Abstract
This study examined the direct and interactive effects of stress reactivity and family adversity on socio-emotional and cognitive development in 338 five-to-six-year-old children. Neurobiological stress reactivity was measured as respiratory sinus arrhythmia and salivary cortisol responses to social, cognitive, sensory, and emotional challenges. Adaptation was assessed using child, parent, and teacher reports of externalizing symptoms, prosocial behaviors, school engagement, and academic competence. Results revealed significant interactions between reactivity and adversity. High stress reactivity was associated with more maladaptive outcomes in the context of high adversity but with better adaption in the context of low adversity. The findings corroborate a reconceptualization of stress reactivity as biological sensitivity to context by showing that high reactivity can both hinder and promote adaptive functioning.
A substantive body of work has established that environmental adversity can have a deleterious effect on children’s functioning (Luthar, 2006; Obradović, Shaffer, & Masten, in press; Rutter, 1983; Sameroff, 2006). Exposure to adverse, stressful events, such as marital conflict, maternal depression, and financial stress, has been linked to socio-emotional behavior problems and cognitive deficits (Boyce, 2007; Boyce et al., 2001; Burchinal, Roberts, Hooper, & Zeisel, 2000; Cummings & Davies, 2002; Essex, Klein, Cho, & Kalin, 2002; Masten & Shaffer, 2006). Nevertheless, not all children are equally susceptible to adverse environmental influences. In accordance with the ‘stress diathesis’ hypothesis, behaviorally or biologically reactive children have been traditionally identified as particularly vulnerable to stressful experiences, showing higher levels of behavioral and health problems than their low reactive peers in the context of environmental risk and adversity (Belsky, Hsieh, & Crnic, 1998; Cummings, El-Sheikh, Kouros, & Keller, 2007; El-Sheik, 2005; Deater-Deckard & Dodge, 1997; Ramos, Guerin, Gottfried, Bathurst, & Oliver, 2005). In recent years, however, a few researchers have challenged this traditional view, arguing that high reactivity, whether measured at the emotional, behavioral, or biological level, is not a unitary, pathogenic response to adversity that invariably leads to maladaptation. Belsky and colleagues (Belsky, Bakermans-Kranenburg, van IJzendoorn, 2007), for example, have posited that temperamentally reactive children may show higher susceptibility to environmental influences “for better and for worse.” That is, reactive children may be more vulnerable to contextual risk, but they may also show more adaptive responses to interventions (Blair, 2002; Klein Velderman, Bakermans-Kranenburg, Juffer, & van IJzendoorn, 2006). Similarly, Boyce and colleagues have proposed a new theory suggesting that stress reactivity is better conceptualized as a high biological sensitivity to context (Boyce, 2007; Boyce & Ellis, 2005). From this theoretical perspective, children with heightened biological sensitivity to context are predicted to be more vulnerable to negative and stressful contextual factors, but also to have greater capacity to benefit from positive environmental influences. Thus, high biological sensitivity may be maladaptive in the context of adversity but adaptive in the context of a nurturing and supportive environment (Boyce, 1996; Boyce et al., 1995, 2006; Ellis, Essex, & Boyce, 2005). This work underlines the importance of understanding more fully the biological processes that interact with environmental influences to shape children’s adaptation, as indexed by competence and psychopathology (Curtis & Cicchetti, 2003; Masten & Obradović, 2006). The current study was designed to examine how early exposure to family adversity interacts with children’s stress reactivity to predict indices of socio-emotional behavior, school engagement, and academic competence.
Human Stress Response
Every day, in all human societies, children are exposed to challenging or stressful events from multiple sources. These events range from normative, potentially positive events, such as forming a new peer group, to adverse events, such as witnessing marital conflict, and truly traumatic events that directly threaten children’s well-being, such as abuse and neglect. Children respond to these stressors with a set of highly integrated, neurobiological stress responses. A “fight-or-flight” response activates the autonomic nervous system (ANS), which initiates, within seconds, an integrated, short-onset repertoire of biobehavioral changes associated with accelerations of heart and respiratory rates, sweat production, and other physiological changes. The ANS comprises both a sympathetic (SNS) branch, which initiates physiological arousal, and a parasympathetic (PNS) branch, which modulates SNS input to the heart and other target organs, regulating recovery and restoring autonomic homeostasis (Berntson, Cacioppo & Quigley, 1993). In a lagged response occurring within minutes, activation of the hypothalamic-pituitary-adrenocortical (HPA) axis via glucocorticoid secretion regulates glucose metabolism, blood pressure, and immunity and counterbalances the effects of the integrated ANS stress response (Sapolsky, Romero, & Munck, 2000). There are numerous ways of assessing children’s stress reactivity. Because of our interest in whether stress reactivity moderates the effects of adversity on indices of psychopathology and competence, we examined two indicators of stress reactivity that are known to play a role in regulating stress-induced arousal and maintaining homeostasis: (1) respiratory sinus arrhythmia (RSA), and (2) salivary cortisol.
Respiratory Sinus Arrhythmia
RSA is a measure of parasympathetic stress response and refers to high frequency heart rate variation controlled by efferent fibers of the vagus nerve during the respiratory cycle. Vagal regulation, in the form of increases and decreases in RSA, has been regarded as an index of children’s capacity to regulate responses to positive and negative environmental demands (Beauchaine, 2001; Beauchaine, Gatzke-Kopp, & Mead, 2007; Porges, 2001, 2003, 2007). High levels of basal RSA have been associated with social competence, empathy, and emotion regulation (Beauchaine, 2001; Eisenberg et al., 1995; Fabes, Eisenberg, & Eisenbud, 1993; Fox & Field, 1989). Low basal RSA, on the other hand, may indicate emotional lability and dysregulation and has been linked to behavior problems in at-risk or clinical samples (Beauchaine, 2001; Field et al., 1996; Beauchaine et al. 2007; Mezzacappa et al., 1997; Pine et al., 1996, 1998). However, it is important to note that recent studies of community, non-clinical samples have found a positive relation, or no significant relation, between resting RSA and externalizing symptoms (Calkins, Graziano, & Keane, 2007; Dietrich et al., 2007).
Further, researchers have examined changes in RSA in response to stressful laboratory challenges. High reactivity, as indexed by decreases in RSA from basal levels (i.e. greater vagal withdrawal), has been associated with more sustained attention, better emotion regulation, and increased engagement during challenging tasks (Calkins, 1997; Suess, Porges, & Plude, 1994). However, in community samples of kindergarten children, higher RSA reactivity to various challenge tasks has been linked to seemingly contradictory indices of adaptation, such as high levels of sociability (Doussard-Roosevelt, Montogomery, & Porges, 2003) and internalizing symptoms (Boyce et al., 2001). Some of the inconsistencies across studies might be explained by the nature of the sample. For example, low RSA reactivity has been associated with externalizing symptoms in normative samples of young children (Boyce et al., 2001; Calkins et al., 2007), whereas high RSA reactivity in response to emotional stimuli has been observed in children with clinical levels of behavior problems (Crowell et al. 2005).
Only a few studies have examined the effect of RSA in the context of both high and low adversity. Several studies of community samples indicate that high basal RSA levels and high RSA reactivity in response to emotion-evoking stimuli (e.g., an adult argument) buffered children from the effects of marital conflict on behavior problems, academic achievement, and health (El-Sheikh, Harger, & Whitson, 2001; El-Sheikh & Whitson, 2006; Katz & Gottman, 1995, 1997). Only children with low baseline RSA and RSA reactivity were at risk for maladaptation in the context of high marital conflict. However, these findings varied across gender, type of marital conflict (e.g., verbal, physical), and children’s outcomes. Similarly, high RSA reactivity to interpersonal stress emerged as a protective factor against the effects of hostile-withdrawn parenting on peer conflict, but not on positive indices of peer interactions (Leary & Katz, 2004). In contrast, in a clinical sample, parental psychopathology had a negative effect on children’s emotional and behavioral problems only in children with high baseline RSA (Shannon, Beauchaine, Brenner, Neuhaus, & Gatzke-Kopp, 2007). Studies examining other indices of ANS stress reactivity (i.e. heart rate, blood pressure, skin conductance) in community samples also indicate that children with high stress reactivity may be at risk for social, cognitive, behavioral, and health problems in the context of high adversity, but may show better developmental outcomes in the context of low adversity (Boyce, 1996; Boyce et al., 1995, 2006; Cummings et al., 2007; El-Sheik, 2005).
In sum, the effect of stress reactivity on adaptation seems to be context-dependent, but the directions of reactivity effects across different contexts remain unclear. Further studies are needed to elucidate the role of RSA reactivity in the development of competence and psychopathology, bearing in mind the limitations of previous research. First, many existing studies of RSA reactivity have important methodological flaws, such as a lack of appropriate controls for respiratory rate, attention and motor activity (Beauchaine, 2001). Second, RSA reactivity has been examined mostly in relation to a single challenge, potentially misrepresenting the comprehensive reactivity to various types of challenges that children may encounter in their daily lives. Third, studies have often focused on a single source of adversity, such as marital conflict, yet children face various kinds of adversities in family environments. Fourth, studies of stress reactivity in general have been largely focused on measures of behavioral and health problems, to the exclusion of more positive and prosocial indicators of adaptation.
Cortisol and Adaptive Functioning
Cortisol is the human glucocorticoid hormone secreted by the adrenal glands and passively diffuses into saliva. Salivary cortisol reflects the levels of unbound and biologically active cortisol circulating in the blood. As with RSA, researchers have linked individual differences in both basal and reactive cortisol expression primarily to indices of behavior problems (Gunnar & Vazquez, 2006). In general, in both clinical and community samples, elevated daily cortisol levels have been associated with internalizing symptoms (Goodyer, Herbert, & Althan, 1998; Klimes-Dougan, Hastings, Granger, Usher, & Zahn-Waxler, 2001), whereas lower levels of daily cortisol have been associated with externalizing symptoms (Hardie, Moss, Vanyukov, Yao, & Kirillovac, 2002; King, Barkley, & Barrett, 1998; McBurnett et al., 1991; McBurnett, Lahey, Rathouz, & Loeber, 2000; Moss, Vanyukov, & Moss, 1995; Shirtcliff, Granger, Booth, & Johnson, 2005). However, in community samples of kindergarten children, basal cortisol secretion was positively related to internalizing symptoms, social wariness, and symptom severity (Essex et al., 2002; Smider et al., 2002).
A few studies have examined cortisol reactivity to specific laboratory challenges. Moderate elevation of cortisol in response to cognitive tasks has been associated with better executive functioning and self-regulation skills in both middle- and low-income samples (Blair, Granger, & Razza, 2005; Davis, Bruce, & Gunnar, 2002). In addition, high cortisol reactivity in response to parent-child conflict tasks has been associated with social problems and internalizing symptoms in clinical samples (Granger, Weisz, McCracken, Ikeda, & Douglas, 1996; Granger, Weisz, & Kauneckis, 1994). However, differences in sample characteristics and stimuli across these studies preclude any conclusions regarding the relation between cortisol reactivity to different challenges and adaptive functioning.
Studies examining cortisol changes over a day in childcare or preschool in community samples have linked elevated cortisol to impulsivity, poor effortful control, peer rejection, and aggression (Dettling, Gunnar, & Donzella, 1999; Dettling, Parker, Lane, Sebanc, & Gunnar, 2000; Gunnar, Sebanc, Tout, Donzella, & van Dulmen, 2003). Similarly, elevated cortisol in response to starting a new school year has been associated with negative affectivity and surgency/extraversion (Davis, Donzella, Krueger, & Gunnar, 1999). However, a more complex picture emerged from a study that measured children’s cortisol response during the first weeks of school, when children forge new peer groups and connections, and again later in the school year, when peer groups have been fully formed (Gunnar, Tout, de Haan, Pierce, & Stansbury, 1997). Children showing elevated cortisol expression during the first weeks of school and normal levels later in the year were rated as extraverted, socially competent, and outgoing, whereas children who showed high levels of cortisol later in the school year showed more solitary and negative behaviors and were seen as less competent and less outgoing.
Although these studies indicate that the relation between cortisol reactivity and adaptation may be context-dependent and may vary with levels of contextual stress, there is a paucity of studies examining the interactive effects of cortisol and adversity on adaptation. We are aware of only one such study, which shows that cortisol reactivity is related to symptom severity only for children with low father involvement in infancy (Boyce et al., 2006). Moreover, studies examining cortisol response to various age-appropriate stressors and in the context of overall family adversity are rare. Thus, it is unknown whether cortisol reactivity may diminish or amplify the effects of adversity on children’s adaptation. In order to better understand the regulatory function of cortisol, researchers need to examine whether cortisol reactivity interacts with contextual factors in predicting both positive and negative developmental outcomes.
Current Study
The main goal of this study was to investigate how the interplay between children’s stress reactivity and overall family adversity influences adaptation. We focused our study on kindergarten children, because understanding processes that subserve adaptation during the salient developmental transition to a school setting is very important, given the reverberatory effects that early behavior problems or academic failure can have on later developmental trajectories. Consequently, we examined adaptation across four domains of functioning known to contribute to early school success and later adjustment: (1) externalizing symptoms, (2) prosocial behaviors toward peers, (3) school engagement, and (4) academic competence.
Based on the broad literature on risk and adversity, we hypothesized a robust negative main effect of family adversity across all indices of adaptation. We also expected to find main effects of stress reactivity on adaptation, but given the simultaneous test of interactive effects, as well as the paucity of studies examining the effects of RSA and cortisol reactivity, especially for positive developmental outcomes, and some inconsistencies within such studies, we did not hypothesize the directions of these main effects. More importantly, in accordance with the theory of biological sensitivity to context (Boyce & Ellis, 2005; Ellis et al., 2005), we expected to find evidence that ANS and HPA reactivity moderate the effects of early family adversity on various domains of functioning. We hypothesized that in high adversity family environments, elevated levels of stress reactivity would be associated with maladaptive outcomes, whereas low stress reactivity would act as a protective factor. In the context of low family adversity, on the other hand, we expected high levels of reactivity to be associated with better adaptation. It is important to note that although biological sensitivity to context should be examined in both positive and negative settings, our assessment focuses on six types of family adversities, and a lack of overall family adversity does not necessarily imply a supportive and nurturing environment. In addition to the hypothesized adversity by stress reactivity interactions, we controlled for children’s sex and tested whether main and interactive effects of adversity and reactivity vary across sex.
Method
Participants
The sample included 338 children (163 females, 175 males) who participated in a longitudinal study of social status, biological responses to adversity, and child mental and physical health. Participants were recruited in three waves from 29 kindergarten classrooms within 6 public schools in the San Francisco Bay Area (Oakland, Albany, and Piedmont Unified school districts) during the falls of 2003, 2004, and 2005. Schools were selected to represent a variety of socio-demographic and ethnic characteristics of the metropolitan area. Families were recruited through a series of efforts that included home mailings, presentations at kindergarten parent welcome nights, and in-person recruitment during drop-off and pick-up. Every family with a child in the target classrooms was invited to participate. However, families who were not fluent in either English or Spanish were excluded to ensure adequate comprehension of study questionnaires. Schools were provided with $20 per child enrolled in the study.
The mean age of the children at the kindergarten entry was 5.32 years (SD = .32; range: 4.75–6.28). The sample was ethnically diverse, and children were identified as 19% African American, 11% Asian, 43% European or White, 4% Latino, 22% Multi-ethnic, and 2% as “other” ethnicity. Family demographic information was not provided by 16 families. Primary caregivers were 87% biological mothers, 9% biological fathers, 2.5% adoptive mothers, 0.6% biological grandmothers, and 0.9% “other” relations. As the vast majority of primary caregivers were parents, these terms are used interchangeably here. Average annual household income ranged from < $10,000 to > $400,000 (M = $60–79,999; Mdn = $80–99,999). Highest level of educational attainment in the household ranged from less than a high school diploma to advanced degrees, with 75% of caregivers having at least a college degree.
Procedures
Data for this study were collected in the fall (time 1) and spring (time 2) during the kindergarten year. Parents’ informed consent and children’s assent were obtained prior to the start of data collection. Parent report of family adversity and children’s functioning was collected through a series of home mailings, and families were compensated with $50 for each completed time point. Teacher report of children’s functioning was collected through questionnaires dropped off and picked up at the child’s school, and teachers were compensated $15 per child for each report returned. Self report of children’s functioning was collected through a series of structured, scripted interviews conducted in a separate, quiet room at elementary schools.
In addition, children participated in the 20-minute reactivity protocol, which included four age-appropriate tasks designed to elicit stress responses to social, cognitive, sensory, and emotional challenges (Boyce et al., 1995; Alkon et al., 2003). Since PNS can be activated by psychomotor activity, such as gesturing, speaking, focused attending, and non-challenging social engagement (Bazhenova & Porges, 1997; Berntson, Cacioppo, & Fieldstone, 1996; Bernardi et al., 2000; Porges, 2001; Porges et al., 2007), RSA assessed during a challenge task reflects both stress reactivity in response to the challenge and reactivity in response to the psychomotor or engagement demands of the challenge task. Recent literature has emphasized the importance of maximizing the mobilization of the psychological response to the particular challenge and minimizing the peripheral triggers of cardiovascular activation (Bush, Alkon, Obradović, Stamperdahl, & Boyce, 2009; Kamarck & Lovallo, 2003). Thus, in order to best measure the psychophysiological response to the four challenges, each challenge task in the reactivity protocol was preceded by a non-challenging “control task” that paralleled the motor and engagement demands of that challenge and levels of autonomic arousal during the control tasks were used as baseline values.
The autonomic reactivity protocol started with an experimenter reading a calming short story (2 minutes). Next, the child completed four sets of paired tasks, each consisting of a control condition and challenge condition. The social challenge task (2 minutes) was a structured interview about the child’s family, friends, and likes/dislikes adapted from the Gesell School Readiness Screening Test (Carlson, 1985). This was preceded by the social control task in which children were asked to name common animals and colors from a picture book to capture arousal associated with speaking, gestures during social speech, and attention involved in social engagement (2 minutes). The cognitive challenge task (2 minutes) was a digit span recitation task derived from the Kaufman Assessment Battery for Children (Kaufman & Kaufman, 1983) in which children were asked to recall sequences of numbers up to six digits in length and received negative verbal feedback after making a mistake. This was preceded by the cognitive control task in which children were asked to repeat simple, one or two-digit number sequences to capture arousal associated with listening, speaking numbers, and social engagement (1 minute). The sensory challenge task (1 minute) was a taste-identification task (Kagan & Snidman, 1991) during which the child was asked to identify two drops of concentrated lemon juice placed on the tongue. This was preceded by the sensory control task in which children were asked to identify two drops of water placed on their tongue to capture arousal associated with mouth opening and swallowing, anticipation, and guessing the content of the liquid (1 minute). The emotional challenge task (2 minutes) consisted of watching a short emotion-evoking movie clip chosen to elicit fear (Eisenberg et al., 1988). This was preceded by the emotion-control task in which children were asked to watch an emotionally-neutral movie clip to capture physiological responding associated with attending to visual stimuli (2 minutes). The autonomic reactivity protocol concluded with the reading of another calming story (2 minutes).
Measures
Stress Reactivity
Children’s stress reactivity was assessed using changes in both respiratory sinus arrhythmia (RSA) and salivary cortisol during the stress reactivity protocol. After the child was familiarized with the equipment, four spot electrodes (two current, two impedance) were placed in the standard tetrapolar configuration on the child’s neck and chest, and ECG electrodes were placed on the right clavicle and lower left rib. A 4 mAmp AC current at 100 kHz was passed through the two current electrodes. RSA was monitored continuously during the protocol. Data were acquired using the Biopac MP150 (Biopac Systems, Santa Barbara, CA) interfaced to a PC-based computer. Analog data were continuously monitored on the computer for signal and noise, and digitized data were stored for off-line analysis. RSA was derived in accordance with recommendations of the Society for Psychophysiological Research committee on heart rate variability (Berntson et al., 1997). The sampling frequency was 1 kHz. Prior to analyses, each waveform was verified, IBIs were visually checked, and artifacts were identified using Berntson et al.’s (1990) algorithm within the MindWare software program (www.mindwaretech.com). RSA was estimated as the natural logarithm of the variance of heart period within the high frequency bandpass associated with respiration at this age (i.e., 0.15 to 0.80 Hz) (Bar-Haim, Marshall, & Fox, 2000; Rudolph, Rudolph, Hostetter, Lister, & Siegel, 2003). Outlier data were checked and verified minute-by-minute if they were > 3 SD from the group mean. Mean RSA magnitude was calculated for each 1 minute interval and averaged within each task (Cacioppo, Uchino, & Bernston, 1994).
RSA reactivity responses during each of the control tasks were used as baseline reference values to create four task RSA reactivity scores. To control for the influence of baseline levels of arousal, we created four standardized residual scores as measures of reactivity to social, cognitive, sensory, and emotional challenges by regressing RSA values during the challenge tasks on the respective control tasks RSA values. Residuals reflect the extent to which an individual’s physiologic response deviated from the regression line derived from sample responses (Manuck, Kasprowicz, & Muldoon, 1990). The four standardized residual scores were then averaged to create one index of RSA stress reactivity. Elevated RSA reactivity was represented by negative residual scores, indicating a decrease in RSA and vagal withdrawal in response to a given challenge. Conversely, low reactivity was represented by positive residual scores, indicating an increase in RSA and vagal activation in response to the challenge.
Descriptive statistics for RSA responses to tasks are presented in Table 1. Average RSA differences between challenge and control tasks were −.25 (SD = .66) for the social challenge, .60 (SD = .67) for the cognitive challenge, −.32 (SD = .84) for the sensory challenge, and .10 (SD = .47) for the emotional challenge task. RSA reactivity levels were significantly different from zero during the social (t = −6.99, p < .001), cognitive (t = 16.40, p < .001), sensory (t = −7.05, p < .001) and emotional tasks (t = 4.04, p < .001). Averaged across the 4 challenge tasks, 47.1% of the sample showed a decrease in RSA from the levels elicited by the control tasks, suggesting that about half of the sample showed vagal withdrawal in response to challenges. Thus, the reactivity protocol effectively captured variability in child RSA responses to challenges.
Table 1.
Descriptive Statistics for Stress Reactivity and Adversity Variables
| Variable | Scale | Min | Max | M | SD |
|---|---|---|---|---|---|
| RSA | |||||
| Social control | 3.29 | 9.65 | 6.81 | 1.12 | |
| Social challenge | 3.47 | 10.26 | 6.56 | 1.10 | |
| Cognitive control | 2.62 | 9.69 | 6.12 | 1.20 | |
| Cognitive challenge | 3.35 | 9.82 | 6.72 | 1.10 | |
| Sensory control | 2.77 | 9.91 | 6.98 | 1.13 | |
| Sensory challenge | 3.04 | 10.90 | 6.66 | 1.20 | |
| Emotional control | 2.74 | 10.15 | 7.03 | 1.12 | |
| Emotional challenge | 2.48 | 10.17 | 7.13 | 1.10 | |
| Cortisol | |||||
| Pre-protocol | 0.10 | 24.40 | 5.10 | 3.51 | |
| Post-protocol | 0.10 | 30.50 | 4.70 | 3.93 | |
| Adversity | |||||
| Financial Stress | 1–5 | 1.00 | 5.00 | 2.42 | 0.93 |
| Parenting Overload | 1–5 | 1.20 | 5.00 | 3.12 | 0.68 |
| Marital Conflict | 1–5 | 1.00 | 3.10 | 1.74 | 0.38 |
| Family Expressiveness | 1–9 | 1.20 | 7.20 | 4.03 | 0.99 |
| Anger Expression | 0–10 | 0.25 | 6.13 | 2.42 | 0.84 |
| Harsh/Restrictive Parenting | 1–7 | 1.76 | 6.56 | 3.66 | 0.75 |
At the beginning and end of the reactivity protocol, salivary cortisol was collected by instructing the child to chew on a cotton roll for 20 to 30 seconds. Wet cotton rolls were deposited in salivette tubes and stored at −7°C until assayed. The cortisol samples were assayed using a commercial immunoassay with chemiluminescence detection (Cortisol Luminescence Immunoassay; IBL-Hamburg, Hamburg, Germany). The detection limit of the assay was 0.41 nmol/L. The mean interassay and intraassay variations were 8.5% and 6.1%, respectively. Cortisol values above 55 nmol/l (less than 1% of samples) were considered unreliable data and were discarded. Ten children in this sample were taking medications, such as human growth hormone and exogenous glucocorticoids, known to alter salivary cortisol levels (Masharani et al., 2005). These children were excluded from analyses of cortisol reactivity. Given that cortisol levels in saliva reach their peak approximately 15 to 20 minutes following stressor onset, cortisol values collected at the beginning of the session were considered baseline reference values, as they indexed children’s cortisol expression in the familiar context of the kindergarten classroom. The average session lasted 27 minutes (SD = 3 minutes, Range: 19 – 38 minutes). Thus, cortisol values collected at the end of the session were considered a measure of reactivity to a novel, mildly stressful context (e.g., strange experimenter, electrodes, challenge tasks), at the midpoint of the reactivity protocol. Descriptive statistics for pre- and post-protocol values are presented in Table 1. We created standardized residual scores by regressing post-protocol cortisol values on pre-protocol, baseline values. Because cortisol collection occurred both in the morning and in the afternoon, we examined whether cortisol reactivity varied across the day, due to the known circadian activity of the HPA system. The time of collection was related to absolute pre-protocol values (r = .18, p < .01) but not to post-protocol values and cortisol reactivity as indexed by standardized residual scores, which is consistent with current literature (Kudielka, Schommer, Hellhammer, & Kirschbaum, 2004). Length of the session also did not significantly relate to cortisol reactivity. Mean cortisol levels pre- and post-protocol were 5.10 nmol/l (SD = 3.51) and 4.70 nmol/l (SD = 3.93), respectively. Mean difference between pre- and post-protocol values was −.40 nmol/l (SD = 3.81). Positive cortisol reactivity values indicate a stress response, and 37.5% of the sample showed such a response to the challenge protocol. The average difference between pre- and post-protocol values for the responder group was 2.58 nmol/l (SD = 3.86).
Adversity
Children’s exposure to adversity was assessed by six indices of potential sources of family stress. All measures of adversity were based on parent report, and reliability statistics are reported for the current sample. Financial Stress was assessed with four items derived from Essex et al. (2002) that measured parents’ thoughts about money problems, difficulty paying bills, and limited opportunities due to lack of finances (α = .81) Parenting Overload was assessed with five items derived from Essex et al. that measured feelings of being overwhelmed with parenting duties, juggling conflicting obligations, and lacking time to rest or pursue desired activities (α = .79). Marital Conflict was assessed using the 10-item O’Leary-Porter Overt Hostility Scale (α = .72) that measured how often parents openly argue, display physical and verbal hostility, and criticize each other in the presence of their children (Johnson & O’Leary, 1987; Porter & O’Leary, 1980). Exposure to Negative/Anger Expressiveness in the family was assessed using both the Family Expressiveness Questionnaire (FAQ; Halberstadt, 1986) and the Anger Expression Inventory (AEI; Spielberger, 1988). The FAQ consists of a 10-item negative dominant subscale (α = .83), measuring the frequency of overt anger, contempt, and hostility among family members, and a 10-item negative subdominant subscale (α = .75), measuring the frequency of passive sulking, crying, and disappointment among family members. The two FAQ subscales were averaged (r = .55, p <.001) to yield one measure of negative family expressiveness. The total AEI score was calculated using three 8-item subscales that assessed parents’ tendency to express overtly towards other people (α = .69), hold angry feelings inside (α = .68), and control the experience and expression of anger (α = .74). The overall scores based on FAQ and AEI were standardized and averaged (r = .47, p <.001) into one indicator of exposure to negative/anger expressiveness. Maternal Depression was assessed with a 20-item Center for Epidemiological Studies-Depression Scale (CES-D; Radloff, 1977) (α = .81). Harsh and Restrictive Parenting was assessed using a questionnaire version of Child-Rearing Practice Report (CRPR, Block, 1965). The 18-item scale (α = .83) was based on previous studies (Deković, Janssens, & Gerris, 1991; Rickel & Biasatti, 1982). Descriptive statistics for adversity measures are shown in Table 1. As we were interested in capturing children’s overall exposure to family adversity, the six indices of adversity were standardized and composited into one adversity index. Although a few adversity measures included positive items, which provided some assessment of positive family environment (e.g., financial security, parental affection, parent who is happy, has time to relax, shows patience, and controls anger), it is important to note that, low levels of adversity on this measure do not necessarily indicate a supportive family environment.
Adaptive Functioning
Adaptive functioning was assessed during the fall and spring of the kindergarten year using parent-, teacher-, and child-reported measures. For the purposes of this study, we focused on four developmentally salient domains—externalizing symptoms, prosocial behaviors, school engagement, and academic competence—which are described in more detail below. Parent and teacher perceptions of the child’s behaviors and skills across these domains were assessed using The MacArthur Health and Behavior Questionnaire (HBQ; Armstrong, Goldstein, & The MacArthur Working Group on Outcome Assessment, 2003). Parent and teacher forms of the HBQ contain parallel scales, described in detail below. Children’s perceptions of their own behavior and skills across four domains were assessed using the Berkeley Puppet Interview, which is designed to elicit children’s responses to statements that parallel the parent and teacher HBQ scales (BPI; Ablow, Measelle, & The MacArthur Working Group on Outcome Assessment, 2003). The BPI combines both structured and clinical interviewing techniques and allows young children to respond either verbally or non-verbally to opposing statements issued by two puppets about their behavior. Interviewers were extensively trained to probe for more information, to deal with unclear, inaudible, indecisive, or alternate responses, and to create a comfortable, engaging, and interactive environment. Videotaped responses were coded using a 7-point scale based on the statement the child chose to endorse and the degree of endorsement. Interclass correlation coefficients (ICC) were calculated based on 35% of double-coded fall BPIs (ICC range: .91–.98) and 29% of double-coded spring BPIs (ICC range: .92–.99), indicating excellent inter-rater reliability. Individual items were averaged into subscales and scales according to the BPI manual. The validity and reliability of HBQ and BPI measures have been tested with children ages 5–6, and all three measures show good measurement properties (for details see Ablow et al., 2003; Armstrong et al., 2003).
All three measures contained the same scales. Externalizing Symptoms were assessed using an Oppositional Defiant scale (e.g., argues, angry, resentful, misbehaves), Conduct Problem scale (e.g., lies, vandalizes, threatens, cheats), and an Overt Hostility scale (e.g., teases, fights, kicks, taunts). Preliminary analyses revealed that indices of stress reactivity were related differently to a fourth Relational Aggression scale in comparison to the other three scales of externalizing symptoms. We believe that this finding deserves further investigation outside the scope of this study; we thus omitted the Relational Aggression scale from our measure of Externalizing Symptoms. Prosocial Behaviors were assessed using a scale measuring the child’s predisposition to help others, share, invite bystanders to play, and be considerate, collaborative, fair and empathic. School Engagement was assessed using a scale measuring the extent to which the child was excited, eager, happy, frustrated, bored, or irritable about school. Academic Competence was assessed with a scale indexing the child’s achievement, innate ability, and difficulties in literacy and math, as well as the child’s overall academic performance in the classroom in comparison to other classmates. The sample’s descriptive and reliability statistics for scales and domains across three informants and two time periods are presented in Table 2.
Table 2.
Adaptive Functioning Measures
| Domains/Scales | Fall | Spring | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| scale | # items | M | SD | α | λ | M | SD | α | λ | |
| Externalizing Behavior Problems | ||||||||||
| P-HBQ | 0.29 | 0.21 | .78 | .71 | 0.30 | 0.23 | .80 | .75 | ||
| Oppositional Defiant | 0–2 | 9 | 0.44 | 0.31 | .77 | 0.46 | 0.33 | .80 | ||
| Conduct Problems | 0–2 | 12 | 0.13 | 0.16 | .70 | 0.14 | 0.18 | .77 | ||
| Overt Hostility | 0–2 | 4 | 0.27 | 0.27 | .56 | 0.28 | 0.30 | .66 | ||
| T-HBQ | 0.19 | 0.30 | .89 | .71 | 0.26 | 0.36 | .91 | .76 | ||
| Oppositional Defiant | 0–2 | 9 | 0.21 | 0.33 | .88 | 0.29 | 0.41 | .92 | ||
| Conduct Problems | 0–2 | 11 | 0.10 | 0.22 | .73 | 0.14 | 0.26 | .88 | ||
| Overt Hostility | 0–2 | 4 | 0.25 | 0.41 | .82 | 0.33 | 0.47 | .85 | ||
| C-BPI | 2.40 | 0.55 | .81 | .67 | 2.36 | 0.51 | .79 | .73 | ||
| Oppositional Defiant | 1–7 | 6 | 2.46 | 0.64 | .49 | 2.41 | 0.63 | .53 | ||
| Conduct Problems | 1–7 | 9 | 2.39 | 0.60 | .65 | 2.35 | 0.55 | .65 | ||
| Overt Hostility | 1–7 | 7 | 2.32 | 0.65 | .72 | 2.30 | 0.64 | .69 | ||
| Prosocial Behaviors | ||||||||||
| P-HBQ | 0–2 | 20 | 1.31 | 0.31 | .86 | .68 | 1.29 | 0.32 | .88 | .70 |
| T-HBQ | 0–2 | 20 | 1.22 | 0.48 | .95 | .64 | 1.25 | 0.51 | .95 | .80 |
| C-BPI | 1–7 | 7 | 5.34 | 0.83 | .64 | .74 | 5.42 | 0.79 | .67 | .59 |
| School Engagement | ||||||||||
| P-HBQ | 1–4 | 8 | 3.73 | 0.35 | .83 | .65 | 3.65 | 0.42 | .87 | .75 |
| T-HBQ | 0–2 | 8 | 1.80 | 0.28 | .81 | .68 | 1.78 | 0.30 | .84 | .65 |
| C-BPI | 1–7 | 8 | 5.24 | 1.03 | .80 | .76 | 5.27 | 0.93 | .77 | .71 |
| Academic Competence | ||||||||||
| P-HBQ | 1–7 | 8 | 5.01 | 1.03 | .92 | .85 | 5.19 | 1.03 | .93 | .89 |
| T-HBQ | 1–5 | 5 | 3.34 | 0.80 | .95 | .75 | 3.58 | 0.92 | .97 | .86 |
| C-BPI | 1–7 | 12 | 5.13 | 0.80 | .71 | .53 | 5.16 | 0.75 | .73 | .52 |
Notes. λ = factor loading; P-HBQ = Parent report on Health and Behavioral Questionnaire; T-HBQ = Teacher report on Health and Behavior Questionnaire; C-BPI = Child report on Berkeley Puppet Interview.
Using HBQ and BPI data, we conducted eight Principal-Component Analyses (PCA) to obtain multi-informant indices of externalizing symptoms, prosocial behaviors, school engagement, and academic competence, separately for the fall and spring assessments. We used a procedure described by Kraemer et al. (2003) for integrating data from multiple informants by triangulating three potentially orthogonal sources of information (i.e. parent, teacher, child). Following this approach, we used parent, teacher, and child report to extract three components for each PCA that conceptually correspond to the following dimensions: (1) trait (i.e. individual differences in adaptation), (2) perspective (i.e. informant characteristics that influence the assessment of the trait), and (3) context (i.e. environmental features that influences expression of the trait). For the purposes of this study, we used only factor scores based on the “trait” component, which weighs each source of information in the same direction, as an overall core measure of each adaptive domain. Thus, each adaptive domain (e.g., externalizing) is measured by standardized factor scores that represent the common variance across three reports of the domain and are largely free of informant and contextual effects. Table 2 shows PCA loadings for the first (trait) component of each adaptation domain in fall and spring.
Data Preparation and Analytic Procedures
Missing data for the six adversity components, the BPI and HBQ scales, pre- and post-protocol cortisol values, and RSA values to the four challenge and four control tasks were handled using the recommended maximum likelihood estimation procedure for missing data, specifically the expectation-maximization (EM) algorithm (Schafer & Graham, 2002). Percentages of missing data were as follows: fall teacher report (0.0%), fall child report (0.9–1.5%), fall parent report (13.3–13.9%), spring teacher report (3.0%), spring child report (3.6–4.4%), spring parent report (16.9–17.5%), adversity components (11.8–13.0%), RSA values (3.6–4.7%), and cortisol values (7.7–9.8%). Extreme values on standardized final composites were truncated to −3.50 or 3.50 standardized values. All analyses were conducted using the SPSS 15.0 program. All hierarchical multiple regression analyses were conducted controlling for the child’s sex and age at kindergarten entry.
Results
Bivariate Correlations
Bivariate correlations among key variables included in this study are presented in Table 3. Age at kindergarten entry was not related to indices of stress reactivity, adversity, or adaptation, and thus was not included in further analyses. Boys in this sample were older than girls, experienced higher levels of adversity, and showed poorer adaptation across all domains. RSA reactivity was significantly related only to fall externalizing, with low reactive children exhibiting higher levels of symptoms. Cortisol reactivity was significantly related to fall externalizing symptoms and to both fall and spring school engagement, with high reactive children exhibiting higher levels of symptoms and lower levels of engagement. Adversity exposure was related to higher externalizing symptoms and lower school engagement across both time points, as well as to lower prosocial behaviors in spring. Lastly, all domains of adaptation showed significant within-time correlations with other domains, as well as significant longitudinal stability from fall to spring.
Table 3.
Bivariate Correlations among Age, Gender, Stress Reactivity and Adaptive Functioning
| 1 Age | 2 Gender | 3 RSA | 4 CORT | 5 ADV | 6 EXT-F | 7 PRO-F | 8 SE-F | 9 ACA-F | 10 EXT-S | 11 PRO-S | 12 SE-S | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 Age | ---- | |||||||||||
| 2 Gender | −.16** | ---- | ||||||||||
| 3 RSA | .02 | .00 | ---- | |||||||||
| 4 CORT | −.06 | .00 | −.02 | ---- | ||||||||
| 5 ADV | .02 | −.15** | .05 | −.02 | ---- | |||||||
| 6 EXT-F | .05 | −.27*** | .12* | .14* | .29*** | ---- | ||||||
| 7 PRO-F | .00 | .35*** | .06 | −.03 | −.09 | −.50*** | ---- | |||||
| 8 SE-F | .00 | .21*** | −.04 | −.13* | −.25*** | −.52*** | .45*** | ---- | ||||
| 9 ACA-F | .00 | .14* | −.07 | −.09 | −.02 | −.17** | .38*** | .35*** | ---- | |||
| 19 EXT-S | .07 | −.31*** | .08 | .10 | .31*** | .75*** | −.46*** | −.46*** | −.14** | ---- | ||
| 11 PRO-S | −.01 | .39*** | .07 | −.06 | −.19*** | −.48*** | .71*** | .43*** | .24*** | −.61*** | ---- | |
| 12 SE-S | .03 | .19*** | .00 | −.14* | −.26*** | −.47*** | .44*** | .59*** | .22*** | −.55*** | .54*** | ---- |
| 13 ACA-S | .04 | .11* | −.05 | −.09 | .01 | .13* | .31*** | .28*** | .74*** | −.16** | .28*** | .30*** |
Notes. p < .05;
p < .01;
p < .001. RSA = RSA reactivity; CORT = Cortisol reactivity; -F = Fall; -S = Spring; EXT = Externalizing symptoms; PRO = Prosocial behavior; SE = School Engagement; ACA = Academic Competence.
Regression Analyses
The main goal of the regression analyses was to examine the interactive effects of adversity and stress reactivity on indices of adaptive functioning. While we also tested the main effects of family adversity and stress reactivity on adaptation, these direct effects can only be interpreted in the context of the significant interactive effects that qualify them. For each measure of stress reactivity (i.e. RSA and cortisol), two sets of regression analyses were conducted to predict (1) concurrent fall adaptation, and (2) longitudinal change in adaptation by predicting spring adaptation while controlling for fall adaptation. Interactive effects were tested following the procedure outlined by Baron and Kenny (1986). Significant interactions were further investigated using the simple slopes technique proposed by Aiken and West (1991) by comparing the effect of high (i.e. 1 SD above the mean) and low (i.e. 1 SD below the mean) adversity in children with high and low stress reactivity. High RSA reactivity (i.e. vagal withdrawal) was defined as 1 SD below the mean, while low RSA reactivity was defined as 1 SD above the mean. In contrast, high cortisol reactivity was defined as 1 SD above the mean, and low cortisol reactivity was defined as 1 SD below the mean. Finally, we examined whether these main and interactive effects varied for boys and girls.
RSA Reactivity
Results of regression analyses examining the effects of RSA reactivity and adversity on adaptation are presented in Table 4. Compared to boys, girls showed better adaptive functioning across all four domains in fall, as well as a decrease in externalizing symptoms and increase in prosocial behaviors across the first year of kindergarten. Lower RSA reactivity, as indexed by positive RSA residual values, significantly predicted higher levels of externalizing in fall. Higher adversity exposure predicted higher levels of externalizing and lower school engagement in fall. In addition, adversity exposure predicted an increase in externalizing symptoms as well as a decrease in prosocial behavior and school engagement from fall to spring. However, these main effects were qualified by significant interactions.
Table 4.
Standardized Regression Coefficients for Models Testing the Effects of RSA Reactivity and Adversity on Adaptation
| Externalizing | Prosocial Behavior | School Engagement | Academic Competence | |||||
|---|---|---|---|---|---|---|---|---|
| Fall | Spring | Fall | Spring | Fall | Spring | Fall | Spring | |
| Fall Adaptation | .69*** | .64*** | .53*** | .73*** | ||||
| Sex | −.22*** | −.11** | .33*** | .15*** | .17** | .06 | .13* | .00 |
| RSA | .11* | −.01 | .08 | .04 | −.03 | .02 | −.04 | .02 |
| ADV | .25*** | .10** | −.05 | −.11** | −.23*** | −.12** | −.02 | .00 |
| RSAxADV | −.11* | −.01 | .11* | .03 | .12* | .05 | .07 | .13*** |
| ADVxSex | −.07 | −.06 | .01 | .02 | .09 | .03 | −.10 | .01 |
| RSAxSex | .03 | −.05 | .02 | .05 | .02 | .00 | .10 | .08* |
| RSAxADVxSex | .02 | −.03 | .03 | .01 | −.02 | −.08 | −.03 | .01 |
| Total R2 | .17*** | .60*** | .14*** | .54*** | .12*** | .37*** | .05* | .57*** |
Notes. p < .05;
p < .01;
p < .001. RSA = Respiratory Sinus Arrhythmia Reactivity; ADV = Adversity; EXT = Externalizing behavior problems; PRO = Prosocial behavior; SE = School engagement; ACA = Academic competence.
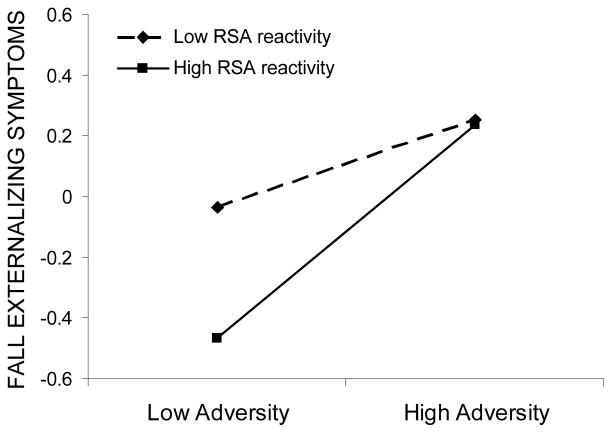
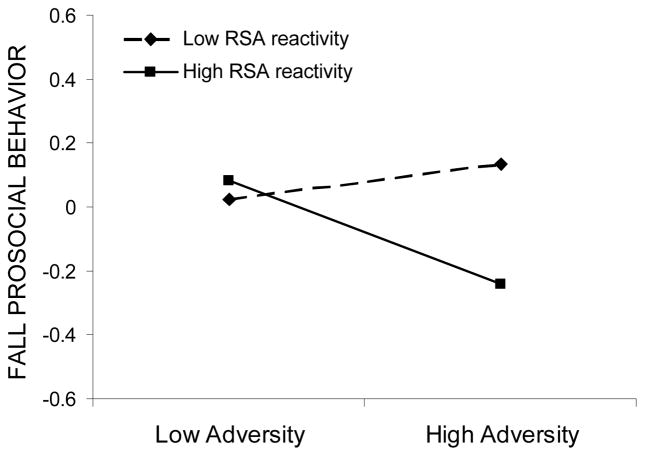
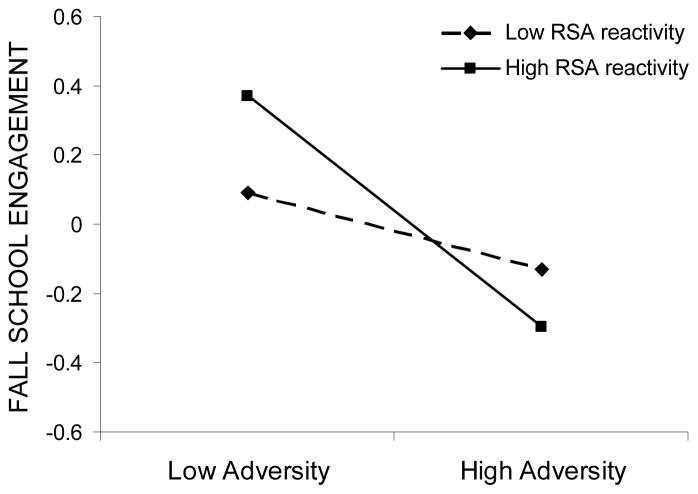
The interaction effect between RSA reactivity and adversity significantly predicted fall adaptation across three out of four domains: externalizing symptoms, prosocial behaviors, and school engagement. Examination of simple slopes revealed that the effect of adversity exposure on adaptation varied across different levels of RSA reactivity. A stronger relation between adversity and externalizing symptoms was found for children with high RSA reactivity (b = .35, p < .001) than for those with low RSA reactivity (b = .15, p < .05). In the context of high adversity, both low and high reactivity groups showed elevated externalizing symptom levels, whereas in the context of low adversity exposure, high reactive children had lower levels of symptoms than low reactive children (see Figure 1). Similarly, adversity exposure was significantly related to prosocial behavior in high reactive children (b = −.16, p < .05), but not in low reactive children (b = .05, p > .05). However, contrary to findings for externalizing symptoms, high reactive children showed lower levels of prosocial behaviors than low reactive children in the context of high adversity but did not differ in the context of low adversity (see Figure 2). Finally, adversity exposure was significantly related to fall school engagement in high reactive children (b = −.33, p < .001) but not in low reactive children (b = −.12, p > .05). In comparison to low reactive peers, high reactive children showed lower levels of fall school engagement in the context of high adversity, but higher levels of school engagement in the context of low adversity (see Figure 3). High reactivity thus operated as a risk factor under conditions of high adversity, but as a promotive, engagement-enhancing factor in circumstances of low adversity.
Figure 1.
Fall externalizing symptoms as a function of adversity exposure and RSA reactivity. Low and high adversity values are graphed at one standard deviation below and above the mean, respectively, whereas low and high RSA reactivity are graphed at one standard deviation above and below the residual score mean, respectively.
Figure 2.
Fall prosocial behaviors as a function of adversity exposure and RSA reactivity. Low and high adversity values are graphed at one standard deviation below and above the mean, respectively, whereas low and high RSA reactivity are graphed at one standard deviation above and below the residual score mean, respectively.
Figure 3.
Fall school engagement as a function of adversity exposure and RSA reactivity. Low and high adversity values are graphed at one standard deviation below and above the mean, respectively, whereas low and high RSA reactivity are graphed at one standard deviation above and below the residual score mean, respectively.
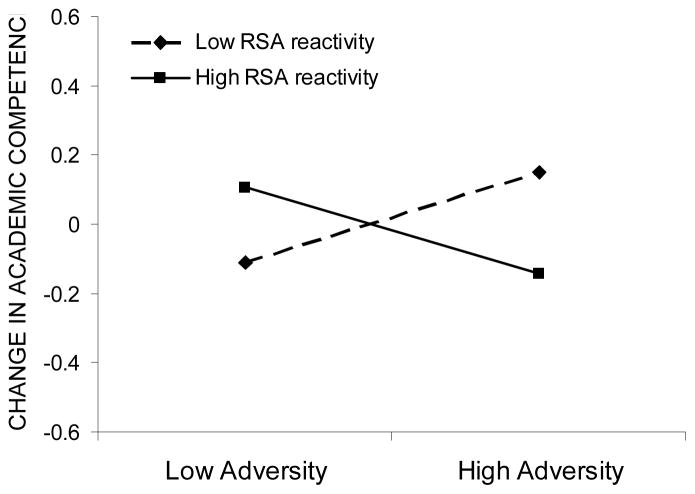
Although the interaction between RSA reactivity and adversity exposure did not predict fall levels of academic competence, it did predict change in academic competence from fall to spring. Analyses of simple slopes revealed that exposure to adversity was related to an increase in academic competence in low reactive children (b = .12, p < .05) but to a decrease in academic competence in high reactive children (b = −.12, p < .05). In the context of high adversity, low reactive children showed higher levels of improvement in academic competence than high reactive children (see Figure 4). However, in the context of low adversity, the reverse was true, with high reactive children showing higher levels of improvement in academic competence than their low reactive peers.
Figure 4.
Change in academic competence as a function of adversity exposure and RSA reactivity. Low and high adversity values are graphed at one standard deviation below and above the mean, respectively, whereas low and high RSA reactivity are graphed at one standard deviation above and below the residual score mean, respectively.
The interaction effects of reactivity and adversity on different domains of adaptation did not vary across children’s sex, as the 3-way interaction effects between sex, reactivity and adversity were not significant (see Table 4). However, a significant 2-way interaction revealed that the main effect of RSA reactivity on 6-month change in academic competence varied for boys and girls. The test of simple slopes indicated a crossover effect such that high reactivity was related to academic improvement in boys (b = −.06, p > .05), whereas low reactivity was related to academic improvement in girls (b = .10, p > .05); however, neither slope reached statistical significance.
Cortisol Reactivity
Results of regression analyses examining the effects of RSA reactivity and adversity on adaptation are presented in Table 5. High cortisol reactivity significantly predicted higher levels of externalizing and lower levels of school engagement and academic competence in fall. Adversity effects were the same as in regression analyses focused on RSA reactivity. However, these main effects are qualified by the significant interactions.
Table 5.
Standardized Regression Coefficients for Models Testing the Effects of Cortisol Reactivity and Adversity on Adaptation
| Externalizing | Prosocial Behavior | School Engagement | Academic Competence | |||||
|---|---|---|---|---|---|---|---|---|
| Fall | Spring | Fall | Spring | Fall | Spring | Fall | Spring | |
| Fall Adaptation | .70*** | .65*** | .54*** | .75*** | ||||
| Sex | −.23*** | −.11** | .32*** | .15*** | .17** | .05 | .13* | .01 |
| CORT | .14** | .00 | −.02 | −.05 | −.13* | −.08 | −.11* | −.02 |
| ADV | .25*** | .09* | −.05 | −.09* | −.24*** | −.10* | .00 | .03 |
| CORTxADV | .05 | .04 | −.13* | −.05 | −.04 | −.06 | −.08 | .03 |
| ADVxSex | −.04 | −.07* | .02 | .03 | .11* | .01 | −.08 | .00 |
| CORTxSex | −.12* | .04 | .02 | −.02 | .07 | .03 | .07 | .00 |
| CORTxADVxSex | −.03 | −.01 | .09 | −.03 | .02 | −.04 | −.06 | .00 |
| Total R2 | .18*** | .59*** | .14*** | .56*** | .14*** | .39*** | .06** | .57*** |
Notes. p < .05;
p < .01;
p < .001. CORT = Cortisol Reactivity; ADV = Adversity; EXT = Externalizing behavior problems; PRO = Prosocial behavior; SE = School engagement; ACA = Academic competence.
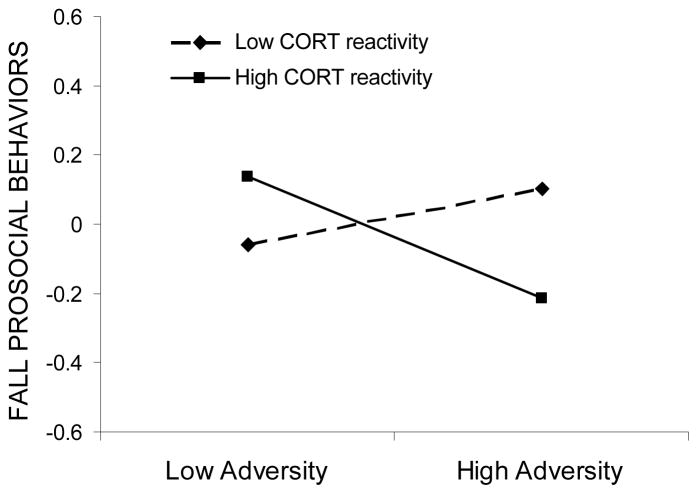
The interaction between cortisol reactivity and adversity significantly predicted fall prosocial behavior. According to tests of simple slopes, adversity exposure was significantly related to fall prosocial behaviors for high reactive children (b = −.16, p < .05), but not in low reactive children (b = .06, p > .05). In comparison to low reactive peers, high reactive children showed lower levels of fall prosocial behavior in the context of high adversity but in the context of low adversity showed slightly higher levels of prosocial behavior (see Figure 5).
Figure 5.
Prosocial behaviors as a function of adversity exposure and cortisol reactivity. Low and high adversity and cortisol reactivity values are graphed at one standard deviation below and above the mean, respectively.
The interaction effect of cortisol reactivity and adversity did not vary across children’s sex, as indicated by non-significant 3-way interactions (see Table 5). However, significant 2-way interactions between sex and adversity emerged for fall school engagement and change in externalizing from fall to spring. Tests of simple slopes indicate that adversity exposure was significantly related to lower school engagement in boys (b = .34, p < .001), but not in girls (b = −.13, p > .05). Likewise, adversity exposure was related to an increase in externalizing symptoms in boys (b = .16, p < .01) but not in girls (b = .01, p > .05). In addition, the main effect of cortisol reactivity on fall externalizing differed for boys and girls, as indicated by a significant 2-way interaction between reactivity and sex in Table 5. Cortisol reactivity was related to higher levels of externalizing in boys (b = .31, p < .001), but not in girls (b = .03, p > .05).
Discussion
The main goal of this study was to examine how stress reactivity and family adversity influence socio-emotional behavior and school readiness skills in children attending kindergarten. Several significant main effects emerged. Consistent with the broad literature on the effects of risk and adversity (e.g., Luthar, 2006), family adversity was associated with concurrent externalizing symptoms and lower school engagement, and with increases in externalizing symptoms, and decreases in prosocial behaviors and school engagement over the kindergarten year. However, the effect of adversity on fall school engagement and change in externalizing symptoms was significant only for boys. Adversity did not have a main effect on early kindergarten indices of academic competence. This discrepancy with previous research could be due to the fact that the majority of children in this study come from highly educated families, which may support academic success despite adversity exposure.
Low RSA reactivity was associated with higher levels of externalizing symptoms in the fall, corroborating previous findings of low stress reactivity and under-arousal in children with externalizing symptoms (Boyce et al., 2001; Calkins et al., 2007; Mezzacappa et al., 1997; Pine et al., 1996, 1998) and the idea that low RSA reactivity is an index of emotional dysregulation and lability (Beauchaine, 2001). On the other hand, high cortisol reactivity was associated with high levels of externalizing behaviors and low levels of school engagement and academic competence. These findings are consistent with studies that have linked elevated cortisol in response to classroom challenges to poor self-regulation, impulsivity, peer rejection and more solitary, negative behaviors (Dettling et al., 1999, 2000; Gunnar et al., 1997; 2003) and the notion that hyperresponsivity of the HPA axis signals a risk for maladaptation (Gunnar & Vazquez, 2006). However, these main effects and the absence of others in these models should be interpreted with caution, as they were conditioned by significant interactions. For example, the effect of RSA reactivity on change in academic competence significantly varied across sex, with high reactivity being promotive for boys but a risk factor for girls. In addition, high cortisol reactivity was related to higher levels of fall externalizing symptoms only in boys. These findings suggest that children’s sex may be important factor to consider when examining the effects of stress reactivity on adaptation.
The study’s most novel and salient findings emerged when adversity and stress reactivity were considered together, as components of interactions between environmental exposures and measures of biological sensitivity. Stress reactivity moderated the negative effect of family adversity across various domains of adaptation. Overall, the findings are consistent with the ‘stress diathesis’ hypothesis that high reactive children show worse adaptive functioning in the context of high adversity. Indeed, such children generally evinced the lowest levels of adaptive functioning of the entire study sample.
However, equally reactive children in settings of low adversity showed the highest levels of adaptation, levels even higher than those of their less reactive counterparts. Specifically, in the context of low family adversity, children who showed high RSA reactivity in response to challenges had the lowest levels of externalizing symptoms and the highest levels of prosocial behaviors and school engagement. Although adaptation showed significant stability from fall to spring, high reactive children showed improvement in academic competence in the context of low adversity and a decline in competence in the context of high adversity, whereas the inverse was true for low reactive children. Similarly, children who showed high cortisol reactivity to the challenge protocol had the highest levels of prosocial behaviors in the context of low adversity. Further, children exhibiting low RSA reactivity in response to challenges were fully or partially buffered against the harmful effects of adversity on externalizing symptoms, prosocial behavior, and school engagement. Likewise, among children who showed low cortisol reactivity, levels of prosocial behaviors did not significantly change across different levels of adversity.
These findings support the biological sensitivity to context theory advanced by Boyce and colleagues (Boyce et al., 2005; Boyce, 2007) and the concept of differential susceptibility to environmental influences proposed by Belsky and colleagues (Belsky, 2005; Belsky et al., 2007). This study illustrates that high reactivity is not merely a pathogenic, risk-amplifying response to adversity but can also promote adaptive functioning. Corroborating Boyce and colleagues’ theoretical perspective, children exhibiting high levels of biological sensitivity to context, as indexed by high autonomic and adrenocortical reactivity, were more susceptible to environmental influences in the context of both low and high family adversity. Thus, biologically sensitive children showed the highest levels of symptoms in the context of high family adversity but the highest levels of competence in the context of low family adversity. However, a lack of family adversity does not necessarily imply the presence of a nurturing family environment. Thus, future studies will need to further examine the role of heightened biological sensitivity to context across both stressful, health-undermining and supportive, health-enhancing contexts.
Together, these findings provide strong support for reframing stress reactivity as a biological sensitivity to context. First, the findings are relatively robust, especially for RSA reactivity, which emerged as a significant moderator of adversity effects across all four domains of adaptation. The evidence was parallel, though less robust, for cortisol reactivity, as the interaction effect was significant only for one domain. Second, each observed interaction was consistent with the biological sensitivity to context theory; high reactivity was promotive in the context of low adversity but a risk factor in the context of high adversity. Third, the interaction effect was found for both positive and negative indices of adaptation. Past studies have rarely examined how interactive processes apply to multiple domains of functioning and have often focused primarily on measures of psychopathology. Fourth, these highly consistent interaction effects were found in a normative, community sample of typically developing children. If stress reactivity moderated the effects of adversity on development in a community sample of children, who on average do not face extreme disadvantage or show clinical levels of problem behavior, the interactive effects are likely to be larger at the extreme ends of such distributions.
Finally, the reported interaction effects complement recent work examining individual differences in markers of behavioral and genetic susceptibility to environmental influences. The current findings are consistent with research on behavioral reactivity indicating that children with high levels of negative affectivity are particularly susceptible to both negative and positive experiences (Belsky, 2005; Belsky et al., 2007; Klein Velderman et al., 2006). In addition, the current findings parallel recent research showing that genetic polymorphisms can moderate the effects on adaptive functioning of family adversity, unresolved trauma, or abusive parenting (Bakermans-Kranenburg & Van IJzendoorn, 2007; Caspi et al., 2002; Taylor et al., 2006; van IJzendoorn & Bakermans-Kranenburg; 2006) and resonate with the observations of Rutter and colleagues on the previously overlooked protective effects of ‘risk-engendering’ genetic variants (Rutter, Moffitt & Caspi, 2006). Future studies should examine whether these behavioral, physiological, and genetic markers of susceptibility to contextual factors represent the same phenomena expressed at different levels of assessment or whether they represent different types of susceptibility that may have a cumulative effect on development.
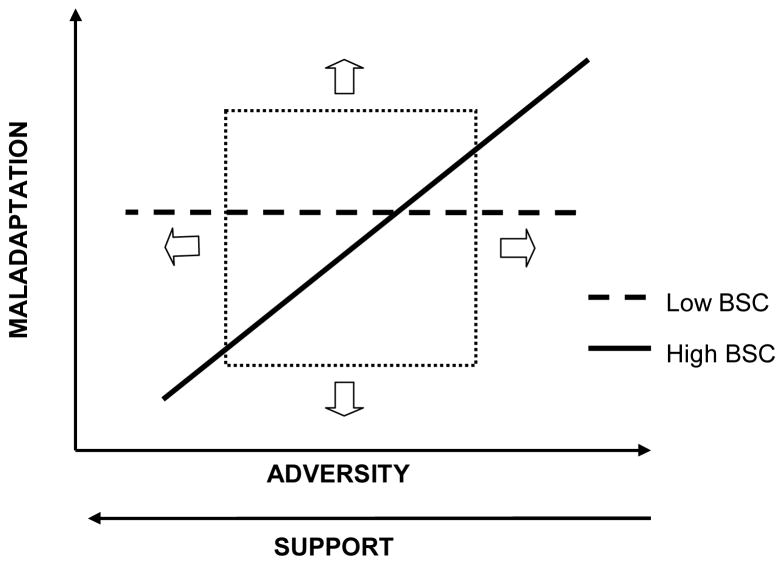
For the purposes of future studies, it is important to note that the nature of the reported interaction effects may depend on several dimensions depicted in the conceptual model of Figure 6. Single studies may be capable of capturing only a portion of the underlying, paradigmatic interaction shown in Figure 6, as the ‘observational window’ shifts from study to study, depending on sampling characteristics, the outcomes of the interest, the measure of reactivity, and the type of challenge. For example, the observational window may shift to the right in studies of highly disadvantaged child populations or upwards in studies of children with severe behavior problems. Similarly, the slopes of the two component lines may be determined by the researchers’ ability to capture the full range of stress reactivity responses and thus distinguish between high and low reactive children. It is critical, therefore, that developmental researchers examine and compare the interactive effects of stress reactivity and context across a range of outcomes and environmental influences and that the full scope of existing literature be taken into account in the interpretation of such effects.
Figure 6.
Conceptual model of the interactive effects between biological sensitivity to context (BSC) and environmental influences.
The relations between stress reactivity and context are also likely to be dynamic and evolving over time and the present study provides only a snapshot into a potentially complex cycle of bi-directional and reverberatory influences. Boyce and colleagues (Boyce, 2006; 2007; Boyce et al., 2005) have argued, for example, that stress reactivity may not only moderate effects of early experience, but may itself be influenced by the quality and character of early experience. While our analyses addressed stress reactivity as a moderator of adversity, given the developmental stage of the participants and the nature of the study data, we are mindful that adversity exposure could also play a role in shaping individuals’ stress reactivity. From the perspective of evolutionary biology (Ellis, Jackson, & Boyce, 2006), children growing up in supportive and nurturing families might be expected to develop high levels of biological sensitivity to context in order to take greater advantage of the positive and stimulating features of their environments. As a result of both high sensitivity and protective environments, these children would show high levels of competence and low rates of mental and health problems. On the other hand, children exposed to high levels of early risk and adversity may also develop high biological sensitivity to context, as a means of sustaining vigilance for environmental threats and hazards. Although such vigilance may be adaptive in the short run, over a longer period of time it could augment children’s vulnerability to the deleterious effects of adversity. It is not surprising then that these children tend to have higher rates of mental and health problems. On the other hand, children who develop low biological sensitivity to context seem to be less affected by both positive and negative environmental influences. These children may consequently demonstrate resilience—adaptation that is better than expected given their adversity exposures—or vulnerability—low levels of adaptation despite growing up in environments abundant with resources and support (Luthar, 2006; Masten & Obradović, 2006). Future studies are needed to examine the processes by which early experiences shape children’s biological stress responses.
Strengths, Limitations and Future Directions
This study had several unique strengths as well as notable limitations that highlight important directions for future improvements. First, the study was based on a large, ethnically diverse sample of kindergarten children; however, a majority of children came from families with relatively high socio-economic status, as indicated by income and parents’ education. Second, the study was unique in that it employed a carefully controlled measure of autonomic reactivity that took into account the possible effects of motor activity and engagement demands elicited by challenge tasks. However, only a third of sample had elevated cortisol in response to stress reactivity protocol, which may have contributed to less robust findings for cortisol reactivity. Future studies should attempt to raise the responder rate, as lack of response to the current protocol does not necessarily indicate a low reactivity to more intense, real-life stressors. Third, we included the various sources of family adversity as an index of early experience. However, future researchers should explicitly assess positive environmental influences in children’s lives by including measures of social support and resources. Another strength of the current study was the use of multiple informants. While it may not always be feasible to use multiple informants when assessing family adversity, given the young age of the participants and the nature of the construct, future studies should, where possible, triangulate the child, parent, and teacher reports of adaptation, thus minimizing informant variance. Fourth, the study was unique in its effort to compare how both indices of autonomic and adrenocortical reactivity interact with early family adversity to predict adaptation. In addition to examining how indices of different types of stress reactivity affect children’s behaviors and skills, future research should examine how different stress response systems interact to produce unique stress reactivity profiles, which may be related to different adaptive patterns across adversity exposure. As discussed above, researchers might use person-focused analytic approaches to examine whether the same children express analogous behavioral, physiological, and genetic sensitivity to contextual factors. Finally, future research should examine transactional relations between stress reactivity and adversity across longer periods of development, as adaptation in this study showed high stability over the 6-month period.
Conclusion
Given the pervasive and long-lasting effects that adversity can have on adaptation (Luthar, 2006; Obradović et al., in press; Sameroff, 2006), it is important to elucidate the processes that buffer or exacerbate these effects. This study represents a rare attempt to examine complex interactions between biological and environmental factors. The findings indicate that children’s biological sensitivity to social context played an important role in moderating the effects of early experiences of family adversity on positive and negative indices of adaptive functioning. We believe that studies such as the one reported here can advance future prevention and intervention efforts, to address more holistically the social disparities in children’s competence and psychopathology.
Acknowledgments
This study was supported by a grant (R01 MH62320) from the National Institute of Mental Health. Dr. Obradović is supported by a Killam Postdoctoral Research Fellowship from the University of British Columbia and is a Junior Fellow in the Canadian Institute for Advanced Research’s Experience-based Brain and Biological Development Program and Junior Fellow Academy. Dr. Boyce is a Fellow of the Canadian Institute for Advanced Research and holds the Sunny Hill Health Centre/BC Leadership Chair in Child Development at the University of British Columbia. The authors acknowledge the substantive contributions made by Dr. Abbey Alkon to the development of our stress reactivity paradigm and the analysis of reactivity data. We are also appreciative of the school principals, teachers, children, and families who participated in the reported study and the numerous research assistants who collected and scored these data.
Contributor Information
Jelena Obradović, University of British Columbia, Vancouver, Canada.
Nicole R. Bush, University of California, San Francisco
Juliet Stamperdahl, University of California, Berkeley.
Nancy E. Adler, University of California, San Francisco
W. Thomas Boyce, University of British Columbia, Vancouver, Canada.
References
- Ablow JC, Measelle JR The MacArthur Working Group on Outcome Assessment. MacArthur Foundation Research Network on Psychopathology and Development. Pittsburgh: University of Pittsburgh Press; 2003. Manual for the Berkeley Puppet Interview: Symptomatology, Social, and Academic Modules (BPI 1.0) [Google Scholar]
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Alkon A, Goldstein L, Smider N, Essex M, Boyce W, Kupfer D. Developmental and contextual influences on autonomic reactivity in young children. Developmental Psychobiology. 2003;42:64–78. doi: 10.1002/dev.10082. [DOI] [PubMed] [Google Scholar]
- Armstrong JM, Goldstein LH . The MacArthur Working Group on Outcome Assessment. Manual for the MacArthur Health and Behavior Questionnaire (HBQ 1.0) In: Kupfer David J., editor. MacArthur Foundation Research Network on Psychopathology and Development. University of Pittsburgh; 2003. [Google Scholar]
- Bakermans-Kranenburg MJ, Van IJzendoorn MH. Genetic vulnerability or differential susceptibility in child development: The case of attachment. Journal of Child Psychology & Psychiatry. 2007;48:1160–1173. doi: 10.1111/j.1469-7610.2007.01801.x. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Marshall P, Fox N. Developmental changes in heart period and high-frequency heart period variability from 4 months to 4 years of age. Developmental Psychobiology. 2000;37:44–56. doi: 10.1002/1098-2302(200007)37:1<44::aid-dev6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bazhenova OV, Porges SW. The Integrative Neurobiology of Affiliation. Annals of the New York Academy of Sciences. 1997;807:469–471. doi: 10.1111/j.1749-6632.1997.tb51940.x. [DOI] [PubMed] [Google Scholar]
- Beauchaine T. Vagal tone, development, and Gray’s motivational theory: toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Beauchaine T, Gatzke-Kopp L, Mead H. Polyvagal theory and developmental psychopathology: Emotion dysregulation and conduct problems from preschool to adolescence. Biological Psychology. 2007;74:174–184. doi: 10.1016/j.biopsycho.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J. Differential susceptibility to rearing influence: An evolutionary hypothesis and some evidence. In: Ellis B, Bjorklund D, editors. Origins of the social mind: Evolutionary psychology and child development. New York: Guilford; 2005. pp. 139–163. [Google Scholar]
- Belsky J, Bakermans-Kranenburg MJ, Van IJzendoorn MH. For better and for worse: Differential susceptibility to environmental influences. Current Directions in Psychological Science. 2007;16:300–304. [Google Scholar]
- Belsky J, Hsieh K, Crnic K. Mothering, fathering, and infant negativity as antecedents of boys’ externalizing problems and inhibition at age 3: Differential susceptibility to rearing influence? Development and Psychopathology. 1998;10:301–319. doi: 10.1017/s095457949800162x. [DOI] [PubMed] [Google Scholar]
- Bernardi L, Wdowczyk-Szulc J, Valenti C, Castoldi S, Passino C, Spadacini G, et al. Effects of Controlled Breathing, Mental Activity and Mental Stress With or Without Verbalization on Heart Rate Variability. Journal of the American College of Cardiology. 2000;35:1462–1469. doi: 10.1016/s0735-1097(00)00595-7. [DOI] [PubMed] [Google Scholar]
- Berntson G, Bigger J, Eckberg D, Grossman P, Kaufman P, Malik M, et al. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Berntson G, Cacioppo J, Fieldstone A. Illusions, arithmetic, and the bidirectional modulation of vagal control of the heart. Biological Psychology. 1996;46:1–17. doi: 10.1016/s0301-0511(96)05197-6. [DOI] [PubMed] [Google Scholar]
- Berntson G, Cacioppo J, Quigley K. Respiratory sinus arrhythmia: Autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology. 1993;30:183–196. doi: 10.1111/j.1469-8986.1993.tb01731.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Quigley KS, Jang J, Boysen ST. An approach to artifact identification: Application to heart period data. Psychophysiology. 1990;27:586–598. doi: 10.1111/j.1469-8986.1990.tb01982.x. [DOI] [PubMed] [Google Scholar]
- Block JH. The child-rearing practices report. University of California; Berkeley: 1965. Unpublished manuscript. [Google Scholar]
- Blair C. Early intervention for low birth weight preterm infants: The role of negative emotionality in the specification of effects. Development and Psychopathology. 2002;14:311–332. doi: 10.1017/s0954579402002079. [DOI] [PubMed] [Google Scholar]
- Blair C, Granger D, Razza RP. Cortisol reactivity is positively related to executive function in preschool children attending head start. Child Development. 2005;76:554–567. doi: 10.1111/j.1467-8624.2005.00863.x. [DOI] [PubMed] [Google Scholar]
- Boyce WT. Biobehavioral reactivity and injuries in children and adolescents. In: Bornstein MH, Genevro J, editors. Child development and behavioral pediatrics: Toward understanding children and health. Mahwah, NJ: Erlbaum; 1996. [Google Scholar]
- Boyce WT. Biology and context: Symphonic causation and the origins of childhood psychopathology. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology: Developmental neuroscience. II. Hoboken, NJ: Wiley & Sons; 2006. pp. 797–817. [Google Scholar]
- Boyce WT. A biology of misfortune: Stress reactivity, social context, and the ontogeny of psychopathology in early life. In: Masten A, editor. Multilevel Dynamics in Developmental Psychopathology: Pathways to the Future. 34. Minneapolis, MN: University of Minnesota; 2007. pp. 45–82. [Google Scholar]
- Boyce WT, Chesney M, Alkon Leonard A, Tschann J, Adams S, Chesterman B, et al. Psychobiologic reactivity to stress and childhood respiratory illnesses: Results of two prospective studies. Psychosomatic Medicine. 1995;57:411–422. doi: 10.1097/00006842-199509000-00001. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Essex MJ, Alkon A, Goldsmith HH, Kraemer HC, Kupfer DJ. Early father involvement moderates biobehavioral susceptibility to mental health problems in middle childhood. Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45:1510–1520. doi: 10.1097/01.chi.0000237706.50884.8b. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Quas J, Alkon A, Smider N, Essex M, Kupfer DJ. Autonomic reactivity and psychopathology in middle childhood. British Journal of Psychiatry. 2001;179:144–150. doi: 10.1192/bjp.179.2.144. [DOI] [PubMed] [Google Scholar]
- Burchinal M, Roberts JE, Hooper S, Zeisel SA. Cumulative risk and early cognitive development: a comparison of statistical risk models. Developmental Psychology. 2000;36:793–807. doi: 10.1037//0012-1649.36.6.793. [DOI] [PubMed] [Google Scholar]
- Bush NR, Alkon A, Obradović J, Stamperdahl J, Boyce WT. Deconstructing psychobiological reactivity: The contributions of psychomotor activity and stress response in five year old children. 2008 Manuscript submitted for publication. [Google Scholar]
- Cacioppo J, Uchino B, Berntson G. Individual differences in the autonomic origins of heart rate reactivity: The psychometrics of respiratory sinus arrhythmia and preejection period. Psychophysiology. 1994;31:412–419. doi: 10.1111/j.1469-8986.1994.tb02449.x. [DOI] [PubMed] [Google Scholar]
- Calkins SD. Cardiac vagal tone indices of temperamental reactivity and behavioral regulation in young children. Developmental Psychobiology. 1997;31:125–135. doi: 10.1002/(sici)1098-2302(199709)31:2<125::aid-dev5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Graziano PA, Keane SP. Cardiac vagal regulation differentiates among children at risk for behavior problems. Biological Psychology. 2007;74:144–153. doi: 10.1016/j.biopsycho.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M, Earls F. Psychological and neuroendocrinological sequelae of early social deprivation in institutionalized children in Romania. Annals of the New York Academy of Sciences. 1997;807:419–428. doi: 10.1111/j.1749-6632.1997.tb51936.x. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt T, Mill J, Martin J, Craig I, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002 August 2;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Crowell S, Beauchaine TP, Gatzke-Kopp L, Sylvers P, Mead H, Chipman-Chacon J. Autonomic correlates of attention-deficit/hyperactivity disorder and oppositional defiant disorder in preschool children. Journal of Abnormal Psychology. 2006;115:174–178. doi: 10.1037/0021-843X.115.1.174. [DOI] [PubMed] [Google Scholar]
- Crowell S, Beauchaine TP, McCauley E, Smith C, Stevens AL, Sylvers P. Psychological, autonomic, and serotonergic correlates of parasuicidal behavior in adolescent girls. Development and Psychopathology. 2005;17:1105–1127. doi: 10.1017/s0954579405050522. [DOI] [PubMed] [Google Scholar]
- Cummings EM, Davies PT. Effects of marital conflict on children: Recent advances and emerging themes in process-oriented research. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2002;43:31–63. doi: 10.1111/1469-7610.00003. [DOI] [PubMed] [Google Scholar]
- Cummings EM, El-Sheikh M, Kouros CD, Keller PS. Children’s skin conductance reactivity as a mechanism of risk in the context of parental depressive symptoms. Journal of Child Psychology and Psychiatry. 2007;48:436–445. doi: 10.1111/j.1469-7610.2006.01713.x. [DOI] [PubMed] [Google Scholar]
- Curtis WJ, Cicchetti D. Moving research on resilience into the 21st century: Theoretical and methodological considerations in examining the biological contributors to resilience. Development and Psychopathology. 2003;15:773–810. doi: 10.1017/s0954579403000373. [DOI] [PubMed] [Google Scholar]
- Davis EP, Bruce J, Gunnar MR. The anterior attention network: Associations with temperament neuroendocrine activity in 6-year-old children. Developmental Psychobiology. 2002;40:43–56. doi: 10.1002/dev.10012. [DOI] [PubMed] [Google Scholar]
- Davis EP, Donzella B, Krueger WK, Gunnar M. The start of a new school year: individual differences in salivary cortisol response in relation to child temperament. Developmental Psychobiology. 1999;35:188–96. doi: 10.1002/(sici)1098-2302(199911)35:3<188::aid-dev3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Deater-Deckard K, Dodge K. Spare the rod, spoil the authors: Emerging themes in research on parenting. Psychological Inquiry. 1997;8:230–235. [Google Scholar]
- Deković M, Janssens JMAM, Gerris JRM. Factor structure and construct validity of the Block child rearing practice report (CRPR) Psychological Assessment: Journal of Consulting & Clinical Psychology. 1991;3:182–187. [Google Scholar]
- Dettling AC, Gunnar MR, Donzella B. Cortisol levels of young children in full-day childcare centers: Relations with age and temperament. Psychoneuroendocrinology. 1999;24:519–536. doi: 10.1016/s0306-4530(99)00009-8. [DOI] [PubMed] [Google Scholar]
- Dettling AC, Parker SW, Lane S, Sebanc A, Gunnar MR. Quality of care and temperament determine changes in cortisol concentrations over the day for young children in childcare. Psychoneuroendocrinology. 2000;25:819–836. doi: 10.1016/s0306-4530(00)00028-7. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Riese H, Sondeijker FEPL, Graces-Lord K, van Roon AM, Ormel J, et al. Externalizing and internalizing problems in relation to autonomic function: A population-based study in preadolescents. Journal of American Academy of Child and Adolescent Psychiatry. 2007;46:378–386. doi: 10.1097/CHI.0b013e31802b91ea. [DOI] [PubMed] [Google Scholar]
- Doussard-Roosevelt JA, Montogomery LA, Porges SW. Short-term stability of physiological measures in kindergarten children: Respiratory sinus arrhythmia, heart period, and cortisol. Developmental Psychobiology. 2003;43:230–242. doi: 10.1002/dev.10136. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Fabes R, Bustamante D, Mathy R, Miller P, Lindholm E. Differentiation of vicariously induced emotional reactions in children. Developmental Psychology. 1988;24:237–246. [Google Scholar]
- Eisenberg N, Fabes RA, Murphy B, Maszk P, Smith M, Karbon M. The role of emotionality and regulation in children’s social functioning: A longitudinal study. Child Development. 1995;66:1360–1384. [PubMed] [Google Scholar]
- Ellis BJ, Essex MJ, Boyce WT. Biological sensitivity to context: II. Empirical explorations of an evolutionary developmental theory. Development and Psychopathology. 2005;17:303–328. doi: 10.1017/s0954579405050157. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Jackson JJ, Boyce WT. The stress response systems: Universality and adaptive individual differences. Developmental Review. 2006;26:175–212. [Google Scholar]
- El-Sheikh M. The role of emotional responses and physiological reactivity in the marital conflict-child functioning link. Journal of Child Psychology and Psychiatry. 2005;46:1191–1199. doi: 10.1111/j.1469-7610.2005.00418.x. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Harger J, Whitson SM. Exposure to interparental conflict and children’s adjustment and physical health: The moderating role of vagal tone. Child Development. 2001;72:1617–1636. doi: 10.1111/1467-8624.00369. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Whitson SA. Longitudinal relations between marital conflict and child adjustment: Vagal regulation as a protective factor. Journal of Family Psychology. 2006;20:30–39. doi: 10.1037/0893-3200.20.1.30. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Klein MH, Cho E, Kalin NH. Maternal stress beginning in infancy may sensitize children to later stress exposure: effects on cortisol and behavior. Biological Psychiatry. 2002;52:776–84. doi: 10.1016/s0006-3223(02)01553-6. [DOI] [PubMed] [Google Scholar]
- Fabes RA, Eisenberg N, Eisenbud L. Behavioral and physiological correlates of children’s reactions to others in distress. Developmental Psychology. 1993;29:655–663. [Google Scholar]
- Field T, Land C, Martinez A, Yando R, Pickens J, Bendell D. Preschool follow-up of infants of dysphoric mothers. Journal of Clinical Child Psychology. 1996;25:272–279. [Google Scholar]
- Fox NA, Field TM. Individual differences in preschool entry behavior. Journal of Applied Developmental Psychology. 1989;10:527–540. [Google Scholar]
- Goodyer IM, Herbert J, Althan P. Adrenal steroid secretion and major depression in 8- to 16-year olds, III. Influence of cortisol/DHEA ratio at presentation on subsequent rates of disappointing life events and persistent major depression. Psychological Medicine. 1998;28:265–273. doi: 10.1017/s0033291797006314. [DOI] [PubMed] [Google Scholar]
- Granger DA, Weisz JR, McCracken JT, Ikeda SC, Douglas P. Reciprocal influences among adrenocortical activation, psychosocial processes, and the behavioral adjustment of clinic-referred children. Child Development. 1996;67:3250–3262. [PubMed] [Google Scholar]
- Granger DA, Weisz JR, Kauneckis D. Neuroendocrine reactivity, internalizing behavior problems, and control-related cognitions in clinic-referred children and adolescents. Journal of Abnormal Psychology. 1994;103:267–276. doi: 10.1037//0021-843x.103.2.267. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Sebanc AM, Tout K, Donzella B, van Dulmen MH. Peer rejection, temperament, and cortisol activity in preschoolers. Developmental Psychobiology. 2003;43:346–358. doi: 10.1002/dev.10144. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Tout K, de Haan M, Pierce S, Stansbury K. Temperament, social competence, and adrenocortical activity in preschoolers. Developmental Psychobiology. 1997;31:65–85. doi: 10.1002/(sici)1098-2302(199707)31:1<65::aid-dev6>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez D. Stress neurobiology and developmental psychopathology. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology. Vol. 2. John Wiley & Sons; Hoboken, NJ: 2006. pp. 533–577. [Google Scholar]
- Halberstadt AG. Family socialization of emotional expression and nonverbal communication styles and skills. Journal of Personality and Social Psychology. 1986;51:827–836. [Google Scholar]
- Hardie T, Moss H, Vanyukov M, Yao J, Kirillovac G. Does adverse family environment or sex matter in the salivary cortisol responses to anticipatory stress? Psychiatry Research. 2002;111:121–131. doi: 10.1016/s0165-1781(02)00182-8. [DOI] [PubMed] [Google Scholar]
- Johnson PL, O’Leary KD. Parental behavior patterns and conduct problems in girls. Journal of Abnormal Child Psychology. 1987;15:573–581. doi: 10.1007/BF00917242. [DOI] [PubMed] [Google Scholar]
- Kagan J, Snidman N. Temperamental factors in human development. American Psychologist. 1991;46:856–862. doi: 10.1037//0003-066x.46.8.856. [DOI] [PubMed] [Google Scholar]
- Kamarck TW, Lovallo WR. Cardiovascular reactivity to psychological challege: Conceptual and measurement considerations. Psychosomatic Medicine. 2003;65:9–21. doi: 10.1097/01.psy.0000030390.34416.3e. [DOI] [PubMed] [Google Scholar]
- Katz LF, Gottman JM. Vagal tone protects children from marital conflict. Development and Psychopathology. 1995;7:83–92. [Google Scholar]
- Katz LF, Gottman JM. Buffering children from marital conflict and dissolution. Journal of Clinical Child Psychology. 1997;26:157–171. doi: 10.1207/s15374424jccp2602_4. [DOI] [PubMed] [Google Scholar]
- Kaufman A, Kaufman N. Kaufman Assessment Battery for Children. Circle Pines, MN: American Guidance Service; 1983. [Google Scholar]
- King J, Barkley RA, Barrett S. Attention-deficit hyperactivity disorder and the stress response. Biological Psychiatry. 1998;44:72–74. doi: 10.1016/s0006-3223(97)00507-6. [DOI] [PubMed] [Google Scholar]
- Klein Velderman M, Bakermans-Kranenburg MJ, Juffer F, van IJzendoorn MH. Effects of attachment-based interventions on maternal sensitivity and infant attachment: Differential susceptibility of highly reactive infants. Journal of Family Psychology. 2006;20:266–274. doi: 10.1037/0893-3200.20.2.266. [DOI] [PubMed] [Google Scholar]
- Klimes-Dougan B, Hastings PD, Granger DA, Usher BA, Zahn-Waxler C. Adrenocortical activity in at-risk and normally developing adolescents: individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Development and Psychopathology. 2001;13:695–719. doi: 10.1017/s0954579401003157. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Measelle JR, Ablow JC, Essex MJ, Boyce WT, Kupfer DJ. A new approach to integrating data from multiple informants in psychiatric assessment and research: mixing and matching contexts and perspectives. American Journal of Psychiatry. 2003;160:1566–1577. doi: 10.1176/appi.ajp.160.9.1566. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Schommer NC, Hellhammer DH, Kirschbaum C. Acute HPA axis responses, heart rate, and mood changes to psychosocial stress (TSST) in humans at different times of day. Psychoneuroendocrinology. 2004;29:983–992. doi: 10.1016/j.psyneuen.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Leary A, Katz LF. Coparenting, family-level processes, and peer outcomes: The moderating role of vagal tone. Development and Psychopathology. 2004;16:593–608. doi: 10.1017/s0954579404004687. [DOI] [PubMed] [Google Scholar]
- Luthar SS. Resilience in development: A synthesis of research across five decades. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology. 2. Vol. 3. Hoboken, NJ: Wiley; 2006. pp. 739–795. [Google Scholar]
- Manuck S, Kasprowicz A, Muldoon M. Behaviorally evoked cardiovascular reactivity and hypertension: Conceptual issues and potential associations. Annals of Behavioral Medicine. 1990;12:17–29. [Google Scholar]
- Masharani U, Shiboski S, Eisner MD, Katz PP, Janson SL, Granger DA, et al. Impact of exogenous glucocorticoid use on salivary cortisol measurements among adults with asthma and rhinitis. Psychoneuroendocrinology. 2005;30:744–752. doi: 10.1016/j.psyneuen.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Masten AS, Obradović J. Competence and resilience in development. Annals of the New York Academy of Sciences. 2006;1094:13–27. doi: 10.1196/annals.1376.003. [DOI] [PubMed] [Google Scholar]
- Masten AS, Shaffer A. How families matter in child development: Reflections from research on risk and resilience. In: Clarke-Stewart A, Dunn J, editors. Families count: Effects on child and adolescent development. Cambridge University Press; 2006. pp. 5–25. [Google Scholar]
- McBurnett KM, Lahey BB, Frick PJ, Risch C, Loeber R, Hart EL, et al. Anxiety, inhibition, and conduct disorder in children: II. Relation to salivary cortisol. Journal of the American Academy of Child and Adolescent Psychiatry. 1991;30:192–6. doi: 10.1097/00004583-199103000-00005. [DOI] [PubMed] [Google Scholar]
- McBurnett KM, Lahey BB, Rathouz PJ, Loeber R. Low salivary cortisol and persistent aggression in boys referred for disruptive behavior. Archives of General Psychiatry. 2000;57:38–43. doi: 10.1001/archpsyc.57.1.38. [DOI] [PubMed] [Google Scholar]
- Mezzacappa E, Tremblay RE, Kindlon D, Saul JP, Arseneault L, Seguin J, et al. Anxiety, antisocial behavior and heart rate regulation in adolescent males. Journal of Child Psychology and Psychiatry. 1997;38:457–469. doi: 10.1111/j.1469-7610.1997.tb01531.x. [DOI] [PubMed] [Google Scholar]
- Moss HB, Vanyukov MM, Martin CS. Salivary cortisol responses and the risk for substance abuse in prepubertal boys. Biological Psychiatry. 1995;38:547–555. doi: 10.1016/0006-3223(94)00382-D. [DOI] [PubMed] [Google Scholar]
- Obradović J, Shaffer A, Masten AS. Adversity and risk in developmental psychopathology: Progress and future directions. In: Mayes LC, Lewis M, editors. The environment of human development: A handbook of theory and measurement. New York: Cambridge University Press; in press. [Google Scholar]
- Pine DS, Wasserman G, Coplan J, Fried J, Sloan R, Myers M, et al. Serotonergic and cardiac correlates of aggression in children. Annals of the New York Academy of Sciences. 1996;794:391–393. doi: 10.1111/j.1749-6632.1996.tb32552.x. [DOI] [PubMed] [Google Scholar]
- Pine DS, Wasserman GA, Miller L, Coplan JD, Bagiella E, Kovelenku P, et al. Heart period variability and psychopathology in urban boys at risk for delinquency. Psychophysiology. 1998;35:521–529. doi: 10.1017/s0048577298970846. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal theory: phylogenetic substrates of a social nervous system. International Journal of Psychophysiology. 2001;42:123–146. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal theory: phylogenetic contributions to social behavior. Physiology and Behavior. 2003;79:503–513. doi: 10.1016/s0031-9384(03)00156-2. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74:174–184. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, Heilman KJ, Bazhenova O, Bal E, Doussard-Roosevelt JA, Koledin M. Does motor activity during psychophysiological paradigms confound the quantification and interpretation of heart rate and heart rate variability measures in young children? Developmental Psychobiology. 2007;49:485–494. doi: 10.1002/dev.20228. [DOI] [PubMed] [Google Scholar]
- Porter B, O’Leary KD. Marital discord and childhood behavior problems. Journal of Abnormal Child Psychology. 1980;8:287–295. doi: 10.1007/BF00916376. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Ramos MC, Guerin DW, Gottfried AW, Bathurst K, Oliver PH. Family conflict and children’s behavior problems: The moderating role of child temperament. Structural Equation Modeling. 2005;12:278–298. [Google Scholar]
- Rickel AU, Biasatti LL. Modification of the Block child rearing practice report. Journal of Clinical Psychology. 1982;38:129–133. [Google Scholar]
- Rudolph CD, Rudolph AM, Hostetter MK, Lister GL, Siegel NJ. Rudolph’s Pediatrics. 21. New York: McGraw Hill Medical; 2003. [Google Scholar]
- Rutter M. Stress, coping, and development: Some issues and some questions. In: Garmezy N, Rutter M, editors. Stress, Coping, and Development in Children. New York: McGraw-Hill; 1983. pp. 1–41. [Google Scholar]
- Rutter M, Moffitt TE, Caspi A. Gene-environment interplay and psychopathology: multiple varieties but real effects. Journal of Child Psychology and Psychiatry. 2006;47:226–261. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- Sameroff A. Identifying risk and protective factors for healthy child development. In: Clark-Stewart A, Dunn J, editors. Families count: Effects on child and adolescent development. Cambridge: Cambridge University Press; 2006. pp. 53–76. [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- Shannon KE, Beauchaine TP, Brenner SL, Neuhaus E, Gatzke-Kopp L. Familial and temperamental predictors of resilience in children at risk for conduct disorder and depression. Development and Psychopathology. 2007;19:701–727. doi: 10.1017/S0954579407000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Booth A, Johnson D. Low salivary cortisol levels and externalizing behavior problems in youth. Development and Psychopathology. 2005;17:167–184. doi: 10.1017/s0954579405050091. [DOI] [PubMed] [Google Scholar]
- Smider NA, Essex MJ, Kalin NH, Buss KA, Klein MH, Davidson RJ, et al. Salivary cortisol as a predictor of socioemotional adjustment during kindergarten: a prospective study. Child Development. 2002;73:75–92. doi: 10.1111/1467-8624.00393. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anger Expression Inventory (STAXI) Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- Suess PE, Porges SW, Plude DJ. Cardiac vagal tone and sustained attention in school-age children. Psychophysiology. 1994;31:17–22. doi: 10.1111/j.1469-8986.1994.tb01020.x. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Way BM, Welch WT, Hilmert CJ, Lehman BJ, Eisenberger NI. Early family environment, current adversity, the serotonin transporter polymorphism, and depressive symptomatology. Biological Psychiatry. 2006;60:671–676. doi: 10.1016/j.biopsych.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Van IJzendoorn MH, Bakermans-Kranenburg MJ. DRD4 7-repeat polymorphism moderates the association between maternal unresolved loss or trauma and infant disorganization. Attachment and Human Development. 2006;8:291–307. doi: 10.1080/14616730601048159. [DOI] [PubMed] [Google Scholar]