Abstract
Purpose
To evaluate the efficacy of early sutureless amniotic membrane transplantation in the management of severe bacterial keratitis to reduce pain, inflammation, and haze, and to promote healing.
Method
A noncomparative case series including 3 eyes of 3 consecutive patients with severe bacterial keratitis exhibiting persistent epithelial defect/ulcer, more than 5 mm in diameter, located within 3mm from the visual axis with infiltration occupying more than 50% of the corneal thickness. They were retrospectively reviewed following early (ie, within 96 hours) sutureless amniotic membrane transplantation via ProKera together with selective topical antibiotics and preservative-free steroid. Pain relief, inflammation, haze, and corneal epithelial healing were monitored.
Results
ProKera was inserted once in 1 eye and twice in the other 2 eyes. Pain was significantly relieved and inflammation was markedly reduced in all cases. The corneal epithelial defect and stromal ulceration rapidly healed while visual acuity improved in 2 of the 3 eyes.
Conclusion
Temporary sutureless amniotic membrane transplantation via ProKera allows easy insertion and replacement of the membrane in the office, as well as early intervention to promote epithelialization, reduce pain, haze and inflammation in cases with severe bacterial keratitis. This result justifies large series controlled studies in the future.
Keywords: amniotic membrane, bacterial keratitis, sutureless, wound healing
Microbial keratitis (caused by bacteria, fungi, viruses, or parasites) is a serious ocular infection resulting in persistent epithelial defect/ulcer at the acute phase but corneal scarring and a permanent loss of vision at the chronic phase. The principal therapeutic goals for infectious keratitis are to eliminate the pathogens and to prevent irreversible corneal structural damage.1 Corneal destruction may be caused directly by infectious agents and/or by the associated inflammatory response. Severe bacterial keratitis requires immediate treatment with intensive topical broad-spectrum antibiotics, which are potentially toxic to the corneal epithelium and contribute to a prolonged corneal epithelial defect.2
Amniotic membrane transplantation (AMT) with sutures has been used as an adjuvant treatment for acute severe infectious keratitis.2–4 It counterbalances the epithelial toxicity of fortified antibiotic eye drops while exerting as-yet unclear antimicrobial actions5,6 and acting as a long-term drug delivery system.2 AMT can also directly promote rapid epithelialization and reduce stromal inflammation and ulceration in experimental bacterial keratitis.7 Therefore, AMT may also allow an earlier use of topical steroids, which may prevent excessive scar formation. Although the benefits of early performed AMT are to be considered in terms of reducing the duration of hospitalization and improving the patient compliance to treatment, it may also add an extra cost when performed in the operating room.4 ProKera, which is a single sheet of cryopreserved amniotic membrane (AM) clipped into a dual polycarbonate ring system, can be inserted in the office to deliver AM's anti-inflammatory action without delay and might thus circumvent the above disadvantages. The ProKera is manufactured with the epithelial side up when in contact with the ocular surface. Herein we reported our early experience in using ProKera in 3 consecutive cases with severe microbial keratitis. Its potential efficacy of avoiding prolonged antimicrobial agents and in adding preservative free steroid is further discussed in an attempt of calling for future controlled studies in developing a new regimen for the treatment of severe infectious keratitis.
PATIENTS AND METHODS
This study was approved by the ethics committee of Ocular Surface Research and Education Foundation (Miami, FL) to retrospectively review the medical records of 3 patients (3 eyes) with severe bacterial keratitis that were seen at Ocular Surface Center (Miami, FL). These cases were considered severe because the ulcer had a diameter of more than 5 mm and a depth of more than 50% of the cornea, was located within 3 mm from the visual axis, or had a moderate to severe anterior chamber reaction.5 As a routine, the standard microbiologic workup included smears and cultures to identify the pathogen. Patients had been treated with a combination of 50 mg/mL vancomycin and 40 mg/mL tobramycin instilled alternatively every hour for 48 hours or until the pathogen was identified, at which time topical antibiotic therapy was modified if necessary based on the microbiologic result including the sensitivity. When the infectious activity appeared to be suppressed while the improvement of wound healing was not apparent, a written consent was obtained to insert ProKera obtained from Bio-Tissue (Miami, FL).
After being thawed in the room temperature for few minutes, ProKera was inserted in the office after topical application of 0.5% proparacaine hydrochloride eye drops (Akron, Buffalo Grove, IL) into the superior fornix while holding the upper eyelid, and slid under the lower lid while the patient looked down. Topical 0.1% preservative free dexamethasone phosphate 4 times daily was initiated 48 hours later and tapered when the healing was complete. Patients were examined daily for the first week, every other day during the second week and once weekly thereafter unless otherwise required. On each visit, patients were asked about changes in their symptoms. Although fluorescein staining can be observed through ProKera, it was removed for photographic documentation of the healing pattern and reinserted afterwards. If the membrane in the ProKera dissolved or became cloudy, a new one was inserted. After significant healing of the ocular surface was noted, topical medications were tapered off and ProKera was switched to a bandage contact lens. The main outcome measures included pain, inflammation, and corneal wound healing.
RESULTS
Insertion, exchange, and removal of ProKera were easily done in all patients without any difficulty. On the first day after insertion, all 3 patients reported a significant relief of pain and light sensitivity. ProKera was inserted once in patient 1 and twice in the other 2 patients. In patient 2, the membrane fastened on ProKera dissolved presumably due to intense corneal infiltration; in patient 3, the membrane of ProKera became cloudy due to accumulation of inflammatory cells, a phenomenon also reported when used to treat acute chemical burns.8 Within few days after insertion, ocular surface inflammation was consistently reduced while corneal ulcers started to heal centripetally in all eyes. The patients' subjective symptoms significantly improved thereafter. The visual acuity increased in 2 cases, but unchanged in the third case because of residual central corneal opacity.
Patient 1: Staphylococcus Keratitis
A 61-year-old woman presented with severe left ocular pain, left hemicranial headache, and loss of vision in her left eye for 2 weeks. She had suffered from dry eye and ocular irritation for the last 8 years after bilateral hyperopic laser-assisted in situ keratomileusis in both eyes, and received flap lifting for epithelial ingrowth in the right eye. She was treated with topical 0.5% loteprednol etabonate (Bausch & Lomb Pharmaceuticals, Tampa, FL), neomycin, and polymyxin eye drops without help. On initial examination, her best corrected visual acuity was 20/30 in the right eye and hand motion in the left eye. The left eye showed diffuse conjunctival inflammation with limbal ciliary injection, a 5-mm corneal ulcer with surrounding stromal haze, and a layered hypopyon to the lower one-quarter of the anterior chamber (Fig. 1A and B). Intraocular pressure was normal in both eyes.
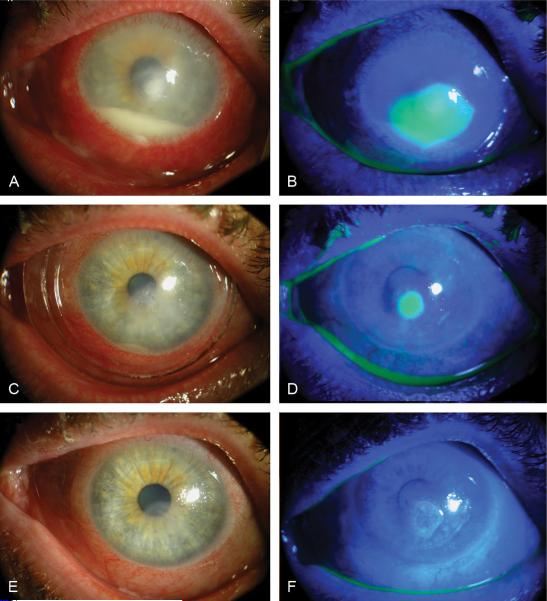
FIGURE 1.
Clinical photographs of patient 1 before insertion (A, B), and at day 4 (C, D) and day 14 (E, F) after ProKera insertion. Diffuse conjunctival inflammation with limbal ciliary injection and a layered hypopyon (A, B) resolved while corneal epithelialization started centripetally from a 5-mm corneal ulcer 4 days (C, D), and completely epithelialized 14 days (E, F) after ProKera insertion.
Because the corneal sensation was decreased, the patient was put on alternating topical 50 mg/mL vancomycin and 40 mg/mL tobramycin eye drops hourly together with oral Acyclovir 200 mg 5 times/day. Corneal scrapping and culture showed Staphylococcus aureus. Two days after treatment, pain and inflammation were getting worse, the epithelial defect was 1 mm larger, and the eye pressure increased. Four days after treatment, repeated culture showed light growth, but the epithelial defect and inflammation remained unchanged. ProKera was inserted under topical anesthesia in the office and the fortified eye drops was replaced with tobramycin every 4 hours. The second day after ProKera insertion, pain was markedly decreased. On day 4 post insertion, conjunctival inflammation was reduced and corneal epithelialization started centripetally (Fig. 1C and D). On day 8, ProKera was replaced with a therapeutic bandage contact lens and tobramycin eye drops was replaced by 0.3% gatifloxacin (Allergan, Irvine, CA) eye drops and 0.1 % preservative-free dexamethasone phosphate eye drops. On day 14, the corneal surface completely healed (Fig. 1E and F) and the visual acuity improved to 20/30. Dexamethasone eye drops were tapered off.
Patient 2: Staphylococcus Keratitis
A 58-year-old woman, a contact lens wearer for about 20 years, presented with severe left ocular pain, photophobia, and loss of vision for 2 weeks. She had been diagnosed as severe bacterial keratitis and treated topically with unknown fortified antibiotic eye drops for 2 weeks. Corneal scrapping for culture and smears showed S. aureus, and was negative for Acanthamoeba and fungal organisms. She was referred for further management due to the lack of improvement. On initial examination, her best corrected visual acuity was 20/20 in the right eye and hand motion in the left eye. The left eye showed diffuse severe inflammation in both bulbar and tarsal conjunctiva, limbal injection, and a 4-mm paracentral corneal ulcer surrounded by 2 mm of whitish scattered ring infiltration encroaching the visual axis, corneal stromal edema, and haziness (Fig. 2A and B). The intraocular pressure was normal in both eyes.
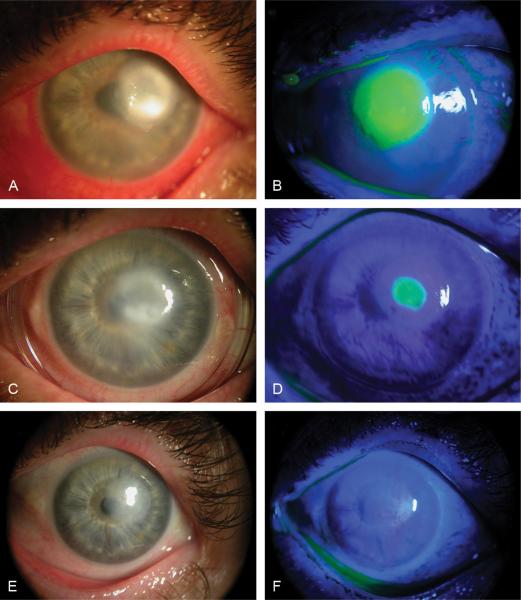
FIGURE 2.
Clinical photographs of patient 2 before insertion (A, B), at day 5 after first insertion (C, D), and at day 14 (E, F) after second ProKera insertion. Severe conjunctival inflammation, limbal injection, and a 4-mm paracentral corneal ulcer surrounded by 2-mm whitish scattered ring infiltration (A, B) were reduced, while corneal epithelialization started centripetally 5 days (C, D) after first ProKera insertion and completely epithelialized 14 days (E, F) after second insertion.
The patient was put on alternating topical 50 mg/mL vancomycin and 40 mg/mL tobramycin eye drops hourly. Four days later, the culture was negative after the antibiotic therapy was suspended for 24 hours. Because the patient still complained of blurry vision, severe pain and light sensitivity, ProKera was inserted in the left eye under topical anesthesia and topical medication was switched to polymyxin eye drops 4 times/day. The pain rapidly subsided on day 1. Conjunctival inflammation was reduced and corneal epithelialization started centripetally on day 3 postinsertion, when preservative-free 0.1% dexamethasone eye drops were added 4 times a day. On day 5, the patient had no pain, ocular surface inflammation was notable reduced, and the epithelial healing progressed centripetally (Fig. 2C and D). On day 7, the amniotic membrane dissolved and ProKera was replaced with a therapeutic bandage contact lens. However, the epithelial defect enlarged on day 9; therefore, a new ProKera was inserted. At day 14 after the second insertion, the corneal surface was healed completely, leaving a 2-mm paracentral faint cornea haziness, and her best corrected visual acuity improved to 20/70 (Fig. 2E and F).
Patient 3: Pseudomonas Keratitis
A 51-year-old man presented with left ocular pain, mild photophobia, and loss of vision in the left eye due to refractory corneal hypopyon ulcer for 2 months. His past history was significant for a left vitrectomy performed 6 months ago for retinal detachment, which was complicated with hypotony and cataract formation. Three months after surgery, he developed left corneal ulcer and was treated empirically with amphotericin B eye drops for 2 weeks, resulting in some improvement. Subsequently, his condition deteriorated; smears and cultures revealed Pseudomonas aeruginosa.
The patient was treated with a combination of 0.3% gatifloxacin (Allergan, Irvine, CA) and 5% natamycin eye drops for 2 weeks without improvement. On initial presentation, visual acuity was 20/40 in the right eye and light perception in the left eye. The left eye showed severe diffuse conjunctival and limbal inflammation, severe corneal edema and haziness, a large 9-mm corneal epithelial defect with necrotic infiltration and thinning mostly at the temporal border of the ulcer, a 360-degree superficial and midstromal corneal neovascularization, and a layered hypopyon up to the lower one-quarter of the anterior chamber (Fig. 3A and B). Anterior segment ischemia with secondary bacterial infection was suspected. All medications were discontinued and preservative-free 0.1% dexamethasone phosphate drops every 2 hours was given for 24 hours and then reduced to 4 times a day.
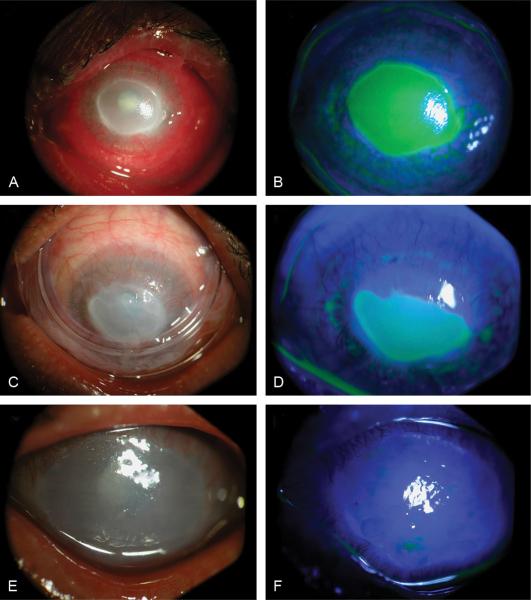
FIGURE 3.
Clinical photographs of patient 3 before insertion (A, B), at day 10 after ProKera insertion (C, D), and at day 8 after AMT (E, F). Diffuse conjunctival and limbal inflammation, severe corneal edema and haziness, and a layered hypopyon (A, B) were resolved while corneal epithelialization for a 9-mm corneal epithelial defect started centripetally few days after ProKera insertion. On day 10, the epithelial healing did not progress because of poor apposition of ProKera to the cornea due to lower lid ectropion (C, D). Eight days after AMT, epithelialization was completed and peripheral corneal vascularization regressed (E, F).
Knowing that the ulcer did not deteriorate or improve after 2 days with 0.3% ofloxacin (Allergan, Irvine, CA) eye drops 4 times a day, ProKera was inserted. On day 2 after insertion, pain disappeared, inflammation and epithelial defect decreased, hypopyon vanished but corneal thinning remained unchanged. On day 7, the inflammation was much reduced, but ProKera became cloudy and necessitated exchange. On day 10, the epithelial healing did not progress because of poor apposition of ProKera to the cornea due to lower lid ectropion (Fig. 3C and D). Therefore, amniotic membrane transplantation using sutures together with small nasal tarsorrhaphy was performed. The same medications were continued after surgery. Eight days later, epithelialization was completed, sutured membrane dissolved, peripheral corneal vascularization regressed (Fig. 3E and F), and his visual acuity remained hand motion. A therapeutic bandage contact lens was inserted and medications were tapered. His corneal surface remained stable 3 months later.
DISCUSSION
After the infectious pathogen is identified by microbiological workup, topical antimicrobial therapies take precedence to sterilize the corneal lesion. The moment when the infectious process appears to be controlled, a dilemma frequently emerges in deciding how to curtail tissue inflammation and destruction, especially for severe sight-threatening keratitis. Theoretically, the corneal tissue can be damaged by enzymes released by microbes as well as by infiltrating inflammatory and immune cells. Subsequent repair involves a complex interplay of resident and infiltrating cells when the infectious agent is eradicated. Several questions remain unanswered. For example, how long should fortified antimicrobials be instituted, knowing that many of them are also cytotoxic to host cells? When could topical steroid be initiated, knowing that it potentially hampers the host immunity against microbes?
Based on the inherent biological actions known to amniotic membrane,9–11 several investigators have explored the clinical efficacy of deploying AMT as an adjunctive therapy to reduce inflammation and to promote healing at an early stage. Together with appropriate antimicrobials, favorable results have been obtained in experimental necrotizing stromal keratitis caused by herpes simplex virus (HSV)-1,12,13 and by Staphylococcus aureus.7 It remains unclear whether change of antibiotics, based on the sensitivity, in such a small sample size may interfere with the interpretation of the healing effect of ProKera on the epithelial defect. This question can only be addressed in future studies with a larger sample size. Additionally, AMT has also been shown to reduce pain significantly and promote healing in human bacterial keratitis,4 and to prevent corneal perforation in human acute fungal keratitis.14 Consistent with all these published results by AMT using sutures,2,4 we also noted that early sutureless AMT via insertion of ProKera facilitated resolution of pain and inflammation and promoted rapid epithelialization in 3 consecutive cases with severe microbial keratitis. Although pain scoring questionnaire was not performed, no pain killers were administered.
Besides the ease of allowing timely administration in the office, hence reducing the overall surgical cost, the sutureless approach via insertion of ProKera offers another important advantage over the sutured method used in experimental7 and human2,4 infectious keratitis. That is, the already inflamed and necrotic corneal tissue may not be amenable to hold sutures, which potentially attract more inflammation and vascularization. We were intrigued by the worsening in patient 2 when switched to a bandage contact lens and subsequently improved by reinserting ProKera. This finding not only suggested that although bandage contact lens, known to provide the protective effect and frequently used in managing nonhealing ulcers, might not be sufficient to exert actions inherently present in AM. One cautious note of using ProKera was illustrated in patient 3, where its effectiveness was hampered when the lower lid ectropion caused its decentration and poor apposition.
Although the exact action mechanism remains to be determined, the aforementioned efficacies may well be associated with AM's antibacterial, anti-inflammatory, and antiscarring properties, and its action of promoting epithelial progenitor cell expansion. 9–11 Interestingly, a rapid relief of pain by AMT has been clinically observed in treating chemical burns8 and Stevens Johnson syndrome with or without toxic epidermal necrolysis,15–17 and at the acute stage, painful bullous keratopathy.18–20 Recently, insertion of a sheet of cryopreserved AM has also been shown to reduce pain that is frequently incurred during irradiation therapy for ocular tumors.21 Although one may attribute AM's effect in relieving pain to its anti-inflammatory action, we suspect that such a rapid (within 24 h) action in pain relief might well be mediated by an as-yet unknown antipain action that deserves further investigation.
When the AM was used as a temporary patch, polymorphonuclear cells rapidly adhere to the stromal side of the AM in rabbit models of alkali burns22 and photorefractive keratectomy by excimer laser ablation,23 and in human patients with chemical burns.8,24 Notably these adherent cells undergo rapid apoptosis.24,25 Similarly, adherent mononuclear cells including lymphocytes and macrophages also undergo rapid apoptosis when adherent onto the AM stroma in a murine model of HSV-induced necrotizing stromal keratitis.12,13 Such a unique anti-inflammatory action of the AM by promoting cellular apoptosis has been recapitulated in an in vitro culturing system using murine macrophages.26 Recently, our laboratory has discovered that such an activity is retained in AM soluble extacts.27 Although the exact molecular mechanism awaits further elucidation, these results indicate that it may be necessary to change the “pus”-accumulated AM during severe acute inflammation to refresh its action. In this regard, the use of ProKera certainly facilitates the ease of such an instant exchange as shown recently in the management of acute chemical burns8 as well as in 2 of our patients in this study.
Clinically, we were assured by the findings that AMT itself does not impede penetration of topical antibiotics,2 and that AM soaked with antibiotics actually prolong the bactericidal effect,28 thus allowing one to use less but avoiding antimicrobial's potential cytotoxic effect. Although we noted that insertion of ProKera as early as 96 hours after intensive fortified antibiotic treatment in patient 1 after the pathogen was identified could deliver AM's desirable actions with success, our small series of 3 patients was still too few to determine whether this is an optimal timing for all infectious keratitis. Similarly, although insertion of ProKera appeared to allow concomitant topical administration of preservative-free steroid in all 3 cases, future studies are still needed to determine whether it is necessary to use steroids at all when ProKera is inserted for managing infectious keratitis. Resolution of these questions through additional controlled studies may allow us to consider the said sutureless AMT as an adjunctive therapy, which for the first time may actively modify corneal wound healing during the course of managing severe infectious keratitis.
Footnotes
Dr. Tseng and his family are more than 5% shareholders of TissueTech, Inc., which owns U.S. patents 6,152,142 and 6,326,019 on the method of preparation and clinical uses of human amniotic membrane and ProKera distributed by Bio-Tissue, Inc. No other author has any proprietary interest in any material mentioned in this study.
REFERENCES
- 1.Slansky HH, Dohlman CH, Berman MB. Prevention of corneal ulcers. Trans Am Acad Ophthalmol. Otolaryngol. 1971;75:1208–1211. [PubMed] [Google Scholar]
- 2.Kim JS, Kim JC, Hahn TW, et al. Amniotic membrane transplantation in infectious corneal ulcer. Cornea. 2001;20:720–726. doi: 10.1097/00003226-200110000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Ma DH-K, Wang SF, Su WY, et al. Amniotic membrane graft for the management of scleral melting and corneal perforation in recalcitrant infectious scleral and corneoscleral ulcers. Cornea. 2002;21:275–283. doi: 10.1097/00003226-200204000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Gicquel JJ, Bejjani RA, Ellies P, et al. Amniotic membrane transplantation in severe bacterial keratitis. Cornea. 2007;26:27–33. doi: 10.1097/ICO.0b013e31802b28df. [DOI] [PubMed] [Google Scholar]
- 5.Talmi YP, Sigler L, Inge E, et al. Antibacterial properties of human amniotic membranes. Placenta. 1991;12:285–288. doi: 10.1016/0143-4004(91)90010-d. [DOI] [PubMed] [Google Scholar]
- 6.Kjaergaard N, Hein M, Hyttel L, et al. Antibacterial properties of human amnion and chorion in vitro. Eur J Obstet Gynecol Reprod Biol. 2001;94:224–229. doi: 10.1016/s0301-2115(00)00345-6. [DOI] [PubMed] [Google Scholar]
- 7.Barequet IS, Habot-Wilner Z, Keller N, et al. Effect of amniotic membrane transplantation on the healing of bacterial keratitis. Invest Ophthalmol Vis Sci. 2008;49:163–167. doi: 10.1167/iovs.07-1005. [DOI] [PubMed] [Google Scholar]
- 8.Kheirkhah A, Johnson DA, Paranjpe DR, et al. Temporary sutureless amniotic membrane patch for acute alkaline burns. Arch Ophthalmol. 2008;126:1059–1066. doi: 10.1001/archopht.126.8.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tseng SCG, Espana EM, Kawakita T, et al. How does amniotic membrane work? Ocular Surface. 2004;2:177–187. doi: 10.1016/s1542-0124(12)70059-9. [DOI] [PubMed] [Google Scholar]
- 10.Dua HS, Gomes JA, King AJ, et al. The amniotic membrane in ophthalmology. Surv Ophthalmol. 2004;49:51–77. doi: 10.1016/j.survophthal.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Bouchard CS, John T. Amniotic membrane transplantation in the management of severe ocular surface disease: indications and outcomes. Ocular Surface. 2004;2:201–211. doi: 10.1016/s1542-0124(12)70062-9. [DOI] [PubMed] [Google Scholar]
- 12.Heiligenhaus A, Meller D, Meller D, et al. Improvement of HSV-1 necrotizing keratitis with amniotic membrane transplantation. Invest Ophthalmol Vis Sci. 2001;42:1969–1974. [PubMed] [Google Scholar]
- 13.Heiligenhaus A, Li H, Hernandez Galindo EE, et al. Management of acute ulcerative and necrotising herpes simplex and zoster keratitis with amniotic membrane transplantation. Br J Ophthalmol. 2003;87:1215–1219. doi: 10.1136/bjo.87.10.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen HC, Tan HY, Hsiao CH, et al. Amniotic membrane transplantation for persistent corneal ulcers and perforations in acute fungal keratitis. Cornea. 2006;25:564–572. doi: 10.1097/01.ico.0000227885.19124.6f. [DOI] [PubMed] [Google Scholar]
- 15.John T, Foulks GN, John ME, et al. Amniotic membrane in the surgical management of acute toxic epidermal necrolysis. Ophthalmology. 2002;109:351–360. doi: 10.1016/s0161-6420(01)00900-9. [DOI] [PubMed] [Google Scholar]
- 16.Di Pascuale MA, Espana EM, Liu DT, et al. Correlation of corneal complications with eyelid cicatricial pathologies in patients with Stevens-Johnson syndrome and toxic epidermal necrolysis syndrome. Ophthalmology. 2005;112:904–912. doi: 10.1016/j.ophtha.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi A, Yoshita T, Sugiyama K, et al. Amniotic membrane transplantation in acute phase of toxic epidermal necrolysis with severe corneal involvement. Ophthalmology. 2006;113:126–132. doi: 10.1016/j.ophtha.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Pires RTF, Tseng SCG, Prabhasawat P, et al. Amniotic membrane transplantation for symptomatic bullous keratopathy. Arch Ophthalmol. 1999;117:1291–1297. doi: 10.1001/archopht.117.10.1291. [DOI] [PubMed] [Google Scholar]
- 19.Espana EM, Grueterich M, Sandoval H, et al. Amniotic membrane transplantation for bullous keratopathy in eyes with poor visual potential. J Cat Refract Surg. 2003;29:279–284. doi: 10.1016/s0886-3350(02)01525-0. [DOI] [PubMed] [Google Scholar]
- 20.Sonmez B, Kim BT, Aldave AJ. Amniotic membrane transplantation with anterior stromal micropuncture for treatment of painful bullous keratopathy in eyes with poor visual potential. Cornea. 2007;26:227–229. doi: 10.1097/01.ico.0000244876.92879.c1. [DOI] [PubMed] [Google Scholar]
- 21.Finger PT. Finger's amniotic membrane buffer technique: protecting the cornea during radiation plaque therapy. Arch Ophthalmol. 2008;126:531–534. doi: 10.1001/archopht.126.4.531. [DOI] [PubMed] [Google Scholar]
- 22.Kim JS, Kim JC, Na BK, et al. Amniotic membrane patching promotes healing and inhibits protease activity on wound healing following acute corneal alkali burns. Exp Eye Res. 2000;70:329–337. doi: 10.1006/exer.1999.0794. [DOI] [PubMed] [Google Scholar]
- 23.Wang MX, Gray TB, Parks WC, et al. Corneal haze and apoptosis is reduced by amniotic membrane matrix in excimer laser photoablation in rabbits. J Cat Refract Surg. 2001;27:310–319. doi: 10.1016/s0886-3350(00)00467-3. [DOI] [PubMed] [Google Scholar]
- 24.Shimmura S, Shimazaki J, Ohashi Y, et al. Antiinflammatory effects of amniotic membrane transplantation in ocular surface disorders. Cornea. 2001;20:408–413. doi: 10.1097/00003226-200105000-00015. [DOI] [PubMed] [Google Scholar]
- 25.Park WC, Tseng SCG. Modulation of acute inflammation and keratocyte death by suturing, blood and amniotic membrane in PRK. Invest Ophthalmol Vis Sci. 2000;41:2906–2914. [PubMed] [Google Scholar]
- 26.Li W, He H, Kawakita T, et al. Amniotic membrane induces apoptosis of interferon-gamma activited macrophages in vitro. Exp Eye Res. 2006;82:282–292. doi: 10.1016/j.exer.2005.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He H, Li W, Chen SY, et al. Amniotic membrane extract suppresses activation and induces apoptosis in RAW264.7 Cells. Invest Ophthalmol Vis Sci. 2008;49:4468–4475. doi: 10.1167/iovs.08-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mencucci R, Menchini U, Dei R. Antimicrobial activity of antibiotic-treated amniotic membrane: An in vitro study. Cornea. 2006;25:428–431. doi: 10.1097/01.ico.0000214207.06952.23. [DOI] [PubMed] [Google Scholar]