Abstract
Purpose
To test if improving optical coherence tomography (OCT) resolution and scanning speed improves the visualization of glaucomatous structural changes as compared with conventional OCT.
Design
Prospective observational case series.
Participants
Healthy and glaucomatous subjects in various stages of disease.
Methods
Subjects were scanned at a single visit with commercially available OCT (StratusOCT) and high-speed ultrahigh-resolution (hsUHR) OCT. The prototype hsUHR OCT had an axial resolution of 3.4 μm (3 times higher than StratusOCT), with an A-scan rate of 24 000 hertz (60 times faster than StratusOCT). The fast scanning rate allowed the acquisition of novel scanning patterns such as raster scanning, which provided dense coverage of the retina and optic nerve head.
Main Outcome Measures
Discrimination of retinal tissue layers and detailed visualization of retinal structures.
Results
High-speed UHR OCT provided a marked improvement in tissue visualization as compared with StratusOCT. This allowed the identification of numerous retinal layers, including the ganglion cell layer, which is specifically prone to glaucomatous damage. Fast scanning and the enhanced A-scan registration properties of hsUHR OCT provided maps of the macula and optic nerve head with unprecedented detail, including en face OCT fundus images and retinal nerve fiber layer thickness maps.
Conclusion
High-speed UHR OCT improves visualization of the tissues relevant to the detection and management of glaucoma.
Glaucoma damage primarily affects the optic nerve, retinal ganglion cell layer, and retinal nerve fiber layer (RNFL).1 Therefore, detection of early glaucomatous changes in these tissues is important for the management of glaucoma patients. A variety of imaging technologies are currently used for this purpose. Optical coherence tomography (OCT) is a noninvasive imaging technology using low-coherence interferometry to generate in vivo high-resolution cross-sectional images of retinal and optic nerve head (ONH) structure by measuring the echo time delay and magnitude of backscattered light.2-4 Images generated by OCT are useful for the identification, monitoring, and quantitative assessment of glaucoma and other ocular diseases.
Commercially available standard time domain OCT (StratusOCT, Carl Zeiss Meditec, Dublin, CA) quantifies reflectance within the tissue with an axial resolution of 8 to 10 μm.5 Previous studies have demonstrated the ability of OCT to detect and quantify glaucomatous retinal damage6-8; however, technological constraints limit the scanning speed of the standard OCT to 400 A-scans per second. Because of the relatively slow scanning speed, Stratus scans are susceptible to motion artifacts, limited by the need to interpolate RNFL thickness in areas between scans, and further limited by challenges in the registration of sequential A-scans.
Recent improvements in OCT technology provide higher acquisition speeds and higher resolution than StratusOCT. Our prototype high-speed ultrahigh-resolution (hsUHR) OCT, constructed at Massachusetts Institute of Technology and the University of Pittsburgh, uses spectral/Fourier domain detection to acquire A-scans at ultrahigh resolution and high speed, and promises to improve the accuracy and details of OCT images.9-13 The fast scanning rate of hsUHR OCT allows the acquisition of novel scanning patterns that are not available by StratusOCT, which may further improve the ability to detect glaucomatous structural changes.
The purpose of this pilot study was to compare StratusOCT and hsUHR OCT images in the detection of RNFL and ONH changes in glaucoma patients.
Materials and Methods
Healthy subjects and glaucoma patients from the University of Pittsburgh Medical Center Eye Center received a comprehensive ocular examination, visual field (VF) test, ONH photographs, and StratusOCT and hsUHR OCT scanning at the same visit. All scans were performed through undilated pupils. Representative samples of a healthy eye and patients at various stages of glaucomatous damage were chosen retrospectively for this study. Institutional review board and ethics committee approval was obtained for the study, and informed consent was obtained from all subjects. This study followed the tenets of the Declaration of Helsinki and was conducted in compliance with the Health Insurance Portability and Accountability Act.
Instrumentation
Visual Field
All the participants had reliable Swedish interactive thresholding algorithm 24-2 standard perimetry. Visual field examinations were considered nonreliable if they had 30% or more fixation losses or false-positive or false-negative responses. Healthy subjects had normal VF, and the glaucoma patients had a reproducible typical glaucomatous VF defect with ≥3 adjacent non-edge points in typically glaucomatous locations, all of which were depressed on the pattern deviation plot at a P<0.05 level and one of which was depressed at a P<0.01 level.
StratusOCT
The commercially available StratusOCT uses a superluminescent diode light source that generates an 850-nm beam with a 25-nm bandwidth. The light is split into 2 beams using a fiber optic coupler. One beam is projected onto the eye (measurement arm), whereas the second beam is projected onto an oscillating mirror (reference arm). An interference signal is created when path lengths of the reference and measurement arms are matched within the coherence length of the light. StratusOCT has an axial resolution in the eye of approximately 8 to 10 μm, with a transverse resolution of 20 μm on the retina. The scanning rate of the StratusOCT is 400 A-scans per second.
High-speed Ultrahigh-Resolution Optical Coherence Tomography
Our hsUHR OCT uses spectral/Fourier domain detection and has been described in detail in previous publications.9,13 Briefly, it uses a multiplexed low-coherence superluminescent diode light source at 840 nm with a broad 100-nm bandwidth (Broadlighter 840T, Superlum, Moscow, Russia). The light is split into 2 beams using a fiber optic coupler. One beam is projected onto the eye, whereas the other is projected onto a stationary reference mirror. Backscattered light from each arm is combined using a coupler, creating an optical interference signal. The interference spectrum is measured by a spectrometer and Fourier transformed to generate A-scan information. This spectral/Fourier domain detection acquires an entire A-scan simultaneously rather than detecting echoes sequentially as in standard OCT with time domain detection. Echo time delay is frequency encoded in the interference pattern. The intensity of the interference pattern at each frequency represents tissue reflectance at the location associated with that frequency. High-speed UHR OCT has an axial resolution of 3.4 μm in the eye and a scanning speed of 24 000 A-scans per second.
The hsUHR OCT scanning patterns used in this study included high-definition scans, consisting of 8000 A-scans evenly spaced along a 6-mm line of retina tissue; circumpapillary scans, consisting of three 3.4-mm-diameter circular scans centered on the ONH; and raster scans, consisting of 180 adjacent line scans, each with 501 pixels (a total of 90 000 transverse points) covering a 6×6-mm area of retina. The circumpapillary scans were similar to those acquired by the fast scanning mode of the StratusOCT, in which the 3 scans are aligned and acquired automatically after the initial positioning by the operator. High-definition scans and circumpapillary scans were acquired in 1.2 seconds, and raster scans were acquired in ~4 seconds.
An en face fundus image was created from the raster scans by summing all pixel intensity values along individual A-scans. The resultant sum along each A-scan was the intensity value used for the pixel corresponding to that A-scan’s location within the 2-dimensional en face hsUHR OCT fundus image. Retinal nerve fiber layer thickness maps were automatically created by segmenting the retina based on detection of the internal limiting membrane and outer RNFL border in each A-scan of each OCT frame.13 The measurements were color coded. Red and yellow denoted a thicker RNFL, and blue indicated a thinner one.
Results
A healthy subject and 3 glaucoma patients were retrospectively chosen with a range of VF abnormalities representing various stages of glaucomatous damage.
Case 1: Healthy Subject
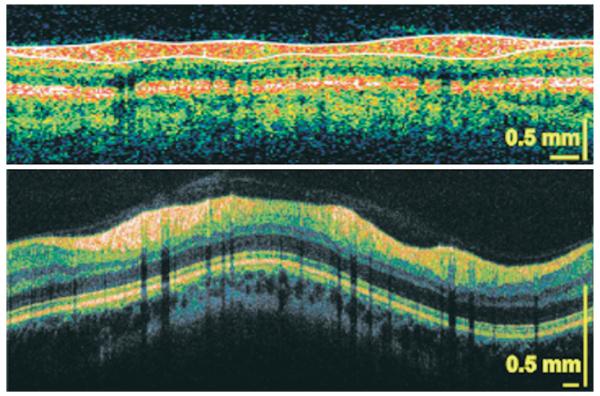
A healthy 35-year-old Caucasian woman with normal left eye examination results and a normal VF was scanned with StratusOCT and hsUHR OCT. Circumpapillary and cross-sectional images of the ONH (Figs 1, 2 [available at http://aaojournal.org]) and macula (Fig 3 [available at http://aaojournal.org]) were obtained. The typical double-hump configuration can be observed in the circumpapillary scans with thickening of the RNFL at the superior and inferior regions. The black vertical lines are shadows of blood vessels. High-speed UHR OCT provided a finer and more detailed cross-sectional image of the retina than the StratusOCT scan. A portion of the choroidal vasculature can be observed near the bottom of the hsUHR OCT scan.
Figure 1.
A circumpapillary StratusOCT scan of a healthy subject (top) and high-speed ultrahigh-resolution optical coherence tomography (bottom). The white lines delineate the nerve fiber layer, and the black vertical lines are shadows cast by the blood vessels.
Figure 2.
A StratusOCT scan of the optic nerve head of a healthy subject scan (top) and high-speed ultrahigh-resolution optical coherence tomography (hsUHR OCT) (bottom). The termination of the retinal pigment epithelium layer (lower red horizontal band) is clearly visible in the hsUHR OCT scan. The arrows point to blood vessels, in which their patent lumen can be observed.
Figure 3.
A StratusOCT macular scan in a healthy subject (top) and high-speed ultrahigh-resolution optical coherence tomography (hsUHR OCT) (bottom). Clear delineation of the retinal layers can be observed in the hsUHR OCT scan. ELM = external limiting layer; GCL = ganglion cell layer; INL = inner nuclear layer; IPL = inner plexiform layer; IS/OS = inner segment/outer segment intersection of photoreceptors; NFL = nerve fiber layer; ONL = outer nuclear layer; OPL = outer plexiform layer; RPE = retinal pigment epithelium.
The current StratusOCT ONH algorithms use the termination of retinal pigment epithelium (RPE) or Bruch’s membrane to determine the border of the disc for quantitative measurements (Fig 2). The RNFL and RPE/Bruch’s membrane tips can easily be distinguished in the hsUHR OCT cross-sectional scan of the ONH. Due to the high resolution and high scanning speed achieved by hsUHR OCT, the lumens of vascular structures within the cup and posterior hyaloid membrane are visible in the hsUHR OCT but not the standard OCT scan (Fig 2).
Numerous layers can be discerned with the hsUHR OCT macular scan that could not be discriminated using StratusOCT. Boundaries of the ganglion cell layer and RNFL are clearly visible throughout the hsUHR OCT scan.
The novel raster scan pattern enabled the creation of an en face OCT fundus image that resembled the clinical appearance of the fundus (Fig 4). A color-coded RNFL thickness map was created from the same raster scan (Fig 4). A thicker RNFL was observed in the superior and inferior regions, with a thinner RNFL temporal to the ONH. The red line on the en face and thickness map images corresponds to the location of the individual cross section (Fig 4) that was obtained during the raster acquisition. Thicker RNFL can be clearly observed on the left side of the cross section image, along with thinner RNFL on the right side corresponding to the thinner temporal RNFL seen on the thickness map.
Figure 4.
A raster optic nerve head—centered scan in a healthy subject, with an en face image (left) and color-coded retinal nerve fiber layer (RNFL) thickness map (center), with red and yellow denoting a thicker layer and blue a thinner one. Right, One of the cross sections that contributed to the construction of the raster scan. The plane of the cross section is marked by the red line in the en face and thickness map.
Case 2: Preperimetric Glaucoma
A 68-year-old Caucasian woman was referred as an untreated glaucoma suspect. On examination, her visual acuity in the right eye was 20/20, and the intraocular pressure (IOP) was 24 mmHg. Pachymetry of the cornea was 578 μm, and the anterior chamber (AC) appearance was normal, with an open angle. There was minimal lenticular nuclear sclerosis and moderate ONH cupping with a thin inferotemporal neuroretinal rim (Fig 5A). The patient had a normal-appearing VF with a normal glaucoma hemifield test result, but there was VF deterioration in the superior arcuate region by the VF glaucoma progression analysis (Fig 5B). The StratusOCT circumpapillary scan showed a thin RNFL inferiorly (Fig 5C), which was more evident with the comparison to the OCT normative database in the thickness profile graph and quadrant analysis (Fig 5D). The clock-hour normative dataset comparison showed an abnormally thin RNFL at the 6-o’clock position and borderline thin RNFL (yellow) at the 7-o’clock position (Fig 5D). The thickness profile showed a second area of focally thin RNFL (Fig 5D) that was not shown in the quadrant or clock-hour analysis. Careful examination of the cross-sectional image revealed this focally thin region (Fig 5C). The circumpapillary scan obtained by hsUHR OCT clearly showed 2 locations with a focally thin RNFL (Fig 5E). The thin area in the inferior sector (right side of the scan) can be appreciated when compared with the RNFL thickness in the superior region (left side of the scan). A localized wedge defect in the temporal inferior sector was observed in the raster scan color-coded thickness map (Fig 5F) corresponding to the neuroretinal rim notch and the finding in the circumpapillary scan. A cross-sectional image (Fig 5F) along the red line that appears in the lower part of the color-coded RNFL thickness map clearly demonstrates the focally thin RNFL.
Figure 5.
A preperimetric glaucoma subject. A, Optic nerve head photograph. B, Visual field pattern deviation map (left) and glaucoma progression analysis (right). C, StratusOCT circumpapillary scan. D, StratusOCT retinal nerve fiber layer (RNFL) thickness profile (left) and the comparison with normative data, which are marked for quadrants and clock hours (right). The green area denotes RNFL thickness within normal limits; yellow, borderline RNFL thickness; and red, outside normal limits. Inf = inferior; nas = nasal; OD = right eye; sup = superior; temp = temporal. E, High-speed ultrahigh-resolution optical coherence tomography (hsUHR OCT) circumpapillary scan. Arrows, location of RNFL thinning in the StratusOCT and hsUHR OCT images. F, Retinal nerve fiber layer thickness is color coded on a map (left), with a wedge defect (arrow) clearly visible in the cross section (right).
Case 3: Moderate Glaucoma
A 68-year-old Caucasian woman had primary open-angle glaucoma (POAG) that was treated with trabeculectomy in each eye and currently required no further medical treatment. Visual acuity was 20/20 in the left eye, with 7-mmHg IOP and corneal thickness of 546 μm. Clinical examination revealed a superior thin-walled elevated filtration bleb, open AC angle (ACA), and pseudophakia. Marked excavation was noted on fundus examination with a vertical cup-to-disc ratio of 0.9 (Fig 6A). The VF showed inferior arcuate and paracentral scotomas and suspected superior and inferior temporal wedges (Fig 6B). StratusOCT showed an overall attenuation of the RNFL with 2 punched-out defects at the temporal inferior and temporal superior regions (Fig 6C, D). An hsUHR OCT circular scan showed 2 locations with substantially thin RNFL (Fig 6E) corresponding to the defects highlighted by StratusOCT. Careful examination of another area that seemed to contain thin RNFL (center of the scan) disclosed a posterior bowing of the tissue but with intact RNFL. The retinal blood vessel lumen and choroidal details can be clearly seen. The RNFL thickness map showed 2 wedge defects, with a more pronounced and larger defect in the superior temporal region corresponding to the inferior VF defect (Fig 6F). A functional abnormality corresponding to the inferior structural defect was not detected on the VF. The cross section through the area denoted by the red line on the retinal map showed the thin RNFL, with the arrows marking the edges of the defect (Fig 6F).
Figure 6.
Moderate glaucoma. A, Optic nerve head photograph. B, Visual field pattern deviation map. C, Circumpapillary StratusOCT scan. D, StratusOCT retinal nerve fiber layer (RNFL) thickness profile (left) and the comparison with normative data are marked for quadrants and clock hours (right). Inf = inferior; nas = nasal; OS = left eye; sup = superior; temp = temporal. E, High-speed ultrahigh-resolution optical coherence tomography (hsUHR OCT) circumpapillary scan. Arrows, location of RNFL thinning in the StratusOCT and hsUHR OCT images. F, Retinal nerve fiber layer thickness color-coded map (left) with 2 wedge defects. Arrows, edges of the upper defect in the map and cross section (right).
Case 4: Moderate Glaucoma
An 89-year-old Caucasian woman with POAG in both eyes was treated medically with dorzolamide, timolol, and bimatoprost. Visual acuity was 20/40 (right eye), with an IOP of 10 mmHg. On examination of the anterior segment, corneal thickness was 589 μm, with an open ACA. The patient was aphakic and had a peripheral iridectomy. The posterior segment revealed near total rim attenuation in the temporal half of the ONH and was most remarkably thin in the temporal superior area (Fig 7A). A small disc hemorrhage was noted at 11-o’clock, and there was circular peripapillary atrophy. In the VF, an inferior nasal defect was noted, as was a superior rim defect (Fig 7B). The StratusOCT circumpapillary RNFL scan showed marked attenuation of RNFL thickness superiorly (Fig 7C, D). Similarly, the hsUHR OCT circumpapillary scan showed a clear delineation of the RNFL defect superiorly, presenting as a thin and weak RNFL signal (Fig 7E). The strong signal throughout the RPE layer was an indication that the scan was good, and the thin RNFL was not an artifact. The hsUHR OCT color-coded retinal thickness map showed substantial attenuation throughout the upper sector that indicated more advanced structural damage than suggested by the VF (Fig 7F).
Figure 7.
Moderate glaucoma. A, Optic nerve head photograph. B, Visual field pattern deviation map. C, Circumpapillary StratusOCT scan. D, StratusOCT retinal nerve fiber layer (RNFL) thickness profile (left) and the comparison with normative data are marked for quadrants and clock hours (right). Arrows, location of RNFL thinning in StratusOCT. Inf = inferior; nas = nasal; OD = right eye; sup = superior; temp = temporal. E, High-speed ultrahigh-resolution optical coherence tomography (hsUHR OCT) circumpapillary scan. Arrows, edges of the RNFL defect. F, Retinal nerve fiber layer thickness color-coded map (left), with general thinning of the RNFL superior to the optic nerve head (left, right).
Discussion
The increased axial resolution and A-scan density of hsUHR OCT allowed the identification of intraretinal structures that were unseen with StratusOCT. These structures include the ganglion cell layer, external limiting membrane, and inner/outer segment junction of the photoreceptors. Previously, UHR OCT imaging using time domain detection, with an axial resolution of 3 μm, also visualized these intraretinal layers.14-18 However, this device had a slow acquisition rate and, thus, was prone to marked eye motion artifacts. In addition to achieving UHR, the hsUHR OCT using Fourier domain detection (the device used in the present study) acquires A-scans approximately 60 times faster than the standard-resolution StratusOCT and 100 times faster than UHR OCT using time domain detection. The StratusOCT image consisting of 512 axial scans is acquired in 1.3 seconds, whereas a time domain UHR OCT image consisting of 600 axial scans takes 4 seconds to acquire. In the present study, hsUHR OCT was able to acquire 2048 A-scans in only ~0.085 seconds. This improvement in scan rate allows the acquisition of high-definition images with high transverse pixel densities. Therefore, the quality of hsUHR OCT images using Fourier domain detection is superior to that of those acquired with either standard resolution or UHR time domain OCT.
The ganglion cell layer is specifically prone to glaucomatous damage. The only in vivo method that is currently available to estimate the structural damage to the ganglion cells is assessment of RNFL thickness or ONH topography. It has been suggested that RNFL thinning may precede VF loss and changes in the ONH by as much as 6 years.19 The accurate measurement of the RNFL with hsUHR OCT may provide earlier detection and treatment of glaucoma. Moreover, the unique ability of hsUHR OCT to delineate the ganglion cell layer might enable the quantification of this layer, leading to further improvements in glaucoma detection.
The images presented in this study demonstrate the ability of hsUHR OCT to provide detailed in vivo retinal information at a level that is similar to histology (Figs 1-3 [available at http://aaojournal.org]). There is a clear delineation of the retinal layers that may be of benefit for detection of a variety of retinal abnormalities. In Figure 4, we present an example of a novel use of the improved performance of the device. The combination of the thickness map and cross-sectional scans was beneficial in visualizing and identifying defects, as presented in Figures 5 to 7. Figure 5 shows an example of a preperimetric defect in which the VF was normal, but the glaucoma progression analysis indicated progressive damage. Despite the lack of a normative database, at this stage in hsUHR OCT technology development, the cross-sectional image clearly showed a localized focal RNFL defect in the OCT fundus image and RNFL thickness map. In addition to RNFL thinning corresponding to the VF progression, hsUHR OCT may have indicated more advanced structural damage than observed in the functional test, as evident by the superior defect without a corresponding VF abnormality. This is also evident in Figure 7 with a thin superior RNFL with limited corresponding VF abnormality. Figures 6 and 7 show the ability of the hsUHR OCT image to clearly define the extent of the RNFL defect in both cross-sectional OCT images and the en face OCT fundus image view. Some of the features that were detected by hsUHR OCT could be retrospectively detected in StratusOCT scans, but due to the lower resolution and eye motion artifacts they were difficult to discern independently.
The high speed of hsUHR OCT minimizes image distortion caused by involuntary eye movements. This in turn allows the use of novel scanning patterns not possible with standard OCT due to eye movement artifacts; however, motion artifacts are still present between subsequent lines within raster scans. This scan pattern is especially valuable for the assessment of focal retinal abnormalities that might be missed by a single poorly positioned OCT line scan. The raster scan also allows a postprocessing cross-sectional sampling in any desired orientation, including a circumpapillary sampling, thus minimizing interscan variability due to image registration problems, as observed with StratusOCT. Sampling errors and the possibility of missing focal pathologies could also be reduced with raster scanning, as the number and density of axial scans on the retina are dramatically increased compared with standard OCT. Recently, a number of manufacturers have introduced OCT instruments using spectral/Fourier domain detection. These instruments achieve speeds of ~24 000 A-scans per second or faster but have axial resolutions of 5 to 6 μm. Although the axial resolution of the commercial devices does not achieve the 3.4-μm axial resolution of the prototype instrument used in this study, we expect that these new instruments would yield similar advantages in visualization compared with standard OCT, albeit with less axial image resolution.
In summary, the improved visualization of retinal layers obtained with hsUHR OCT may improve the clinical utility of this technology in the management of glaucoma.
Acknowledgments
Supported in part by the National Institutes of Health, Bethesda, Maryland (grant nos. R01-EY013178-7, RO1-EY11289-21, P30-EY008098); National Science Foundation, Arlington, Virginia (grant no. BES-0522845); Air Force Office of Scientific Research and Medical Free Electron Laser Program, Arlington, Virginia (contract no. FA9550-040-1-0046); and unrestricted grants from Research to Prevent Blindness, Inc., New York, New York, and the Eye and Ear Foundation, Pittsburgh, Pennsylvania.
Footnotes
Presented in part at: American Glaucoma Society Annual Meeting, March 2006, Charleston, South Carolina, and International Society for Imaging of the Eye Annual Meeting, May 2006, Ft. Lauderdale, Florida.
Drs Schuman and Fujimoto receive royalties for intellectual property licensed by Massachusetts Institute of Technology to Carl Zeiss Meditec.
References
- 1.Harwerth RS, Carter-Dawson L, Shen F, et al. Ganglion cell losses underlying visual field defects from experimental glaucoma. Invest Ophthalmol Vis Sci. 1999;40:2242–50. [PubMed] [Google Scholar]
- 2.Hee MR, Izatt JA, Swanson EA, et al. Optical coherence tomography of the human retina. Arch Ophthalmol. 1995;113:325–32. doi: 10.1001/archopht.1995.01100030081025. [DOI] [PubMed] [Google Scholar]
- 3.Puliafito CA, Hee MR, Lin CP, et al. Imaging of macular diseases with optical coherence tomography. Ophthalmology. 1995;102:217–29. doi: 10.1016/s0161-6420(95)31032-9. [DOI] [PubMed] [Google Scholar]
- 4.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178–81. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujimoto JG, Hee MR, Huang D, et al. Principals of optical coherence tomography. In: Schuman JS, Puliafito CA, Fujimoto JG, editors. Optical Coherence Tomography of Ocular Diseases. 2nd ed SLACK Inc.; Thorofare, NJ: 2004. pp. 3–8. [Google Scholar]
- 6.Schuman JS, Hee MR, Puliafito CA, et al. Quantification of nerve fiber layer thickness in normal and glaucomatous eyes using optical coherence tomography. Arch Ophthalmol. 1995;113:586–96. doi: 10.1001/archopht.1995.01100050054031. [DOI] [PubMed] [Google Scholar]
- 7.Wollstein G, Ishikawa H, Wang J, et al. Comparison of three optical coherence tomography scanning areas for detection of glaucomatous damage. Am J Ophthalmol. 2005;139:39–43. doi: 10.1016/j.ajo.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 8.Bowd C, Weinreb RN, Williams JM, Zangwill LM. The retinal nerve fiber layer thickness in ocular hypertensive, normal, and glaucomatous eyes with optical coherence tomography. Arch Ophthalmol. 2000;118:22–6. doi: 10.1001/archopht.118.1.22. [DOI] [PubMed] [Google Scholar]
- 9.Srinivasan VJ, Wojtkowski M, Witkin AJ, et al. High-definition and 3-dimensional imaging of macular pathologies with high-speed ultrahigh-resolution optical coherence tomography. Ophthalmology. 2006;113:2054–65. doi: 10.1016/j.ophtha.2006.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wojtkowski M, Leitgeb R, Kowalczyk A, et al. In vivo human retinal imaging by Fourier domain optical coherence tomography. J Biomed Opt. 2002;7:457–63. doi: 10.1117/1.1482379. [DOI] [PubMed] [Google Scholar]
- 11.Fercher AF, Hitzenberger CK, Kamp G, El-Zaiat SY. Measurement of intraocular distances by backscattering spectral interferometry. Opt Commun. 1995;117:43–8. [Google Scholar]
- 12.Nassif N, Cense B, Park BH, et al. In vivo human retinal imaging by ultrahigh-speed spectral domain optical coherence tomography. Opt Lett. 2004;29:480–2. doi: 10.1364/ol.29.000480. [DOI] [PubMed] [Google Scholar]
- 13.Wojtkowski M, Srinivasan V, Fujimoto JG, et al. Three-dimensional retinal imaging with high-speed ultrahigh-resolution optical coherence tomography. Ophthalmology. 2005;112:1734–46. doi: 10.1016/j.ophtha.2005.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drexler W, Sattmann H, Hermann B, et al. Enhanced visualization of macular pathology with the use of ultrahigh-resolution optical coherence tomography. Arch Ophthalmol. 2003;121:695–706. doi: 10.1001/archopht.121.5.695. [DOI] [PubMed] [Google Scholar]
- 15.Wollstein G, Paunescu LA, Ko TH, et al. Ultrahigh-resolution optical coherence tomography in glaucoma. Ophthalmology. 2005;112:229–37. doi: 10.1016/j.ophtha.2004.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drexler W, Morgner U, Ghanta RK, et al. Ultrahigh-resolution ophthalmic optical coherence tomography. Nat Med. 2001;7:502–7. doi: 10.1038/86589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wollstein G, Schuman JS, Price LL, et al. Optical coherence tomography (OCT) macular and peripapillary retinal nerve fiber layer measurements and automated visual fields. Am J Ophthalmol. 2004;138:218–25. doi: 10.1016/j.ajo.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 18.Ko TH, Fujimoto JG, Schuman JS, et al. Comparison of ultrahigh- and standard-resolution optical coherence tomography for imaging macular pathology. Ophthalmology. 2005;112:1922–35. doi: 10.1016/j.ophtha.2005.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sommer A, Katz J, Quigley HA, et al. Clinically detectable nerve fiber atrophy precedes the onset of glaucomatous field loss. Arch Ophthalmol. 1991;109:77–83. doi: 10.1001/archopht.1991.01080010079037. [DOI] [PubMed] [Google Scholar]