Abstract
Background:
Cognitive event-related potential (P300) is an index of cognitive processing time. It was found to be prolonged in dementia, renal, and hepatic encephalopathies, but was not extensively assessed in respiratory failure.
Objective:
To evaluate P300 changes in patients with respiratory failure, and especially those with mild or subclinical hypoxic–hypercapnic encephalopathy.
Methods:
Auditory event-related evoked potential P300 latency was measured using an oddball paradigm in patients with respiratory failure due to any cause (partial pressure of oxygen in arterial blood (PO2) should be 75 mm/Hg or less). Apart from blood gases measurement, patients underwent the Mini-Mental State Examination (MMSE). Patient performances were compared with that of matched normal control. Patients were admitted into the study from outpatient clinics and wards at King Khalid University Hospital and Sahara Hospital.
Results:
Thirty-four patients (12 women, 22 men) were admitted to the study. Ages ranged from 19–67 years with a mean of 46.1 years. Respiratory failure was severe or very severe in 11 patients (33%), and mild or moderate in the rest (66%). Mean value for PO2 and partial pressure of carbon dioxide in arterial blood (PCO2) were 63.7 and 45.2 mm/Hg, respectively. pH mean was 7.4 and O2 saturation was 90.7%. P300 latency ranged from 218 to 393 milliseconds, with a mean of 338.4 milliseconds. In comparison with control (309.9 milliseconds), there was a significant difference (P = 0.007). P300 amplitude differences were not significant. No significant difference in MMSE was noted between mild and severe respiratory failure. Results of detailed neuropsychological assessment were clearly abnormal but were limited by the small number of tested patients. P300 latency changes correlated significantly with age as well as severity of respiratory failure. P300 was also significantly delayed whether hypoxia occurred with or without hypercapnia.
Conclusion:
Results show a significant delay of P300 latency in patients with severe and mild respiratory failure. This was associated with subclinical encephalopathy in most patients, evidenced by a near-normal MMSE score. Apart from confirming the importance of P300 latency measurement as a marker of respiratory encephalopathy, this study asserts the causal relationship between hypoxemia and cognitive derangement. Furthermore, it promotes the early use of oxygen therapy in a selected group of patients with mild or moderate respiratory failure, who have responsibilities which involve taking rapid critical decisions.
Keywords: event-related evoked potentials, hypoxic–hypercapnic encephalopathy, respiratory failure, chronic respiratory encephalopathy
Introduction
Chronic hypoxic–hypercapnic states occur in many pulmonary and cardiac diseases. These states affect the central nervous system causing well-described nonspecific clinical manifestations including headache, dullness of mentation and drowsiness, confusion progressing in severe cases to coma with papilledema, asterixis, action tremor, and muscular twitching.1 When these features appear in the context of a severe respiratory failure, the association is clear, and electroencephalography (EEG) shows marked slowing of brain activity.2,3 On the other hand, mild chronic hypoxic conditions cause subtle or subclinical changes including inattention, reduction in psychomotor activity, forgetfulness, slight decrease of intelligence, slowing of reaction time, and abnormalities in constructional drawings.4–6 Grant and colleagues administered the Halstead–Reitan neuropsychological test battery to patients with chronic obstructive pulmonary disease (COPD), and found their scores to be significantly lower than controls in all tests.5 COPD patient performance, particularly in tasks requiring sustained attention, was poor. In these instances, abnormalities of partial pressure of oxygen in arterial blood (PO2) and partial pressure of carbon dioxide in arterial blood (PCO2) can be easily confirmed by a simple blood test. On the contrary, there is no similar readily available objective tests for the assessment of associated cognitive derangement as structured neuropsychological tests are too complex and timely to be included in the clinical assessment of most patients. Heaton and Pendleton have strongly argued that many of these subtle cognitive impairment affects the quality of various daily activities.7 It is expected that such subtle cognitive dysfunction may have serious consequences in situations where patients are involved in activities requiring rapid critical decisions such as operators of complex machines.
Visual-, auditory-, and somatosensory-evoked potentials are well established neurophysiological tests and are widely used in investigating various neurological disorders.8–11 Although its value in assessing acute hypoxic states is well established,12,13 its benefit in assessing the effect of chronic hypoxic–hypercapnic states on the nervous system was doubtful.14–19 Cognitive event-related evoked potentials (EREPs) are long-latency potentials obtained during information processing tasks which involve attention, stimulus discrimination, memory, and related processes.20 Among the many EREP components, the P300 potential is the most widely studied and used. It is a positive-going potential with a modal latency of 300 ms and centroparietal scalp distribution, obtained usually by the oddball paradigm.21 P300 latency is an index of cognitive processing time and was shown to be prolonged in normal aging, confusional states, and dementia.22–24 P300 latency has been found also to be a sensitive marker of subclinical encephalopathy in hepatic and renal failure.25–28 The effect of acute hypoxia on EREP was studied in an experiment that utilized a simulated high altitude environment, and P300 latency was found to be delayed.29 So far, few studies have described P300 latency abnormalities in chronic respiratory insufficiency.16,17,30 A group of 19 patients with mild or moderate respiratory failure (RF) of different etiologies were studied by Barbieri and colleagues16 and another group of 17 patients with variable severity chronic RF by Nakano and colleagues.17 Although their results show a tendency for P300 changes, these were not significant. Umahara and colleagues, on the other hand, in a selected group of 14 patients with post pulmonary tuberculosis RF, were able to detect a significant delay in P300.30 Taking into account the large variability of P300 latency, these studies are limited by the relatively small number of studied subjects, besides the insignificant results in the first two. This study is designed to examine cognitive EREP in RF in a large group of patients and compare it with mental assessment results and measurement of blood gases. In particular, we aim to determine the sensitivity of P300 changes in detecting mild or subclinical cognitive impairment (ie, subclinical respiratory encephalopathy).
Patients and methods
Thirty-four patients, 12 females (35.3%) and 22 males (64.7%) were admitted to the study from both King Khalid University Hospital and Sahara Hospital out- and inpatient services. All had chronic RF with PCO2 of 75 mm/Hg or less, which could be either type I in the presence of hypoxia alone, or type II when combined with hypercapnia. All patients should be able to conduct all required assessments and investigations. Patients with any cause of mental dysfunction apart from RF were excluded. The severity of RF was defined according to PO2 as mild (75–65 mm/Hg), moderate (64–60 mm/Hg), severe (59–50 mm/Hg), and very severe (<50 mm/Hg). Patients underwent the following assessments and investigations:
- Clinical:
- Full neurological assessment
- Mini-Mental State Examination (MMSE)
- Arterial blood gases:
- PO2, PCO2, PH, and O2 saturation.
- Neurophysiological:
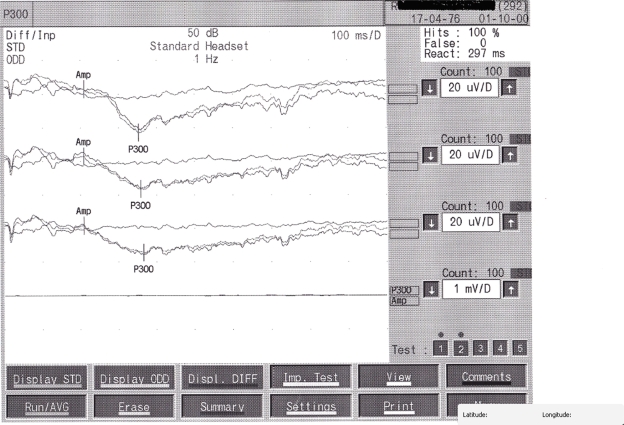
- P300 in two-tone oddball paradigm (Figure 1).
Figure 1.
P300 in two-tone oddball paradigm.
Event-related potential recordings were performed using standard methods recommended by the International Federation of Clinical Neurophysiology (IFCN).31
Data analysis
The StatPac Gold (StatPac Inc., Bloomington, MN) statistical analysis package was utilized to analyze the results.
Results
Patient ages ranged from 19 to 67 years with a mean of 46.4 ± 11.6 years. Eight were below the age of 40 years (25.8%), 22 (61.3%) were aged 40–59 years, and four (12.9%) were older than 60 years. Biodata, P300 latency and amplitude, blood gases results, and MMSE scores are included in Table 1.
Table 1.
Biodata and results of blood gases, EREP, and MMSE in 34 patients
| Serial no | File no | Sex | Age | PO2 | PCO2 | PH | O2 Sat % | Fz Cz Pz Latency ms | Fz Cz Pz Amplitude μv | MMSE | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 466650 | F | 43 | 47.2 | 71.4 | 7.360 | 80.9 | 327 | 328 | 329 | 1.17 | 0.10 | 0.85 | 24 |
| 2 | 508904 | M | 53 | 39.7 | 35.3 | 7.464 | 78.2 | 326 | 332 | 344 | 10.40 | 8.05 | 7.11 | 25 |
| 3 | 596577 | F | 31 | 51.2 | 49.2 | 7.401 | 86.0 | 304 | 302 | 306 | 5.57 | 8.99 | 9.27 | 24 |
| 4 | 346571 | M | 62 | 75.2 | 44.5 | 7.400 | 95.0 | 359 | 358 | 358 | 9.27 | 8.93 | 11.00 | 23 |
| 5 | 596731 | M | 39 | 56.9 | 40.6 | 7.442 | 91.8 | 322 | 326 | 329 | 11.40 | 19.40 | 18.20 | 24 |
| 6 | 055464 | M | 45 | 32.0 | 64.2 | 7.353 | 63.2 | 332 | 332 | 332 | 4.97 | 4.36 | 6.69 | 26 |
| 7 | 543238 | M | 54 | 64.6 | 46.5 | 7.378 | 93.2 | 362 | 368 | 368 | 15.50 | 11.40 | 9.48 | 25 |
| 8 | 460117 | M | 53 | 47.2 | 49.7 | 7.362 | 84.2 | 392 | 393 | 397 | 52.80 | 20.80 | 11.60 | 24 |
| 9 | 416512 | M | 65 | 52.2 | 43.7 | 7.424 | 88.2 | 383 | 382 | 382 | 9.88 | 6.06 | 7.77 | 27 |
| 10 | 147927 | M | 67 | 49.6 | 52.2 | 7.379 | 83.6 | 549 | 558 | 554 | 20.30 | 12.10 | 7.36 | 28 |
| 11 | 414386 | M | 55 | 69.9 | 34.6 | 7.443 | 93.7 | 299 | 302 | 10.60 | 15.90 | 30 | ||
| 12 | 159095 | M | 30 | 52.1 | 49.3 | 7.378 | 84.1 | 304 | 283 | 282 | 16.20 | 13.50 | 7.71 | 27 |
| 13 | 206853 | M | 36 | 40.8 | 49.2 | 7.373 | 81.5 | 251 | 268 | 268 | 3.98 | 9.64 | 14.50 | 24 |
| 14 | 606009 | M | 46 | 62.5 | 40.6 | 7.450 | 92.5 | 294 | 295 | 306 | 7.89 | 15.60 | 12.80 | 27 |
| 15 | 611416 | M | 36 | 74.5 | 41.2 | 7.397 | 97.6 | 202 | 208 | 11.30 | 9.80 | 3.35 | 25 | |
| 16 | 096204 | M | 57 | 65.5 | 43.1 | 7.398 | 93.0 | 352 | 352 | 352 | 23.60 | 18.30 | 14.90 | 29 |
| 17 | 111112 | F | 37 | 57.7 | 48.3 | 7.349 | 88.4 | 340 | 329 | 320 | 8.62 | 7.86 | 5.79 | 26 |
| 18 | 618472 | F | 50 | 54.0 | 65.5 | 7.398 | 86.8 | 368 | 372 | 367 | 9.23 | 16.70 | 10.80 | 25 |
| 19 | 612457 | M | 46 | 68.5 | 38.9 | 7.413 | 93.0 | 329 | 332 | 338 | 15.80 | 16.40 | 15.40 | 27 |
| 20 | 005619 | F | 40 | 67.4 | 39.8 | 7.431 | 96.6 | 311 | 308 | 312 | 15.60 | 20.40 | 17.00 | 28 |
| 21 | 345696 | F | 50 | 75.7 | 37.9 | 7.409 | 96.0 | 387 | 391 | 398 | 10.40 | 8.05 | 7.16 | 26 |
| 22 | 229859 | M | 38 | 57.9 | 39.5 | 7.423 | 92.6 | 378 | 374 | 374 | 4.99 | 9.01 | 10.40 | 25 |
| 23 | 055464 | M | 45 | 75.7 | 79.8 | 7.338 | 95.0 | 378 | 362 | 245 | 14.00 | 10.40 | 13.20 | 28 |
| 24 | 252915 | M | 38 | 58.5 | 47.1 | 7.407 | 90.3 | 351 | 332 | 331 | 6.59 | 3.10 | 2.54 | 24 |
| 25 | 344947 | F | 65 | 75.9 | 37.4 | 7.449 | 95.0 | 355 | 358 | 358 | 3.29 | 3.10 | 1.18 | 25 |
| 26 | 393969 | F | 50 | 75.2 | 44.0 | 7.394 | 96.8 | 336 | 336 | 338 | 18.10 | 16.70 | 17.40 | 26 |
| 27 | 535671 | F | 52 | 75.2 | 44.1 | 7.400 | 96.1 | 345 | 341 | 338 | 16.00 | 11.20 | 7.99 | 30 |
| 28 | 591569 | M | 19 | 73.7 | 38.2 | 7.374 | 94.5 | 309 | 308 | 305 | 8.05 | 16.40 | 20.60 | 27 |
| 29 | 596133 | M | 46 | 66.1 | 35.1 | 7.443 | 94.0 | 327 | 336 | 348 | 21.10 | 18.30 | 16.80 | 28 |
| 30 | 472146 | M | 58 | 72.8 | 30.9 | 7.463 | 95.5 | 330 | 334 | 334 | 101.60 | 68.50 | 53.60 | 29 |
| 31 | 568092 | F | 40 | 65.6 | 44.2 | 7.351 | 91.9 | 318 | 318 | 319 | 22.20 | 25.30 | 14.60 | 24 |
| 32 | 016557 | F | 58 | 70.3 | 40.7 | 7.396 | 94.1 | 311 | 312 | 312 | 0.73 | 6.76 | 10.10 | 22 |
| 33 | 514339 | F | 40 | 75.9 | 33.6 | 7.394 | 96.6 | 390 | 395 | 395 | 21.90 | 10.70 | 5.65 | 28 |
| 34 | 550413 | M | 26 | 64.8 | 38.7 | 7.421 | 93.0 | 319 | 309 | 309 | 6.49 | 7.86 | 3.59 | 27 |
Abbreviations: EREP, event-related evoked potentials; MMSE, Mini-Mental State Examination; PO2, partial pressure of oxygen in arterial blood; PCO2, partial pressure of carbon dioxide in arterial blood.
Thirty-six controls matched for age, sex, and education level were included. Their demographic data and results of P300 latency and amplitude measurements are included in Table 2.
Table 2.
Biodata and results of P300 in 36 normal controls
| Serial no | Hospital no | Age | Sex | Education/Work | P300 Latency ms | P300 Amplitude μv |
|---|---|---|---|---|---|---|
| 1 | 111111 | 44 | M | College level | 372 | 18.1 |
| 2 | 580776 | 41 | F | College level | 313 | 13.2 |
| 3 | 068562 | 68 | F | College level | 328 | 14.9 |
| 4 | 170476 | 57 | F | College level | 356 | 19.3 |
| 5 | 155210 | 38 | F | College level | 308 | 16.3 |
| 6 | 224721 | 57 | F | College level | 309 | 28.2 |
| 7 | 382234 | 42 | M | College level | 288 | 26.3 |
| 8 | 103774 | 49 | F | College level | 334 | 20.0 |
| 9 | 050455 | 58 | F | College level | 290 | 23.3 |
| 10 | 312645 | 55 | M | College level | 220 | 14.5 |
| 11 | 149844 | 53 | F | College level | 283 | 24.9 |
| 12 | 339360 | 45 | F | College level | 361 | 11.0 |
| 13 | 123784 | 51 | F | College level | 295 | 19.6 |
| 14 | 219041 | 56 | F | College level | 344 | 5.83 |
| 15 | 469789 | 43 | M | Secondary level | 316 | 10.7 |
| 16 | 338830 | 50 | F | College level | 258 | 10.3 |
| 17 | 344239 | 53 | F | College level | 301 | 26.9 |
| 18 | 123456 | 54 | F | Secondary level | 305 | 21.1 |
| 19 | 041768 | 45 | F | Secondary level | 312 | 9.05 |
| 20 | 001020 | 63 | M | Secondary level | 333 | 16.2 |
| 21 | 015408 | 55 | M | Secondary level | 290 | 23.9 |
| 22 | 035526 | 62 | M | Secondary level | 306 | 10.1 |
| 23 | 002477 | 58 | M | Secondary level | 302 | 22.4 |
| 24 | 028034 | 48 | M | Secondary level | 274 | 5.98 |
| 25 | 070112 | 48 | M | Secondary level | 308 | 33.8 |
| 26 | 018549 | 57 | M | College level | 309 | 7.06 |
| 27 | 396058 | 46 | M | College level | 328 | 15.3 |
| 28 | 712658 | 62 | M | College level | 355 | 22.8 |
| 29 | 851708 | 56 | M | College level | 308 | 38.3 |
| 30 | 417765 | 55 | M | Secondary level | 301 | 13.1 |
| 31 | 123456 | 52 | M | Secodary level | 333 | 21.1 |
| 32 | 026199 | 50 | M | Secondary level | 338 | 8.9 |
| 33 | 123456 | 44 | M | College level | 274 | 20.7 |
| 34 | 000440 | 47 | M | College level | 319 | 15.1 |
| 35 | 000112 | 47 | M | College level | 323 | 15.4 |
| 36 | 000000 | 40 | F | Secondary level | 308 | 13.1 |
Fourteen patients (40%) had severe or very severe RF, and 60% had mild to moderate severity RF.
The mean value for PO2 and PCO2 were 63.7 ± 14.3 mm/Hg (range from 32–90.7 mm/Hg), and 45.26 ± 10.68 mm/Hg (range from 30–79.8 mm/Hg) respectively. Mean value for pH was 7.4 ± 0.34 and 90.7% ± 7.2% for O2 saturation. MMSE scores ranged from 22–30 with a mean of 26.1 ± 2.02. Table 3 summarizes these results. No significant difference in MMSE could be found between different subtypes of RF.
Table 3a.
Summary of results blood gases and MMSE in 34 patients with respiratory failure
| Blood gas N = 34 | Minimum | Maximum | Mean | Median | Standard deviation |
|---|---|---|---|---|---|
| PO2 | 32.0 | 90.7 | 63.72 | 65.15 | 14.34 |
| PCO2 | 30.0 | 79.8 | 45.26 | 43.4 | 10.78 |
| pH | 7.34 | 7.46 | 7.4 | 7.4 | 0.34 |
| O2 saturation | 63.2 | 99.6 | 90.71 | 93.0 | 7.36 |
| MMSE | 22.0 | 30.0 | 26.09 | 26.0 | 2.02 |
Abbreviations: MMSE, Mini-Mental State Examination; PO2, partial pressure of oxygen in arterial blood; PCO2, partial pressure of carbon dioxide in arterial blood.
Table 3b.
Data of neuropsychological findings in five patients who underwent a full battery of neuropsychological assessments
| Test | Mean | Standard deviation | Assessment |
|---|---|---|---|
| Digit span (forward) | 3.2 | 1.78 | Very poor |
| Verbal immediate recall | 8 | 2.9 | Acceptable, but slightly low |
| Verbal delayed recall | 6.4 | 2.6 | Poor |
| Verbal fluency | 26.1 | 1.64 | Good |
| Raven’s test | 19.0 | 6.44 | Poor |
P300 (Cz) latencies in patients ranged from 268 to 393 ms, with a mean of 339.29 ± 54.26 ms. P300 amplitude was extremely variable at 13.52 ± 11.3 μv. The mean P300 latency in controls was 309.96 ± 29.15 ms, ranging from 220–372 ms, and P300 amplitude mean was 15.08 ± 6.43 μv. EREP results for patients and controls are summarized in Table 4.
Table 4.
Summary of results of EREPs in 34 patients with chronic respiratory failure and their controls
| EREP | Cases (X ± SD) | Controls (X ± SD) | t |
|---|---|---|---|
| Fz (latency) | 339.29 ± 54.26 ms | 309.97 ± 27.08 | 0.005* |
| Cz (latency) | 339.06 ± 54.88 ms | 310.49 ± 31.11 | 0.008* |
| Pz (latency) | 338.06 ± 56.72 ms | 309.43 ± 31.17 | 0.01* |
| Fz (amplitude) | 15.28 ± 17.91 μv | 15.08 ± 7.64 | 0.95 |
| CZ (amplitude) | 13.52 ± 11.30 μv | 16.68 ± 7.39 | 0.165 |
| PZ (amplitude) | 11.41 ± 9.14 μv | 13.48 ± 6.05 | 0.263 |
| Mean P300 latency | 338.44 ± 53.99 ms | 309.96 ± 29.15 | 0.007* |
| Mean P300 amplitude | 13.42 ± 12.9 μv | 15.08 ± 6.43 | 0.471 |
Abbreviations: EREP, event-related evoked potentials; P300, cognitive event-related potential; SD, standard deviation.
P300 latency (Cz) was higher among patients than controls (339.29 ± 54.26 ms, vs 309.97 ± 27.08 ms), and was also higher for Pz measurements (339.06 ± 54.88 ms, vs 310.49 ± 31.11 ms). The differences were statistically significant for both measurements (P = 0.005 and 0.008, respectively).
The mean P300 latency from all measurements was also significantly higher in patients than controls (338.44 ± 53.99 ms vs 309.96 ± 29.15 ms; P = 0.007).
P300 amplitude at Cz and Pz were lower in patients than controls (16.68 ± 7.39 μv vs 13.52 ± 11.3 μv and 13.48 ± 6.05 μv vs 11.41 ± 9.14 μv, respectively), but were not statistically significant. Also the mean P300 amplitude difference was not statistically significant (13.42 ± 12.2 μv vs 15.08 ± 6.43 μv, respectively) between both groups. The mean P300 latency in patients changed from 324.2 ms in mild RF to 367.3 ms in very severe RF.
There is a moderate correlation between age and the mean P300 (latency) in cases (r = 0.54, P = 0.001), but not in controls (r = 0.06, P = 0.71). The correlation between age and mean P300 amplitude was weak for cases and controls (r = 0.17, P = 0.32 and r = 0.07, P = 0.67, respectively).
Discussion
This study clearly confirm the occurrence of P300 latency delay in association with RF. This is consistent with results obtained by Umahara and colleagues in their selected group of 14 patients with postpulmonary tuberculosis RF.30 P300 latency is already experimentally proven as marker of cognitive function (central processing time), and its delay has already been documented in other metabolic encephalopathies. Of special importance is the fact that significant P300 latency changes were observed in association with various degrees of RF, including the mild or subclinical states. Most patients with mild RF in this study had no or some nonspecific complaints, such as headache, concentration, and memory difficulties, which usually do not raise significant clinical concern. Furthermore MMSE was found to be insensitive in most of these patients. P300 latency may be of special importance in this group of patients that demonstrate the presence of definite, though subtle, cognitive derangement. This is because patients at this stage may be executing or undertaking complicated or risky tasks unaware that their mental performance is compromised. Misjudgments or miscalculated decisions may have serious consequences, increase the burden of stress, precipitate work and home difficulties, and possibly emotional disturbances, including depression. Different studies has been able to document subtle cognitive derangements in subclinical respiratory encephalopathy using detailed neuropsychological assessments.4–6 In such patients, P300 measurement will be an objective method in assessing cognitive derangement and confirming the presence of ‘subclinical’ respiratory encephalopathy.
On another front, P300 derangement occurs in both types of RF, and Its latency correlated well with severity of hypoxemia in both types. In type 2 RF, it may be partially responsible, however, in type 1 it is the only culprit. The question which arises here is whether correction of hypoxemia, however mild, may be of help in correcting the associated cognitive derangements. In cases of overt clinical respiratory encephalopathy associated with severe hypoxemia, the use of oxygen led to clear mental improvement, which formed the basis for portable oxygen therapy. Should portable oxygen be offered to patients with a milder degree of RF, especially those undertaking sophisticated, specialized professions? Results of the present study clearly goes in favor of such a decision, as the use of P300 latency as the marker of processing time was found to be prolonged in most of these patients. However a more definite answer to this question is expected from a prospective interventional study, where P300 latency is assessed before and after administering oxygen.
Finally, P300 latency varies widely in normal subjects, making measurements of individual patients of little use generally. Although we were able to establish a normal P300 latency value with a two standard-error range in this study, the practical use of these absolute values in individual patients will require prolonged experience. The main benefit of this potential will be in assessing the benefit of a therapeutic modality, ie, oxygen, in a group of patients with respiratory encephalopathy. Or conversely, assessing the effect of any medicine used for other reasons on exacerbating encephalopathy. Taking into account the fact that all patients in the group of mild encephalopathy do not use oxygen therapy, it is reasonable to suggest that at least some of those patients may benefit from an earlier introduction of such treatment, especially a subgroup of patients holding critical responsibilities, in whom cognitive dysfunction may affect their optimum performance.
Acknowledgments
The authors would like to acknowledge the generous grant of King Abdulaziz City for Science and Technology (KACST) for this project (Limited Grant Project No. 3–25). Without such support this work would not have been completed. The authors are also grateful to the help of other staff including technicians, secretaries, and research assistants at King Khalid University Hospital and Sahara Hospital.
References
- 1.Adams R, Victor M. Principles of Neurology. New York, NY: McGraw Hill; 1993. The acquired metabolic disorders of the neuron systems; pp. 5pp. 877–902. [Google Scholar]
- 2.Labram C, Bursauxe M, Baudouin R, Gaillot MJ. L’encephalopathie respiratoire. Hypertension intracanienne ave oedeme papillaire et hemorragies retiniennes au cours d’une insuffisance respiratoire chroniique meconnue. Presse Med. 1966;74:2465–2468. [PubMed] [Google Scholar]
- 3.Meyer JS, Gotoh H, Tomita A. Acute respiratory acidemia correlation of jugular blood composition and electroencephalogram during CO2 narcosis. Neurology. 1969;16:463–474. doi: 10.1212/wnl.16.5.463. [DOI] [PubMed] [Google Scholar]
- 4.Flenley DC. Clinical hypoxia: causes, consequences and correction. Lancet. 1978;1:542–546. doi: 10.1016/s0140-6736(78)90564-0. [DOI] [PubMed] [Google Scholar]
- 5.Grant I, Heaton RK, McSweeny AJ, Adams KM, Timms RM. Neuropsychologic findings in hypoxemic chronic obstructive pulmonary disease. Arch Intern Med. 1982;142:1470–1476. [PubMed] [Google Scholar]
- 6.Prigatano GP, Parsons O, Wright E, Levin DC, Hawryluk G. Neuropsychologic test performance in mildly hypoxemic patients with chronic obstructive pulmonary disease. J Cons Clin Psychol. 1983;51:108–116. doi: 10.1037//0022-006x.51.1.108. [DOI] [PubMed] [Google Scholar]
- 7.Heaton RK, Pendleton MG. Use of neuropsychological test to predict adult patient’s everyday functioning. J Cons Clin Psychol. 1981;49:807–821. doi: 10.1037//0022-006x.49.6.807. [DOI] [PubMed] [Google Scholar]
- 8.Celesia GG, Brigell MG. Pattern visual stimulation in pre-chiasmatic lesions. Visual Evoked Potentials. 1990:76. [Google Scholar]
- 9.Kono, Yeda Y, Nakajima K, Araki K, Kagawa K, Kashima K. Subcortical impairment in subclinical hepatic encephalopathy. J Neurol Sci. 1994;126(2):162–167. doi: 10.1016/0022-510x(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 10.Aminoff MJ. Brainstem auditory evoked potentials in neurology: Methodology, interpretation, and clinical application. In: Stockard J, Pope-Stockard JE, Sharbrough FW, editors. Electrodiagnosis in Clinical Neurology. 1992. pp. 503–536. [Google Scholar]
- 11.Eisen A. The use of somatosensory evoked potentials for the evaluation of the peripheral nervous system. Neurol Clin. 1988;6:825–838. [PubMed] [Google Scholar]
- 12.Tsuyama N, Tsuzuki N, Kurokawa T, Imai T. Clinical application of spinal cord action potential measurement. Int Orthop. 1978;2:39–44. [Google Scholar]
- 13.Iwayama K, Mori K, Sakai S, Yamashiro K, Iwamoto K. The changes of somatosensory evoked potentials accompanying ischaemia and hypoxia in cats. Neurol Res. 1986;8:157–163. doi: 10.1080/01616412.1986.11739748. [DOI] [PubMed] [Google Scholar]
- 14.Mabin D, Borsotti JP, Le Mevel JC, Tea S, Calvier J. Value of visual and somatosensory evoked potentials and the electroencephalogram in chronic respiratory insufficiency. Rev Electroencephalogr Neurophysiology Clin. 1985;15:53–57. doi: 10.1016/s0370-4475(85)80035-6. [DOI] [PubMed] [Google Scholar]
- 15.Zeitlhofer J, Graf M, Mamoli B, Schilc K, Kummer F, Haber P. Neurophysiologic studies in Pickwickian syndrome. Nervenarzt. 1986;57:262–268. [PubMed] [Google Scholar]
- 16.Barbieri S, Fayoumi XZ, Berardinelli P, et al. Evidence of a subclinical involvement of central nervous system in mild or moderate chronic respiratory insufficiency. Electromyogr Clin Neurophysiol. 1996;36:67–72. [PubMed] [Google Scholar]
- 17.Nakano S, Inamura S, Tokunaga K, Tsuji S, Hashimoto I. Evoked potentials in patients with chronic respiratory insufficiency. Intern Med. 1997;36:270–275. doi: 10.2169/internalmedicine.36.270. [DOI] [PubMed] [Google Scholar]
- 18.Paquereau J, Maurice JC, Neau JP, Ingrand P, Patte F. Auditory brain stem responses (ABRs) in sleep respiratory disorders. Eur J Clin Invest. 1994;24:156–160. doi: 10.1111/j.1365-2362.1994.tb00981.x. [DOI] [PubMed] [Google Scholar]
- 19.Barlieri S, Fayoumi Z, Berardinelli P, et al. Evidence for a subclinical involvement of the central nervous system in mild or moderate chronic respiratory insufficiency. Electroenceph Clin Neurophysiol. 1996;36:67–72. [PubMed] [Google Scholar]
- 20.Polich J. P300 in clinical applications: meaning, method, and measurement. In: Neidemeyer E, Da Silva FL, editors. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. 3rd edition. Philadelphia, PA: Lippincott Williams & Wilkins; 1993. pp. 1005–1018. [Google Scholar]
- 21.Polich J, Kok A. Cognitive and biological determinants of P300: an integrative review. Biol Psychol. 1995;41:103–146. doi: 10.1016/0301-0511(95)05130-9. [DOI] [PubMed] [Google Scholar]
- 22.Goodin DS, Starr A, Chippendale T, Squires KC. Sequential changes in the P3 component of the auditory evoked potential in confusional states and dementing illnesses. Neurology. 1983;33:1215–1218. doi: 10.1212/wnl.33.9.1215. [DOI] [PubMed] [Google Scholar]
- 23.Slaets JPJ, Fortgene C. On the value of P300 event-related potentials in the different diagnosis of dementia. Br J Psychiatry. 1984;145:652–656. doi: 10.1192/bjp.145.6.652. [DOI] [PubMed] [Google Scholar]
- 24.Polich J, Ehlens CL, Otis S. P300 latency reflects the degree of cognitive decline in dementing illness. Electroencephalogr Clin Neurophysiol. 1986;63:138–144. doi: 10.1016/0013-4694(86)90007-6. [DOI] [PubMed] [Google Scholar]
- 25.Davies MG, Rowan MJ, Feely J. EEG and event-related potentials in hepatic encephalopathy. Metab Brain Dis. 1991;6:175–186. doi: 10.1007/BF00996917. [DOI] [PubMed] [Google Scholar]
- 26.Kyler CF, Lotterer E, Petter J, et al. Visual event-related P300 potentials in early portosystemic encephalopathy. Gastroenterology. 1992;103:302–310. doi: 10.1016/0016-5085(92)91127-p. [DOI] [PubMed] [Google Scholar]
- 27.Davies MG, Rowan MJ, MacMathuna P, Keeling PW, Weir OG, Feely J. The auditory P300 event-related potential: an objective marker of the encephalopathy of chronic liver disease. Hepatology. 1990;12:688–689. doi: 10.1002/hep.1840120412. [DOI] [PubMed] [Google Scholar]
- 28.Ruzicka E, Tesar V, Jelinkova E, Mmerta M, Nevsimalova S, Kucerova O. Event-related potentials in evaluation of metabolic encephalopathies. Schweiz Arch Neurol Psychiatr. 1993;144:378–384. [PubMed] [Google Scholar]
- 29.Wissensten NJ, Crowley J, Balkin T, et al. Effects of stimulated high altitude exposure on long latency event-related brain potentials and performance. Aviat Space Environ Med. 1993;64:30–36. [PubMed] [Google Scholar]
- 30.Umahara T, Kiuchi A, Kobayashi Y, Iwamoto T, Takasaki M. P300 latency in patients with respiratory insufficiency due to sequelae of pulmonary tuberculosis. No To Shinkei. 1992;44:19–23. [PubMed] [Google Scholar]
- 31.IFCN Committee IFCN recommended standards for long-term auditory event-related potentials. Electroencephalogr Clin Neurophysiol. 1994;91:18–20. doi: 10.1016/0013-4694(94)90014-0. [DOI] [PubMed] [Google Scholar]