Abstract
Rationale
increased sympathetic nerve activity has been linked to the pathogenesis of hypertension in humans and animal models. Enhanced peripheral chemoreceptor sensitivity which increases sympathetic nerve activity has been observed in established hypertension but has not been identified as a possible mechanism for initiating an increase in SNA prior to the onset of hypertension.
Objective
we tested this hypothesis by measuring the pH sensitivity of isolated carotid body glomus cells from young spontaneously hypertensive rats (SHR) prior to the onset of hypertension and their control normotensive Wistar Kyoto (WKY) rats.
Methods and Results
we found a significant increase in the depolarizing effect of low pH in SHR versus WKY glomus cells which was caused by overexpression of two acid-sensing non-voltage gated channels. One is the amiloride-sensitive acid-sensing sodium channel (ASIC3) which is activated by low pH and the other is the two-pore domain acid sensing K+ channel (TASK1) which is inhibited by low pH and blocked by quinidine. Moreover we found that the increase in sympathetic nerve activity in response to stimulation of chemoreceptors with sodium cyanide was markedly enhanced in the still normotensive young SHR compared to control WKY rats.
Conclusions
our results establish a novel molecular basis for increased chemotransduction that contributes to excessive sympathetic activity prior to the onset of hypertension.
Keywords: carotid body glomus cells, pH sensitivity, ion channels, sympathetic nerve activity, prehypertensive SHR
Increased sympathetic nerve activity (SNA) has been linked to the pathogenesis of hypertension in humans with essential hypertension (1-4), with borderline hypertension (5) and with obstructive sleep apnea (6). Similarly, exaggerated sympathoadrenal drive is a major determinant of the elevated arterial pressure in the spontaneously hypertensive rat (SHR) model of genetic hypertension (7, 8). There is also evidence that in young SHR there are regional increases in sympathetic activity (9) and changes in norepinephrine levels (10) that occur at 4 to 5 weeks of age prior to the onset of hypertension. In young SHR sympathectomy and prazosin abrogate the subsequent development of hypertension in adult rats (10). Recent studies by Simms et al. (11) also support the view that increased SNA is already present in neonatal SHR prior to the onset of hypertension as evident in the significant enhancement of respiratory-coupled bursts of sympathetic activity compared to WKY rats. Our goal was to identify mechanisms that could result in the initiation of an early increase in SNA in the SHR prior to hypertension.
We considered the possibility that enhanced chemoreceptor activity may be such a mechanism for two reasons. First, the chemoreceptor reflex is enhanced in established hypertension (12-15) and, it increases SNA and blood pressure when activated by hypoxemia, hypercapnia and acidosis (12, 13, 16–19). Second, the increases in SNA reported in the young SHR (9, 10) have been associated with disturbances in acid-base balance prior to the onset of hypertension (20, 21). Such changes in acid-base balance may activate peripheral chemoreceptors, and exaggerate sympathetic drive.
Therefore, in this study we tested the hypothesis that carotid body chemoreceptors are hypersensitive in SHR prior to the onset of hypertension. Cellular and molecular studies on isolated carotid body glomus cells allowed us to define an enhanced responsiveness of specific acid-sensing ion channels and their over-expression in SHR. Additionally, functional studies revealed augmented SNA in response to chemoreceptor stimulation with intraarterial NaCN in prehypertensive SHR compared to WKY rats. The results establish a novel molecular basis for increased chemotransduction that contributes to the initiation of excessive SNA.
Materials and Methods
The work was done on SHR and WKY rats, 4–6 weeks of age. All animal handling and experimental protocols were approved by the animal care and use committee of the University of Iowa. Published protocols for isolation of glomus cells from rat carotid bodies were followed (22, 23). Conventional whole-cell perforated patch clamp recordings from glomus cells provided data on membrane currents and voltage potentials during fast exchanges of various pH solutions extracellularly while intracellular pH was buffered at pH 7.2. Carotid body mRNA and proteins were measured at 1 month of age using real-time RT-PCR, Western blots and immuno-histochemistry to determine the expression of ASICs and TASK channels as reported (23). Using the working heart-brainstem preparation as previously described (11), responses of thoracic sympathetic and phrenic nerve activities to intraaortic sodium cyanide (NaCN) were measured.
An expanded Materials and Methods section is in the Online Supplement Material. This section includes Online Figure I showing age dependent increases in blood pressure in SHR and WKY; Online Table I showing the PCR primer sequences; and Online Table II showing the antibodies used in the study.
The Online Supplement Material also describes results, which include Online Figure II showing a blockade of pH-induced rapid inward currents with amiloride; Online Figure III showing voltage-dependent outward currents blocked by low pH and quinidine; Online Table III showing selective effects of amiloride and quinidine on low pH-induced depolarizations; and Online Table IV showing hemodynamic, autonomic and respiratory responses to chemoreceptor stimulation with NaCN.
Results
There was no significant difference in the size (cell diameter), membrane capacitance, membrane resistance and resting membrane potential between SHR and WKY glomus cells. Values were 9.1±0.2 μm, 4.8±0.3 pF, 3.0±0.3 GΩ and −60±1mV (n=28) in SHR, and 9.1±0.1 μm, 4.7±0.3 pF, 2.6±0.3 GΩ and −57±1mV (n=36) in WKY.
Acid-evoked rapid inward ASIC-like currents
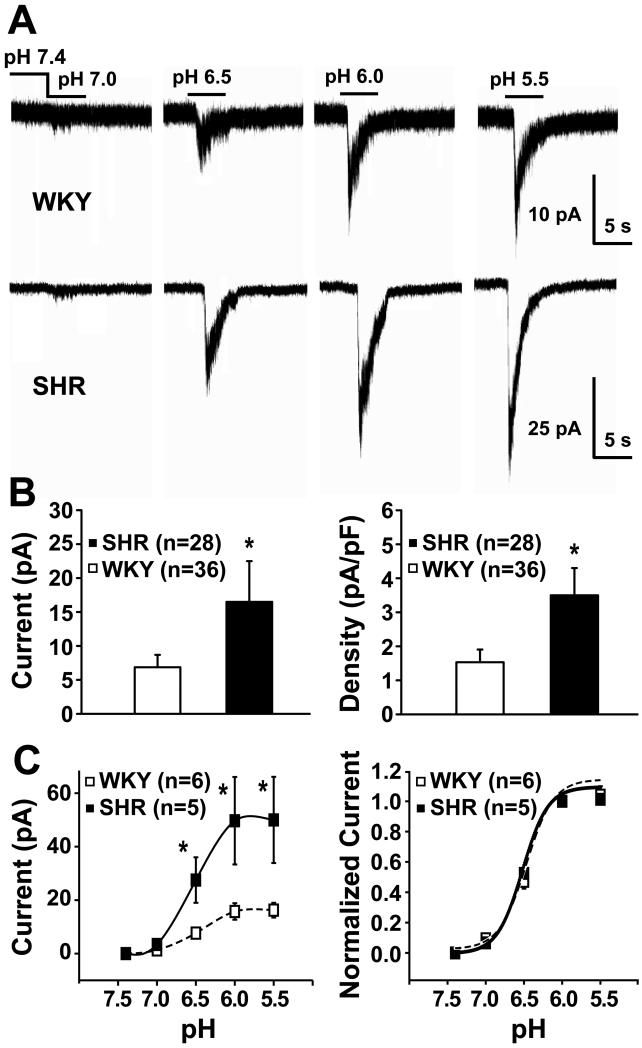
Extracellular solutions at pH 7.0, 6.5, 6.0 and 5.5 triggered pH-dependent initial rapid inward currents that were markedly enhanced in SHR compared with WKY glomus cells (Fig. 1A). Both the amplitude and density of these currents were significantly higher in SHR (Fig 1B). The threshold for activation occurred at about pH 7 or higher, and the currents were half-activated at pH 6.5 (Fig. 1A and 1C). In addition, the rapid inward currents in both SHR and WKY glomus cells were significantly blocked by the ASIC blocker amiloride (200 μmol/L) to 4±0.3% and 13±2% of control values and recovered after amiloride to 98±3% and 95±2% of control values respectively (please refer to the Supplement Material, Online Fig. II).
Figure 1. Comparison of extracellular acid-evoked rapid inward currents in glomus cells from WKY rats vs. SHRs.
A) Representative recordings show incremental acid-evoked currents that are pH-dependent and much greater in a SHR glomus cell than in a WKY cell (note different current scales); B) Mean maximum currents and mean current densities (pA/pF) of pH 6.0-evoked currents were significantly (* P<0.05) enhanced in glomus cells from SHR compared to WKY. Values are means ± SE. There was marked variability in responses among glomus cells and several WKY cells had no response to pH6; C) Highly responsive cells were selected from the upper tertile of each group to test threshold, pH dependence, and sensitivity of the evoked currents. Left: pH-evoked current amplitudes were pH-dependent and significantly greater in SHR (*P<0.05). Right: currents were normalized to the peak response induced by pH 6.0; curves are best fit of Hill equation, I = I0+A/ (1 + ([H+]50/[H+])b), half-activation values were pH 6.5 for both groups; Hill coefficients were 2.6 and 2.4 for SHR and WKY cells, respectively. Values are means ± SE.
Acid-inhibited TASK-like outward background potassium currents
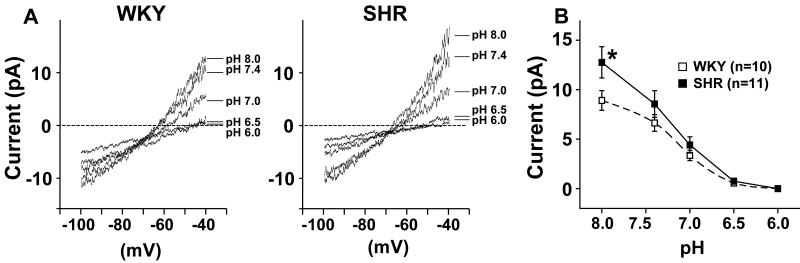
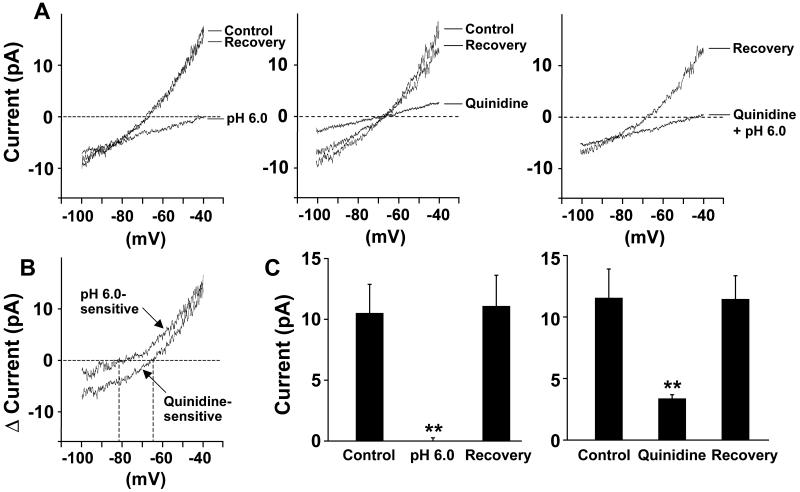
A slow ramp depolarization from −100 mV to −40 mV over a period of 2 seconds was used to record the background outward current (Fig. 2). Outward currents recorded at pH 7.4 in glomus cells from a SHR and a WKY rat were increased by an alkaline pH of 8.0, and were progressively inhibited by acidic pHs of 7.0, 6.5, and 6.0 (Fig 2A and B). At −40 mV and at pH 8.0 the currents were significantly higher in SHR cells compared to WKY and the decrease in current as pH was lowered from 8.0 to 6.5 was significantly greater in SHR vs. WKY cells (ANOVA) (Fig 2B). The TASK channel blocker quinidine (1 mmol/L) reversibly inhibited the outward currents during ramp depolarizations as did low pH (Fig. 3A and 3C). A residual outward current after quinidine was completely inhibited by quinidine plus low pH (Fig. 3A). Fig. 3B shows the pH-inhibited and quinidine-inhibited currents during the ramp depolarizations. The calculated reversal potentials of the pH-inhibited currents in both SHR and WKY coincide with the known reversal potential for K+ channels. Limited contributions by voltage-gated currents to pH responses are discussed and presented in the Online Supplement Material (Online Fig. III).
Figure 2. Effect of pH on TASK-like outward background leak current seen with slow ramp depolarization from −100 to −40 mV.
A) Representative recordings show pH-dependent inhibition of the currents from a WKY and a SHR glomus cell with progressive reductions in pH. The pH-sensitive currents were calculated by subtracting values obtained at pH 6.0 from those at pH 8.0. The reversal potentials of these pH-inhibited currents averaged −80.3±4.2 mV (n=10) and −87.2±4.5 mV (n=11) for WKY and SHR respectively and were not statistically different. These values coincide with the reversal potential of the outward leak K+ currents that have a theoretical reversal potential of −85.7 mV; B) Mean ±SE of outward background currents recorded at −40 mV during exposure to progressively lower levels of pH ranging from pH 8.0 to pH 6.0. The acid-inhibited outward current was significantly enhanced in SHR cells compared to WKY (ANOVA) and the current at pH 8.0 was significantly larger in SHR than in WKY (Bonferroni). Values are means ± SE. * P<0.05 for post-hoc comparison (Bonferroni) between SHR and WKY cells.
Figure 3. Effect of quinidine on the pH sensitive outward background leak current (TASK).
A) Representative recordings from an SHR glomus cell show that both pH 6.0 (left tracing) and 1 mmol/L quinidine (middle tracing) reversibly inhibited the outward background currents during ramp depolarization from −100 to −40 mV. The small residual outward current seen after quinidine was completely inhibited by quinidine plus low pH (right tracing). The rightward shift of the curve with pH 6.0 compared to control is not seen with quinidine, suggesting the presence of a pH-insensitive, but quinidine-sensitive, inward current that is blocked by quinidine.
B) The tracings represent a pH-sensitive current calculated by subtracting values obtained during the ramp depolarizations at pH 6.0 from those obtained at pH 8.0; and the quinidine-sensitive current calculated by subtracting the values during ramp depolarizations at pH 7.4 after quinidine from those before quinidine. The reversal potential of the pH-sensitive current was −82 mV, which coincides with that of K+ current. The quinidine-sensitive net current had a reversal potential of −64 mV reflecting blockade of both the outward K+ current and an inward current which is not sensitive to pH. The mean group value for the quinidine-sensitive current reversal potential was 63.4±2.4 mV (n=5). C) The bar graphs show that the outward currents measured at −40 mV (mean ± SE) are completely inhibited by pH 6.0 followed by recovery (left panel) and markedly reduced by quinidine followed by recovery (right panel). (** P<0.01 for differences from control and from recovery.)
Acid-induced depolarizations
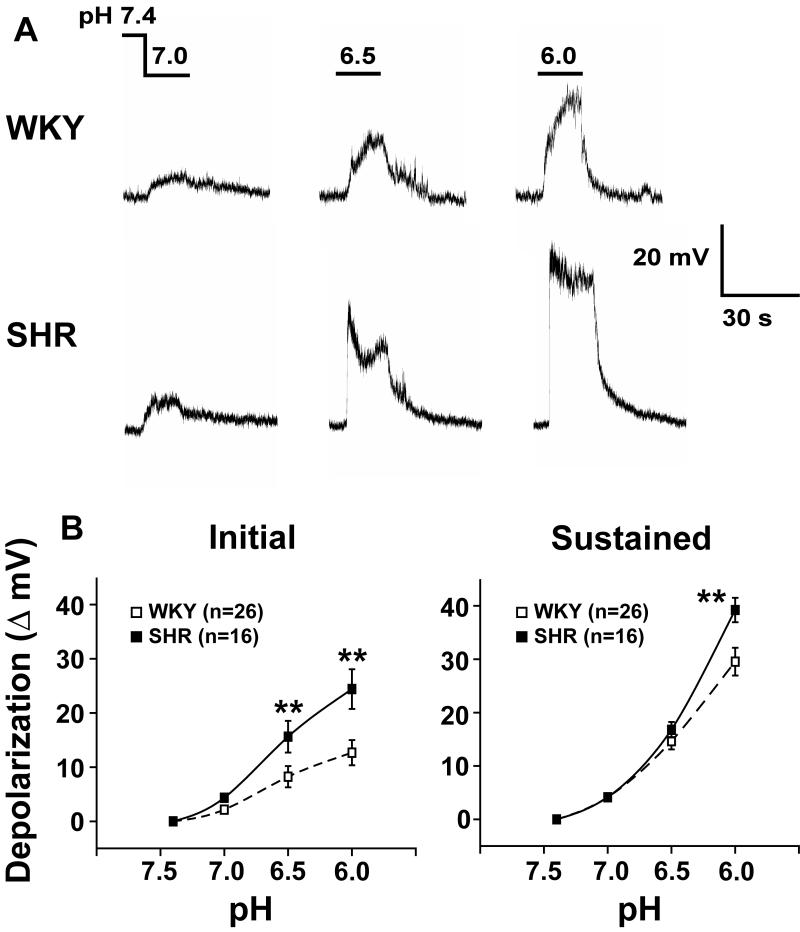
The activation of inward ASIC currents and inhibition of outward TASK currents caused early rapid and subsequent sustained depolarizations, respectively. Under current-clamp conditions, graded pH levels of 7.0, 6.5, and 6.0 were applied. The rapid “initial depolarization” which peaked within 5 seconds was followed by a more “sustained depolarization” that reached a plateau over 15-20 seconds (Fig. 4A). Both the initial and the sustained depolarizations were pH-dependent and were significantly enhanced in glomus cells from SHR vs. WKY rats (ANOVA, Fig.4A and B).
Figure 4. Acid-evoked rapid and sustained depolarizations in glomus cells from WKY rats and SHRs.
A) Tracings show pH dependent depolarizations in a WKY (top) and a SHR (bottom) glomus cell. An abrupt “initial depolarization” peaks rapidly and is followed by a more “sustained depolarization”; B) Both initial rapid (left) and sustained (right) depolarizations were significantly enhanced in SHR compared with WKY (ANOVA, repeated measure, P<0.05). Values are means ± SE. ** P<0.01 for post-hoc comparison (Bonferroni) between SHR and WKY cells.
The ASIC blocker amiloride (200 μmol/L) did not alter resting membrane potential of glomus cells of SHR (−1.5 ± 0.3 mV, n=6) and WKY (−0.8 ± 0.6 mV, n=6) rats, but it specifically and reversibly inhibited (P<0.01) the initial rapid depolarization in response to low pH leaving the slower sustained depolarization intact (Fig 5 and Online Supplement Material, Online Table III). In contrast to amiloride, the TASK blocker quinidine (1 mmol/L) caused depolarizations of the resting membrane potential that were greater in SHR glomus cells (Δ20.6 ± 1.7 mV, n=5) compared to WKY (Δ 16.0 ± 0.8 mV, n=5) (P<0.05) (Fig. 5). Moreover, quinidine reversibly inhibited (P<0.05) the sustained depolarization in response to low pH while the initial depolarization with low pH remained intact (Fig. 5 and Online Supplement Material, Online Table III). The inhibitory effects of both blockers were similar in SHR and WKY.
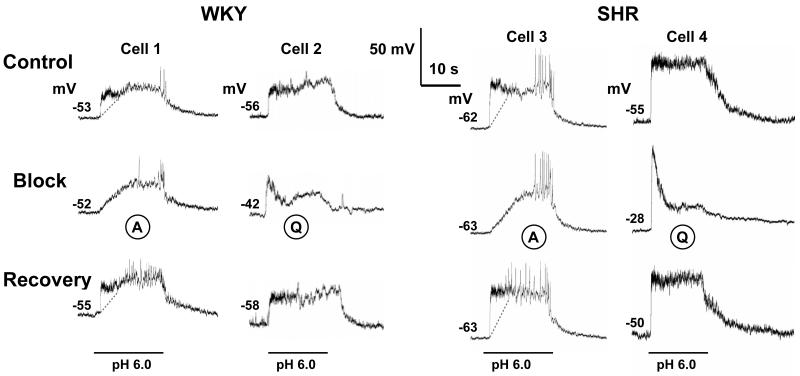
Figure 5. Effects of amiloride and quinidine on initial rapid and sustained depolarizations of glomus cells evoked by pH 6.0 (horizontal bars).
Tracings represent depolarizations of four glomus cells, 2 from WKY (Cells 1 & 2) and 2 from SHR (Cells 3 & 4). Acid-evoked depolarizations were greater in SHR cells. Amiloride (A) did not alter the resting membrane potential but quinidine (Q) (1 mmol) depolarized the cells to a greater degree in SHR (Cell 4 from −55 to −28 mV) than in WKY (Cell 2 from −56 to −42 mV). In addition, amiloride blocked selectively and reversibly the ASIC-mediated initial depolarizations in both WKY (Cell 1) and SHR (Cell 3) while the sustained depolarizations were preserved (the dotted lines delineate the rapid and sustained depolarizations). In contrast, quinidine reduced significantly the sustained depolarization that is TASK-mediated in Cell 2 (WKY) and Cell 4 (SHR), while the initial depolarizations were preserved. Resting membrane potentials measured at pH 7.4 are indicated above baseline traces.
Expression of ASIC and TASK channels in carotid bodies
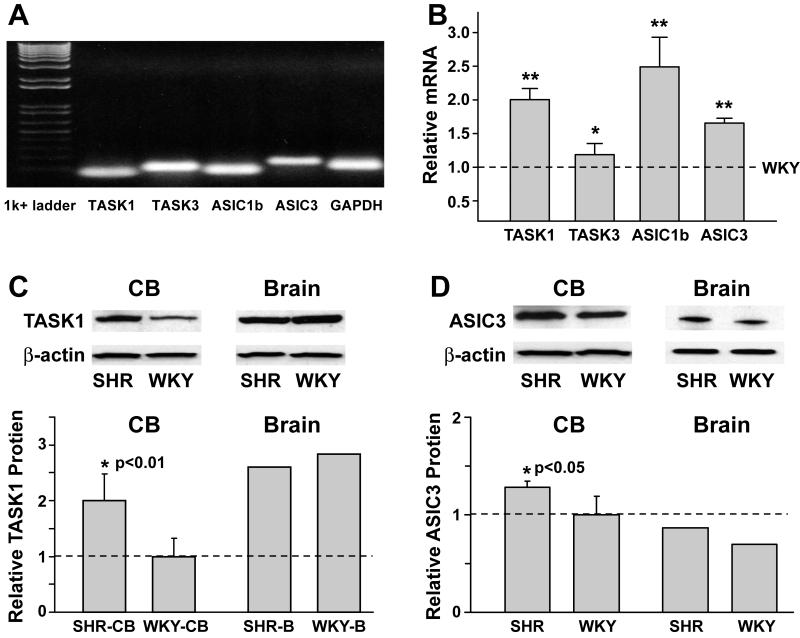
Carotid bodies were obtained from 1 month old rats. Fig. 6A shows the bands of real time PCR products of carotid bodies of WKY rats. Only one band in each lane was observed verifying the specificity of the PCR primers for each gene expressed. mRNA expression of TASK1, TASK3, ASIC1b and ASIC3 were significantly higher in SHR than in WKY carotid bodies (Fig. 6B).
Figure 6. A & B: mRNA expression of ASIC and TASK channels in carotid bodies from 1 month old WKY and SHR.
A) mRNA expression of TASK1, TASK3, ASIC1b and ASIC3 in carotid bodies from WKY rats. GAPDH was used as control.
B) Expression levels of ASIC and TASK mRNAs were higher in SHR carotid bodies compared to WKY. The results from SHR carotid bodies (n=5) are expressed relative to those from WKY carotid bodies (n=5) for corresponding channels (dashed line). (**P<0.01 and *P<0.05, SHR vs. WKY)
The relative mRNA expression of ASIC3 was confirmed when β-actin and 18s were used as housekeeping genes in addition to GAPDH. Comparison of ASIC3 expression obtained with GAPDH vs. β-actin; GAPDH vs. 18s, and β-actin vs. 18s gave P values of 0.81, 0.99, and 0.79 respectively.
C & D: Comparison of protein expression of ASIC and TASK channels in carotid bodies (CB) and brain from WKY and SHR at 1 month of age.
Carotid bodies of WKY were used as reference. Protein expressions were detected by Western blotting from carotid bodies and brain of WKY and SHR. There was a selective increase in expression of both TASK1 and ASIC3 in carotid bodies of SHR vs. WKY rats (P<0.01 and <0.05 respectively) but not of ASIC1. Expression did not differ in brain from SHR and WKY rats.
Protein expressions were detected by Western blotting. Carotid bodies from rats were pooled and homogenized as one sample for extracting membrane proteins. Three such samples were analyzed from SHR and four from WKY rats. TASK1 and ASIC3 protein expression were each increased significantly in SHR over WKY carotid bodies by 100±48% and 36±0.4% respectively (Fig. 6C and D), but the brain values of both channels were not different between SHR and WKY rats. Despite increased mRNA levels of ASIC1b in SHR vs. WKY, the protein expression was not significantly increased (data not presented).
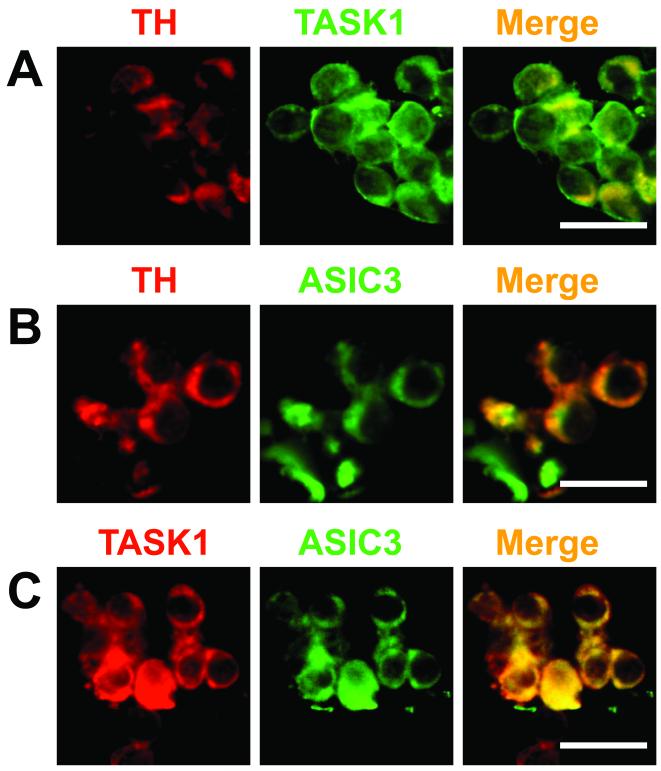
Clusters of glomus cells from SHR are shown in Fig. 7. The expression of tyrosine hydroxylase (TH) identified type I glomus cells. TH, TASK1 and ASIC3 were detected from the immunofluorescence signals. TASK1 and ASIC3 were each expressed in type I glomus cells, and each colocalized with TH (Fig. 7, A and B). The majority of cells expressing TASK1 and ASIC3 were also TH positive and ASIC3 and TASK1 were colocalized in several cells (Fig 7, C).
Figure 7. Immunocytochemical localization of tyrosine hydroxylase (TH), TASK1 and ASIC3 proteins in clusters of glomus cells from two SHRs.
Panels A and B show localization of TASK1 and ASIC3 respectively in TH containing cells. Panel C shows co-localization of ASIC3 and TASK1. Only a qualitative assessment of the distribution of fluorescence can be obtained, which shows occasional TASK1 or ASIC3 fluorescence in the absence of TH. Scale bar = 10 μm.
Increased SNA in response to NaCN in SHR vs. WKY rats
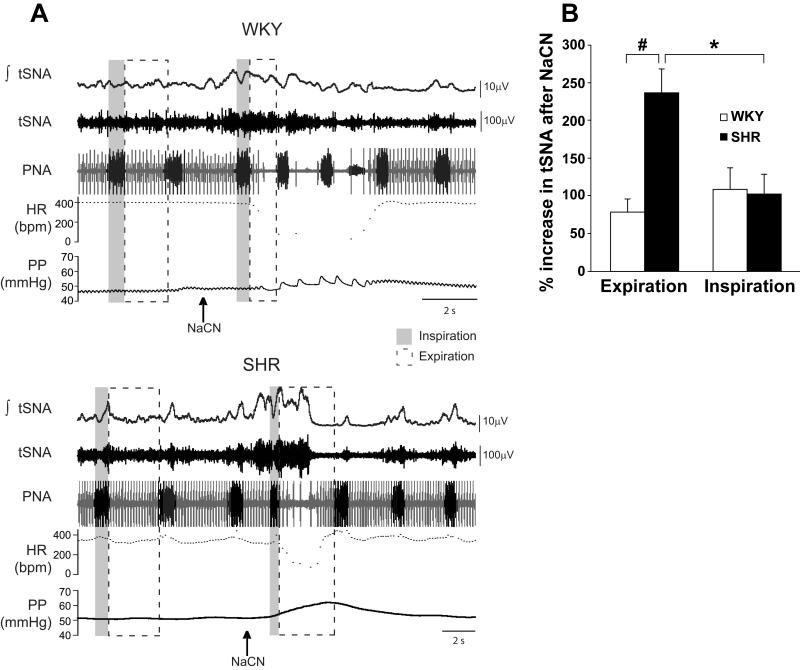
Using the working heart-brainstem preparation (11), the changes in thoracic SNA, heart rate, perfusion pressure and phrenic nerve activity were measured in response to stimulation of carotid chemoreceptors with intraarterial injections of NaCN. NaCN increased SNA in both SHR and WKY rats (Fig 8A). The increase was significantly enhanced in the expiration phase of respiration in SHR (P<0.001) (Fig. 8B and Online Supplement Material, Online Table IV). In SHR, the bradycardia was less pronounced than in WKY rats (P<0.05) while the increased frequency of phrenic nerve activity bursts and perfusion pressure were not significantly different in the two groups (Fig. 8A and Online Supplement Material, Online Table IV).
Figure 8.
A: Responses to peripheral chemoreceptor stimulation with cyanide (NaCN) in young prehypertensive SHR and normotensive WKY rats recorded in situ. The arrows indicate the time of intraarterial injection of NaCN. The vertical shaded bars coincide to phrenic nerve activity during inspiration before and immediately after NaCN. The subsequent expirations are bracketed within the dashed rectangles. The response of thoracic SNA (tSNA) was analyzed separately for inspiratory and expiratory phases. Note the greater expiratory related increases in tSNA in the SHR compared to the WKY rat. The bradycardia in beats per min (HR-bpm) was less pronounced in SHR than in WKY. PNA=phrenic nerve activity; PP=perfusion pressure
B: Mean grouped data showing the enhanced thoracic sympathetic nerve activity (tSNA) response evoked following peripheral chemoreceptor stimulation during the expiratory versus inspiratory respiratory phase in the SHR compared to the WKY rat as recorded in situ. (#P<0.001, *P<0.05)
Discussion
It is increasingly evident that exaggerated SNA is an essential component of the pathogenesis of hypertension in humans and in animal models (1–10). Two important issues have been unclear. One is the mechanism that leads to excessive SNA and the second is whether the increase in SNA occurs prior to the onset of hypertension.
We addressed these two issues in a genetic model of neurogenic hypertension, the SHR, because it exhibits early signs of increased SNA prior to the onset of hypertension at 4–6 weeks of age (9–11) and there is evidence that chemical-sympathectomy plus prazosin abrogate the development of hypertension in the adult rats (10).
Our results show that in young SHR compared to WKY: 1) carotid body glomus cells are hyperresponsive to low pH; 2) Activation of amiloride-sensitive ASIC-like inward sodium currents and inhibition of quinidine-sensitive TASK-like outward potassium currents by low pH are enhanced, resulting in greater rapid and sustained depolarizations respectively; 3) carotid body mRNA and protein expression of ASIC3 and TASK1 are significantly increased at 1 month of age; and 4) Activation of peripheral chemoreceptors with intraarterial NaCN causes significantly greater increases in thoracic SNA.
These results demonstrate exaggerated SNA responses to peripheral chemoreceptor activation prior to the development of hypertension and provide a molecular basis for enhanced chemoreceptor activity. We speculate that the genetic overexpression of ASIC and TASK channels in carotid bodies results in increases in SNA that may initiate increases in vascular resistance and contribute to structural changes that are seen in chronic hypertension.
In this discussion, we address: 1) the relative roles of ASIC and TASK channels vs. other ion channels and molecular modulators of chemoreceptors; 2) the mechanisms that may be involved in overexpression of acid-sensing ion channels vs. other genetic factors that may contribute to the initiation of hypertension in young prehypertensive SHR; and 3) the functional implications of enhanced chemoreceptor activity and consequent increases in SNA.
Ionic Mechanisms of Chemoreceptor Activation
The depolarization of glomus type I cells in carotid bodies is recognized as the primary event in the chemotransduction of hypoxemia, hypercapnia, and acidosis (13, 16, 24). The resulting increase in intracellular Ca2+ causes the release of neurotransmitters (ATP and acetylcholine) that activate the adjacent carotid nerve endings triggering both hyperventilation and sympathetic activation (25–29). Hypoxemia has been reported to induce closure of K+ channels including the large-conductance Ca2+ activated potassium (BK) channels (30–33). The transduction of acidosis includes a drop in intracellular pH which may inhibit voltage-gated K+ channels (13, 19, 35) but may also be mediated by extracellular pH-sensitive ion conductances such as Cl-currents (36) and the non-voltage-gated TASK-like currents (37). We have reported that extracellular acidosis activates the non-voltage-gated acid-sensing sodium channels (ASICs) causing a rapid early depolarization of glomus cells followed by inhibition of the TASK channels that results in a sustained depolarization (23). The capacity of lactate to facilitate proton-gating of ASICs by chelating extracellular Ca2+ suggests that ASICs may be particularly important pH sensors during metabolic acidosis (38). Although extracellular acidosis may directly inhibit L-type Ca2+ currents and possibly indirectly inhibit BK (39, 40), we found that iberiotoxin (the specific blocker of BK) had no influence on the acid-evoked inward current and depolarizations (23). This is in contrast with the effect of hypoxia which may induce depolarization by inhibition of BK (33), as well as inhibition of voltage-gated KV02 (12), and of TASK-like currents (17, 37).
A clear definition of ion channels involved in the transduction of hypoxia, hypercapnia and acidosis remains a challenge because of the complexity of channel interactions. It appears, however, that extracellular acidosis involves predominately non-voltage-gated ASIC3 and TASK-like currents.
Assessment of contributions of the ASIC and TASK currents to the depolarization of glomus cells
Role of ASICs
The rapid kinetics of activation and inactivation of ASIC (41–43) may limit its functional contribution to acid chemosensing. However the complementary contribution of TASK channels and the coexpression of both ASIC and TASK in glomus cells allow the rapid early depolarization by ASICs to be followed by the slower more sustained depolarization by TASKs in response to low pH. The ASIC desensitization which is much slower in the glomus cells than reported in e.g. dorsal root ganglion neurons may also be modulated under certain conditions to sustain a more persistent response to prolonged stimuli as was reported in myocardial ischemia (38, 44,). Although there is a significant variability in the ASIC response to low pH, the percentage of responsive glomus cells is enhanced significantly in the presence of low Ca2+ and high lactate, and in glomus cells from SHR. Moreover, the current density is high especially in SHR glomus cells as is the ASIC message and protein expression with immunofluorescence of a majority of cells.
Role of TASK
Buckler and colleagues were the first to point to the role of TASK-like non-voltage gated K+ channels in the pH response of neonatal rat glomus cells (37, 45, 46). Biophysical and metabolic regulation of this potassium channel in glomus cells indicates that it provides a background leak outward current to maintain a more negative resting membrane potential (47, 48). Reduction of this background current by low oxygen or low pH depolarizes and activates the glomus cell. Also the quinidine-induced depolarization of glomus cells may be explained in part by inhibition of the TASK leak current. In contrast, ASICs are not active at resting membrane potentials and their inhibition by amiloride does not alter the resting membrane potential.
In this study we were able to quantify the magnitude of the outward leak background current with a slow ramp depolarization from −100 mV to −40 mV. There was a significant pH-dependent regulation of the TASK outward current with enhancement at pH 8.0 and progressive inhibition as pH was lowered to 6.0. The calculation of pH-inhibited outward current during the ramp depolarization revealed a reversal potential of −82.0 mV (Fig. 3B) that coincides with that of a selective K+ channel (i.e. −85.7 mV). That reversal potential was similar in SHR (−87.2±4.5 mV) and WKY (−80.3±4.2 mV) suggesting the engagement of comparable pH-sensitive outward currents in both SHR and WKY, although the magnitude of the inhibited current was greater in SHR. In contrast, the calculated quinidine-sensitive net current during ramp depolarization had a reversal potential of −64.0 mV which is significantly less negative than that of K+ indicating a contribution of an inward current that is pH-insensitive, but quinidine-sensitive.
The concentration of quinidine used in the present study (1mmol/L) was insufficient to block totally the sustained depolarization. A higher concentration of 2-5 mmol/L of quinidine was also tested. The higher doses of quinidine caused additional inhibition of the depolarization but often disturbed the stability of membrane potential and its recovery (data not shown). Thus, quinidine effects on the sustained depolarization may not be specific solely to its inhibition of TASK.
Role of voltage-gated currents
Using various blockers of voltage-gated currents in our previous report (23) we concluded that the primary contributions to pH-induced depolarizations in glomus cells was determined by the activity of the two non-voltage-gated ion channels ASICs and TASKs. In this study we pursued further the possible role of voltage-gated channels in the pH-sensitivity of SHR and WKY glomus cells. The results are presented and discussed in the Online Supplement Material (Online Fig. III).
Briefly, we used patch-clamp measurements of outward currents during the slow ramp depolarizations of glomus cells from −100 mV to 0 mV as well as during rapid step (50ms) depolarizing pulses from a holding potential of −60 mV to −40 mV and beyond up to +20mV. We did detect large outward currents at very low negative potentials as well as at positive potentials. These were equally inhibited by low pH in SHR and WKY. We question their functional significance since glomus cells rarely develop action potentials and the outward currents were most pronounced during marked slow ramp depolarizations or step depolarizations to positive membrane potentials (refer to Online Supplement Material, Online Fig. III).
Mechanisms involved in overexpression of acid-sensing ion channels
Several mechanisms have been identified as potential regulators of expression of ASICs. Inflammation has been shown to induce large increases in ASIC expression in sensory neurons through proinflammatory mediators nerve growth factor, serotonin, interleukin-1, and bradykinin (49, 50). An analysis of the promoter region of the ASIC3 encoding gene reveals that gene transcription is controlled by nerve growth factor and serotonin (50). It is interesting that the tissue expression of both nerve growth factor and bradykinins are increased in young SHR (51, 52). Moreover, the disturbance of acid-base balance in young SHR might affect the expression of ASICs since chronic metabolic acidosis has been found to up-regulate expression of ENaCs, another member of the DEG/ENaC superfamily, as well as other genes involved in acid-base, sodium, and water transport and in cell proliferation (20, 53, 54).
Over 25 transcripts encoding proteins involved in urine acidification were co-regulated during acidosis (54). We speculate that the adjustment to metabolic acidosis in the young SHR may not only include the kidney, but the cardiovascular system through up-regulation of acid-sensing ion channels in the glomus cells of the peripheral chemoreceptors, which are the critical sites for reflex adjustments to pH changes.
Functional significance and mechanisms of enhanced chemoreceptor activity
The peripheral chemoreceptor reflex contributes to exercise hyperpnea in humans (55) and provides a respiratory compensation for the metabolic acidosis of exercise (56). Enhanced peripheral chemoreceptor sensitivity and sympathetic activity are often coupled with a reduction in baroreceptor activity (57, 58) and are found in patients with borderline hypertension (5), in animal models of hypertension such as SHR (14), and chronic intermittent hypoxia (15), and in heart failure (59).
In congestive heart failure, the enhanced SNA is thought to be a consequence of the heart failure but it also contributes greatly to the morbidity and mortality (60, 61). The reported mechanisms involved in increased carotid body nerve activity in heart failure include overexpression of AT1 receptors (62), enhanced NADPH oxidase-derived superoxide anion production (63), inhibition of voltage-gated potassium channels (KVO) (64), and the downregulation of carbon monoxide and nitric oxide in carotid body (69). The blunted outward Kvo current in CB glomus cells and the chemoreceptor sensitivity to hypoxia have been normalized by gene transfer of CuZn superoxide dismutase to carotid body (66).
In hypertension, enhanced SNA is recognized as an important component of the disease that sustains hypertension (1–4) and, an exaggerated chemoreceptor activity contributes to the process (5, 6). In contrast to heart failure, the molecular mechanisms involved in the exaggerated chemoreceptor activity in hypertension are less clear (12). Chronic intermittent hypoxia enhances carotid body chemoreceptor discharge (67) leading to increased SNA and elevated arterial pressure in normal rats (68, 69).
In the SHR, a genetic model of neurogenic hypertension, the carotid body nerve discharge during hypoxia is augmented (14, 70), the carotid bodies are enlarged (71), and minute ventilation is increased (72). More importantly, however, increased SNA and norepinephrine release in skeletal muscle and adipose tissue occur early in young SHR prior to the onset of hypertension and may therefore contribute to its initiation (9–11). Chronic hyperventilation, a hypermetabolic state and metabolic acidosis also occur before hypertension (20, 21) and are compatible with increased chemoreceptor activity. The potential contribution of this enhanced peripheral chemoreceptor sensitivity to an early prehypertensive increase in SNA which we now report had not been examined previously.
We have herein provided support for that scenario. The significant increase in SNA in the young prehypertensive SHR following intraarterial injection of NaCN supports the conclusion that the chemoreceptor reflex is enhanced. Cyanide is a potent stimulus of chemoreceptors which simulates a hypoxic response by inhibition of mitochondrial oxidative respiration (73). Its inhibitory effect on TASK-like currents in glomus cells causing their depolarization (74) is one of the mechanisms by which it stimulates chemoreceptors possibly through a drop in intracellular pH. Our in vivo findings of chemoreceptor hypersensitivity with direct recordings of SNA, coupled with evidence of enhanced glomus cell depolarization and overexpression of acid-sensitive channels in the prehypertensive stage strongly support our hypothesis that activated chemoreceptors may provide a genetically determined sympatho-excitatory signal prior to the development of hypertension.
Other Genetic factors that may contribute to the development of hypertension in SHR
Several other abnormalities in gene expression have been described in prehypertensive SHR and may be linked to the development of hypertension. Young SHR demonstrate an increase in sodium retention. Enhanced expressions of AT1R mRNA (75) and Na+K+AT Pase (76) were found in proximal renal tubule of 4 week-old SHR compared to WKY. Vascular smooth muscle cells derived from aortas of 4-week-old SHR also show exaggerated growth, increased production of Ang II, and increased expression of several growth factors, adhesion molecules and cytokines (77). Phospholipase C isozymes (PLC-beta 1 and delta 1) and phospholipase activity are also up-regulated in systemic and renal vasculatures in 6 week-old SHR (78).
Functional adhesion molecule-1 is differentially expressed in multiple regions of the brain and in all peripheral vascular beds. More specifically, its expression in the nucleus tractus solitarius was significantly higher in SHR both at 3 weeks of age (prehypertensive) as well as in the adults (15 weeks old) suggesting a prohypertensive role for this protein in the brain stem (79).
Limitation of interpretations
Our results provide the first demonstration of chemoreceptor hyperactivity in young prehypertensive SHR reflected in increased SNA in response to intraarterial NaCN. The overexpression of acid sensing channels in glomus cells is likely an important factor in the initiation of this carotid body hyperactivity. Since we showed that this hyperactivity leads to increased SNA and others have reported that the elimination of SNA at this stage (10) prevents the development of hypertension, future studies may consider if targeted inhibition of expression of ASIC and TASK in the carotid body will suppress the development of hypertension in SHR.
A particular interest has been the recognition of the wide variability in responses between glomus cells, which we report in Fig. 1. Early reports on ultrastructure of Type 1 glomus cells (80-82) reveal that some had very large dense microvesicles while others had less dense or clear vesicles, and some were heavily innervated and others were not and may explain the wide variability in responses to low pH. These differences led some investigators to classify Type 1 glomus cells into 2 or more subtypes. The differences in structure and innervation may denote different transmitters, the expression of different ion channels, and certainly suggest selective sensitivities to different chemical stimuli such as pH, hypoxia or hypercapnia.
A quantitative correlation between the varying electrophysiological responses of individual glomus cells and the magnitude of gene or protein expressions in the whole carotid bodies with its diverse composition of glomus cells of different types, other cells, vascular tissue, axons or neurons would be very unlikely. Moreover, the ASIC channel is probably a heterotrimer and although ASIC3 is the predominately acid-sensitive subunit, the association with other subunits, rather than the amounts of ASIC3 protein, may determine the current magnitude.
Although the glomus cell is the site of initial chemotransduction and the enhanced chemoreceptor reflex may certainly reflect the carotid body hyperactivity, neural interactions at other sites involving afferent, central and efferent neurons may also contribute to the enhanced reflex. The coupling of the enhanced SNA to the expiration phase of respiration (Fig. 8) demonstrates such an interaction.
Novelty and Significance.
Increased sympathetic nerve activity (SNA) has been linked to the pathogenesis of hypertension; however, two important issues have been unclear. First – what the cause of increased SNA is, and second - whether the increase in SNA contributes to hypertension. We report for the first time that in genetically hypertensive rats (SHR) hypersensitivity of chemoreceptors causes a greater increase in SNA at a very young age prior to the onset of hypertension. We have found that the isolated glomus Type 1 cells of the carotid body, where chemotransduction occurs, have enhanced depolarization in response to low pH in the prehypertensive SHR compared to normotensive WKY because of increased expression of two ion channels. One, which conducts an inward sodium current, is a member of the acid sensing ion channels (ASIC) family and it opens at low pH. The other, which conducts an outward potassium leak current, is a two-pore domain acid sensitive channel (TASK) that closes at low pH. Thus, early overexpression of these molecular determinants of hypersensitivity of chemoreceptors causing increased SNA may be important in the initiation and development of hypertension in SHR. The results strongly support our hypothesis that activated chemoreceptors provide a genetically enhanced sympatho-excitatory signal prior to the onset of hypertension. Future basic and translational studies need to consider if targeted inhibition of expression of ASIC and TASK in the carotid bodies by genetic or pharmacologic means suppresses the development of hypertension.
What is known?
Increased SNA is an essential component of the pathogenesis of hypertension.
Chemoreceptor hyperactivity can increase SNA in hypertension.
New information contributed by this study ?
Increased chemoreceptor sensitivity is a cause of increased SNA in a genetic model of hypertension (the SHR) prior to the onset of hypertension.
The cellular mechanism involves a hypersensitivity of the chemotransducing carotid body Type 1 glomus cell to low pH.
The molecular determinants include the overexpression of acid sensitive ion channels ASIC and TASK in the carotid bodies which initiate a prehypertensive increase in SNA and may cause the development of hypertension.
Supplementary Material
Acknowledgments
The studies on the effects of intra-arterial NaCN on SNA in young normotensive SHR and WKY rats were carried out by co-author Annabel E. Simms in the laboratory of Dr. Andrew Allen, Department of Physiology at the University of Melbourne, Melbourne, Australia.
We thank Sarah Porter and Courtney Cable of the Department of Internal Medicine, University of Iowa, for assistance preparing the manuscript. We thank Shawn Roach of the Department of Internal Medicine, University of Iowa, for assistance in creating and formatting the figures for this paper, and the Central Microscopy Core Facility of the Carver College of Medicine for providing the confocal microscope used in these studies.
Sources of Funding The work at the University of Iowa was supported by NIH grant HL14388.
The work at the University of Melbourne was supported by the Australian National Health and Medical Research Council (Project grant number 454432).
Nonstandard abbreviations and acronyms
- ASIC
acid-sensing ion channel
- SNA
sympathetic nerve activity
- TASK
two pore-domain acid-sensitive K+
- SHR
spontaneously hypertensive rat
- TH
tyrosine hydroxylase
- WKY
Wistar-Kyoto
- NaCN
sodium cyanide
- BK
large-conductance Ca(2+)-activated potassium
Footnotes
Disclosures None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abboud FM. The Sympathetic System in Hypertension: State-of-the-Art Review. Hypertension. 1984;4(Suppl II):208–225. [PubMed] [Google Scholar]
- 2.Esler M, Rumantir M, Kaye D, Jennings G, Hastings J, Socratous F, Lampert G. Sympathetic nerve biology in essential hypertension. Clin Exp Pharmacol Physiol. 2001;28:986–989. doi: 10.1046/j.1440-1681.2001.03566.x. [DOI] [PubMed] [Google Scholar]
- 3.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 4.Mancia G, Grassi G, Giannattasio C, Serravalle G. Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Hypertension. 1999;34:724–728. doi: 10.1161/01.hyp.34.4.724. [DOI] [PubMed] [Google Scholar]
- 5.Somers VK, Mark AL, Abboud FM. Potentiation of sympathetic nerve responses to hypoxia in borderline hypertensive subjects. Hypertension. 1988;11:608–612. doi: 10.1161/01.hyp.11.6.608. [DOI] [PubMed] [Google Scholar]
- 6.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J. Clin. Invest. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Judy WV, Farrell SK. Arterial baroreceptor reflex control of sympathetic nerve activity in the spontaneously hypertensive rat. Hypertension. 1979;1:605–614. doi: 10.1161/01.hyp.1.6.605. [DOI] [PubMed] [Google Scholar]
- 8.Lundin S, Ricksten SE, Thoren P. Renal sympathetic activity in spontaneously hypertensive rats and normotensive controls, as studied by three different methods. Acta Physiol Scand. 1984;120:265–272. doi: 10.1111/j.1748-1716.1984.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 9.Cabassi A, Vinci S, Calzolari M, Bruschi G, Borghetti A. Regional sympathetic activity in prehypertensive phase of spontaneously hypertensive rats. Life Sci. 1998;62:1111–1118. doi: 10.1016/s0024-3205(98)00034-4. [DOI] [PubMed] [Google Scholar]
- 10.Korner P, Bobik A, Oddie C, Friberg P. Sympathoadrenal system is critical for structural changes in genetic hypertension. Hypertension. 1993;22:243–252. doi: 10.1161/01.hyp.22.2.243. [DOI] [PubMed] [Google Scholar]
- 11.Simms AE, Paton JFR, Pickering AI, Allen AM. Amplified respiratory-sympathetic coupling in the spontaneously hypertensive rat: does it contribute to hypertension? J Physiol. 2009;587(3):597–610. doi: 10.1113/jphysiol.2008.165902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schultz HD, Li YL, Ding Y. Arterial chemoreceptors and sympathetic nerve activity: implications for hypertension and heart failure. Hypertension. 2007;50:6–13. doi: 10.1161/HYPERTENSIONAHA.106.076083. [DOI] [PubMed] [Google Scholar]
- 13.Prabhakar NR, Peng YJ. Peripheral chemoreceptors in health and disease. J Appl Physiol. 2004;96:359–366. doi: 10.1152/japplphysiol.00809.2003. [DOI] [PubMed] [Google Scholar]
- 14.Fukuda Y, Sato A, Trzebski A. Carotid chemoreceptor discharge responses to hypoxia and hypercapnia in normotensive and spontaneously hypertensive rats. J Auton Nerv Syst. 1987;19:1–11. doi: 10.1016/0165-1838(87)90139-1. [DOI] [PubMed] [Google Scholar]
- 15.Prabhakar NR, Dick TE, Nanduri J, Kumar GK. Systemic, cellular and molecular analysis of chemoreflex-mediated sympathoexcitation by chronic intermittent hypoxia. Exp Physiol. 2007;92:39–44. doi: 10.1113/expphysiol.2006.036434. [DOI] [PubMed] [Google Scholar]
- 16.Lahiri S, Roy A, Baby SM, Hoshi T, Semenza GL, Prabhakar NR. Oxygen sensing in the body. Prog Biophys Mol Biol. 2006;91:249–286. doi: 10.1016/j.pbiomolbio.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Buckler KJ. TASK-like potassium channels and oxygen sensing in the carotid body. Respir Physiol Neurobiol. 2007;157:55–64. doi: 10.1016/j.resp.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Lopez JR, Perez-Garcia MT. Oxygen sensitive Kv channels in the carotid body. Respir Physiol Neurobiol. 2007;157:65–74. doi: 10.1016/j.resp.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 19.Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO(2) and acid signaling in chemosensitive neurons. Am J Physiol Cell Physiol. 2004;287:C1493–1526. doi: 10.1152/ajpcell.00282.2004. [DOI] [PubMed] [Google Scholar]
- 20.Lucas PA, Lacour B, McCarron DA, Drueke T. Disturbance of acid-base balance in the young spontaneously hypertensive rat. Clin Sci (Lond) 1987;73:211–215. doi: 10.1042/cs0730211. [DOI] [PubMed] [Google Scholar]
- 21.Lucas PA, Lacour B, Comte L, Drueke T. Pathogenesis of abnormal acid-base balance in the young spontaneously hypertensive rat. Clin Sci. 1988;75:29–34. doi: 10.1042/cs0750029. [DOI] [PubMed] [Google Scholar]
- 22.Lopez-Lopez JR, Gonzalez C, Perez-Garcia MT. Properties of ionic currents from isolated adult rat carotid body chemoreceptor cells: effect of hypoxia. J Physiol. 1997;499:429–441. doi: 10.1113/jphysiol.1997.sp021939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan ZY, Lu Y, Whiteas CA, Benson CJ, Chapleau MW, Abboud FM. Acid-sensing ion channels contribute to transduction of extracellular acidosis in rat carotid body glomus cells. Circ Res. 2007;101:1009–1019. doi: 10.1161/CIRCRESAHA.107.154377. [DOI] [PubMed] [Google Scholar]
- 24.Pallot DJ. The mammalian carotid body. Adv Anat Embryol Cell Biol. 1987;102:1–91. doi: 10.1007/978-3-642-71857-1. [DOI] [PubMed] [Google Scholar]
- 25.Eyzaguirre C, Fidone SJ. Transduction mechanisms in carotid body: glomus cells, putative neurotransmitters, and nerve endings. Am J Physiol. 1980;239:C135–52. doi: 10.1152/ajpcell.1980.239.5.C135. [DOI] [PubMed] [Google Scholar]
- 26.Weiss N, Donnelly DF. Depolarization is a critical event in hypoxia-induced glomus cell secretion. Adv Exp Med Biol. 1996;410:181–187. doi: 10.1007/978-1-4615-5891-0_26. [DOI] [PubMed] [Google Scholar]
- 27.Nurse CA. Neurotransmission and neuromodulation in the chemosensory carotid body. Auton Neurosci. 2005;120:1–9. doi: 10.1016/j.autneu.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 28.Prabhakar NR. O2 sensing at the mammalian carotid body: why multiple O2 sensors and multiple transmitters? Exp Physiol. 2006;91:17–23. doi: 10.1113/expphysiol.2005.031922. [DOI] [PubMed] [Google Scholar]
- 29.Somers VK, Mark AL, Zavala DC, Abboud FM. Contrasting effects of hypoxia and hypercapnia on ventilation and sympathetic activity in humans. J Appl Physiol. 1989;67:2101–2106. doi: 10.1152/jappl.1989.67.5.2101. [DOI] [PubMed] [Google Scholar]
- 30.Williams SE, Wootton P, Mason HS, Bould J, Iles DE, Riccardi D, Peers C, Kemp PJ. Hemoxygenase-2 is an oxygen sensor for a calcium-sensitive potassium channel. Science. 2004;306:2093–2097. doi: 10.1126/science.1105010. [DOI] [PubMed] [Google Scholar]
- 31.Pardal R, Lopez-Barneo J. Carotid body thin slices: responses of glomus cells to hypoxia and K+-channel blockers. Resp. Physiol. & Neurobio. 2002;132:69–79. doi: 10.1016/s1569-9048(02)00050-2. [DOI] [PubMed] [Google Scholar]
- 32.Lopez-Barneo J, Lopez-Lopez JR, Urena J, Gonzalez C. Chemotransduction in the carotid body: K+ current modulated by PO2 in type I chemoreceptor cells. Science. 1988;241:580–582. doi: 10.1126/science.2456613. [DOI] [PubMed] [Google Scholar]
- 33.Peers C. Hypoxic suppression of K+ currents in type I carotid body cells: selective effect on the Ca2(+)-activated K+ current. Neurosci Lett. 1990;119:253–256. doi: 10.1016/0304-3940(90)90846-2. [DOI] [PubMed] [Google Scholar]
- 34.Deleted in proof.
- 35.Peers C, Green FK. Inhibition of Ca(2+)-activated K+ currents by intracellular acidosis in isolated type I cells of the neonatal rat carotid body. J Physiol. 1991;437:589–602. doi: 10.1113/jphysiol.1991.sp018613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petheo GL, Molnar Z, Roka A, Makara JK, Spat A. A pH-sensitive chloride current in the chemoreceptor cell of rat carotid body. J Physiol. 2001;535:95–106. doi: 10.1111/j.1469-7793.2001.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buckler KJ, Williams BA, Honore E. An oxygen-, acid- and anaesthetic-sensitive TASK-like background potassium channel in rat arterial chemoreceptor cells. J Physiol. 2000;525:135–142. doi: 10.1111/j.1469-7793.2000.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Immke DC, McCleskey EW. Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat Neurosci. 2001;4:869–870. doi: 10.1038/nn0901-869. [DOI] [PubMed] [Google Scholar]
- 39.Summers BA, Overholt JL, Prabhakar NR. CO(2) and pH independently modulate L-type Ca(2+) current in rabbit carotid body glomus cells. J Neurophysiol. 2002;88:604–612. doi: 10.1152/jn.2002.88.2.604. [DOI] [PubMed] [Google Scholar]
- 40.Klockner U, Isenberg G. Intracellular pH modulates the availability of vascular L-type Ca2+ channels. J Gen Physiol. 1994;103:647–663. doi: 10.1085/jgp.103.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krishtal O. The ASICs: signaling molecules? Modulators? Trends Neurosci. 2003;26:477–483. doi: 10.1016/S0166-2236(03)00210-8. [DOI] [PubMed] [Google Scholar]
- 42.Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci. 2006;29:578–586. doi: 10.1016/j.tins.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 43.Immke DC, McCleskey EW. Protons open acid-sensing ion channels by catalyzing relief of Ca2+ blockade. Neuron. 2003;37:75–84. doi: 10.1016/s0896-6273(02)01130-3. [DOI] [PubMed] [Google Scholar]
- 44.Yagi J, Wenk HN, Naves LA, McCleskey EW. Sustained currents through ASIC3 ion channels at the modest pH changes that occur during myocardial ischemia. Circ Res. 2006;99:501–509. doi: 10.1161/01.RES.0000238388.79295.4c. [DOI] [PubMed] [Google Scholar]
- 45.Buckler KJ, Vaughan-Jones RD, Peers C, Lagadic-Gossmann D, Nye PC. Effects of extracellular pH, PCO2 and HCO3- on the intracellular pH in isolated type-I cells of the neonatal rat carotid body. J Physiol. 1991;444:703–721. doi: 10.1113/jphysiol.1991.sp018902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buckler KJ, Vaughan-Jones RD. Effects of hypercapnia on membrane potential and intracellular calcium in rat carotid body type I cells. J Physiol. 1994;478:157–171. doi: 10.1113/jphysiol.1994.sp020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams BA, Buckler KJ. Biophysical properties and metabolic regulation of a TASK-like potassium channel in rat carotid body type 1 cells. Am J Physiol Lung Cell Mol Physiol. 2004;286:L221–L230. doi: 10.1152/ajplung.00010.2003. [DOI] [PubMed] [Google Scholar]
- 48.Xu F, Tse FW, Tse A. Pituitary adenylate cyclase-activating polypeptide (PACAP) stimulates the oxygen sensing type I (glomus) cells of rat carotid bodies via reduction of a background TASK-like K+ current. J Neurochem. 2007;101:1284–1293. doi: 10.1111/j.1471-4159.2007.04468.x. [DOI] [PubMed] [Google Scholar]
- 49.Voilley N, de Weille J, Mamet J, Lazdunski M. Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. J. Neurosci. 2001;21:8026–8033. doi: 10.1523/JNEUROSCI.21-20-08026.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mamet J, Baron A, Lazdunski M, Voilley N. Proinflammatory mediators, stimulators of sensory neuron excitability via the expression of acid-sensing ion channels. J. of Neuroscience. 2002;22:10662–10670. doi: 10.1523/JNEUROSCI.22-24-10662.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ueyama T, Hamada M, Hano T, Nishio I, Masuyama Y, Furukawa S. Increased nerve growth factor levels in spontaneously hypertensive rats. J. Hypertens. 1992;10:215–219. doi: 10.1097/00004872-199203000-00003. [DOI] [PubMed] [Google Scholar]
- 52.Campbell DJ, Duncan AM, Kladis A, Harrap SB. Increased levels of bradykinin and its metabolites in tissues of young spontaneously hypertensive rats. J. Hypertens. 1995;13:739–746. [PubMed] [Google Scholar]
- 53.Faroqui S, Sheriff S, Amlal H. Metabolic acidosis has dual effects on sodium handling by rat kidney. AJP-Renal. 2006;291:F322–F331. doi: 10.1152/ajprenal.00338.2005. [DOI] [PubMed] [Google Scholar]
- 54.Cheval L, Morla L, Elalouf JM, Doucet A. Kidney collecting duct acid-base “regulon.”. Physiol. Genomics. 2006;27:271–281. doi: 10.1152/physiolgenomics.00069.2006. [DOI] [PubMed] [Google Scholar]
- 55.Kobayashi T, Sakakibara Y, Masuda A, Ohdaira T, Honda Y. Contribution of peripheral chemoreceptor drive in exercise hyperpnea in humans. Appl Human Sci. 1996;15:259–266. doi: 10.2114/jpa.15.259. [DOI] [PubMed] [Google Scholar]
- 56.Rausch SM, Whipp BJ, Wasserman K, Huszczuk A. Role of the carotid bodies in the respiratory compensation for the metabolic acidosis of exercise in humans. J Physiol. 1991;444:567–578. doi: 10.1113/jphysiol.1991.sp018894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heistad DD, Abboud FM, Mark AL, Schmid PG, Brooks LA, Swanson J. Interaction of baroreceptor and chemoreceptor reflexes. Modulation of the chemoreceptor reflex by changes in baroreceptor activity. J. Clin. Invest. 1974;53:1226–1236. doi: 10.1172/JCI107669. with technical assistance of. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Somers VK, Mark AL, Abboud FM. Interaction of baroreceptor and chemoreceptor reflex control of sympathetic nerve activity in normal humans. J. Clin. Invest. 1991;87:1953–1957. doi: 10.1172/JCI115221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun SY, Wang W, Zucker IH, Schultz HD. Enhanced peripheral chemoreflex function in conscious rabbits with pacing-induced heart failure. J Appl Physiol. 1999;86:1264–1272. doi: 10.1152/jappl.1999.86.4.1264. [DOI] [PubMed] [Google Scholar]
- 60.Zucker IH, Schultz HD, Li YF, Wang Y, Wang W, Patel KP. The origin of sympathetic outflow in heart failure: the roles of angiotensin II and nitric oxide. Prog Biophys Mol Biol. 2004;84:217–232. doi: 10.1016/j.pbiomolbio.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 61.Schultz HD, Sun SY. Chemoreflex function in heart failure. Heart Fail Rev. 2000;5:45–56. doi: 10.1023/A:1009846123893. [DOI] [PubMed] [Google Scholar]
- 62.Li YL, Xia XH, Zheng H, Gao L, Li YF, Liu D, Patel KP, Wang W, Schultz HD. Angiotension II enhances carotid body chemoreflex control of sympathetic outflow in chronic heart failure rabbits. Cardiovasc Res. 2006;71:129–138. doi: 10.1016/j.cardiores.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 63.Li YL, Gao L, Zucker IH, Schultz HD. NADPH oxidase-derived superoxide anion mediates angiotensin II-enhanced carotid body chemoreceptor sensitivity in heart failure rabbits. Cardiovasc Res. 2007;75:546–554. doi: 10.1016/j.cardiores.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li YL, Schultz HD. Enhanced sensitivity of Kv channels to hypoxia in the rabbit carotid body in heart failure: role of angiotensin II. J Physiol. 2006;575:215–227. doi: 10.1113/jphysiol.2006.110700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ding Y, Li YL, Schultz HD. Downregulation of carbon monoxide as well as nitric oxide contributes to peripheral chemoreflex hypersensitivity in heart failure rabbits. J Appl Physiol. 2008;105:14–23. doi: 10.1152/japplphysiol.01345.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ding Y, Li YL, Zimmerman MC, Davisson RL, Schultz HD. Role of CuZn superoxide dismutase on carotid body function in heart failure rabbits. Cardiovasc Res. 2009;81:678–685. doi: 10.1093/cvr/cvn350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peng Y, Kline DD, Dick TE, Prabhakar NR. Chronic intermittent hypoxia enhances carotid body chemoreceptor response to low oxygen. Adv Exp Med Biol. 2001;499:33–38. doi: 10.1007/978-1-4615-1375-9_5. [DOI] [PubMed] [Google Scholar]
- 68.Zoccal DB, Simms AE, Bonagamba LGH, Braga VA, Pickering AE, Paton JFR, Machado BH. Increased sympathetic outflow in juvenile rats submitted to chronic intermittent hypoxia correlates with enhanced expiratory activity. J Physiol. 2008;586:3253–3265. doi: 10.1113/jphysiol.2008.154187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lesske J, Fletcher EC, Bao G, Unger T. Hypertension caused by chronic intermittent hypoxia-influence of chemoreceptors and sympathetic nervous system. J Hypertens. 1997;15:1593–1603. doi: 10.1097/00004872-199715120-00060. [DOI] [PubMed] [Google Scholar]
- 70.Weil JV, Stevens T, Pickett CK, Tatsumi K, Dickinson MG, Jacoby CR, Rodman DM. Strain-associated differences in hypoxic chemosensitivity of the carotid body in rats. Am J Physiol Lung Cell Mol Physiol. 1998;274:L767–L774. doi: 10.1152/ajplung.1998.274.5.L767. [DOI] [PubMed] [Google Scholar]
- 71.Honig A, Habek JO, Pfeiffer C, Schmidt M, Huckstorf C, Rotter H, Eckermann P. The carotid bodies of spontaneously hypertensive rats (SHR): a functional and morphological study. Acta Biol Med Ger. 1981;40:1021–1030. [PubMed] [Google Scholar]
- 72.Jennings DB, Lockett HJ. Angiotensin stimulates respiration in spontaneously hypertensive rats. Am J Physiol Regulatory Integrative Comp Physiol. 2000;278:R1125–R1133. doi: 10.1152/ajpregu.2000.278.5.R1125. [DOI] [PubMed] [Google Scholar]
- 73.Krylov SS, Anichkov SV. The effect of metabolic inhibitors on carotid chemoreceptors. In: Torrance RW, editor. Arterial Chemoreceptors. Blackwell; Oxford: 1968. pp. 103–109. [Google Scholar]
- 74.Wyatt CN, Buckler KJ. The effect of mitochondrial inhibitors on membrane currents in isolated neonatal rat carotid body type 1 cells. J Physiol. 2004;556:175–191. doi: 10.1113/jphysiol.2003.058131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cheng H-F, Wang J-L, Vinson GP, Harris RC. Young SHR express increased type 1 angiotensin II receptors in renal proximal tubule. Am. J. of Physiol. 1998;43:F10–F17. doi: 10.1152/ajprenal.1998.274.1.F10. [DOI] [PubMed] [Google Scholar]
- 76.Hinojos CA, Doris PA. Altered subcellular distribution of Na+, K+-ATPase in proximal tubules in young spontaneously hypertensive rats. Hypertension. 2004;44:95–100. doi: 10.1161/01.HYP.0000132557.16738.92. [DOI] [PubMed] [Google Scholar]
- 77.Hu W-Y, Fukuda N, Kanmatususe K. Growth characteristics, angiotensin II generation, and microarray-determined gene expression in vascular smooth muscle cells from young spontaneously hypertensive rats. J. Hypertens. 2002;20:1323–1333. doi: 10.1097/00004872-200207000-00019. [DOI] [PubMed] [Google Scholar]
- 78.Peng Z, Dang A, Arendshorst WJ. Increased expression and activity of phospholipase C in renal arterioles of young spontaneously hypertensive rats. Am. J. Hypertens. 2007;20:38–43. doi: 10.1016/j.amjhyper.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 79.Waki H, Liu B, Miyake M, Katahira K, Murphy D, Kasparov S, Paton JF. Junctional adhesion molecule-1 is upregulated in spontaneously hypertensive rats: evidence for a prohypertensive role within the brain stem. Hypertension. 2007;49:1321–1327. doi: 10.1161/HYPERTENSIONAHA.106.085589. [DOI] [PubMed] [Google Scholar]
- 80.Helström S. Morphometric studies of dense-cored vesicles in Type I cells of rat carotid body. J. Neurocytol. 1975a;4:77–86. doi: 10.1007/BF01099097. [DOI] [PubMed] [Google Scholar]
- 81.McDonald DM, Mitchell RA. The innervation of glomus cells, ganglion cells and blood vessels in the rat carotid body: A quantitative ultrastructural analysis. J. Neurocytol. 1975a;4:177–230. [Google Scholar]
- 82.Morita E, Chiocchio SR, Tramezzani JH. Four types of main cells in the carotid body of the cat. J Ultrastruct Res. 1969;28:399–410. doi: 10.1016/s0022-5320(69)80029-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.