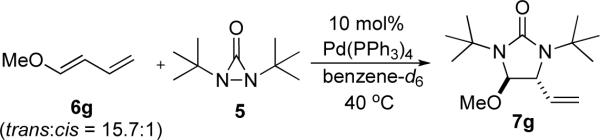
Abstract
Various dienes and a triene can be regioselectively diaminated at the internal double bond with good yields and high diastereoselectivity using di-tert-butyldiaziridinone (5) as nitrogen source and Pd(PPh3)4 (1–10 mol%) as catalyst. Kinetic studies with 1H NMR spectroscopy show that the diamination is first-order in total Pd catalyst and inverse first-order in PPh3. For reactive dienes, such as 1-methoxybutadiene (6g) and alkyl 1,3-butadienes (6a, 6j), the diamination is first order in di-tert-butyldiaziridinone (5) and zero-order in the olefin. For olefins with relatively low reactivity, such as (E)-1-phenyl-butadiene (6b) and (3E,5E)-1,3,5-decatriene (6i), similar diamination rates were observed when 3.5 equivalents of olefins were used. Pd(PPh3)2 is likely to be the active species for the insertion of Pd(0) into the N-N bond of di-tert-butyldiaziridinone (5) to form a four-membered Pd(II) complex (A), which can be detected by NMR spectroscopy. Olefin complex (B), formed from intermediate A via ligand exchange between the olefin substrate and the PPh3, undergoes migratory insertion and reductive elimination to give the diamination product and regenerate the Pd(0) catalyst.
Introduction
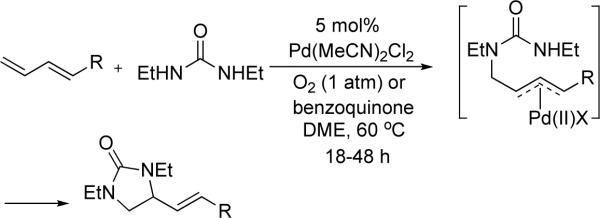
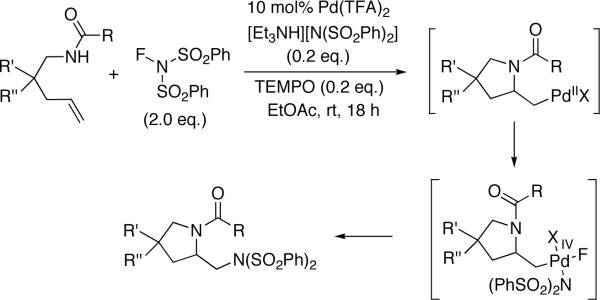
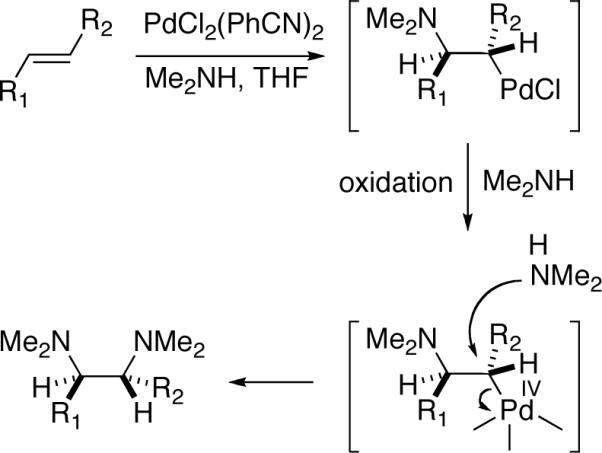
Vicinal diamines are very important functional moieties which are present in various biologically active compounds, chemical materials, and chiral catalysts.1 Diamination of olefins presents an attractive strategy to introduce vicinal nitrogen atoms. Various metal-free,1,2 metal-mediated1,3,4 or metal-catalyzed5,6 processes have been developed. Palladium has been one of the most important metals explored for the diamination process. In a pioneering study, Bäckvall reported stereospecific diamination of olefins via trans-aminopalladation, oxidation of Pd(II) to possible Pd(IV) with oxidants such as mCPBA, and subsequent nucleophilic displacement of Pd by an amine (Scheme 1).7 In 2005, Lloyd-Jones, Booker-Milburn, and coworkers reported a Pd(II)-catalyzed intermolecular diamination of conjugated dienes with ureas (Scheme 2).8 The diamination occurred regioselectively at the less-substituted double bond of dienes. The reaction is likely to proceed via a Pd(II)-promoted aza-Wacker-type process (trans-aminopalladation)9 to form a π-allyl Pd complex, followed by the displacement of Pd with N. The resulting Pd(0) is oxidized by O2 or benzoquinone to regenerate the Pd(II) catalyst. In 2005, Muñiz and coworkers reported a Pd(II)-catalyzed intramolecular diamination of olefins tethered with ureas (Scheme 3).10 The reaction was proposed to proceed via aminopalladation to form a Pd(II) intermediate, which is oxidized to Pd(IV) species, followed by the replacement of the C-Pd(IV) bond with a C-N bond to give the diamination product and regenerate the Pd(II) catalyst.10c,11,12,13 Very recently, Michael and coworkers reported the diamintion of olefins tethered with amide groups using N-fluorobenzenesulfonimide as electrophilic nitrogen source (Scheme 4).14 The diamination is proposed to proceed via a Pd(II)-promoted aminopalladation, oxidative addition of N-fluorobenzenesulfonimide to the Pd(II) species, and subsequent reductive elimination.
Scheme 1.
Scheme 2.
Scheme 3.
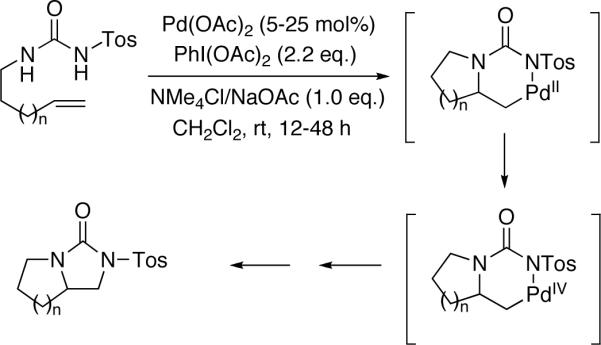
Scheme 4.
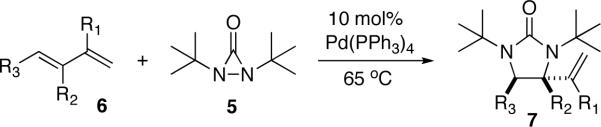
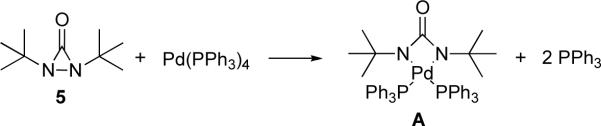
As part of our general interest in the oxidation of olefins,15 we have explored a possible diamination of olefins via the activation of a N-N bond (Scheme 5). It was hoped that a suitable metal catalyst could oxidatively insert into the N-N bond of dinitrogen compound 1 (cyclic or acylic) to form diamido species 2, which would then undergo migratory insertion into a double bond to form 3,16 followed by reductive elimination to give diamination product 4. Our studies started with the investigation of the feasibility of diaziridines and related analogues as nitrogen source and various metals as catalyst. It was found that conjugated dienes can be effectively diaminated at the internal double bond with di-tert-butyldiaziridinone (5)17,18 as nitrogen source and Pd(PPh3)4 as catalyst (Scheme 6).19 In subsequent studies, the reaction parameters such as ligand and solvent have been examined to improve the reaction process. The catalyst loading can be reduced to 1–2 mol% from the original 10 mol% (Scheme 6).17b The reaction mechanism has also been investigated via NMR spectroscopy and kinetic studies, providing a better understanding of the reaction process.20–22 Herein, we wish to report our detailed studies on this subject.23–26
Scheme 5.
Scheme 6.
Results and Discussion
1. Reaction Condition Studies
Several solvents were screened for the diamination using (E)-1,3-decadiene (6a) as substrate, 10 mol% Pd(PPh3)4 as catalyst, and di-tert-butyldiaziridinone (5) as nitrogen source at 65 °C. As shown in Table 1, a number of solvents27 are suitable for the diamination except CDCl3 and CH3CN. Since Pd(PPh3)4 was found to be less soluble in solvents such as hexanes or toluene, benzene was then typically used for the diamination study.
Table 1.
Solvent study on the Pd(PPh3)4-catalyzed diaminationa
| Entry | Solvent | Conv. (%)b |
|---|---|---|
| 1 | CDCl3 | 0 |
| 2 | C6D6 | 100 |
| 3 | toluene | 100 |
| 4 | THF | 100 |
| 5 | hexanes | 100 |
| 6 | DCM | 100 |
| 7 | CH3CN | 0 |
All reactions were carried out with olefin 6a (0.20 mmol), di-tert-butyldiaziridinone (5) (0.24 mmol), Pd(PPh3)4 (0.020 mmol) at 65 °C in dry solvent (0.60 mL) with stirring under argon for 1.5 h.
The conversion was determined by crude 1H NMR spectroscopy based on the olefin.
A series of phosphorus ligands were examined for the diamination of (E)-1,3-decadiene (6a) at 65 °C in dry benzene-d6 using 10 mol% Pd complex as catalyst. Catalysts were prepared in situ by treating Pd(OAc)2 with n-BuLi and phosphorus ligands at room temperature.28 Studies showed that monodentate triarylphosphines with exception of bulkier ones, were effective ligands for the diamination (Table 2, entries 1–6 vs entries 7–10). However, phosphines containing one or more alkyl groups, bidentate phosphine ligands, and phosphites were found to be poor ligands for this diamination (Table 2, entries 11–19). Overall, PPh3 became the ligand of choice for the diamination due to its effectiveness and low cost.
Table 2.
Ligand study on the Pd (0)-catalyzed diaminationa
| Entry | Ligand | Conv. (%)b |
|---|---|---|
| 1 | PPh3 | 100 |
| 2 | P(4-tolyl)3 | 100 |
| 3 | P(4-MeOC6H4)3 | 100 |
| 4 | P(4-CF3C6H4)3 | 91 |
| 5 | P(3-tolyl)3 | 100 |
| 6 | P(2-furyl)3 | 92 |
| 7 | P(2-tolyl)3 | 15 |
| 8 | P[3,5-(CF3)2C6H3]3 | 3 |
| 9 | P[2,6-(MeO)2C6H3]3 | 0 |
| 10 | P(2,4,6-Me3C6H2)3 | 0 |
| 11 | PPh2Me | 0 |
| 12 | PMe2Ph | 0 |
| 13 | P(n-Bu)3 | 0 |
| 14 | P(c-hexyl)3 | 10 |
| 15c | dppe | 0 |
| 16c | dppb | 0 |
| 17 | P(OMe)3 | 0 |
| 18 | P(OEt)3 | 4 |
| 19 | P(OPh)3 | 18 |
All reactions were carried out with olefin (6a) (0.20 mmol), di-tert-butyldiaziridinone (5) (0.25 mmol), and Pd catalyst (0.020 mmol) [prepared in situ from Pd(OAc)2 (0.020 mmol), phosphorus ligand (0.080 mmol), n-butyllithium (0.040 mmol, 0.025 mL, 1.6 M in hexanes) at room temperature] in benzene-d6 (0.20 mL) at 65 °C under argon for 2 h unless otherwise stated.
The conversion was determined by crude 1H NMR spectroscopy based on olefin.
Phosphorus ligand (0.040 mmol).
The previously reported diamination procedure requires 10 mol% Pd(PPh3)4.19 Only 20% conversion was obtained for (E)-1-phenylbutadiene (6b) when the catalyst loading was reduced to 5 mol%. It was surmised that the relatively high concentration of di-tert-butyldiaziridinone (5) in the reaction system might cause the deactivation of the Pd catalyst, and that slow addition of 5 could lower its concentration and might be beneficial for this diamination. It was indeed the case. As shown in Table 3, the catalyst could be reduced to 2 mol% (even to 1 mol% as illustrated in entry 7)17b when di-tert-butyldiaziridinone (5) was added slowly via syringe pump (Method B). Various dienes and a triene can be efficiently diaminated at 65 °C to give diamination products with good to excellent yields (79–95%) in high regio- and diastereoselectivity. Essentially only one regio- and stereoisomer was obtained. Other regio- and stereoisomers were barely detectable by 1H NMR spectroscopy of the crude reaction mixture if there was any. The yields obtained with 2 mol% Pd(PPh3)4 (Method B) are comparable to the ones obtained with the previous conditions (Method A) in most cases. However, electron-deficient diene 6h, was not an effective substrate for Method B (Table 3, entry 8).
Table 3.
Diamination of Conjugated Dienes and a Triene with Pd(PPh3)4
| Entry | Substrate (6) | Product (7) | Methoda | Yield (%)b |
|---|---|---|---|---|
| 1 |

|

|
A B |
90 92 |
| 2 | 6c, R = p-MeOPh | A B |
94 95 |
|
| 3 |

|

|
A B |
78 80 |
| 4 |

|

|
A | 91 |
| 5 | 6a, R = n-C6H13 | B | 86 | |
| 6 |

|

|
A B |
72 82 |
| 7c |

|

|
A B |
95 91(81d) |
| 8 |

|

|
A | 62 |
| 9 |

|

|
A B |
81 79 |
Method A (ref. 19): All reactions were carried out with di-tert-butyldiaziridinone (5) (0.20 mmol), olefin (E isomer, 0.24 mmol), and Pd(PPh3)4 (0.02 mmol) in benzene-d6 (0.6 mL) in an NMR tube at 65 °C under argon for 0.25–5 h unless otherwise stated. For entry 3, diene (E/Z = 1/1, E isomer: 0.24 mmol). For more examples, see ref. 19. Method B: All reactions were carried out with olefin (0.80 mmol), di-tert-butyldiaziridinone (5) (0.90 mmol, slow addition at a rate of 0.3 mmol/h ), Pd(PPh3)4 (0.016 mmol) at 65 °C under solvent-free conditions and argon for 4 h unless otherwise stated. For entry 2, benzene-d6 (0.10 mL) was used to dissolve diene.
Isolated yield based on 5 for Method A and based on olefin for Method B.
Containing <10% Z isomer, diamination product 7g is acid sensitive and was purified on less acidic silica gel (Iatrobeads 6RS-8060, Mitsubishi Kagaku Iatron, Inc. Japan).
1 mol % Pd(PPh3)4 (0.008 mmol) was used.
2. Monitoring the diamination reaction by 1H, 13C, and 31P NMR Spectroscopy
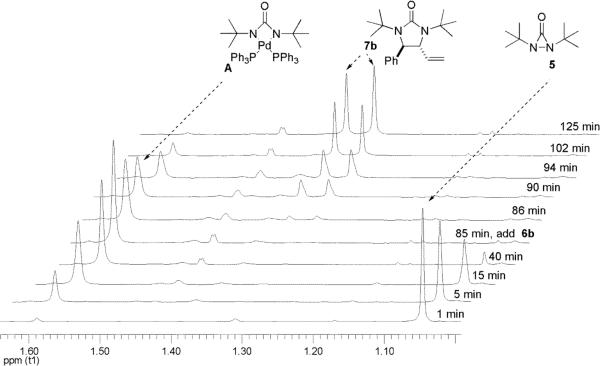
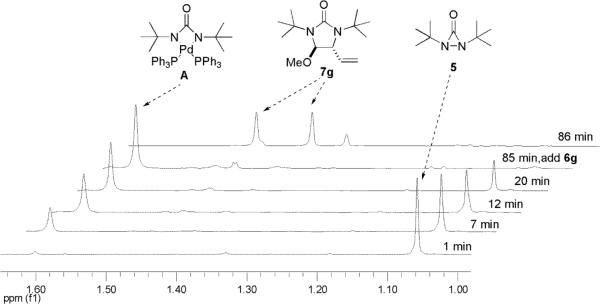
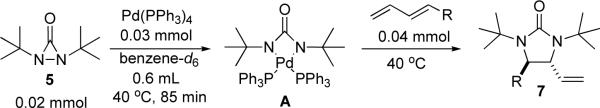
In an effort to gain a better understanding of the diamination, the reaction was monitored by 1H NMR spectroscopy. As shown in Figure 1, a new singlet peak at 1.59 ppm appeared when di-tert-butyldiaziridinone (5) was treated with Pd(PPh3)4 in dry benzene-d6 at 40 °C. This singlet peak (1.59 ppm) is corresponding to the signal of the tert-butyl groups of intermediate A [the signal of the tert-butyl groups of di-tert-butyldiaziridinone (5) appeared as a singlet at 1.05 ppm (Figure 1)]. After 85 min at 40 °C, all the di-tert-butyldiaziridinone (5) was consumed, and the singlet peak at 1.59 ppm reached its highest point. At that point, (E)-1-phenylbutadiene (6b) was added, the signals of the tert-butyl groups of diamination product 7b appeared immediately as two singlet peaks at 1.32 and 1.36 ppm and gradually increased while the singlet peak at 1.59 ppm gradually decreased. The diamination was practically complete 40 min after the addition of diene substrate 6b. As shown in Figure 2, more electron-rich 1-methoxybutadiene (6g) was much more reactive toward this diamination. The reaction between the intermediate and the diene was finished in less than one minute. These results indicate a two-step mechanism for the Pd(PPh3)4-catalyzed diamination. Di-tert-butyldiaziridinone (5) first reacts with Pd(PPh3)4 to form an active intermediate with a singlet peak at 1.59 ppm (corresponding to the signal of its tert-butyl groups), which subsequently reacts with the olefin substrate to give the diamination product. The singlet peak at 1.59 ppm is consistent with the symmetry of four-membered Pd(II) species A, whose structure is also supported by its 13C NMR spectrum (177.3 ppm for C=O, 58.1 ppm for CMe3, and 33.6 ppm for Me, see Supporting Information).
Figure 1.
Monitoring of the reaction of Pd(PPh3)4 with di-tert-butyldiaziridinone (5) and the subsequent diamination of (E)-1-phenylbutadiene (6b) by 1H NMR spectroscopy. Pd(PPh3)4 (0.030 mmol) reacted with di-tert-butyldiaziridinone (5) (0.020 mmol) in dry benzene-d6 (0.6 mL) in an NMR tube under argon atmosphere at 40 °C for 85 min, followed by the addition of (E)-1-phenylbutadiene (6b) (0.040 mmol). The NMR spectra were recorded at 40 °C.
Figure 2.
Monitoring of the reaction of Pd(PPh3)4 with di-tert-butyldiaziridinone (5) and the subsequent diamination of (E)-1-methoxybutadiene (6g) by 1H NMR spectroscopy. Pd(PPh3)4 (0.030 mmol) reacted with di-tert-butyldiaziridinone (5) (0.02 mmol) in dry benzene-d6 (0.6 mL) in an NMR tube under argon atmosphere at 40 °C for 85 min, followed by the addition of (E)-1-methoxybutadiene (6g) (E:Z = 15.7:1, E isomer: 0.040 mmol). The NMR spectra were recorded at 40 °C.
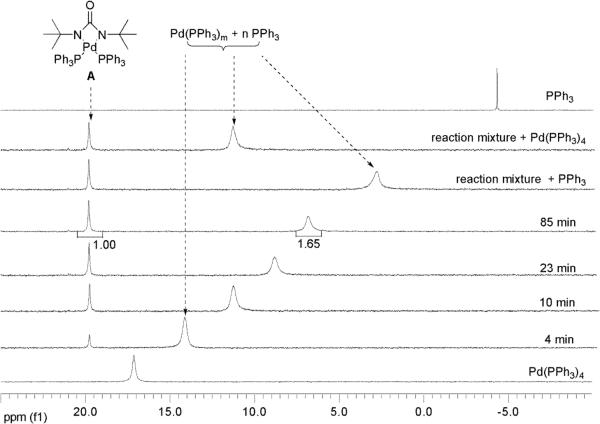
The reaction between Pd(PPh3)4 and di-tert-butyldiaziridinone (5) (Scheme 7) was also monitored in situ by 31P NMR spectroscopy. The reaction was carried out in benzene-d6 at 40 °C in an NMR tube, and 31P NMR spectra were collected at room temperature for higher quality spectra. As shown in Figure 3, a new singlet at 19.8 ppm appeared and increased gradually as more di-tert-butyldiaziridinone (5) was consumed. A broad singlet between −4.3 pm and 17.1 ppm appeared as an averaged spectrum of Pd(PPh3)m and PPh3 as a result of rapid ligand exchange between Pd(PPh3)m and free PPh3 [PPh3 at −4.3 ppm, Pd(PPh3)4 at 17.1 ppm)].29 As the reaction proceeded, and more free PPh3 was released, the broad singlet moved upfield in the direction of PPh3, and its intensity decreased gradually. After 85 min, di-tert-butyldiaziridinone (5) was completely consumed as judged by 1H NMR spectroscopy, and the new singlet at 19.8 ppm in the 31P NMR spectrum reached the highest intensity. The integration ratio of the singlet at 19.8 ppm to the broad singlet at 7.2 ppm is 1:1.65 as shown in Figure 3, which is smaller than the theoretical value (1:1). A possible reason is that the partial decomposition of di-tert-butyldiaziridinone (5) under the reaction conditions led to incomplete conversion of Pd(PPh3)4 to intermediate A when 5 was consumed. At that point, additional PPh3 or Pd(PPh3)4 was added to the reaction mixture to monitor any changes of the signals. It was observed that the broad 31P NMR singlet moved further upfield in the direction of PPh3 upon addition of PPh3 and moved downfield in the direction of Pd(PPh3)4 upon addition of Pd(PPh3)4. The signal intensity increased in similar magnitude in both of the cases as expected. The singlet at 19.8 ppm is consistent with the PPh3 signal of symmetric four-membered Pd(II) intermediate A, generated from Pd(PPh3)4 and di-tert-butyldiaziridinone (5).
Scheme 7.
Figure 3.
Monitoring of the reaction between di-tert-butyldiaziridinone (5) (0.030 mmol) and Pd(PPh3)4 (0.030 mmol) by 31P NMR spectroscopy. The reaction was carried out at 40 °C, and 31P NMR spectra were collected at room temperature (for higher quality spectra). “reaction mixture + PPh3”: additional PPh3 (0.040 mmol) was added to the reaction mixture after 85 min. “reaction mixture + Pd(PPh3)4”: additional Pd(PPh3)4 (0.010 mmol) was added to the reaction mixture after 85 min in a separate experiment.
3. Kinetic Model for the diamination
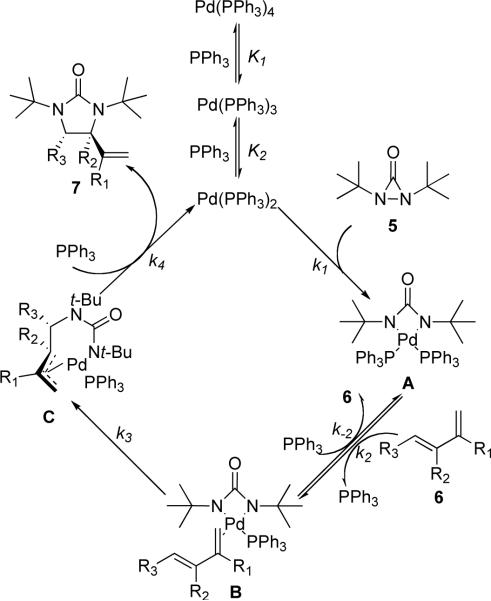
Based on the above NMR spectroscopy studies, a detailed catalytic cycle for the Pd(PPh3)4-catalyzed diamination is proposed in Scheme 8. The 14e species Pd(PPh3)2, generated from Pd(PPh3)4 by loss of two PPh3 ligands,29,30 inserts into the N–N bond of di-tert-butyldiaziridinone (5), giving four-membered Pd(II) species A.31,32 Olefin-complex B, resulting from intermediate A via the ligand exchange between olefin 6 and the PPh3, then undergoes migratory insertion to form π-allyl Pd intermediate C,33,34,35 followed by reductive elimination to give diamination product 736 and regenerate the active catalyst Pd(PPh3)2.
Scheme 8.
Proposed catalytic cycle for Pd(0)-catalyzed diamination of olefin.
Ligand exchange between the Pd(PPh3)m and free PPh3 in solution is very fast even at room temperature.29 Therefore, it is reasonable to assume that there are two fast pre-equilibria among Pd(PPh3)4, Pd(PPh3)3, and Pd(PPh3)2.30 According to the literature, Pd(PPh3)4 in solution mainly exists as Pd(PPh3)3.29,30 Using steady-state approximation, the reaction rate for the diamination can be expressed as equation 1, which indicates that the diamination is first-order in total Pd(PPh3)4 and inverse first-order in PPh3 (for derivation of equation 1, see: Supporting Information). If the Pd(0) insertion into the N-N bond of di-tert-butyldiaziridinone (5) is the rate-determining step, the reaction rate can be expressed with [Pd(PPh3)2] and [5] (equation 2). Based on the quick pre-equilibria among Pd(PPh3)2, Pd(PPh3)3, and Pd(PPh3)4, [Pd(PPh3)2] can be expressed with [Pd(PPh3)4]0 and [PPh3]. Therefore, the reaction rate can be further expressed with [Pd(PPh3)4]0, [PPh3], and [5] (equation 2) (for detailed derivation, see Supporting Information), indicating first-order in total Pd(PPh3)4 and di-tert-butyldiaziridinone (5) respectively, zero-order in olefin 6, and inverse first-order in PPh3.
| (1) |
| (2) |
4. Kinetic studies for the Pd(PPh3)4-catalyzed diamination with 1-methoxybutadiene (6g)
Using 1H NMR spectroscopy, kinetics studies of the diamination were carried out in benzene-d6 with 10 mol% Pd(PPh3)4 as catalyst using Si(SiMe3)4 as internal standard (Scheme 9). The diamination was run at 40 °C so that the reaction was slow enough to allow the data collection. 1-Methoxybutadiene (6g) was chosen as substrate for the kinetic experiment to avoid any interference with the tert-butyl signals of di-tert-butyldiaziridinone (5) and the diamination product (7g) in the 1.0–2.0 ppm region. The concentrations of 5 and diamination product 7g were determined by integration of the signals corresponding to the respective tert-butyl groups. As judged by 1H NMR spectroscopy, this diamination was very clean and no side-reaction was observed under the reaction conditions.
Scheme 9.
(a) Reaction order in di-tert-butyldiaziridinone (5) for the diaminations
The diamination of 1-methoxybutadiene (6g) was monitored by 1H NMR spectroscopy at 40 °C. The concentrations of di-tert-butyldiaziridinone (5) and diamination product (7g) at different reaction times were obtained via integration of the signals corresponding to the respective tert-butyl groups. The plots of the concentrations of 5 and 7g against the reaction time are shown in Figure 4. No induction period was observed for this reaction, suggesting that the active catalyst species Pd(PPh3)2 is generated very quickly, then enters the catalytic cycle. The sum of the concentrations of 5 and 7g remained almost constant over the reaction time (Figure 4), indicating that di-tert-butyldiaziridinone (5) was quantitatively converted to diamination product 7g. The 31P NMR spectrum of the reaction mixture is similar to that of Pd(PPh3)4, and no reaction intermediates were observed from the 1H NMR spectrum, indicating that the concentrations of Pd intermediates in the catalytic cycle were very low during the reaction. A plot of ln([5]0/[5]) against the reaction time gives a straight line with a slope, kobs = 8.2 × 10−4 s−1 (Figure 5), indicating first-order kinetics in di-tert-butyldiaziridinone (5) for the diamination of 1-methoxybutadiene (6g).
Figure 4.
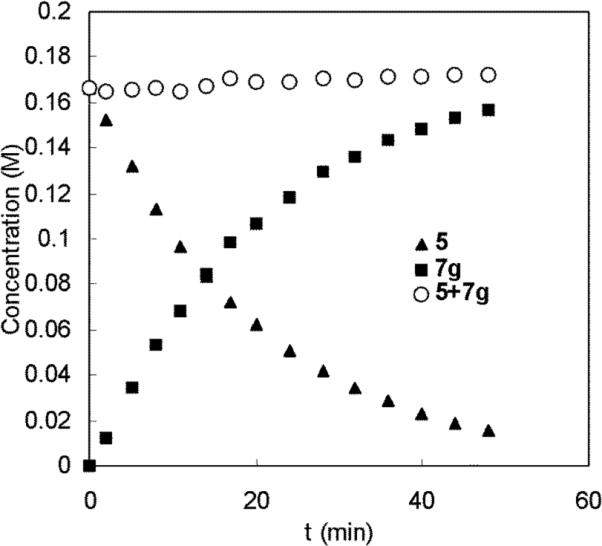
Plots of concentrations of di-tert-butyldiaziridinone (5), diamination product (7g), and their sum (5+7g) against the reaction time (min) for the diamination of 1-methoxybutadiene (6g) (E:Z = 15.7:1, E isomer: 0.24 mmol) with 5 (0.20 mmol) and Pd(PPh3)4 (0.020 mmol) in dry benzene-d6 (1.2 mL) at 40 °C in an NMR tube.
Figure 5.
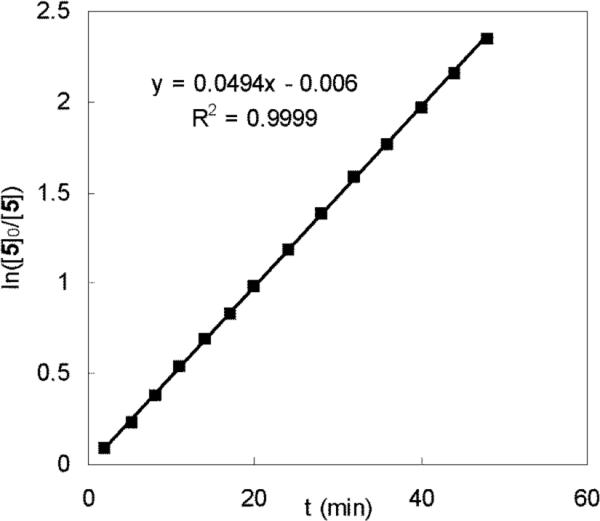
Plot of ln([5]0/[5]) against the reaction time for the diamination of 1-methoxybutadiene (6g) (E:Z = 15.7:1, E isomer: 0.24 mmol) with 5 (0.20 mmol) and Pd(PPh3)4 (0.020 mmol) in dry benzene-d6 (1.2 mL) in an NMR tube at 40 °C. [5]0 stands for the initial concentration of 5 in M and [5] stands for the concentration of 5 in M at a particular reaction time.
(b) Effect of 1-methoxybutadiene (6g) concentration
Diaminations with different amounts of 6g were investigated with 10 mol% Pd(PPh3)4 at 40 °C. As shown in Figure 6, these diaminations have similar reaction rates, indicating zero-order kinetics in olefin 6g. All these results show that the diamination is first-order in di-tert-butyldiaziridinone (5) and zero-order in diene 6g as expressed in equation 2, which indicates that the Pd(0) insertion into the N-N bond of di-tert-butyldiaziridinone (5) is the rate-determining step for the diamination of 1-methoxybutadiene (6g).
Figure 6.
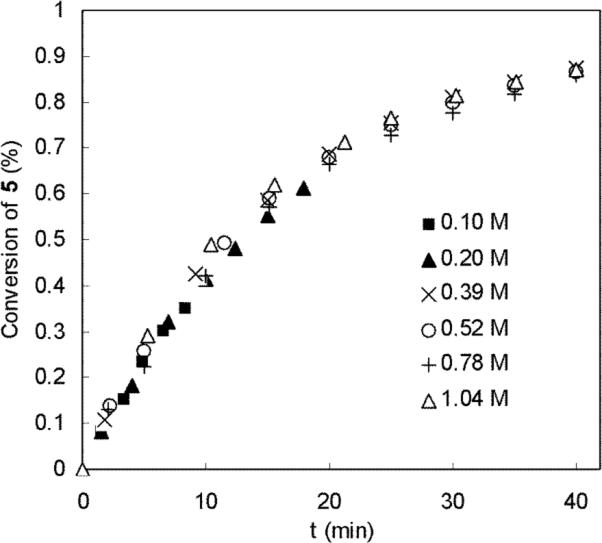
Plots of the conversion of di-tert-butyldiaziridinone (5) against the reaction time (min) for the diamination of 1-methoxybutadiene (6g) with different initial olefin concentrations. The diaminations were carried out with 6g (E:Z = 15.7:1, E isomer: 0.12 mmol, 0.24 mmol, 0.47 mmol, 0.62 mmol, 0.94 mmol, or 1.25 mmol), di-tert-butyldiaziridinone (5) (0.40 mmol), and Pd(PPh3)4 (0.040 mmol) in dry benzene-d6 (1.2 mL) in an NMR tube at 40 °C.
(c) Effect of PPh3 concentration
According to equation 2, the observed rate constant of the diamination (kobs) would be inversely proportional to [PPh3]. The diaminations were carried out at 40 °C with 10 mol% Pd(PPh3)4 and varying amounts of PPh3. Since Pd(PPh3)3 is the main Pd species in the solution of Pd(PPh3)4,29,30 the concentration of free PPh3 in the reaction mixture should approximately equal to the sum of the initial [Pd(PPh3)4] and [PPh3] added.30a The plot of 1/kobs against the concentration of PPh3 was found to be a straight line as shown in Figure 7, indicating the diamination is inverse first-order in PPh3 ligand and also validating the approximation of the value of [PPh3]free. This result is consistent with equation 2, which further supports the reaction mechanism proposed in Scheme 8.
Figure 7.
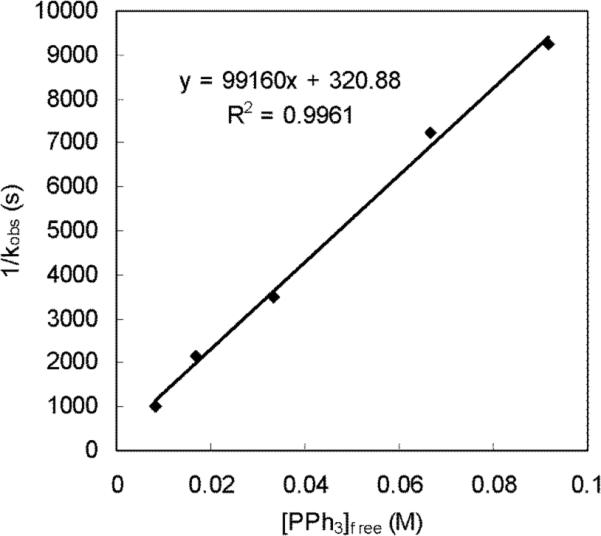
The plot of 1/kobs against the concentration of free PPh3 for the diamination of 1-methoxybutadiene (6g) (E:Z = 15.7:1, E isomer: 0.12 mmol) with di-tert-butyldiaziridinone (5) (0.10 mmol), Pd(PPh3)4 (0.010 mmol), and added PPh3 (0, 0.010, 0.030, 0.070, and 0.10 mmol, respectively) in dry benzene-d6 (1.2 mL) in an NMR tube at 40 °C. The value of [PPh3]free is corresponding to [PPh3]added + [Pd(PPh3)4]0.
(d) Effect of total Pd concentration
Equation 2 indicates that the diamination should be first-order in the total Pd catalyst. In order to investigate the influence of Pd catalyst, the diamination of 1-methoxybutadiene (6g) was investigated with different amounts of Pd(PPh3)4 and fixed concentration of free PPh3. The plot of kobs against [Pd(PPh3)4]0 was found to be a straight line as shown in Figure 8, indicating the diamination is first-order in total Pd catalyst as expressed in equation 2.
Figure 8.
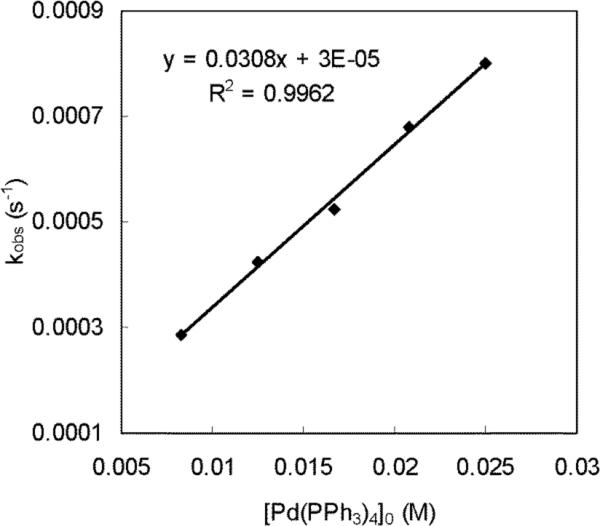
The plot of kobs against [Pd(PPh3)4]0 for the diamination of 1-methoxybutadiene (6g) (E:Z = 15.7:1, E isomer: 0.12 mmol) with di-tert-butyldiaziridinone (5) (0.10 mmol), various amounts of Pd(PPh3)4 (0.010–0.030 mmol), and fixed amount of free PPh3 (0.040 mmol) in dry benzene-d6 (1.2 mL) in an NMR tube at 40 °C. Additional PPh3 (0.030–0.010 mmol) was added to maintain the amount of PPh3 (0.040 mmol) in each reaction [PPh3 (added) = 0.040 mmol – Pd(PPh3)4 (added)].
5. Effect of olefin structure
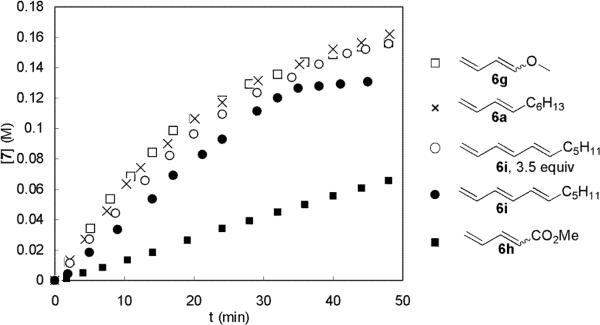
Thus far, studies have been mainly focused on the diamination of 1-methoxybutadiene (6g), which is zero-order in 6g under the diamination conditions. Several other dienes and a triene were also examined, and the results are shown in Figure 9. Alkyl diene 6a has a diamination rate similar to that of 6g. The diamination was found to also be first-order in 5. The diamination of triene 6i was found to be a little slower but with some unidentified side reaction(s) under the same reaction conditions. The reaction was no longer first order in di-tert-butyldiaziridinone (5) possibly due to the side reaction(s) and/or lower substrate reactivity. When more substrate was used (3.5 equiv), the diamination rate of 6i was similar to that of 6g. Significantly slower diamination was observed for electron-deficient olefin 6h.
Figure 9.
Plots of [7] against the reaction time for the diaminations of different dienes or triene with di-tert-butyldiaziridinone (5) (0.20 mmol), Pd(PPh3)4 (0.020 mmol) in dry benzene-d6 (1.2 mL) in an NMR tube at 40 °C. For 1-methoxybutadiene (6g): E:Z = 15.7:1, E isomer: 0.24 mmol; for (E)-1,3-decadiene (6a): 0.24 mmol; for (3E,5E)-undeca-1,3,5-triene (6i): 3.5 equiv (0.70 mmol), 1.2 equiv (0.24 mmol); for methyl 1,3-butadiene-1-carboxylate (6h): E:Z = 8.3:1, E isomer: 0.24 mmol.
6. Studies on the reaction of olefins with Pd(II) intermediate A
To investigate the olefin reactivity toward intermediate A (Scheme 8), the diaminations of various olefins were carried out with preformed Pd(II) species A by treating di-tert-butyldiaziridinone (5) with Pd(PPh3)4 (1.5 equiv) in dry benzene-d6 in an NMR tube at 40 °C under argon atmosphere for 85 min (Scheme 10). The reaction was monitored by 1H NMR spectroscopy at 40 °C upon addition of olefins (2.0 equiv). As shown in Figure 10, the reaction was influenced significantly by the electronic and steric properties of the olefin. Electron-rich olefins were more reactive toward complex A. 1-Methoxybutadiene (6g) was the most reactive for the diamination and the reaction was finished in several seconds at 40 °C. Alkyl dienes such as 6j and 6a were more reactive than aryl dienes such as 6b and aliphatic triene 6i.
Scheme 10.
Figure 10.
Plots of [7] against the reaction time for the reactions betwen Pd(II) intermediate A and different dienes or triene (6) (E isomer: 0.040 mmol) in dry benzene-d6 (0.6 mL) at 40 °C in an NMR tube. For 1-methoxybutadiene (6g): E:Z = 15.7:1, E isomer: 0.040 mmol; for (E)-1,3-pentadiene (6j): 0.040 mmol; for (E)-1,3-decadiene (6a): 0.040 mmol; for (E)-1-phenylbutadiene (6b): 0.040 mmol; for (3E,5E)-undeca-1,3,5-triene (6i): 0.040 mmol; for methyl 1,3-butadiene-1-carboxylate (6h): E:Z = 8.3:1, E isomer: 0.040 mmol. Compound A was prepared by reacting di-tert-butyldiaziridinone (5) (0.020 mmol) with Pd(PPh3)4 (0.030 mmol) in dry benzene-d6 (0.6 mL) at 40 °C in an NMR tube for 85 min.
In the aforementioned kinetic studies with 1-methoxybutadiene (6g), little information was obtained about the details for the reaction between the four-membered Pd(II) species A and olefins because the rate-determining step was the insertion of Pd(0) into the N-N bond of di-tert-butyldiaziridinone (5) and the C-N bond formation occurred after the rate-determining step. In order to get some insight into C-N bond formation, an olefin with relatively low activity was needed for the kinetic studies so that the rate of C-N bond formation is slower than or competitive to the rate of the insertion of Pd into the N-N bond of 5. (3E, 5E)-Undeca-1,3,5-triene (6i) showed a relatively low activity in the reaction with intermediate A (Figure 10). Intermediate A could be detected by 1H NMR spectroscopy during the reaction when 6i was subjected to the catalytic diamination conditions, which provides an opportunity to get some insight into the C-N bond formation. Using pre-equilibrium and steady-state approximations, the concentration of intermediate A for the diamination is expressed in equation 3 (see Suporting Information). The corresponding reciprocal of equation 3 is shown as equation 4, which indicates that the plot of 1/[A] against [6]/[5] should be a straight line and PPh3 would have little impact on the slope. Diamination of 6i was carried out with 20 mol% Pd(PPh3)4 and varying amounts of PPh3 added in an NMR tube at 40 °C. The data of [A], [5], and [6i] were collected for the plot after the reaction reached steady state (~20% conversion of 5). As shown in Figure 11, the plot of 1/[A] against [6i]/[5] gives three almost overlapped straight lines with different amounts of PPh3 present in the reaction, illustrating that PPh3 has little impact on [A] as expected based on equation 4. These results support that the reaction between intermediate A and the olefin proceeds via the pathway proposed in Scheme 8. One PPh3 ligand in intermediate A is substituted by olefin 6 via ligand exchange to form olefin complex B before the C-N formation. This proposed pathway is consistent with the observation that no diamination was observed with bidentate phosphorus ligands (Table 2, entries 15 and 16). According to the proposed pathway, one ligand is still attached to the Pd during the C-N bonds formation, providing opportunities for asymmetric induction with a chiral ligand. High enentioselectivity has indeed been achieved for this diamination with a BINOL-tetramethylpiperidine based phosphorus amidite ligand.23a
| (3) |
| (4) |
Figure 11.
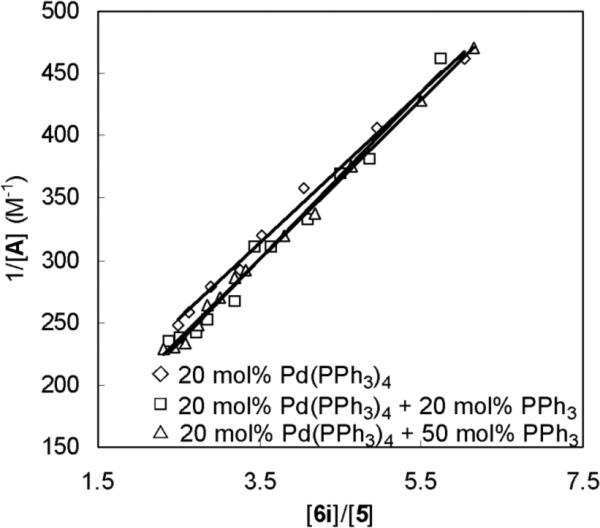
Plots of 1/[A] against [6i]/[5] for the diaminations of (3E,5E)-undeca-1,3,5-triene (6i) (0.20 mmol) with di-tert-butyldiaziridinone (5) (0.10 mmol), Pd(PPh3)4 (0.020 mmol), and different amounts of PPh3 added (0 mmol; 0.020 mmol; 0.050 mmol) in dry benzene-d6 (1.2 mL) at 40 °C in an NMR tube.
7. Diamination with Urea
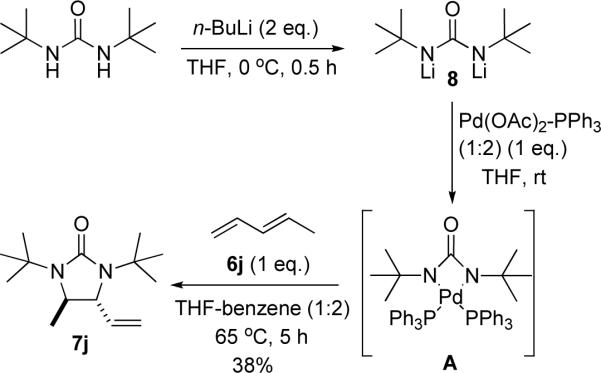
The above studies show that the four-membered Pd(II) species A resulting from the Pd(0) insertion into the N-N bond of di-tert-butyldiaziridinone 5 is an active intermediate for the diamination of dienes. It has been reported that some four-membered Pd(II) species can be generated by reacting PdX2 with dilithium salts of ureas,37 which prompted us to investigate if such a process could be used for diamination. The dilithium salt 8 generated from di-tert-butylurea and n-BuLi was treated with a mixture of Pd(OAc)2-PPh3 (1:2) and diene 6j (Scheme 11). At the end of the reaction, the palladium complex was removed by filtration, and diamination product 7j was isolated by flash chromatography on silica gel in 38% yield. The diamination occurred regioselectively at the internal double bond, suggesting that the reaction proceeded via four-membered Pd(II) intermediate A. The regioselectivity obtained in this case is different from the Pd(II)-catalyzed terminal diamination of diene with urea as previously reported by Lloyd-Jones, Booker-Milburn, and coworkers,8 illustrating that the current diamination procceds via a completely different reaction mechanism. While the reaction efficiency needs to be improved and a catalytic process needs to be developed, the diamination process using a urea salt described in Scheme 11 is very encouraging, which could open up a new approach to the diamination. Further study on this subject is in progress.
Scheme 11.
Conclusions
Various conjugated dienes and a triene can be efficiently diaminated with high yields and high regio- and diastereoselectivity using Pd(PPh3)4 as catalyst and di-tert-butyldiaziridinone (5) as nitrogen source. The catalyst loading was reduced to 1–2 mol% by slowly adding di-tert-butyldiaziridinone (5) under solvent-free conditions. A detailed catalytic pathway has been proposed based on the NMR spectroscopy and kinetic studies (Scheme 8). The Pd(0) [likely Pd(PPh3)2] first inserts into the N-N bond of di-tert-butyldiaziridinone (5) to form a symmetric four-membered Pd(II) intermediate (A), which can be detected by 1H, 13C, and 31P NMR spectroscopy. This four-membered Pd(II) intermediate forms an olefin complex (B) via ligand exchange between the olefin substrate and the PPh3. The resulting Pd-diene complex (B) undergoes migratory insertion into the internal double bond to form a π-allyl Pd intermediate (C), which gives the diamination product and regenerates the Pd(0) catalyst after reductive elimination. For reactive substrates such as 1-methoxybutadiene (6g) and alkyl dienes, kinetic studies show that the diamination is first-order in di-tert-butyldiaziridinone (5) and the Pd(0) catalyst, zero-order in the olefin substrate, and inverse first-order in PPh3, which is consistent with the fact that the Pd(0) insertion into the N-N bond of di-tert-butyldiaziridinone (5) is the rate-determining step. Further studies show that the four-membered Pd(II) complex generated from Pd(OAc)2 and dilithium salts of di-tert-butylurea can also regioselectively diaminate the internal double bond of a diene although the reaction efficiency needs to be improved. This result further validates the four-membered Pd(II) intermediate in the proposed catalytic pathway, and opens up new opportunities for diamination using urea salts. The studies presented in this work provide a better understanding of the Pd(0)-catalyzed diamination process and will facilitate further development of more effective diamination systems.
Supplementary Material
Acknowledgment
We are grateful to the generous financial support from the General Medical Sciences of the National Institutes of Health (GM083944-02).
Footnotes
Supporting Information Available: The procedures for diamination, NMR spectroscopy studies, and kinetic studies along with the kinetic analysis (18 pages). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).For leading reviews, see: Lucet D, Gall TL, Mioskowski C. Angew. Chem., Int. Ed. 1998;37:2580. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2580::AID-ANIE2580>3.0.CO;2-L.. Mortensen MS, O'Doherty GA. Chemtracts: Org. Chem. 2005;18:555.. Kotti SRSS, Timmons C, Li G. Chem. Biol. Drug Des. 2006;67:101. doi: 10.1111/j.1747-0285.2006.00347.x.. Kizirian J-C. Chem. Rev. 2008;108:140. doi: 10.1021/cr040107v.. Lin G-Q, Xu M-H, Zhong Y-W, Sun X-W. Acc. Chem. Res. 2008;41:831. doi: 10.1021/ar7002623.. de Figueiredo RM. Angew. Chem., Int. Ed. 2009;48:1190. doi: 10.1002/anie.200804362.. Cardona F, Goti A. Nat. Chem. 2009;1:269. doi: 10.1038/nchem.256..
- (2).For leading references on recent Ritter-type diamination see: Li G, Kim SH, Wei H-X. Tetrahedron Lett. 2000;41:8699.. Booker-Milburn KI, Guly DJ, Cox B, Procopiou PA. Org. Lett. 2003;5:3313. doi: 10.1021/ol035374m..
- (3).For examples of metal-mediated diaminations, see: Tl: Aranda VG, Barluenga J, Aznar F. Synthesis. 1974:504.. Os: Chong AO, Oshima K, Sharpless KB. J. Am. Chem. Soc. 1977;99:3420.. Muñiz K. Eur. J. Org. Chem. 2004:2243.. Hg: Barluenga J, Alonso-Cires L, Asensio G. Synthesis. 1979:962.. Co: Becker PN, White MA, Bergman RG. J. Am. Chem. Soc. 1980;102:5676.. Mn: Fristad WE, Brandvold TA, Peterson JR, Thompson SR. J. Org. Chem. 1985;50:3647..
- (4).For recent Cu(II)-mediated intramolecular diamination, see: Zabawa TP, Kasi D, Chemler SR. J. Am. Chem. Soc. 2005;127:11250. doi: 10.1021/ja053335v.. Zabawa TP, Chemler SR. Org. Lett. 2007;9:2035. doi: 10.1021/ol0706713..
- (5).For Rh(II), Cu(I), and Fe(III)-catalyzed diamination with TsNCl2 or TsNBr2, see: Li G, Wei H-X, Kim SH, Carducci MD. Angew. Chem., Int. Ed. 2001;40:4277. doi: 10.1002/1521-3773(20011119)40:22<4277::AID-ANIE4277>3.0.CO;2-I.. Wei H-X, Kim SH, Li G. J. Org. Chem. 2002;67:4777. doi: 10.1021/jo0200769.. Han J, Li T, Pan Y, Kattuboina A, Li G. Chem. Biol. Drug. Des. 2008;71:71. doi: 10.1111/j.1747-0285.2007.00604.x..
- (6).For a recent Au(I)-catalyzed intramolecular diamination of allenes via dihydroamination, see: Li H, Widenhoefer RA. Org. Lett. 2009;11:2671. doi: 10.1021/ol900730w..
- (7).Bäckvall J-E. Tetrahedron Lett. 1978:163. [Google Scholar]
- (8).Bar GLJ, Lloyd-Jones GC, Booker-Milburn KI. J. Am. Chem. Soc. 2005;127:7308. doi: 10.1021/ja051181d. [DOI] [PubMed] [Google Scholar]
- (9).For leading reviews, see: Hegedus LS. Tetrahedron. 1984;40:2415.. Müller TE, Beller M. Chem. Rev. 1998;98:675. doi: 10.1021/cr960433d..
- (10).(a) Streuff J, Hövelmann CH, Nieger M, Muñiz K. J. Am. Chem. Soc. 2005;127:14586. doi: 10.1021/ja055190y. [DOI] [PubMed] [Google Scholar]; (b) Muñiz K. J. Am. Chem. Soc. 2007;129:14542. doi: 10.1021/ja075655f. [DOI] [PubMed] [Google Scholar]; (c) Muñiz K, Hövelmann CH, Streuff J. J. Am. Chem. Soc. 2008;130:763. doi: 10.1021/ja075041a. [DOI] [PubMed] [Google Scholar]; (d) Hövelmann CH, Streuff J, Brelot L, Muñiz K. Chem. Commun. 2008:2334. doi: 10.1039/b719479j. [DOI] [PubMed] [Google Scholar]; (e) Muñiz K, Hövelmann CH, Campos-Gómez E, Barluenga J, González JM, Streuff J, Nieger M. Chem. Asian J. 2008;3:776. doi: 10.1002/asia.200700373. [DOI] [PubMed] [Google Scholar]; (f) Muñiz K, Streuff J, Chávez P, Hövelmann CH. Chem. Asian J. 2008;3:1248. doi: 10.1002/asia.200800148. [DOI] [PubMed] [Google Scholar]
- (11).For a theoretical study, see: Yu Y, Shen W, Zhang J, He R, Li M. J. Phys. Org. Chem. 2008;21:979.. Yu H, Fu Y, Guo Q, Lin Z. Organometallics. 2009;28:4507..
- (12).For a related Ni(II)-catalyzed diamination, see: Muñiz K, Streuff J, Hövelmann CH, Núñez A. Angew. Chem., Int. Ed. 2007;46:7125. doi: 10.1002/anie.200702160.. Muñiz K, Hövelmann CH, Streuff J, Campos-Gómez E. Pure Appl. Chem. 2008;80:1089..
- (13).For additional leading references on oxidation of Pd(II) to Pd(IV) and subsequent displacement of Pd with heteroatom nucleophiles, see: Bäckvall J-E. Acc. Chem. Res. 1983;16:335.. Yoneyama T, Crabtree RH. J. Mol. Catal. A. 1996;108:35.. Dick AR, Hull KL, Sanford MS. J. Am. Chem. Soc. 2004;126:2300. doi: 10.1021/ja031543m.. Desai LV, Hull KL, Sanford MS. J. Am. Chem. Soc. 2004;126:9542. doi: 10.1021/ja046831c.. Alexanian EJ, Lee C, Sorensen EJ. J. Am. Chem. Soc. 2005;127:7690. doi: 10.1021/ja051406k.. Giri R, Liang J, Lei J-G, Li J-J, Wang D-H, Chen X, Naggar IC, Guo C, Foxman BM, Yu J-Q. Angew. Chem., Int. Ed. 2005;44:7420. doi: 10.1002/anie.200502767.. Dick AR, Kampf JW, Sanford MS. J. Am. Chem. Soc. 2005;127:12790. doi: 10.1021/ja0541940.. Wang D-H, Hao X-S, Wu D-F, Yu J-Q. Org. Lett. 2006;8:3387. doi: 10.1021/ol061384m.. Hull KL, Anani WQ, Sanford MS. J. Am. Chem. Soc. 2006;128:7134. doi: 10.1021/ja061943k.. Wan X, Ma Z, Li B, Zhang K, Cao S, Zhang S, Shi Z. J. Am. Chem. Soc. 2006;128:7416. doi: 10.1021/ja060232j.. Liu G, Stahl SS. J. Am. Chem. Soc. 2006;128:7179. doi: 10.1021/ja061706h.. Deprez NR, Sanford MS. Inorg. Chem. 2007;46:1924. doi: 10.1021/ic0620337.. Whitfield SR, Sanford MR. J. Am. Chem. Soc. 2007;129:15142. doi: 10.1021/ja077866q.. Furuya T, Ritter T. J. Am. Chem. Soc. 2008;130:10060. doi: 10.1021/ja803187x.. Wasa M, Yu J-Q. J. Am. Chem. Soc. 2008;130:14058. doi: 10.1021/ja807129e.. Mei T-S, Wang X-S, Yu J-Q. J. Am. Chem. Soc. 2009;131:10806. doi: 10.1021/ja904709b.. Ball ND, Sanford MS. J. Am. Chem. Soc. 2009;131:3796. doi: 10.1021/ja8054595.. Wang X, Mei T-S, Yu J-Q. J. Am. Chem. Soc. 2009;131:7520. doi: 10.1021/ja901352k.. Racowski JM, Dick AR, Sanford MS. J. Am. Chem. Soc. 2009;131:10974. doi: 10.1021/ja9014474..
- (14).Sibbald PA, Michael FE. Org. Lett. 2009;11:1147. doi: 10.1021/ol9000087. [DOI] [PubMed] [Google Scholar]
- (15).(a) Shi Y. Acc. Chem. Res. 2004;37:488. doi: 10.1021/ar030063x. [DOI] [PubMed] [Google Scholar]; (b) Wong OA, Shi Y. Chem. Rev. 2008;108:3958. doi: 10.1021/cr068367v. [DOI] [PubMed] [Google Scholar]
- (16).For examples of cis-aminopalladation, see: Isomura K, Okada N, Saruwatari M, Yamasaki H, Taniguchi H. Chem. Lett. 1985:385.. Ney JE, Wolfe JP. Angew. Chem., Int. Ed. 2004;43:3605. doi: 10.1002/anie.200460060.. Brice JL, Harang JE, Timokhin VI, Anastasi NR, Stahl SS. J. Am. Chem. Soc. 2005;127:2868. doi: 10.1021/ja0433020.. (d) ref. 13k. Fritz JA, Nakhla JS, Wolfe JP. Org. Lett. 2006;8:2531. doi: 10.1021/ol060707b..
- (17).For the preparation of di-tert-butyldiaziridinone (5), see: Greene FD, Stowell JC, Bergmark WR. J. Org. Chem. 1969;34:2254.. Du H, Zhao B, Shi Y. Org. Synth. 2009;86:315..
- (18).For a leading review on diaziridinones, see: Heine HW. In: The Chemistry of Heterocyclic Compounds. Hassner A, editor. John Wiley & Sons, Inc; New York: 1983. p. 547..
- (19).Du H, Zhao B, Shi Y. J. Am. Chem. Soc. 2007;129:762. doi: 10.1021/ja0680562. [DOI] [PubMed] [Google Scholar]
- (20).For leading reviews on mechanistic studies on transition-metal catalysis, see: Masel RI. Chemical Kinetics and Catalysis. Wiley-Interscience; New York: 2001. . Bhaduri S, Mukesh D. Homogeneous Catalysis: Mechanisms and Industrial Applications. Wiley-Interscience; New York: 2000. . Heaton B. Mechanisms in Homogeneous Catalysis: a Spectroscopic Approach. Wiley-VCH; Weinheim: 2005. . Blackmond DG. Angew. Chem., Int. Ed. 2005;44:4302. doi: 10.1002/anie.200462544..
- (21).For a leading review on mechanistic studies of Pd-catalyzed reactions, see: Amatore C, Jutand A. Acc. Chem. Res. 2000;33:314. doi: 10.1021/ar980063a..
- (22).For leading references on mechanistic studies of Pd-catalyzed reactions, see: Hartwig JF, Paul F. J. Am. Chem. Soc. 1995;117:5373.. Goodson FE, Wallow TI, Novak BM. J. Am. Chem. Soc. 1997;119:12441.. Marcone JE, Moloy KG. J. Am. Chem. Soc. 1998;120:8527.. Alcazar-Roman LM, Hartwig JF, Rheingold AL, Liable-Sands LM, Guzei IA. J. Am. Chem. Soc. 2000;122:4618.. Shultz CS, Ledford J, DeSimone JM, Brookhart M. J. Am. Chem. Soc. 2000;122:6351. doi: 10.1021/ja0160896.. Rosner T, Bars JL, Pfaltz A, Blackmond DG. J. Am. Chem. Soc. 2001;123:1848. doi: 10.1021/ja003191e.. McGuinness DS, Saendig N, Yates BF, Cavell KJ. J. Am. Chem. Soc. 2001;123:4029. doi: 10.1021/ja003861g.. Rosner T, Pfaltz A, Blackmond DG. J. Am. Chem. Soc. 2001;123:4621. doi: 10.1021/ja005872f.. Stambuli JP, Bühl M, Hartwig J,F. J. Am. Chem. Soc. 2002;124:9346. doi: 10.1021/ja0264394.. Singh UK, Strieter ER, Blackmond DG, Buchwald SL. J. Am. Chem. Soc. 2002;124:14104. doi: 10.1021/ja026885r.. de K. Lewis AK, Caddick S, Cloke FGN, Billingham NC, Hitchcock PB, Leonard J. J. Am. Chem. Soc. 2003;125:10066. doi: 10.1021/ja035565k.. Strieter ER, Blackmond DG, Buchwald SL. J. Am. Chem. Soc. 2003;125:13978. doi: 10.1021/ja037932y.. Steinhoff BA, Guzei IA, Stahl SS. J. Am. Chem. Soc. 2004;126:11268. doi: 10.1021/ja049962m.. Mueller JA, Goller CP, Sigman MS. J. Am. Chem. Soc. 2004;126:9724. doi: 10.1021/ja047794s.. Shekhar S, Ryberg P, Hartwig JF. Org. Lett. 2006;8:851. doi: 10.1021/ol0528890.. Nakhla JS, Kampf JW, Wolfe JP. J. Am. Chem. Soc. 2006;128:2893. doi: 10.1021/ja057489m.. Rubina M, Conley M, Gevorgyan V. J. Am. Chem. Soc. 2006;128:5818. doi: 10.1021/ja060085p.. Shekhar S, Ryberg P, Hartwig JF, Mathew JS, Blackmond DG, Strieter ER, Buchwald SL. J. Am. Chem. Soc. 2006;128:3584. doi: 10.1021/ja045533c.. Cochran BM, Michael FE. J. Am. Chem. Soc. 2008;130:2786. doi: 10.1021/ja0734997.. Erhardt S, Grushin VV, Kilpatrick AH, Macgregor SA, Marshall WJ, Roe DC. J. Am. Chem. Soc. 2008;130:4828. doi: 10.1021/ja078298h.. Desai LV, Stowers KJ, Sanford MS. J. Am. Chem. Soc. 2008;130:13285. doi: 10.1021/ja8045519.. Gordillo Á, de Jesús E, López-Mardomingo C. J. Am. Chem. Soc. 2009;131:4584. doi: 10.1021/ja900333x.. Alvaro E, Hartwig JF. J. Am. Chem. Soc. 2009;131:7858. doi: 10.1021/ja901793w.. Barrios-Landeros F, Carrow BP, Hartwig JF. J. Am. Chem. Soc. 2009;131:8141. doi: 10.1021/ja900798s..
- (23).For asymmetric process of this diamination, see: Du H, Yuan W, Zhao B, Shi Y. J. Am. Chem. Soc. 2007;129:11688. doi: 10.1021/ja074698t.. Xu L, Shi Y. J. Org. Chem. 2008;73:749. doi: 10.1021/jo702167u..
- (24).For related Pd(0)-catalyzed intermolecular allylic and homoallylic C-H diamination of terminal olefins using di-tert-butyldiaziridinone, see: Du H, Yuan W, Zhao B, Shi Y. J. Am. Chem. Soc. 2007;129:7496. doi: 10.1021/ja072080d.. Du H, Zhao B, Shi Y. J. Am. Chem. Soc. 2008;130:8590. doi: 10.1021/ja8027394..
- (25).For Pd(0)-catalyzed dehydrogenative diamination of terminal olefins using di-tert-butylthiadiaziridine 1,1-dioxide, see: Wang B, Du H, Shi Y. Angew. Chem., Int.Ed. 2008;47:8224. doi: 10.1002/anie.200803184..
- (26).For Cu(I)-catalyzed intermolecular diamination with di-tert-butyldiaziridinone and related compounds, see: Yuan W, Du H, Zhao B, Shi Y. Org. Lett. 2007;9:2589. doi: 10.1021/ol071105a.. Zhao B, Yuan W, Du H, Shi Y. Org. Lett. 2007;9:4943. doi: 10.1021/ol702061s.. Zhao B, Du H, Shi Y. Org. Lett. 2008;10:1087. doi: 10.1021/ol702974s.. Du H, Zhao B, Yuan W, Shi Y. Org. Lett. 2008;10:4231. doi: 10.1021/ol801605w.. Wen Y, Zhao B, Shi Y. Org. Lett. 2009;11:2365. doi: 10.1021/ol900808z..
- (27).All the solvents were dried according to standard drying procedures. It was found that moisture in the solvent could lead to the decomposition of di-tert-butyldiaziridinone (5) and deactivation of the Pd catalyst.
- (28).Trost BM, Mignani S. J. Org. Chem. 1986;51:3435. [Google Scholar]
- (29).(a) Tolman CA, Seidel WC, Gerlach DH. J. Am. Chem. Soc. 1972;94:2669. [Google Scholar]; (b) Mann BE, Musco A. J. Chem. Soc. Dalton Trans. 1975:1673. [Google Scholar]
- (30).(a) Fauvarque J-F, Pflüger F, Troupel M. J. Organomet. Chem. 1981;208:419. [Google Scholar]; (b) Amatore C, Pflüger F. Organometallics. 1990;9:2276. [Google Scholar]
- (31).For leading references on metal insertion into N-N bonds, see: Erker G, Froemberg W, Krueger C, Raabe E. J. Am. Chem. Soc. 1988;110:2400.. Kitamura M, Yanagisawa H, Yamane M, Narasaka K. Heterocycles. 2005;65:273.. Hoover JM, DiPasquale A, Mayer JM, Michael FE. Organometallics. 2007;26:3297.. Hoover JM, Freudenthal J, Michael FE, Mayer JM. Organometallics. 2008;27:2238..
- (32).The insertion of Ni and Pd to the N-N bond of diaziridinone has been reported, see: Komatsu M, Tamabuchi S, Minakata S, Ohshiro Y. Heterocycles. 1999;50:67.. However, to the best of our knowledge, the reaction of the resulting complex with an olefin has not been reported.
- (33).For a recent leading review on π-allyl Pd chemistry, see: Trost BM. J. Org. Chem. 2004;69:5813. doi: 10.1021/jo0491004..
- (34).For a recent book on Pd, see: Tsuji J. Palladium Reagents and Catalysts: New Perspective for the 21st Century. John Wiley & Sons Ltd; 2004. .
- (35).The precise understanding for the first C-N formation and the origin of the observed high regioselectivity awaits further studies.
- (36).It appears that the cyclization for the five-membered ring is kinetically favored with no seven-membered ureas being observed.
- (37).For a leading reference, see: Paul F, Fischer J, Ochsenbein P, Osborn JA. C. R. Chimie. 2002;5:267..
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.