. On the day of the visit, women were instructed to eat 1.5 hours prior to the visit but not thereafter. Self-report psychosocial questionnaires were completed upon arrival at the laboratory at each visit. A brief ultrasound scan was administered to determine fetal position followed by 50 minutes of undisturbed fetal recording.
The psychosocial questionnaires included measures of general anxiety and pregnancy specific stress, both administered at each visit. The former was evaluated by the Spielberger Trait Anxiety Scales (Y-2; STAI) (Spielberger, 1983), one of the most commonly used and extensively validated self-administered measures of anxiety. This questionnaire includes twenty 4-point items; items were reversed as necessary and summed such that higher scores indicate greater trait anxiety. Pregnancy-specific stress was assessed by a shortened form of the Pregnancy Experiences Scale (PES). The original version was previously validated (DiPietro, Ghera, Costigan, & Hawkins, 2004b) and contains 41 items specific to pregnancy, each rated on 4-point scales and scored in terms of intensity and frequency. The revised version was developed for the recurring and frequent administration of the current design. The PES-Brief includes the 10 most frequently endorsed hassles and uplifts from the full PES, each rated on the original 4-point scale, and averaged. Higher values reflect greater perceived intensity of either negative or positive stressors during pregnancy. Comparable reliability and stability to the original have been demonstrated (DiPietro, Christensen, & Costigan, submitted).
Fetal data were collected using a Toitu (MT320) fetal actocardiograph. This monitor detects fetal movement and fetal heart rate with a single wide array transabdominal Doppler transducer and processes this signal through a series of autocorrelation techniques. The actograph detects fetal movements by preserving the remaining signal after bandpassing frequency components of the Doppler signal that are associated with FHR and maternal somatic activity. Reliability studies comparing actograph based versus ultrasound visualized fetal movements have found the performance of this monitor to be highly accurate in detecting both fetal motor activity and quiescence (Besinger & Johnson, 1989; DiPietro, Costigan, & Pressman, 1999; Maeda, Tatsumura, & Utsu, 1999).
Fetal data were digitized and analyzed off-line using software developed in our laboratory (GESTATE; James Long Company, Caroga Lake NY). Fetal heart rate data underwent error rejection procedures based on moving averages of acceptable values as needed. Variable extraction included fetal heart rate variability (standard deviation of each 1-min epoch of fetal heart rate averaged over the full recording). Fetal movement data represent raw voltage values generated from the actograph calibrated by multiplying by a conversion factor and scaled from 0 to 100 in arbitrary units (a.u.s). Fetal motor activity was computed as the mean signal values generated per minute, averaged over the entire recording. The relation between fetal heart rate and motor activity (hereafter, FM-FHR coupling), was calculated as the proportion of discrete fetal movement bouts, defined as commencing when an actograph signal attained an amplitude of 15 units and ending with a cessation of signal for at least 10 s, that were associated with excursions in fetal heart rate ≥ 5 bpm over baseline within 5 s before the start of a movement or within 15 s after the start of a movement, consistent with previously developed criteria (Baser, Johnson, & Paine, 1992; DiPietro et al., 1996),
Postnatal
Infants were examined using the Dubowitz neurological examination of the newborn (Dubowitz et al., 1998) within the first two weeks after birth during a home visit conducted by a pediatric nurse practitioner. The assessment is a more intensive version of exams routinely used to assess status and gestational age immediately postpartum. It contains 34 items distributed into 6 clusters: Tone (e.g., posture and head control), Tone Patterns (e.g., extensor vs flexor), Reflexes (e.g., Moro, placing), Motor (e.g., spontaneous movements and head raising), Abnormal Signs (e.g., tremoring), and Behavior (e.g., orientation, irritability). Scoring proceeded based on optimality criteria, developed by validation across gestational age (Dubowitz et al., 1998). That is, raw scores are coded along an optimality continuum such that a higher score on an item is not necessarily coded as more optimal.
A second visit was scheduled to collect brainstem auditory evoked potential (BAEP). Initially, the protocol was designed to accomplish both at one visit; however, this interfered with obtaining BAEP data of sufficient quality. A licensed audiologist was subsequently dispatched to conduct a second visit but this change was not implemented until the 21st participant. BAEP measurements were undertaken utilizing single-channel differential recording with an active electrode attached to the high-forehead and a reference electrode attached to the mastoid. The contralateral electrode served as ground. Testing relied on a portable BAEP unit (Smart USB Lite; Intelligent Hearing Systems, Miami, FL) connected to a laptop computer through a USB port to a single channel amplifier. Infants were generally swaddled and placed in a supine position or in their mothers’ arms. Recording took place while infants were in a sleep state. Stimuli were rarefaction clicks presented at a rate of 27.7/s through ER3 insert phones at a level of 70 dB nHL. Responses to two trials of 2048 clicks were filtered (30–1500 Hz) and amplified (100K). A 12 ms recording window was utilized. The analysis was undertaken offline (SmartEP System, Version 3.82, Intelligent Hearing Systems) and latencies for waves I, III, and V were assessed for each trial and each ear. Both trials were averaged. No significant differences in values generated between right and left ears were detected, so these were further averaged. Interpeak latency intervals were computed by subtracting the earlier wave from the later. The following variables were used in the analysis: Wave V latency (i.e., stimulus presentation to Wave V peak) and interpeak intervals I–III, III–V, and I–V.
Analysis Plan
Variables were examined for skewness and outliers. Hierarchical linear models were estimated using SPSS Mixed (version 15.0) to examine growth in fetal neurobehavioral development across the second half of gestation. Models incorporated two levels: Level 1 models the change in the dependent variable over time for each individual in the sample, while Level 2 relates individual growth parameters to predictors of interest (Bryk & Raudenbush, 1987; Singer & Willett, 2003). Two advantages emerge from this approach. First, individuals are not directly compared based on the dependent variables of interest, but on the parameters describing their developmental slope across gestation. Therefore, week of gestation at observation was allowed to vary across participants and is accounted for in the Level 1 model. Second, the number of data points per subject can also vary as long as there is sufficient information to describe the developmental trajectory for that individual. Unbalanced data sets, including those with planned missing data as generated by the current design, are thus acceptable in these models (Singer & Willett, 2003).
Restricted maximum likelihood (REML) was used in reporting model parameters when assessing the significance of the random effects; degrees of freedom were estimated using the Satterthwaite method. The 95% confidence interval (CI) for random variation around each fixed effect was calculated as ± 2 standard deviations of its accompanying random variance term. To best describe growth trajectories, linear, polynomial, and piecewise (spline) terms were examined during model-fitting. Models were centered at 38 weeks, such that the intercept represented individual differences in fetal neurobehaviors (fetal heart rate variability, motor activity, and FM-FHR coupling) at the end of gestation. Models were initially specified with random intercepts and the effects of including random slopes on model fit were subsequently evaluated. The fixed effects of each predictor (e.g., fetal sex, maternal distress) on the intercept and change across gestation, calculated as slope from 24 to 38 weeks, were tested sequentially in the whole group. Model fit was assessed with likelihood deviance difference tests for nested models (Singer & Willett, 2003). This process evaluates the significance of the change in model fit following the addition of a new parameter(s) (e.g., a quadratic term) to a previously tested model (e.g., a linear model).
Exploratory analyses were used to identify potential covariates. No outliers in postnatal measures were detected. Random intercepts and slopes exported from the HLM models described above were used in correlation and regression analyses. Pearson correlations were computed between fetal and infant measures. Regression models were constructed by entering covariates at the initial step (e.g., gestational age) followed by entry of the fetal predictor (i.e., intercept or slope for FHR variability, FM-FHR coupling, and fetal motor activity).
Results
Fetal Neurobehavioral Development from 24- to -38 Weeks Gestation
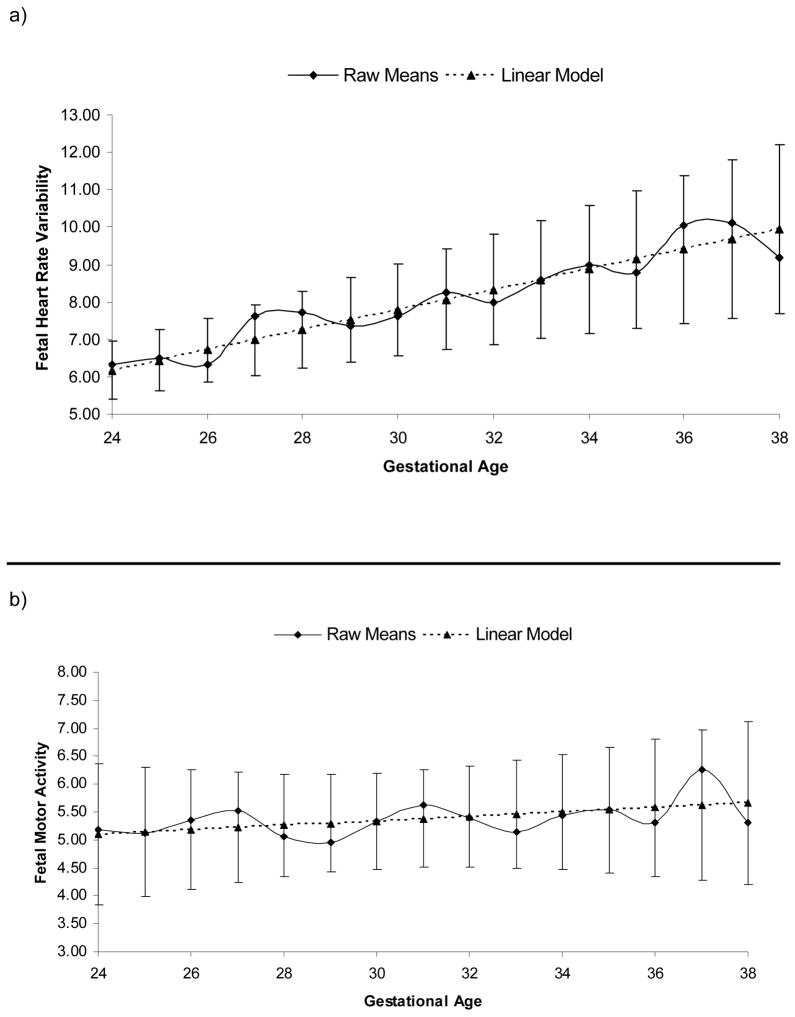
Nearly half (49.1%) of the participants completed all 5 data visits; 38.4% completed 4 visits; and 12.5% completed 3 visits. Of those with missed visits, 13 (22.8%) were a result of delivery of infant prior to the last (i.e., 36 to 38 week) scheduled visit. Scheduling problems associated with the assessment schedule of 3 week intervals were the most common reason for the remaining missed visits. Hierarchical linear models were fit to produce estimates of individual differences in fetal neurobehaviors at 38 weeks (intercept) and growth across the second half of gestation. Model parameters are given in Table 1. Unadjusted raw means, best fit models, and confidence intervals based on individual values are presented in Figure 1. No sex differences in intercept or developmental slope were detected for fetal measures.
Table 1.
Hierarchical Linear Models Describing Change in Fetal Heart Rate Variability, Fetal Motor Activity, and FM-FHR Coupling Across the Second Half of Gestation
| Fetal Heart Rate Variability |
Fetal Motor Activity |
FM- FHR Coupling |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Fixed Effects | Estimate | SE | T | Estimate | SE | t | Estimate | SE | t |
| Intercept at 38 weeks | 9.95 | .26 | 38.48*** | 5.66 | .15 | 38.40*** | .308 | .011 | 28.19*** |
| Linear Slope | .27 | .02 | 11.93*** | .04 | .02 | 2.43* | .014 | .002 | 8.25*** |
| Slope Deviation at 32 Weeks | −.010 | .004 | −2.90** | ||||||
| Random Effects | Estimate | SE | Wald Z | Estimate | SE | Wald Z | Estimate | SE | Wald Z |
| Residual | 3.00 | .25 | 11.81*** | 1.34 | .12 | 11.38*** | .006 | .000 | 11.74*** |
| Intercept Variance | 4.61 | 1.03 | 4.49*** | 1.06 | .33 | 3.23** | .005 | .001 | 3.39* |
| Linear Slope Variance | .02 | .01 | 1.97* | .01 | .00 | 2.28* | .000 | .000 | 1.13 |
| Intercept-Linear Slope Covariance | .26 | .08 | 3.16** | .07 | .03 | 2.28* | .000 | .000 | 2.15* |
p < .05,
p < .01,
p < .001
Figure 1.
Raw means and best fit models with 95% confidence intervals based on individual values for fetal neurobehaviors across the second half of gestation. Fetal heart rate variability (a) and motor activity (b) increased linearly from 24 to 38 weeks gestation. Fetal heart rate-movement coupling (c) increased from 24 to 32 weeks, followed by a decline in rate after 32 weeks.
Fetal heart rate variability
Fetal heart rate variability increased linearly from 24 to 38 weeks gestation (p < .001) by .27 bpm (4.5%) per week (95% CI: .22 to .31) without any significant changes in slope (Figure 1a), to reach an average of 9.95 bpm by 38 weeks (95% CI: 9.44 to 10.47).
Fetal motor activity
Ten visits were excluded for fetal motor activity due to technical difficulties with the fetal movement signal and an additional motor activity outlier (+3 SD) was removed at 33 weeks. The amplitude of fetal motor activity increased gradually at a rate of .04 a.u.s. (0.7%) per week (95% CI: .01 to .07) with advancing gestation (p < .05; Figure 1b). By 38 weeks, mean fetal motor activity reached a level of 5.66 a.u.s. (9.5% CI: 5.37 to 5.95 a.u.s.).
FM-FHR coupling
A single outlier (+3 SD) was excluded from the FM-FHR coupling analysis at 34 weeks. FM-FHR coupling increased steadily from 24 through 32 weeks gestation (p < .001), with a significant decline in slope after 32 weeks (32 week spline term, p < .01; Figure 1c). The coupling index initially increased at a rate of .014 (8.0%) per week of gestation (95% CI: .011 to .017). After 32 weeks, the rate of increase declined by .010 per week (95% CI: −.003 to −.017) to 29% of the original slope. By 38 weeks, the mean FM-FHR coupling index had reached .309 (95% CI: .287 to .330).
Random linear slopes improved the fit for fetal heart rate variability, motor activity, and FM-FHR coupling models (ps < .05). Additional piecewise models were generated for FM-FHR coupling to obtain individual differences in linear slopes before and after 32 weeks.
Postnatal Assessment
Infant data, stratified by sex, are presented in Table 2. Infants were normally grown and none were low birth weight. There were 4 instances of mildly preterm delivery (2 each at 35 and 36 weeks gestation); all were discharged from the hospital on routine schedules. Twenty-four percent (n = 27) were delivered by Caesarian section. Six infants were determined to be too sick to visit/test (e.g., thrombocytopenia; sepsis) during the evaluation period. There were no sex differences on birth outcome measures.
Table 2.
Descriptive Birth and Neonatal Assessment Values
| Boys | Girls | |||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |
| Birth characteristics (n = 112; 55 boys, 57 girls) | ||||||
| Gestational age (weeks) | 39.4 | 1.2 | 39.4 | 1.4 | 39.5 | 1.1 |
| Birth weight (g) | 3443.5 | 435.6 | 3439.0 | 468.3 | 3447.9 | 405.8 |
| Head circumference (cm) | 34.2 | 1.5 | 34.1 | 1.5 | 34.3 | 1.7 |
| 5-minute Apgar | 8.9 | .4 | 8.9 | .5 | 8.9 | .3 |
| Dubowitz optimality scores (n= 97; 47 boys, 50 girls) | ||||||
| Tone | 8.6 | 1.5 | 8.4 | 1.7 | 8.9 | 1.2 |
| Tone Patterns | 4.4 | .7 | 4.3 | .8 | 4.5 | .6 |
| Reflexes | 5.3 | .6 | 5.3 | .7 | 5.2 | .5 |
| Behavior | 5.6 | 1.4 | 5.5 | 1.4 | 5.6 | 1.3 |
| Motor | 2.2 | 1.0 | 2.0 | 1.1 | 2.3 | 1.0 |
| Brainstem auditory evoked potential (n = 47; 24 boys, 23 girls) | ||||||
| Axillary temperature | 97.7 | .7 | 97.6 | .5 | 97.7 | .8 |
| Wave V | 7.3 | .3 | 7.4 | .3 | 7.1 | .3a |
| Interpeak interval I–V | 5.2 | .3 | 5.3 | .3 | 5.0 | .3a |
| Interpeak interval I–III | 2.8 | .2 | 2.9 | .2 | 2.7 | .2a |
| Interpeak interval III–V | 2.4 | .2 | 2.4 | .2 | 2.4 | .2 |
Signifies significant sex difference; see text for details.
Dubowitz neurological exam
Ninety-seven of the remaining 106 newborns received the Dubowitz neurological exam during the home visit (91.5%). Reasons for lack of visiting included nurse unavailability (n = 5), residence relocation out of local area (n = 2) or declining of evaluation (n = 2). Infants were examined between days 2 and 9 postpartum (M = 5.0, sd = 1.2); 78% were tested on days 4, 5, or 6. There were no significant, or near significant correlations between age at test and any optimality cluster, rs (95) range from −.10 to .06. One of the 6 optimality cluster scores (i.e., Abnormal Signs) did not have sufficient variability to analyze so was discarded. Remaining cluster values are presented in Table 2; there were no sex differences.
Fetal motor activity level was positively associated with optimality in the Motor and Reflex clusters, r = .27, p < .01 and r = .20, p < .05, respectively. Exploratory analysis revealed that a number of birth characteristics were also associated with some of the exam clusters, including gestational age and delivery type (vaginal vs Caesarian). In general, infants with longer gestations scored more optimally while those delivered by Caesarian section scored less optimally. Regression results, controlling for gestational age, delivery type, and infant sex are presented in Table 3. In general, more active fetuses displayed more optimal motor and reflex patterns as neonates. In addition to level, analysis of associations between the slope of fetal motor activity during gestation indicated positive associations with Motor cluster optimality, r (95) = .20, p < .05, but the r2Δ did not remain significant in the regression model, F (4,92) = 3.04, p < .10. However, a significant negative association between Tone Pattern optimality and fetal activity slope was also detected, r (95) = −.23, p < .05, and contributed additional significant variance to the regression model, multiple R = .47, r2Δ = .06, F (4,92) = 6.57, p < .01; there was no association between Tone Pattern and fetal activity level.
Table 3.
Multiple Regression Models Predicting Newborn Motor and Reflex Optimality From Fetal Motor Activity Level (N = 97)
| Motor Optimality | Reflex Optimality | |||||||
|---|---|---|---|---|---|---|---|---|
| β | t | p | Multiple R | β | t | p | Multiple R | |
| Constant | −9.68 | 1.63 | ||||||
| Infant sex | .25 | 1.26 | ns | −.16 | −1.43 | ns | ||
| Delivery type | −.65 | −2.75 | <.01 | −.12 | −.88 | ns | ||
| Gestational age | .25 | 3.06 | <.01 | .37 | .07 | 1.55 | ns | .20 |
| R2= .13, F (3, 93) = 4.83** | R2= .04, F (3, 93) = 1.35 | |||||||
| Fetal motor activity | .39 | 2.88 | <.01 | .45 | .16 | 2.07 | <.05 | .29 |
| R2Δ = .07, F (4, 92) = 8.29** | R2Δ = .04, F (4, 92) = 4.28* | |||||||
p < .05;
p < .01
The fetal motor measure used in the modeling that generated these gestational values is an indicator of overall motor signal output and does not capture all characteristics of fetal motor behavior. Supplemental correlations were computed between four additional measures of motor behavior generated at the last fetal assessment and the Motor and Reflex optimality scores to determine the generalizability of the motor results. Fetal motor measures were contributed by 86 fetuses at this assessment, of those, 77 had a postnatal exam. Significant associations were detected for three of these measures with postnatal Motor optimality: mean duration of individual movements, the longest movement bout during the recording, and the total time, in minutes, spent moving, rs (75) = .25, ps < .05. The number of movement bouts was unrelated to Motor optimality scores, and no cross-sectional motor characteristics at term, other than the longitudinally modeled measure, were related to Reflex optimality.
Significant, negative associations were detected between the level and developmental slope of FHR variability with Tone Optimality, rs (95) = −.24, ps < .05. Both level, multiple R = .32, R2Δ = .047, F (4, 92) = 4.85, p < .05, and slope, multiple R = .31, R2Δ = .045, F (4, 92) = 4.63, p < .05 contributed significant unique variance to the regressions. FM-FHR coupling intercept and slope were unrelated to Optimality scores.
Brainstem auditory evoked potential (BAEP)
As described in Procedures, dedicated BAEP visits commenced partway into the protocol. Testing occurred between postpartum days 3 and 14 (M = 8.6, sd = 2.8; M interim between first and second visits = 3.5 days, sd = 2.7). There were 70 visits in which BAEP data collection was attempted. One parent declined the procedure, and poor signal quality or recording problems (e.g., persistent 60 cycle noise, lack of infant sleep state, motor artifact, or electrical interference from household sources that could not be determined) resulted in inability to collect complete data (i.e., two trials of 2048 stimuli presentations per ear) at 22 of the visits, resulting in 47 infants with BAEP data. Axillary temperature was recorded for each infant at the start of the visit, because of an existing known relation between body temperature and BAEP conductance (Bastuji, Larrea, Bertrand, & Mauguiere, 1988; Litscher, 1995). Infants on whom BAEP data could and could not be collected did not differ on birth characteristics (e.g., birth weight, gestational age, delivery method), sex, or Dubowitz cluster scores. The only significant difference was postpartum age at which the data collection was attempted: successful recordings were more likely to be generated on slightly older infants (M = 9.4 vs 7.6 days; t (65) = 2.67, p < .01). Mean values are presented in Table 2.
Gestational age, postnatal age at testing, and measures of size taken at the prior visit were unrelated to BAEP waves, with one modest exception. Greater head circumference was negatively associated with interpeak latency III–V, r (45) = −.32, p < .05. However, boys showed significantly slower BAEP latencies for Wave V, t (45) = 3.03, p < .01, and interpeak intervals I–V, t (45) = 2.93, p < .01 and I–III, t (45) = 2.81, p < .01. Infants with higher temperatures tended to have longer latencies, rs ranged from .21 to .32 for Wave V and interpeak latencies I–III and I to V, although only the last value attained significance (p < .05). Table 4 presents partial correlation coefficients, controlling for temperature, between FHR variability and FM-FHR coupling intercepts and slopes for each BAEP measure. In general, higher levels and steeper developmental trajectories for fetal heart rate variability and FM-FHR coupling were predictive of shorter interpeak intervals from Waves I and III to Wave V. Fetal motor activity was unrelated to BAEP.
Table 4.
Partial Correlations Between Fetal Neurobehavioral Measures And BAEP Values Controlling for Infant Temperature (N = 47)
| Inter-peak latencies |
||||
|---|---|---|---|---|
| Wave V | I–III | III–V | I–V | |
| FHR variability | ||||
| Level | −.36* | −.13 | −.30* | −.32* |
| Slope | −.36* | −.15 | −.29* | −.32* |
| FM-FHR coupling | ||||
| Level | −.29* | .00 | −.42** | −.28+ |
| Slope < 32 weeks | −.11 | −.07 | −.34* | −.12 |
| Slope ≥ 32 weeks | −.36* | .14 | −.42** | −.34* |
p < .10;
p < .05;
p < .01
Correspondence Between Dubowitz Exam and BAEP Assessment
No association between any cluster score of the Dubowitz neurologic exam and any BAEP variable was detected.
Associations Between Maternal Psychological Distress and Development
Fetal Neurobehavioral Development
HLM models did not reveal any change over gestation for either pregnancy specific hassles (estimate = .00, SE = .00, t = 1.58, ns) or trait anxiety (estimate = .02, SE = .04, t = .61, ns). Pregnancy-specific uplifts gradually increased (estimate = .01, SE = .00, t = 3.11, p < .01). Variables were centered at the grand mean and average scores were used in analysis of associations with fetal neurobehavioral measures. Means are as follows: PES intensity of hassles M = 1.41, sd = .39; PES intensity of uplifts M = 2.37, sd = .41; trait anxiety M = 31.52, sd = 7.13.
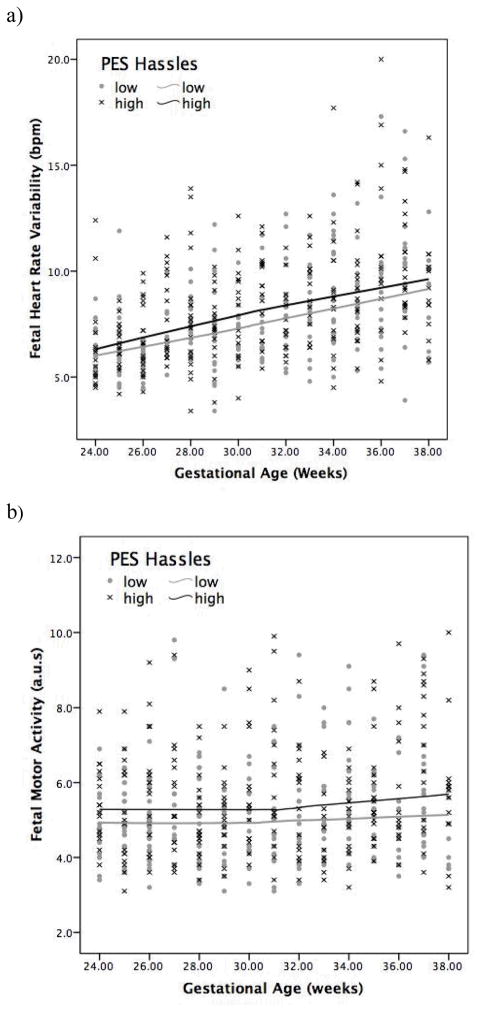
Trait anxiety and pregnancy uplifts were not associated with level or change for any fetal neurobehavior. Because chest wall motions generated by fetal hiccups can generate significant artifact in the motor signal, the presence of fetal hiccups in 59 of the visits was controlled for by inclusion of a dummy variable in these analyses. A greater intensity of pregnancy hassles (1 SD above the mean) was predictive of 2.8% higher fetal heart rate variability (95% CI: 0.2% to 5.4%), t = 2.17, p < .05, and marginally higher motor activity (2.5%; 95% CI: −0.0% to 5.2%), t = 1.86, p = .065 in models centered at 38 weeks (Figure 2a and b). These associations were confirmed by correlations between these two fetal measures and the mean score for intensity of hassles, rs (110) = .19, ps < .05. In addition, more intense pregnancy hassles (+1 SD above the mean) were also associated with an increase in the slope of FM-FHR coupling after 32 weeks by .010 per +1 SD per week (95% CI: .001 to .019), t = 2.10, p < .05, augmenting it to twice the post 32-week slope. Conversely, less intense pregnancy hassles (−1 SD below the mean) were associated with a decline of .010 per 1 SD per week, resulting in a nearly flat FM-FHR coupling slope after 32 weeks (i.e., .0001 increase per week).
Figure 2.
Fetuses of mothers reporting a greater intensity of pregnancy-specific hassles (1 SD above the mean; solid black line) show significantly higher heart rate variability (a) and more fetal motor activity (b) during the second half of gestation.
Postnatal maturation
PES-Brief hassles, uplifts, and STAI anxiety scores were unrelated to the motor and tone-related optimality clusters of the Dubowitz exam. However, exploratory data analyses indicated significant associations between PES-Brief hassles intensity and three components of the Behavior optimality cluster: irritability, consolability, and crying, but not with items related to alertness or orientation performance. A composite score for irritability was derived from these three items; the association between it and PES hassles was significant, r (95) = .28, p < .01. Separate analysis by sex revealed that this association was significant for boys r (45) = .45, p < .01 but not girls r (48) = .08. The coefficients were significantly different, Z = 1.75, p < .05, one-tailed.
There was a significant pattern of associations between PES-Brief hassle intensity scores and BAEP values as follows: Wave V, r (45) = −.35, p < .05, interpeak latencies I–III, r (45) = −.27, p < .10 and I–V, r (45) = −.30, p < .05. There were no significant associations for PES-Brief uplifts or STAI anxiety scores. Analyses conducted separately by sex indicated augmentation of these associations for boys, rs (22) from −.35 to −.45. In contrast, none of the associations with PES hassles was significant for girls. However, significant associations emerged for anxiety scores, Wave V and interpeak intervals I–V, III–V, rs (21) range from −.48 to −.59.
Discussion
The current study provides confirmation of the three working hypotheses and underscores the value of the fetal period as providing the foundation for postnatal development. The observed pattern of associations were consistent with the prediction that fetal motor activity would be associated with newborn maturation as assessed by a traditional examination, but that fetal heart rate variability and somatic-cardiac coupling would differentially predict BAEP. With respect to the first of these, the current results support the long-standing speculation that prenatal motor activity is an ontogenic adaptation that prepares the fetus for postnatal life (Prechtl, 1984). There are at least two interpretations of the observed associations. The first is that the relation between greater fetal motor activity and more optimal neonatal motor and reflex performance simply reveals conservation in motor functioning from the prenatal to postnatal periods, and reflects an intrinsic characteristic of the individual perhaps as a result of a genetic or other constitutional contribution. The second possibility is that greater fetal motor activity provides a practice effect that develops the musculature and potentiates the neural circuitry associated with more optimal neonatal motor performance and reflex responsivity. Based on psychobiological observations of the epigenetic determination of prenatal motor behavior in animal preparations (Smotherman & Robinson, 1987), it is likely that both interpretations contribute to the observed findings. There was an unexpected negative association between steeper developmental slopes for motor activity and lower newborn Tone Pattern. Examination of the scale scores indicates that lower Tone Pattern scores were generally reflective of hyper-responsivity in flexor or extensor tone. Given that there was no association with fetal motor activity level, this suggests that an unusually steep developmental trajectory of motor activity may reflect an inoptimal developmental progression that results in excess tone. Similarly, we have no ready explanation for the negative associations between fetal heart rate variability, a measure that is typically regarded as an indicator of autonomic integration, and Tone optimality.
The second set of findings relates measures of fetal neurobehavioral functioning that have been conceptually linked to maturation of the nervous system with measurement of neural conductivity. Higher levels and steeper slopes of both fetal heart rate variability and FM-FHR coupling during development were associated with shorter neural conduction times for wave V latency in general as well as the wave III–V interpeak interval. The intervals between the component waves provide an estimate of the rate at which signals progress from peripheral to more central levels of the auditory pathway. Given their anatomical origins, and parallels with the progression of development throughout the nervous system, latencies of the later components of the BAEP (i.e., waves III and V) are the least mature in newborn infants. This reflects incomplete neural myelination of the fibers in the auditory pathway, reduced axon diameter, and immaturity in synaptic function (Eggermont & Salamy, 1988; Folsom & Wynne, 1987) which mature over the first 18 months of life.
The associations detected in the current study between fetal measures and conduction of Wave V and the III–V interval reflect activity focused in the later maturing regions of the brainstem auditory pathway. The relative degree of immaturity in these components in the newborn ensures greater detection of inter-individual variation. The notion that BAEP provides a marker of functional integrity of the nervous system beyond the confines of the auditory pathway has been supported by studies that find reductions in auditory pathway conduction following prenatal exposures known to negatively impact postnatal development, such as lead (Rothenberg et al., 2000) and accelerated conductivity following postnatal exposures that enhance development, such as supplementation with docosahexaenoic acid (DHA) (Unay et al., 2004). BAEP also corresponds to neurodevelopmental pathologies (Jiang et al., 2008). With respect to the current findings, it should be noted that the auditory brainstem pathway lies in close proximity to neural units that control heart rate and breathing (Amin, Charafeddine, & Guillet, 2005). More importantly, the predicted absence of any associations with fetal motor activity, and the consistent associations with both FM-FHR coupling and fetal heart rate variability, confirm speculation that the latter measures better reflect neural integrity of the developing fetus while fetal motor activity may more accurately provide an indication of temperament. However, this discussion needs to be qualified by the fact that less that half the original sample generated BAEP data so findings should be regarded as more preliminary than definitive. Home recording of evoked potentials with portable equipment is a particularly challenging undertaking since BAEPs consist of bioelectrical events in the microvolt range and highly susceptible to interference from ambient and electrical noise in the home environment. Nonetheless, the fairly consistent findings provide support for conservation and/or prediction of neonatal neural maturation from fetal indicators.
The prenatal models generated from this study also serve to extend our prior work documenting normal ontogeny of fetal neurobehavioral development by providing continuously generated gestational data in weekly intervals. In general, these confirmed developmental trajectories that had previously been generated by data collected in monthly intervals. These include significant increases in fetal heart rate variability and FM-FHR coupling and little or no change in fetal motor activity (DiPietro et al., 2004a). There was one exception; in prior studies, significant changes in slope were detected at 32 and 28 weeks gestation for FM-FHR coupling and fetal heart rate variability respectively, such that the rate of development slowed near the end of gestation. The current study confirmed the developmental transition for FM-FHR coupling but not variability. This suggests that the longer interval between sampling in the prior report, which was 4 weeks, may have contributed to the perception of discontinuity in contrast to the more gradual change revealed by weekly measurement.
The third aim of the current study was to investigate the contributory role of maternal stress and anxiety on both the fetus and newborn. Stress is a broad construct to operationalize; in this study we focused on the types of stressors, both positive and negative, that are specific to pregnancy, since these have particular salience to the pregnant woman. For the sample as a whole, significant associations were detected with maternal stress but not anxiety. Fetuses of women with higher levels of maternal pregnancy-specific stress displayed higher levels of fetal heart rate variability and steeper incline in somatic-cardiac coupling as term approached. Variability in heart rate has been well-documented as an indicator of parasympathetic maturation in both the prenatal (Martin, 1978) and postnatal (Bar-Haim, Marshall, & Fox, 2000; Porges, 1983) periods. Increases in synchronous activation of somatic and cardiac processes is a conspicuous feature of neurobehavioral development during gestation and has been similarly implicated as a marker for neural integration (Johnson et al., 1992) and fetal well-being (Baser et al., 1992). Thus, contemporaneous maternal stress appears to have facilitative effects on fetal neurodevelopment.
In addition, fetuses of women with higher stress were found to be marginally more active; replicating an earlier report using the same measure of maternal stress (DiPietro, Hilton, Hawkins, Costigan & Pressman, 2002). Unlike the other two fetal measures, there is no compelling conceptual basis to regard variation in fetal motor activity along a continuum of maturation such that higher activity is either better or worse than lower activity. Instead, as in infants, fetal motor activity may best be considered as an indicator of temperament (Eaton & Saudino, 1992). Two studies have reported that maternal cortisol is positively correlated with fetal activity level (DiPietro, Kivlighan, Costigan, & Laudenslager, submitted; Field, Diego, Hernandez-Reif, Gil, & Vera, 2005) suggesting a potential mechanism for the effects observed here.
In the postnatal period, maternal prenatal stress was positively associated with neonatal irritability. This finding partially supports others that detect associations between higher maternal prenatal stress or anxiety and less infant behavioral regulation and/or greater reactivity to novelty measured by observational methods (Davis et al., 2004; Gutteling et al., 2005; Huizink et al., 2002) although another study reports the reverse association (Mohler, Parzer, Brunner, Wiebel, & Resch, 2006). However, in the current study, prenatal stress was also associated with faster neural conduction for three of the four BAEP latencies. In a prior report on a different sample, we found that maternal prenatal stress was associated with higher Bayley Scales of Infant Development scores at age 2, after controlling for postnatal maternal psychological measures (DiPietro et al., 2006). However, there is significant concern regarding the degree to which indicators of mental and motor development as measured by standard developmental assessments adequately reflect information processing, which is at the core of early brain development. Thus BAEP results in the present study provide perhaps more compelling support for the maturative role of maternal prenatal stress on development since BAEP was measured closer to birth and is unambiguously representative of neural processing of information. Together, the postnatal findings suggest that dimensions of information processing and temperament should be regarded as fairly distinct systems, and efforts to measure the former should be careful to exclude confounding influences of the latter.
The current literature detailing maternal stress effects on human development is long on theories of why maternal stress is damaging to the fetus but short on empirical data. Most conceptualization is based on animal models, which use an experimental approach to delivering repetitive, stressful events. These are quite different from human models which necessarily rely on an observational approach to maternal appraisal of elements within their lives, which in turn is confounded by dispositional features of maternal temperament (DiPietro, 2004). Although evidence from animal models often supports deleterious consequences in experimental paradigms (e.g., Weinstock, 2001), functional deficits are not uniformly found. For example, rodents subjected to mild stress showed better spatial learning coupled with facilitated differentiation in neuronal morphology (Fujioka et al., 2001) and more exploratory behavior (Meek, Burda & Paster, 2000). Moreover, methodologies routinely used in human studies have significant limitations. Some of the most widely cited studies in support of an adverse role of maternal stress on behavioral or cognitive development include the following design problems: reliance on maternal report of child outcomes, as opposed to observational data collection, thereby inextricably confounding the dependent and independent (i.e., maternal psychological distress) measures in a direction that promotes detection of positive associations; failure to adequately control for postnatal maternal psychological distress which confers well-known, independent effects on child development as a result of environmental, as opposed to biological, mediation; and lack of control for disparities in social class within samples when it is linked to both exposure and outcomes.
Although the findings reported herein may be counter-intuitive to some readers, they are consistent with the orientation that the human brain requires sufficient, but not excessive, stress to promote neural development both before (Amiel-Tison et al., 2004) and after birth (Huether, 1998). It is important to note that the sample in this study is comprised of well-educated, financially stable women with well-nourished pregnancies and as such, the findings may not generalize to populations of women with psychopathology or those that experience the types of chronic stressors associated with poverty. In terms of a recently proposed lexicon for the consequences of stress exposure on child development, fetal exposures to stress in this sample would likely fall into the categories of “positive” (i.e., moderate, short-lived exposures) or “tolerable” (i.e., time-limited and within a supportive environment) as opposed to “toxic” levels which exceed the capabilities of full recovery (Shonkoff, 2006).
No sex differences in development during the fetal period were evident, confirming findings reported on other samples of similar size (DiPietro et al., 2004a; Robles de Medina, Visser, Huizink, Buitelaar, & Mulder, 2003). In the postnatal period, however, boys had significantly slower BAEP conduction in three of the four latency measures, suggesting slower neural maturation. Given the equivalence in anthropometric indicators of growth between boys and girls, these findings cannot be attributed to conductance differences secondary to size. Instead, they support the long-standing recognition that girls are more mature than boys at birth (Tanner, 1978). In addition, there was differential effect of pregnancy-specific stress exposure on both types of newborn outcomes such that the positive associations between stress and neonatal irritability and neural conduction scores were greater for boys than girls. A pattern of differential responsiveness in neonatal maturity to maternal stress hormones between boys and girls has recently been shown (Ellman et al., 2008). These results suggest that features of the intrauterine environment may interact with fetal sex in ways that remain largely unknown and unexplored.
In summary, the current results provide normative data regarding predictors of neonatal maturation in a sample of healthy women with normally progressing pregnancies. This information provides a basis for future examination of the manner in which early experiences associated with adversity in the maternal environment, intrauterine stress or exposure to potentially deleterious substances may exert influence on the fetus, and in turn, the child.
Acknowledgments
This research was supported by NIH/NICHD award R01 HD27592 to the first author. Newborn evoked potential data collection was supported by a research grant equipment loan from Intelligent Hearing Systems, Miami, FL. We thank Rafael Irizarry, Department of Biostatistics, for his contribution to the original design of the fetal protocol and Kristen Byrnes, for her assistance with Dubowitz training and administration. We remain indebted to the diligent and generous support of our study families, without which this work would not be possible.
References
- Alder J, Fink N, Bitzer J, Hosli I, Holzgreve W. Depression and anxiety during pregnancy: A risk factor for obstetric, fetal and neonatal outcome? A critical review of the literature. Journal of Maternal-Fetal & Neonatal Medicine. 2007;20:189–209. doi: 10.1080/14767050701209560. [DOI] [PubMed] [Google Scholar]
- Almli CR, Ball RH, Wheeler ME. Human fetal and neonatal movement patterns: Gender differences and fetal-to-neonatal continuity. Developmental Psychobiology. 2001;38:252–273. doi: 10.1002/dev.1019. [DOI] [PubMed] [Google Scholar]
- Als H. Toward a synactive theory of development: Promise for the assessment and support of infant individuality. Infant Mental Health Journal. 1982;3:229–243. [Google Scholar]
- Amiel-Tison C, Gosselin J, Kurjak A. Neurosonography in the second half of fetal life: A neonatologist’s point of view. Journal of Perinatal Medicine. 2006;34:437–446. doi: 10.1515/JPM.2006.088. [DOI] [PubMed] [Google Scholar]
- Amin SB, Charafeddine L, Guillet R. Transient bilirubin encephalopathy and apnea of prematurity in 28 to 32 weeks gestational age infants. Journal of Perinatology. 2005;25:386–390. doi: 10.1038/sj.jp.7211295. [DOI] [PubMed] [Google Scholar]
- Amin SB, Orlando MS, Dalzell LE, Merle KS, Guillet R. Morphological changes in serial auditory brain stem responses in 24 to 32 weeks’ gestational age infants during the first week of life. Ear and Hearing. 1999;20:410–418. doi: 10.1097/00003446-199910000-00004. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Marshall PJ, Fox NA. Developmental changes in heart period and high frequency heart period variability from 4 months to 4 years of age. Developmental Psychobiology. 2000;37:44–56. doi: 10.1002/1098-2302(200007)37:1<44::aid-dev6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Barker DJP. Adult consequences of fetal growth restriction. Clinical Obstetrics and Gynecology. 2006;49:270–283. doi: 10.1097/00003081-200606000-00009. [DOI] [PubMed] [Google Scholar]
- Baser I, Johnson TRB, Paine LL. Coupling of fetal movement and fetal heart rate accelerations as an indicator of fetal health. Obstetrics and Gynecology. 1992;80:62–66. [PubMed] [Google Scholar]
- Bastuji H, Larrea L, Bertrand O, Mauguiere F. Baep latency changes during nocturnal sleep are not correlated with sleep stages but with body temperature variation. Electroencephalography and Clinical Neurophysiology. 1988;70:9–15. doi: 10.1016/0013-4694(88)90189-7. [DOI] [PubMed] [Google Scholar]
- Besinger RE, Johnson TRB. Doppler recordings of fetal movement: Clinical correlation with real-time ultrasound. Obstetrics and Gynecology. 1989;74:277–280. [PubMed] [Google Scholar]
- Bryk AS, Raudenbush SW. Application of hierarchical linear models to assessing change. Psychological Bulletin. 1987;101:147–158. [Google Scholar]
- Buitelaar J, Huizink A, Mulder E, Robles de Medina P, Visser G. Prenatal stress and cognitive development and temperament in infants. Neurobiology of Aging. 2003;24:S53–S60. doi: 10.1016/s0197-4580(03)00050-2. [DOI] [PubMed] [Google Scholar]
- Davis E, Snidman N, Wadhwa P, Glynn L, Dunkel-Schetter C, Sandman C. Prenatal maternal anxiety and depression predict negative behavioral reactivity in infancy. Infancy. 2004;6:319–331. [Google Scholar]
- DiPietro JA. The role of prenatal maternal stress in child development. Current Directions in Psychological Science. 2004;13:71–74. [Google Scholar]
- DiPietro JA, Bornstein MH, Costigan KA, Pressman EK, Hahn CS, Painter K, Smith BA, Yi LJ. What does fetal movement predict about behavior during the first two years of life? Developmental Psychobiology. 2002;40:358–371. doi: 10.1002/dev.10025. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Bornstein MH, Hahn CS, Costigan KA, Achy-Brou A. Fetal heart rate and variability: Stability and prediction to developmental outcomes in early childhood. Child Development. 2007;78:1788–1798. doi: 10.1111/j.1467-8624.2007.01099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPietro JA, Caulfield LE, Costigan KA, Merialdi M, Nguyen RHN, Zavaleta N, Gurewitsch E. Fetal neurobehavioral development: A tale of two cities. Developmental Psychology. 2004a;40:445–456. doi: 10.1037/0012-1649.40.3.445. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Christensen A, Costigan KA. The pregnancy experience scale - brief version. doi: 10.1080/01674820802546220. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPietro JA, Costigan KA, Pressman EK. Fetal movement detection: Comparison of the toitu actograph with ultrasound from 20 weeks gestation. Journal of Maternal-Fetal Medicine. 1999;8:237–242. doi: 10.1002/(SICI)1520-6661(199911/12)8:6<237::AID-MFM1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Ghera MM, Costigan KA, Hawkins M. Measuring the ups and downs of pregnancy. Journal of Psychosomatic Obstetrics and Gynecology. 2004b;25:189–201. doi: 10.1080/01674820400017830. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Hodgson DM, Costigan KA, Hilton SC, Johnson TRB. Development of fetal movement- fetal heart rate coupling from 20 weeks through term. Early Human Development. 1996;44:139–151. doi: 10.1016/0378-3782(95)01704-6. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Irizarry RA, Hawkins M, Costigan KA, Pressman EK. Cross-correlation of fetal cardiac and somatic activity as an indicator of antenatal neural development. American Journal of Obstetrics and Gynecology. 2001;185:1421–1428. doi: 10.1067/mob.2001.119108. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Kivlighan KT, Costigan KA, Laudenslager ML. Fetal motor activity is associated with maternal cortisol. doi: 10.1002/dev.20389. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPietro JA, Novak MF, Costigan KA, Atella LD, Reusing SP. Maternal psychological distress during pregnancy in relation to child development at age two. Child Development. 2006;77:573–587. doi: 10.1111/j.1467-8624.2006.00891.x. [DOI] [PubMed] [Google Scholar]
- Dubowitz L, Mercuri E, Dubowitz V. An optimality score for the neurologic examination of the term newborn. Journal of Pediatrics. 1998;133:406–416. doi: 10.1016/s0022-3476(98)70279-3. [DOI] [PubMed] [Google Scholar]
- Eaton W, McKeen N, Campbell D. The waxing and waning of movement: Implications for psychological development. Developmental Review. 2001;21:205–223. [Google Scholar]
- Eaton WO, Saudino KJ. Prenatal activity level as a temperament dimension? Individual differences and developmental functions in fetal movement. Infant Behavior and Development. 1992;15:57–70. [Google Scholar]
- Eggermont JJ, Salamy A. Development of abr parameters in a preterm and a term born population. Ear and Hearing. 1988;9:283–289. doi: 10.1097/00003446-198810000-00009. [DOI] [PubMed] [Google Scholar]
- Ellman LM, Schetter CD, Hobel CJ, Chicz-DeMet A, Glynn LM, Sandman CA. Timing of fetal exposure to stress hormones: Effects on newborn physical and neuromuscular maturation. Developmental Psychobiology. 2008;50:232–241. doi: 10.1002/dev.20293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T, Diego M, Hernandez-Reif M, Gil K, Vera Y. Prenatal maternal cortisol, fetal activity, and growth. International Journal of Neuroscience. 2005;115:423–429. doi: 10.1080/00207450590521082. [DOI] [PubMed] [Google Scholar]
- Folsom RC, Wynne MK. Auditory brainstem responses from human adults and infants: Wave V tuning curves. Journal of the Acoustical Society of America. 1987;81:412–417. doi: 10.1121/1.394906. [DOI] [PubMed] [Google Scholar]
- Freeman RK, Garite TJ, Nageotte MP. Physiologic basis of fetal monitoring. In: Freeman RK, Garite TJ, Nageotte MP, editors. Fetal heart rate monitoring. 2. Baltimore, MD: Williams & Wilkins; 1991. pp. 7–20. [Google Scholar]
- Gingras JL, O’Donnell KJ. State control in the substance-exposed fetus: I. The fetal neurobehavioral profile: An assessment of fetal state, arousal, and regulation competency. Annals of the New York Academy of Sciences. 1998;846:262–276. [PubMed] [Google Scholar]
- Groome L, Swiber M, Holland S, Bentz L, Atterbury J, Trimm R. Spontaneous motor activity in the perinatal infant before and after birth: Stability in individual differences. Developmental Psychobiology. 1999;35:15–24. doi: 10.1002/(sici)1098-2302(199907)35:1<15::aid-dev3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Gutteling B, de Weerth C, Willemsen-Swinkels S, Huizink A, Mulder E, Visser G, Buitelaar J. The effects of prenatal stress on temperament and problem behavior of 27-month-old toddlers. European Child and Adolescent Psychiatry. 2005;14:41–51. doi: 10.1007/s00787-005-0435-1. [DOI] [PubMed] [Google Scholar]
- Hepper PG. Fetal behavior and neural functioning. In: Lecanuet JP, Fifer WP, Krasnegor NA, Smotherman WP, editors. Fetal development: A psychobiological perspective. Hillsdale, NJ: Lawrence Erlbaum Associates; 1995. pp. 405–417. [Google Scholar]
- Hepper PG, Shahidullah S. Habituation in normal and Down’s syndrome fetuses. Quarterly Journal of Experimental Psychology. 1992;44:305–317. doi: 10.1080/02724999208250617. [DOI] [PubMed] [Google Scholar]
- Horimoto N, Koyangi T, Maeda H, Satoh S, Takashima T, Minami T, Nakano H. Can brain impairment be detected by in utero behavioural patterns? Archives of Disease in Childhood. 1993;69:3–8. doi: 10.1136/adc.69.1_spec_no.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizink A, Robles de Medina P, Mulder E, Visser G, Buitelaar J. Psychological measures of prenatal stress as predictors of infant temperament. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:1078–1085. doi: 10.1097/00004583-200209000-00008. [DOI] [PubMed] [Google Scholar]
- Jiang ZD, Liu XY, Shi BP, Lin L, Bu CF, Wilkinson AR. Brainstem auditory outcomes and correlation with neurodevelopment after perinatal axphyxia. Pediatric Neurology. 2008;39:189–195. doi: 10.1016/j.pediatrneurol.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Jiang ZD, Xu X, Brosi DM, Shao XM, Wilkinson A. Sub-optimal function of the auditory brainstem in term infants with transient low Apgar scores. Clincal Neurophysiology. 2007;118:1088–1096. doi: 10.1016/j.clinph.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Johnson TRB, Besinger RE, Thomas RL, Strobino DM, Niebyl JR. Quantitative and qualitative relationships between fetal heart rate accelerations and fetal movement. Journal of Maternal-Fetal Medicine. 1992;1:251–253. [Google Scholar]
- Kainer F, Prechtl H, Engele H, Einspieler C. Assessment of the quality of general movements in fetuses and infants of women with type-i diabetes mellitus. Early Human Development. 1997;50:13–25. doi: 10.1016/s0378-3782(97)00089-3. [DOI] [PubMed] [Google Scholar]
- Khedr E, Farghaly W, El-DinAmry E, Osman A. Neural maturation of breastfed and formula-fed infants. Acta Paediatrica. 2004;93:734–738. doi: 10.1111/j.1651-2227.2004.tb03011.x. [DOI] [PubMed] [Google Scholar]
- Krasnegor N, Fifer W, Maulik D, McNellis D, Romero R, Smotherman W. Fetal behavioral development: A transdisciplinary perspective for assessing fetal well-being and predicting outcome. Prenatal and Neonatal Medicine. 1998;3:185–190. [Google Scholar]
- Laplante D, Barr R, Brunet A, DuFort G, Meaney M, Saucier J, Zelazo P, King S. Stress during pregnancy affects general intellectual and language functioning in human toddlers. Pediatric Research. 2004;56:400–410. doi: 10.1203/01.PDR.0000136281.34035.44. [DOI] [PubMed] [Google Scholar]
- Litscher G. Continuous auditory evoked potential measurements during nocturnal sleep. International Journal of Neuroscience. 1995;87:113–117. doi: 10.3109/00207459508994297. [DOI] [PubMed] [Google Scholar]
- Littleton H, Breitkopf C, Berenson A. Correlates of anxiety symptoms during pregnancy and association with perinatal outcomes: A meta-analysis. American Journal of Obstetrics and Gynecology. 2007;196:424–432. doi: 10.1016/j.ajog.2007.03.042. [DOI] [PubMed] [Google Scholar]
- Maeda K, Morokuma S, Yoshida S, Ito T, Pooh R, Serizawa M. Fetal behavior analyzed by ultrasonic actocardiogram in cases with central nervous system lesions. Journal of Perinatal Medicine. 2006;34:398–403. doi: 10.1515/JPM.2006.079. [DOI] [PubMed] [Google Scholar]
- Maeda K, Tatsumura M, Utsu M. Analysis of fetal movements by doppler actocardiogram and fetal B-mode imaging. Clinics in Perinatology. 1999;26:829–851. [PubMed] [Google Scholar]
- Martin C. Regulation of the fetal heart rate and genesis of FHR patterns. Seminars in Perinatology. 1978;2:131–146. [PubMed] [Google Scholar]
- Mohler E, Parzer P, Brunner R, Wiebel A, Resch F. Emotional stress in pregnancy predicts human infant reactivity. Early Human Development. 2006;82:731–737. doi: 10.1016/j.earlhumdev.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Moller AR, Jannetta PJ. Evoked potentials from the inferior colliculus in man. Electroencephalography and Clinical Neurophysiology. 1982;53:612–620. doi: 10.1016/0013-4694(82)90137-7. [DOI] [PubMed] [Google Scholar]
- Montandon PB, Cao MH, Engel RT, Grajew T. Auditory nerve and brainstem responses inthe newborn and in preschool children. Acta Oto-Laryngologica. 1979;87:279–286. doi: 10.3109/00016487909126421. [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. Journal of Neuroscience. 2008;28:9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder EJ, Morssink LP, van der Schee T, Visser GH. Acute maternal alcohol consumption disrupts behavioral state organization in the near term fetus. Pediatric Research. 1998;44:774–779. doi: 10.1203/00006450-199811000-00022. [DOI] [PubMed] [Google Scholar]
- Nijhuis IJM, ten Hof J. Development of fetal heart rate and behavior: Indirect measures to assess the fetal nervous system. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 1999;87:1–2. doi: 10.1016/s0301-2115(99)00143-8. [DOI] [PubMed] [Google Scholar]
- Nijhuis IJM, ten Hof J, Mulder EJ, Nijhuis JG, Narayan H, Taylor D, Visser GHA. Fetal heart rate in relation to its variation in normal and growth retarded fetuses. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 2000;89:27–33. doi: 10.1016/s0301-2115(99)00162-1. [DOI] [PubMed] [Google Scholar]
- O’Brien P, Wheeler T, Barker D, editors. Fetal programming: Influences on development and disease in later life. London: RCOG Press; 1999. [Google Scholar]
- Poblano A, Belmont A, Sosa J, Ibarra J, Vargas A, Limon G, Martinez C. Amikacin alters auditory brainstem conduction time in newborns. Journal of Perinatology. 2003;31:237–241. doi: 10.1515/JPM.2003.032. [DOI] [PubMed] [Google Scholar]
- Porges SW. Heart rate patterns in neonates: A potential diagnostic window to the brain. In: Field T, Sostek A, editors. Infants born at risk: Physiological, perceptual, and cognitive processes. New York: Grune & Stratton; 1983. pp. 3–22. [Google Scholar]
- Prechtl HFR. Continuity and change in early neural development. In: Prechtl H, editor. Continuity in neural functions from prenatal to postnatal life. Philadelphia, PA: J.B. Lippincott Co; 1984. pp. 1–15. Vol. Clinics in Developmental Medicine No. 94. [Google Scholar]
- Robles de Medina P, Visser G, Huizink A, Buitelaar J, Mulder E. Fetal behaviour does not differ between boys and girls. Early Human Development. 2003;73:17–26. doi: 10.1016/s0378-3782(03)00047-1. [DOI] [PubMed] [Google Scholar]
- Romanini C, Rizzo G. Fetal behaviour in normal and compromised fetuses: An overview. Early Human Development. 1995;43:117–131. doi: 10.1016/0378-3782(95)01667-8. [DOI] [PubMed] [Google Scholar]
- Roncagliolo M, Garrido M, Walter T, Peirano P, Lozoff B. Evidence of altered central nervous system development in infants with iron deficiency anemia at 6 mo: Delayed maturation of auditory brainstem responses. American Journal of Clinical Nutrition. 1998;68:683–690. doi: 10.1093/ajcn/68.3.683. [DOI] [PubMed] [Google Scholar]
- Rothenberg S, Poblano A, Schnaas L. Brainstem auditory evoked response at five years and prenatal and postnatal blood lead. Neurotoxicology and Teratology. 2000;22:503–510. doi: 10.1016/s0892-0362(00)00079-9. [DOI] [PubMed] [Google Scholar]
- Rotteveel JJ, de Graaf R, Colon EJ, Stegeman DF, Visco YM. The maturation of the central auditory conduction in preterm infants until three months post term. II The auditory brainstem responses (ABRs) Hearing Research. 1987;26:21–35. doi: 10.1016/0378-5955(87)90033-5. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Wadhwa P, Hetrick W, Porto M, Peeke H. Human fetal heart rate dishabituation between thirty and thirty-two weeks gestation. Child Development. 1997;68:1031–1040. [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis. New York: Oxford University Press; 2003. [Google Scholar]
- Smotherman WP, Robinson SR. Prenatal influences on development: Behavior is not a trivial aspect of fetal life. Development and Behavioral Pediatrics. 1987;8:171–175. [PubMed] [Google Scholar]
- Sontag LW, Richards TW. Monographs of the Society for Research in Child Development. 4. Vol. 3. 1938. Studies in fetal behavior: I. Fetal heart rate as a behavioral indicator; pp. 1–67. Serial No. 17. [Google Scholar]
- Spielberger C. Manual for the state-trait anxiety inventory (form y) Palo Alto, CA: Mind Garden, Inc; 1983. [Google Scholar]
- Tanner JM. Foetus into man: Physical growth from conception to maturity. Cambridge, MA: Harvard University Press; 1978. [Google Scholar]
- Timor-Tritsch IE, Dierker LJ, Zador I, Hertz RH, Rosen MG. Fetal movements associated with fetal heart rate accelerations and decelerations. American Journal of Obstetrics and Gynecology. 1978;131:276–280. doi: 10.1016/0002-9378(78)90600-2. [DOI] [PubMed] [Google Scholar]
- Unay B, Sarici S, Ulas U, Akin R, Alpay F, Gokcay E. Nutritional effects on auditory brainstem maturation in healthy term infants. Archives of Disease in Childhood. 2004;89:F177–179. doi: 10.1136/adc.2002.021014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bergh B, Mennes M, Oosterlaan J, Stevens V, Stiers P, Marcoen A, Lagae L. High antenatal maternal anxiety is related to impulsivity during performance on cognitive tasks in 14- and 15-year-olds. Neuroscience and Biobehavioral Reviews. 2005;29:259–269. doi: 10.1016/j.neubiorev.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Vintzileos AM, Campbell WA, Nochinson D. Relation between fetal heart rate accelerations, fetal movements, and fetal breathing movements. American Journal of Perinatology. 1986;3:16–18. doi: 10.1055/s-2007-999823. [DOI] [PubMed] [Google Scholar]
- Weinstock M. Alterations induced by gestational stress in brain morphology and behavior of the offspring. Progress in Neurobiology. 2001;65:427–451. doi: 10.1016/s0301-0082(01)00018-1. [DOI] [PubMed] [Google Scholar]
- Yamasaki M, Shono H, Oga M, Ito Y, Shimomura K, Sugimori H. Changes in auditory brainstem responses of normal neonates immediately after birth. Biology of the Neonate. 1991;60:92–101. doi: 10.1159/000243393. [DOI] [PubMed] [Google Scholar]
- Young J. Programming of sympathoadrenal function. Trends in Endocrinology and Metabolism. 2002;13:381–385. doi: 10.1016/s1043-2760(02)00661-6. [DOI] [PubMed] [Google Scholar]