Abstract
The Botryosphaeriaceae includes several species that are serious canker and leaf pathogens of Proteaceae. In the present study, sequence data for the ITS nrDNA region were used in conjunction with morphological observations to resolve the taxonomy of species of Botryosphaeriaceae associated with Proteaceae. Neofusicoccum luteum was confirmed from Buckinghamia and Banksia in Australia, and on Protea cynaroides in South Africa. A major pathogen of Banksia coccinea in Australia was shown to be N. australe and not N. luteum as previously reported. Neofusicoccum protearum was previously reported on Proteaceae from Australia, Madeira, Portugal and South Africa, and is shown here to also occur in Hawaii and Tenerife (Canary Islands). Furthermore, several previous records of N. ribis on Proteaceae were shown to be N. parvum. Saccharata capensis is described as a new species that is morphologically similar to S. proteae. There is no information currently available regarding its potential importance as plant pathogen and pathogenicity tests should be conducted with it in the future.
Keywords: Botryosphaeria, Fusicoccum, Neofusicoccum, Saccharata
INTRODUCTION
The Proteaceae (proteas) is a prominent Southern Hemisphere plant family consisting of approximately seven subfamilies, 60 genera and 1 400 species (Rebelo 2001). Most proteas are trees or shrubs that can survive under very dry conditions. Several genera are successfully cultivated in tropical, subtropical and temperate regions, in many cases as introduced non-natives. Amongst the commonly cultivated species are the South African Protea, Leucadendron and Leucospermum, which are farmed for fresh cut-flowers, dried flowers and dried foliage. These species are traded globally and are in high demand. Any disease on these products has a direct influence on international and domestic trade and markets. Although many pathogens are associated with proteas (Crous et al. 2004a), some of the most important pathogens from a phytosanitary standpoint are species of the Botryosphaeriaceae. This is chiefly because they exist as latent pathogens in healthy plant tissues, causing serious disease when plants are stressed (Denman et al. 2000, 2003). The species of Botryosphaeriaceae occurring on Proteaceae have recently been circumscribed (Denman et al. 2003), and guidelines to the management and control of the diseases with which they are associated have been published (Crous et al. 2004a, Denman et al. 2004).
Species of Botryosphaeriaceae have a worldwide distribution and they occur on a wide diversity of plant hosts (Denman et al. 2000). They also occupy a wide range of niches and can be primary or opportunistic pathogens, endophytes or saprobes (Denman et al. 2000, Swart et al. 2000, Taylor et al. 2001a, b, c, Denman 2002, Crous et al. 2004a, Slippers & Wingfield 2007). Ten lineages in the Botryosphaeriaceae were recognised based on sequence data of 28S rDNA (Crous et al. 2006b), with one recently added lineage representing the anamorph genus Aplosporella (Damm et al. 2007b). Botryosphaeria spp. and similar species are prevalent on proteas under environmental stress, causing stem cankers, dieback or leaf blight (Crous et al. 2004a). A total of 19 species have thus far been reported to be associated with proteas (Table 1), although there are undoubtedly more awaiting discovery (Crous et al. 2006a). Since the DNA-based phylogenetic study conducted on the Botryosphaeriaceae infecting Proteaceae by Denman et al. (2003), several additional isolates have been obtained from Proteaceae cultivated in South Africa and elsewhere in the world. The aim of this study was, therefore, to clarify the taxonomic status of these newly collected isolates by comparing them with reference strains using comparisons of DNA sequence data for the ITS nrDNA region. Furthermore, we aimed to resolve the status of isolates that appeared morphologically distinct from species presently known from this family.
Table 1.
Species of Botryosphaeriaceae reported to be associated with the Proteaceae.
| Species | Clade1 | Host2 | Locality |
|---|---|---|---|
| Botryosphaeria dothidea (anamorph: Fusicoccum aesculi) | Clade 2 | GrevilleaP, LeucadendronP, LeucospermumP, ProteaP, TelopeaP | Guatemala, Hawaii, South Africa, USA: California, Zimbabwe |
| Botryosphaeria gaubae | Petrak 1967 | GrevilleaP | Australia |
| Botryosphaeria sp. | Taylor et al. 2001b | GrevilleaP | USA: Florida |
| ‘Botryosphaeria’ quercuum | Clade 8 | GrevilleaP | USA: Florida |
| Diplodia seriata (teleomorph: ‘Botryosphaeria’ obtusa) | Clade 1 | ProteaP, E | South Africa |
| Diplodia sp. | Clade 1 | ProteaS | South Africa |
| Diplodia sp. | Clade 1 | GrevilleaP | USA: Florida |
| Dothiorella banksiae | Clade 5 | BanksiaP | Australia |
| Dothiorella sp. | Clade 5 | LeucadendronS | South Africa |
| Dothiorella sp. | Clade 5 | LeucadendronP, ProteaP | Hawaii |
| Fusicoccum spp. | Taylor et al. 2001a, b | LeucospermumP, ProteaP, TelopeaP | Hawaii, USA: California |
| Lasiodiplodia theobromae (teleomorph: ‘Botryosphaeria’ rhodina) | Clade 1 | BanksiaP, GrevilleaP, LeucospermumP, ProteaP, TelopeaP | Australia, Cuba3, Hawaii, India3, Madeira, |
| Malawi3, Uganda | |||
| Neofusicoccum australe (teleomorph: ‘Botryosphaeria’ australis) | Clade 6 | BanksiaP, ProteaP | Australia, South Africa |
| Neofusicoccum luteum (teleomorph: ‘Botryosphaeria’ lutea) | Clade 6 | BanksiaP, BuckinghamiaP, ProteaP | Australia, South Africa |
| Neofusicoccum protearum (teleomorph: ‘Botryosphaeria’ protearum) | Clade 6 | LeucadendronP,E, ProteaP,E | Australia, Hawaii, Madeira, South Africa |
| Neofusicoccum cf. protearum | Clade 6 | LeucadendronS, LeucospermumS, ProteaS | South Africa |
| Neofusicoccum ribis (teleomorph: ‘Botryosphaeria’ ribis) | Clade 6 | BanksiaP, BuckinghamiaP, GrevilleaP, LeucadendronP, LeucospermumP, MacadamiaP, ProteaP, TelopeaP | Australia, Hawaii, Malawi, South Africa, Zimbabwe |
| Saccharata capensis | Clade 9 | LeucospermumS, MimetesS | South Africa |
| Saccharata proteae (anamorph: ‘Fusicoccum’ proteae) | Clade 9 | LeucadendronS, LeucospermumP,S, ProteaP,S,E | Australia5, Hawaii, Madeira5, Portugal5, South Africa4, Tasmania, USA: California |
1 Clade number corresponds to that of Crous et al. (2006b).
2 S = saprobe, P = pathogen, E = endophyte.
3 Published as Botryodiplodia theobromae.
4 Published as Phyllachora proteae.
5 Published as Botryosphaeria proteae.
MATERIALS AND METHODS
Isolates
Cultures were obtained by making single spore isolations from mature fruiting bodies present in diseased material as well as by isolating fungi directly from stem cankers and leaf spots. Isolates obtained from asymptomatic protea leaves presumably as endophytes, were also included. Plant tissue was surface disinfested by placing samples in 70 % ethanol for 30 s, 1 % NaOCl for 1 min, 30 s in 70 % ethanol and rinsing in sterile water for 1 min. Spores were allowed to germinate on 2 % malt extract agar (MEA; Sigma-Aldrich Chemie, Zwijndrecht, The Netherlands) plates following the protocols described by Crous (1998).
DNA phylogeny
Genomic DNA was isolated from fungal mycelium grown on MEA, using the FastDNA kit (BIO101, Carlsbad, CA, USA) according to the manufacturer’s protocols. The primers V9G (de Hoog & Gerrits van den Ende 1998) and ITS4 (White et al. 1990) were used to amplify the internal transcribed spacer region (ITS) of the nuclear ribosomal RNA operon, including the 3′ end of the 18S rRNA gene, the first ITS region, the 5.8S rRNA gene; the second ITS region and the 5′ end of the 28S rRNA gene. To resolve taxa in the N. ribis complex (Slippers et al. 2004a) the primers EF1-728F and EF1-986R (Carbone & Kohn 1999) were used to amplify part of the translation elongation factor 1-α gene (TEF1) as described in Crous et al. (2004b) where applicable. Sequences for the internal transcribed spacers and 5.8S rDNA of the Botryosphaeriaceae isolates from Proteaceae were subjected to a megablastn search of NCBI’s GenBank nucleotide database. Identical and closely related sequences were downloaded manually aligned and added to the outgroup sequences using Sequence Alignment Editor v. 2.0a11 (Rambaut 2002) to create the alignment. Phylogenetic analyses of sequence data were made using PAUP (Phylogenetic Analysis Using Parsimony) v. 4.0b10 (Swofford 2003) and consisted of parsimony analyses with alignment gaps treated as a fifth character state and all characters were unordered and of equal weight. Maximum parsimony analysis was performed using the heuristic search option with 100 random taxa additions and tree bisection and reconstruction (TBR) as the branch-swapping algorithm. Branches of zero length were collapsed and all multiple, equally parsimonious trees were saved. The robustness of the tree(s) obtained was evaluated by 1 000 bootstrap replications (Hillis & Bull 1993). Other measures calculated included tree length, consistency index, retention index and rescaled consistency index (TL, CI, RI and RC). The resulting trees were printed with TreeView v. 1.6.6 (Page 1996). Sequences were deposited in GenBank (Table 2) and the alignment and tree in TreeBASE (www.treebase.org). The TEF1 sequences were compared with the sequences available in NCBI’s GenBank nucleotide database using a megablastn search.
Table 2.
Isolates investigated in this study.
| Fungus | Culture accession No. | GenBank No. |
Host1 | Locality | Collector | Reference | |
|---|---|---|---|---|---|---|---|
| ITS | TEF1 | ||||||
| Diplodia seriata | CPC 4373 | AF452556 | Protea magnifica | South Africa | S. Denman | Denman et al. 2003 | |
| Lasiodiplodia theobromae | CBS 111530 = CPC 2095 | FJ150695 | Leucospermum sp. | Hawaii | J.E. Taylor | Present study | |
| Neofusicoccum australe | CBS 115185 = CPC 5182 | FJ150696 | Protea cynaroides | Spain | S. Denman | Present study | |
| CPC 4393 | AF452548 | Protea cynaroides | South Africa | L. Swart | Denman et al. 2003 (as N. luteum) | ||
| CPC 13783 | FJ150697 | Protea sp. | Tenerife | P.W. Crous | Present study | ||
| Neofusicoccum parvum | CBS 111523 = CPC 2051 | AF452526 | Leucospermum sp. | Hawaii | P.W. Crous | Denman et al. 2003 | |
| CBS 111524 = CPC 2057 | AF452524 | FJ150709 | Protea cynaroides | Hawaii | P.W. Crous | Denman et al. 2003 | |
| CBS 114472 = CPC 2055 | AF452523 | FJ150710 | Leucadendron cv. Safari Sunset | Hawaii | P.W. Crous | Denman et al. 2003 | |
| CPC 4381 | AF452522 | Protea cynaroides | Zimbabwe | C. Saywood | Denman et al. 2003 | ||
| Neofusicoccum protearum | CBS 111496 = CPC 1772 | FJ150698 | Protea sp. | South Africa | J.E. Taylor | Present study | |
| CBS 111502 = CPC 1771 | FJ150699 | Protea sp. | South Africa | J.E. Taylor | Present study | ||
| CBS 113071 = CPC 5172 | FJ150700 | Protea cynaroides | Portugal | S. Denman | Present study | ||
| CBS 113076 = CPC 5186 | FJ150701 | Leucadendron cv. Safari Sunset | Portugal | S. Denman | Present study | ||
| CBS 113079 = CPC 5180 | FJ150702 | Protea cv. Pink Ice | Tenerife | S. Denman | Present study | ||
| CBS 114176 = CPC 1775 | AF452539 | Leucadendron cv. Silvan Red | South Africa | S. Denman | Denman et al. 2003 | ||
| CBS 115177 = CPC 4357 | FJ150703 | Protea magnifica | South Africa | S. Denman | Present study | ||
| CBS 115480 = CPC 4398 | AF452531 | Leucadendron sp. | Portugal | S. Denman | Denman et al. 2003 | ||
| CBS 115481 = CPC 4397 | AF452530 | Leucadendron tinctum | Madeira | S. Denman | Denman et al. 2003 | ||
| CBS 115499 = CPC 5171 | FJ150704 | Leucadendron sp. | Portugal | S. Denman | Present study | ||
| CBS 119220 = CMW 20464 | EU552144 | Leaf litter of Leucospermum conocarpodendron | South Africa | S. Marincowitz | Marincowitz et al. 2008 | ||
| CPC 2147 | AF452534 | Protea cynaroides | Hawaii | P.W. Crous | Denman et al. 2003 | ||
| CPC 2930 | AF452528 | Leucadendron sp. | Australia | P.W. Crous | Denman et al. 2003 | ||
| CPC 2988 | AF452537 | Protea magnifica | Australia | P.W. Crous | Denman et al. 2003 | ||
| CPC 4360 | AF195774 | Protea eximia | South Africa | S. Denman | Denman et al. 2000 | ||
| CPC 4361 | AF196295 | Protea magnifica | South Africa | S. Denman | Denman et al. 2000 | ||
| CPC 4367 | AF452544 | Protea neriifolia | South Africa | S. Denman | Denman et al. 2003 | ||
| CPC 4369 | AF452536 | Protea repens | South Africa | S. Denman | Denman et al. 2003 | ||
| CPC 4384 | AF452535 | Protea cynaroides | South Africa | S. Denman | Denman et al. 2003 | ||
| CPC 13780 | FJ150705 | Protea sp. | Tenerife | P.W. Crous | Present study | ||
| CBS 119220 = CMW 20464 | EU552144 | Twig litter and senescent cone of Leucadendron xanthoconus | South Africa | S. Marincowitz | Marincowitz et al. 2008 | ||
| Neofusicoccum sp. | CBS 115184 = CPC 4379 | AF452525 | FJ150711 | Protea cynaroides | Zimbabwe | C. Saywood | Denman et al. 2003 |
| Saccharata capensis | CBS 122693 = CPC 13699 = CMW 22200 | EU552130 | Leaf litter of Mimetes cucullata | South Africa | S. Marincowitz | Present study; Marincowitz et al. 2008 (as Saccharata sp.) | |
| CBS 122694 = CPC 13698 = CMW 22197 | EU552129 | Leaf litter of Leucospermum conocarpodendron subsp. viridum | South Africa | S. Marincowitz | Present study; Marincowitz et al. 2008 (as Saccharata sp.) | ||
| Saccharata proteae | CBS 114569 = CPC 2169 | FJ150706 | FJ150712 | Protea sp. | Hawaii | P.W. Crous | Present study |
| CBS 114570 = CPC 2273 | FJ150707 | Protea cv. Lady Di | Hawaii | P.W. Crous | Present study | ||
| CBS 115206 = CPC 4378 | AF452560 | Protea sp. | Australia (USDA interception) | M.E. Palm | Denman et al. 2003 | ||
| CBS 119218 = CMW 20003 | EU552145 | FJ150713 | Leaf litter of Protea lepidocarpodendron | Betty’s Bay, South Africa | S. Marincowitz | Marincowitz et al. 2008 | |
| CPC 2269 | AF452563 | Protea laurifolia | Hawaii | P.W. Crous | Denman et al. 2003 | ||
| CPC 2271 | AF452562 | Protea cv. Lady Di | Hawaii | P.W. Crous | Denman et al. 2003 | ||
| CPC 4355 | AF196301 | Protea repens | South Africa | S. Denman | Denman et al. 2000 | ||
| CPC 4358 | AF196299 | Protea cynaroides | South Africa | L. Swart | Denman et al. 2000 | ||
| CPC 4399 | AF452557 | Protea cynaroides | Madeira | S. Denman | Denman et al. 2003 | ||
| CPC 4400 | AF452559 | Protea repens | Portugal | S. Denman | Denman et al. 2003 | ||
| CPC 14856 | FJ150708 | Protea sp. | South Africa | P.W. Crous | Present study | ||
1 cv. Safari Sunset = Leucadendron salignum × Leucadendron laureolum, cv. Silvan Red = Leucadendron laureolum × Leucadendron salignum, cv. Lady Di = Protea magnifica × Protea compacta.
Morphology
Colony colours (surface and reverse) were assessed after growth on MEA and oatmeal agar (OA, Gams et al. 2007) using the colour charts of Rayner (1970). Microscopic observations were made from colonies cultivated on MEA and OA. Preparations were mounted in lactic acid and studied under a light microscope (× 1 000 magnification). The 95 % confidence intervals were derived from 30 observations of spores formed on MEA or OA, with extremes given in parentheses. All cultures obtained in this study are maintained in the culture collection of the Centraalbureau voor Schimmelcultures (CBS) in Utrecht, the Netherlands, and duplicates have been stored in the culture collection (CMW) of the Forestry and Agricultural Biotechnology Institute, Pretoria, South Africa or the working collection (CPC) of P.W. Crous (Table 2). Nomenclatural novelties and descriptions were deposited in MycoBank (www.MycoBank.org) and ITS barcodes and DNA sequence trace files in BOLD (www.barcodinglife.org).
RESULTS
DNA phylogeny
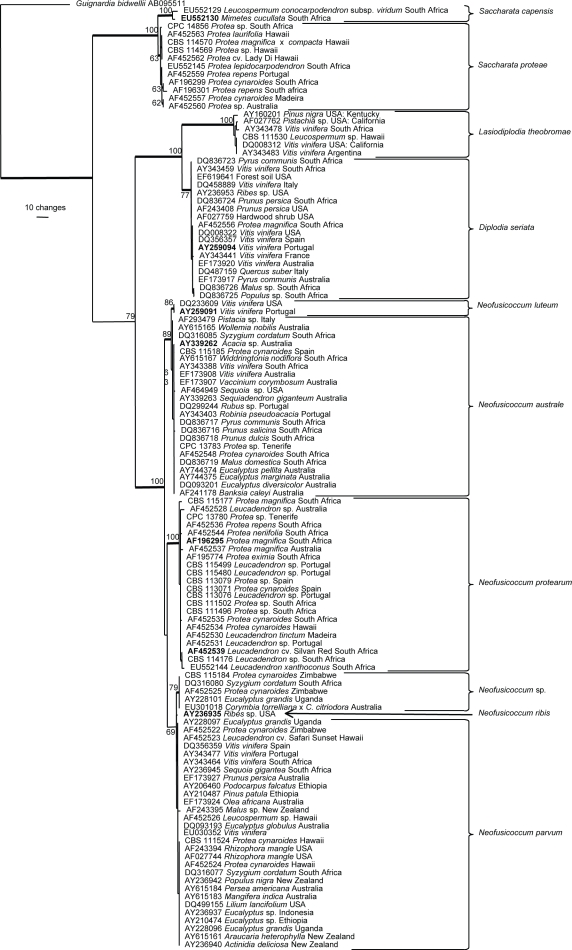
The manually adjusted ITS alignment contained 120 sequences (including the outgroup sequence) and 525 characters were used in the phylogenetic analysis; of these 162 were parsimony-informative, 72 were variable and parsimony-uninformative and 291 were constant. Only the first 1 000 equally most parsimonious trees, one of which is shown in Fig. 1, were saved from the parsimony analysis. Taxonomic novelties are described below and specific taxa are highlighted in the Discussion.
Fig. 1.
One of 1 000 equally most parsimonious trees (TL = 426 steps, CI = 0.847, RI = 0.983, RC = 0.833) obtained from a parsimony analysis using ITS sequence data of members of the Botryosphaeriaceae and allied genera. The bar indicates 10 changes. The numbers at the nodes represent bootstrap support values (higher than 60 %) based on 1 000 resamplings and bootstrap support values within species clades are not shown. Thickened lines indicate branches that are present in the strict consensus tree. The accession numbers of ex-type isolates are printed in bold face. The sequence of Guignardia bidwellii (GenBank accession AB095511) was included as outgroup.
Taxonomy
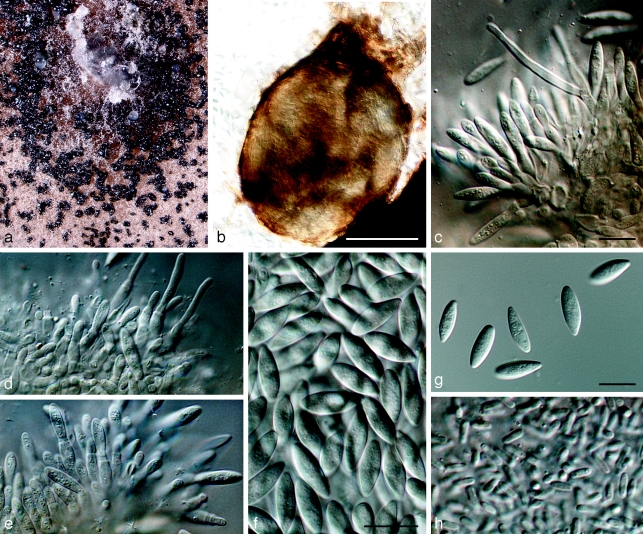
Saccharata capensis Crous, Marinc. & M.J. Wingf., sp. nov. — MycoBank MB512395; Fig. 2
Fig. 2.
Saccharata capensis on oatmeal agar (OA). a. Colony sporulating on OA; b. pycnidial conidioma; c, d. conidiogenous cells and branched paraphyses; d, e. conidiogenous cells showing percurrent proliferation; f, g. fusoid to ellipsoid conidia; h. spermatia. — Scale bars: b = 250 μm, all others = 10 μm.
Saccharata proteae simile, sed conidiis minoribus, (13–)15–16(−18) × (3.5–)4–5(−5.5) μm, differens.
Etymology. Name refers to the Cape Province of South Africa, where the fungus occurs.
Conidiomata pycnidial, black, up to 250 μm diam, opening by a single, central ostiole, up to 20 μm diam; wall consisting of 2–3 layers of pale dark brown textura angularis. Conidiophores hyaline, smooth, subcylindrical, branched, lining the inner layer of the cavity, 1–3-septate, 10–20 × 3.5–5 μm, intermingled with hyaline, smooth, subcylindrical paraphyses, 2–3 μm wide, with obtuse ends, extending slightly above the conidia. Conidiogenous cells phialidic with minute periclinal thickening, or 1–3 apical, percurrent proliferations, subcylindrical with slight apical taper, 7–12 × 3.5–4.5 μm. Conidia hyaline, smooth, thin-walled, aseptate, granular, fusoid-ellipsoid, apex subobtuse, base subtruncate, widest in the middle of the conidium, (13–)15–16(−18) × (3.5–)4–5(−5.5) μm (av. 15.5 × 4.5 μm). Spermatia formed in same conidioma as conidia, bacilliform, hyaline with rounded ends, 3–5 × 1–1.5 μm.
Cultural characteristics — Colonies sporulating profusely on OA, aerial mycelium sparse to absent, olivaceous-black with zones of grey-olivaceous in outer region; colonies flat, spreading, with irregularly crenate margins.
Specimens examined. South Africa, Western Cape Province, Kleinmond Nature Reserve, leaf litter of Mimetes cucullata, 11 July 2000, S. Marincowitz, holotype CBS H-20077, culture ex-type CBS 122693 = CMW 22200 = CPC 13699; Kogelberg Nature Reserve, leaf litter of Leucospermum conocarpodendron subsp. viridum, 11 July 2000, S. Marincowitz, CBS H-20078, culture CBS 122694 = CMW 22197 = CPC 13698.
Notes — Saccharata capensis is only the second species to be described in this genus (Crous et al. 2004a) and it is most easily distinguished from S. proteae by its smaller conidia. When it was originally isolated, a diplodia-like synanamorph, which is also typical of S. proteae, was observed in culture. With time, however, the cultures lost the ability to form this synanamorph and hence only the dominant anamorph state could be described here.
DISCUSSION
The present study is the first to revisit the taxonomy of Botryosphaeriaceae since Denman et al. (2003) treated the taxa that occur on Proteaceae. The most significant change to the taxonomy of this group subsequent to the study of Denman et al. (2003) was presented by Crous et al. (2006b). These authors employed LSU sequence data to reveal that the family consists of at least 10 distinct lineages, correlating to a diversity of different anamorphs and teleomorphs, and restricting Botryosphaeria to a rather small clade containing B. dothidea and B. corticis. This study was recently supplemented by Phillips et al. (2008), who used a similar approach to resolve the dark-spored genera of the Botryosphaeriaceae.
When they characterised the members of Botryosphaeriaceae occurring on proteas, Denman et al. (2003) reported the presence of some taxa that have since been shown to represent species complexes. The most significant of these, Neofusicoccum luteum (as Fusicoccum luteum), was reported from Buckinghamia and Banksia in Australia (Slippers et al. 2004b), and on Protea cynaroides in South Africa. Shearer et al. (1995) described a serious disease of Banksia coccinea caused by N. ribis (as B. ribis) along the south-western coast of Australia, which Denman et al. (2003) believed was N. luteum rather than N. ribis. In light of the present findings (Fig. 1) it appears that these isolates are more correctly treated as N. australe rather than the closely related N. luteum.
Neofusicoccum protearum was reported on Proteaceae from Australia, Madeira, Portugal and South Africa, and it is shown here to also occur on this host in Hawaii and Tenerife (Canary Islands). The exclusive association with South African Proteaceae led Denman et al. (2003) to hypothesise that N. protearum was indigenous to South Africa. In this case it would have been introduced into these other countries along with protea planting material, which is very plausible because the pathogen exists as endophyte in asymptomatic Proteaceae (Denman 2002, Denman et al. 2004). Neofusicoccum protearum causes leaf blight disease of Proteaceae, with lesions extending down the stems (Denman et al. 2003).
Denman et al. (2003) and Crous et al. (2004b) recorded N. ribis, from South African and Australian Proteaceae cultivated in Hawaii, and from P. cynaroides in Zimbabwe. This report, was largely based on the ITS sequence data available at the time, and the broad morphological circumscription applied to N. ribis. In a subsequent study, Slippers et al. (2004a) recollected and epitypified N. ribis, and showed that this species could be distinguished from the morphologically similar N. parvum, only by means of DNA sequence comparisons of TEF1. Results of the present study using these techniques showed clearly that these isolates from Proteaceae represent N. parvum and not N. ribis as initially reported. In fact, none of the previous reports of N. ribis from Proteaceae such as those on Grevillea robusta in Guatemala (Schieber & Zentmyer 1978) and Leucadendron in South Africa (Olivier 1951), have been confirmed and they need to be viewed with some circumspection. Results of this study have also shown that the cryptic Neofusicoccum species closely related to N. parvum and N. ribis, occurring on Eucalyptus in Uganda, Protea cynaroides in Zimbabwe, Corymbia in Australia and Syzygium cordatum in South Africa, probably represent yet another, undescribed component of the N. ribis species complex, which will be resolved elsewhere (B. Slippers, pers. comm.).
Other unconfirmed and doubtful records on Proteaceae include Botryosphaeria dothidea, which is reported to cause cankers, leaf infections and seedling dieback or blight of proteas (Crous et al. 2004a). Because of the lack of cultures and sequence data, we cannot at present confirm that B. dothidea occurs on Proteaceae. Although Lasiodiplodia theobromae, which is associated with dieback and stem cankers of proteas (Crous et al. 2004a) is confirmed from Hawaii (Fig. 1), records from elsewhere remain doubtful. Recent papers focusing on this pathogen have revealed it to represent a species complex (Pavlic et al. 2004, Burgess et al. 2006, Damm et al. 2007a, Alves et al. 2008), again casting doubt on the identity of the species of Lasiodiplodia associated with Proteaceae. While substantial progress has been made towards understanding and managing Botryosphaeriaceae diseases of Proteaceae in recent years (Denman et al. 2004), additional collections from various locations and hosts in this family are required to fully resolve the status of these pathogens on this economically and ecologically important family of plants.
Saccharata proteae appears to be highly host-specific and has been found associated only with South African Proteaceae (Crous et al. 2000b, Taylor et al. 2001a, b). Saccharata proteae, which is a well-established endophyte (Swart et al. 2000, Taylor et al. 2001c), causes leaf spots and leaf tip dieback, which is usually associated with insect wounds (Denman et al. 1999, Crous et al. 2004a). The ecology of S. capensis, which is newly described here from Proteaceae leaf litter, is unknown. However, it is quite possible that isolates of S. capensis have in the past been confused with those of S. proteae, and that it could have a similar ecological habitat. Furthermore, there is some variation in the DNA sequence data between the two collections of S. capensis (12 nt different), which could suggest that further collections may reveal yet more cryptic taxa in this complex.
Diplodia seriata (= ‘Botryosphaeria’ obtusa), Saccharata proteae and Neofusicoccum protearum all have an endophytic habitat (Crous et al. 2004a). Although it is not clear whether Diplodia seriata is a protea pathogen, there are reports of this species associated with serious stem cankers on fruit trees and grapevines (Denman et al. 2003, van Niekerk et al. 2004, Damm et al. 2007a). Several species of Botryosphaeriaceae that are pathogens have also been isolated from Protea litter, including S. proteae, N. protearum and species of Diplodia and Dothiorella (Marincowitz et al. 2008). It is, therefore, not possible to disregard S. capensis as a potential pathogen of Proteaceae until pathogenicity studies have been conducted.
Acknowledgments
We thank Miss Marjan Vermaas for help in preparing the photographic plate, and Arien van Iperen for preparing the fungal cultures for examination.
REFERENCES
- Alves A, Crous PW, Correia A, Phillips AJL. 2008. Morphological and molecular data reveal cryptic speciation in Lasiodiplodia theobromae. Fungal Diversity 28: 1 – 13 [Google Scholar]
- Burgess TI, Barber PA, Mohali S, Pegg G, Beer W de, Wingfield MJ. 2006. Three new Lasiodiplodia spp. from the tropics, recognized based on DNA sequence comparisons and morphology. Mycologia 98: 423 – 435 [DOI] [PubMed] [Google Scholar]
- Carbone I, Kohn LM. 1999. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553 – 556 [Google Scholar]
- Crous PW. 1998. Mycosphaerella spp. and their anamorphs associated with leaf spot diseases of Eucalyptus. Mycologia Memoir 21: 1 – 170 [Google Scholar]
- Crous PW, Denman S, Taylor JE, Swart L, Palm ME. 2004a. Cultivation and diseases of Proteaceae: Leucadendron, Leucospermum and Protea. CBS Biodiversity Series 2: 1 – 228 [Google Scholar]
- Crous PW, Groenewald JZ, Pongpanich K, Himaman W, Arzanlou M, Wingfield MJ. 2004b. Cryptic speciation and host specificity among Mycosphaerella spp. occurring on Australian Acacia species grown as exotics in the tropics. Studies in Mycology 50: 457 – 469 [Google Scholar]
- Crous PW, Rong IH, Wood A, Lee S, Glen H, Botha W, Slippers B, Beer WZ de, Wingfield MJ, Hawksworth DL. 2006a. How many species of fungi are there at the tip of Africa? Studies in Mycology 55: 13 – 33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WFO, Philips AJL, Alves A, Burgess T, Barber P, Groenewald JZ. 2006b. Phylogenetic lineages in the Botryosphaeriaceae. Studies in Mycology 55: 235 – 253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Taylor JE, Bullock S. 2000b. Fungi occurring on Proteaceae in Australia: selected foliicolous species. Australasian Plant Pathology 29: 267 – 278 [Google Scholar]
- Damm U, Crous PW, Fourie PH. 2007a. Botryosphaeriaceae as potential pathogens of Prunus in South Africa, with descriptions of Diplodia africana and Lasiodiplodia plurivora sp. nov. Mycologia 99: 664 – 680 [DOI] [PubMed] [Google Scholar]
- Damm U, Fourie PH, Crous PW. 2007b. Aplosporella prunicola, a novel species of anamorphic Botryosphaeriaceae. Fungal Diversity 27: 35 – 43 [Google Scholar]
- Denman S. 2002. Botryosphaeria diseases of Proteaceae. PhD thesis, Department of Plant Pathology, University of Stellenbosch, South Africa: [Google Scholar]
- Denman S, Crous PW, Groenewald JZ, Slippers B, Wingfield BD, Wingfield MJ. 2003. Circumscription of Botryosphaeria species associated with Proteaceae based on morphology and DNA sequence data. Mycologia 95: 294 – 307 [PubMed] [Google Scholar]
- Denman S, Crous PW, Sadie A, Wingfield MJ. 2004. Evaluation of fungicides for the control of Botryosphaeria protearum on Protea magnifica in the Western Cape Province of South Africa. Australasian Plant Pathology 33: 97 – 102 [Google Scholar]
- Denman S, Crous PW, Taylor JE, Kang J-C, Pascoe I, Wingfield MJ. 2000. An overview of the taxonomic history of Botryosphaeria, and a re-evaluation of its anamorphs based on morphology and ITS rDNA phylogeny. Studies in Mycology 45: 129 – 140 [Google Scholar]
- Denman S, Crous PW, Wingfield MJ. 1999. A taxonomic reassessment of Phyllachora proteae, a leaf pathogen of Proteaceae. Mycologia 91: 510 – 516 [Google Scholar]
- Gams W, Verkleij GJM, Crous PW. 2007. CBS Course of Mycology, 5th ed Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands: [Google Scholar]
- Hillis DM, Bull JJ. 1993. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Systematic Biology 42: 182 – 192 [Google Scholar]
- Hoog GS de, Gerrits van den Ende AHG. 1998. Molecular diagnostics of clinical strains of filamentous Basidiomycetes. Mycoses 41: 183 – 189 [DOI] [PubMed] [Google Scholar]
- Marincowitz S, Crous PW, Groenewald JZ, Wingfield MJ. 2008. Microfungi occurring on Proteaceae in the fynbos. CBS Biodiversity Series 7: 1 – 166 [Google Scholar]
- Niekerk JM van, Crous PW, Groenewald JZ, Fourie PH, Hallen F. 2004. DNA phylogeny and morphological characterization of Botryosphaeria species occurring on grapevines. Mycologia 96: 781 – 798 [PubMed] [Google Scholar]
- Olivier D. 1951. Progress in the study of the silver tree disease. The Journal of the Botanical Society of South Africa 37: 18 – 19 [Google Scholar]
- Page RDM. 1996. TREEVIEW: An application to display phylogenetic trees on personal computers. Computer Applications in the Biosciences 12: 357 – 358 [DOI] [PubMed] [Google Scholar]
- Pavlic D, Slippers B, Coutinho TA, Gryzenhout M, Wingfield MJ. 2004. Lasiodiplodia gonubiensis sp. nov., a new Botryosphaeria anamorph from native Syzygium cordatum in South Africa. Studies in Mycology 50: 313 – 322 [Google Scholar]
- Petrak VF. 1967. Über eine neue Botryosphaeria-Art der australischen flora. Sydowia 21: 235 – 239 [Google Scholar]
- Phillips AJL, Alves A, Pennycook SR, Johnston PR, Ramaley A, Akulov A, Crous PW. 2008. Resolving the status of dark-spored teleomorph genera in the Botryosphaeriaceae. Persoonia 21: 29 – 55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaut A. 2002. Sequence Alignment Editor. Version 2.0 Department of Zoology, University of Oxford, United Kingdom: [Google Scholar]
- Rayner RW. 1970. A mycological colour chart Commonwealth Agricultural Bureau, Kew, United Kingdom: [Google Scholar]
- Rebelo T. 2001. SASOL Proteas: A field guide to the Proteas of Southern Africa. 2nd edn Fernwood Press, South Africa: [Google Scholar]
- Schieber E, Zentmyer GA. 1978. An important canker disease of Grevillea in Guatemala. Plant Disease Reporter 62: 923 – 924 [Google Scholar]
- Shearer BL, Fairman RG, Bathgate JA. 1995. Cryptodiaporthe melanocraspeda canker as a threat to Banksia coccinea on the south coast of Western Australia. Plant Disease 79: 637 – 641 [Google Scholar]
- Slippers B, Crous PW, Denman S, Coutinho TA, Wingfield BD, Wingfield MJ. 2004a. Combined multiple gene genealogies and phenotypic characters differentiate several species previously identified as Botryosphaeria dothidea. Mycologia 96: 83 – 101 [PubMed] [Google Scholar]
- Slippers B, Vermeulen G, Crous PW, Coutinho TA, Wingfield BD, Wingfield MJ. 2004b. Multiple gene sequences delimit Botryosphaeria australis sp. nov. from B. lutea. Mycologia 96: 1030 – 1041 [PubMed] [Google Scholar]
- Slippers B, Wingfield MJ. 2007. The Botryosphaeriaceae as endophytes and latent pathogens of trees: Identification, ecology and potential impact. Fungal Biology Reviews 21: 90 – 106 [Google Scholar]
- Swart L, Crous PW, Petrini O, Taylor JE. 2000. Fungal endophytes of Proteaceae, with particular emphasis on Botryosphaeria proteae. Mycoscience 41: 123 – 127 [Google Scholar]
- Swofford DL. 2003. PAUP*: Phylogenetic Analyses using Parsimony (* and other methods), Version 4.0b10, Sinauer, Sunderland, USA: [Google Scholar]
- Taylor JE, Crous PW, Palm ME. 2001a. Foliar and stem fungal pathogens of Proteaceae in Hawaii. Mycotaxon 78: 449 – 490 [Google Scholar]
- Taylor JE, Crous PW, Swart L. 2001b. Foliicolous and caulicolous fungi associated with Proteaceae cultivated in California. Mycotaxon 78: 75 – 103 [Google Scholar]
- Taylor JE, Denman S, Crous PW. 2001c. Endophytes isolated from three species of Protea in a nature reserve in the Western Cape, South Africa. Sydowia 53: 247 – 260 [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (eds), PCR Protocols: a guide to methods and applications: 315–322 Academic Press, USA: [Google Scholar]