Abstract
Since first being described in the fruit fly Drosophila melanogaster, Toll-like receptors (TLRs) have proven to be of great interest to immunologists and investigators interested in the molecular basis to inflammation. They recognize pathogen-derived factors and also products of inflamed tissue, and trigger signaling pathways that lead to activation of transcription factors such as nuclear factor-κB and the interferon regulatory factors. These in turn lead to induction of immune and inflammatory genes, including such important cytokines as tumor necrosis factor-α and type I interferon. Much evidence points to a role for TLRs in immune and inflammatory diseases and increasingly in cancer. Examples include clear roles for TLR4 in sepsis, rheumatoid arthritis, ischemia/reperfusion injury, and allergy. TLR2 has been implicated in similar pathologic conditions and also in systemic lupus erythematosus (SLE) and tumor metastasis. TLR7 has also been shown to be important in SLE. TLR5 has been shown to be radioprotective. Recent advances in our understanding of signaling pathways activated by TLRs, structural insights into TLRs bound to their ligands and antagonists, and approaches to inhibit TLRs (including antibodies, peptides, and small molecules) are providing possiblemeans by which to interfere with TLRs clinically. Here we review these recent advances and speculate about whether manipulating TLRs is likely to be successful in fighting off different diseases.
I. Introduction
The discovery of Toll-like receptors (TLRs1) heralded the renaissance of interest in innate immunity for immunologists, who, despite extensive studies having been carried out in the area (mainly by those investigators interested in inflammation), previously thought it to be relatively crude, nonspecific, and somewhat unpromising for specific therapeutic targets for infectious and inflammatory diseases. Extensive analysis of TLRs, however, has revealed specificity in terms of ligand recognition, expression in different cell types and tissues, and, importantly, a role for TLRs in the pathogenesis of multiple diseases involving both the innate and adaptive immune systems. There are 10 TLRs in humans and they recognize different microbial ligands during infection (O'Neill and Bowie, 2007). There is also a growing body of evidence to indicate that certain TLRs also sense products of damaged tissue. Both pathogen-derived factors and also damaged tissue will provoke inflammation; it has therefore been hypothesized that TLRs initiate the inflammatory response in both cases. Also of interest are the different signaling pathways activated by TLRs. Five different adapter proteins are recruited in different combinations to different TLRs, allowing for tailored responses to each pathogen (O'Neill and Bowie, 2007). There are several protein kinases downstream of these adapters, notably the IL-1 receptor-associated kinase (IRAK) family and TBK-1. These activate pathways leading to the activation of the respective transcription factors nuclear factor κB (NFκB) and interferon regulatory factor 3 (IRF3), which in turn induce various immune and inflammatory genes. The human TLRs, their ligands, and the signals they activate are shown in Fig. 1.
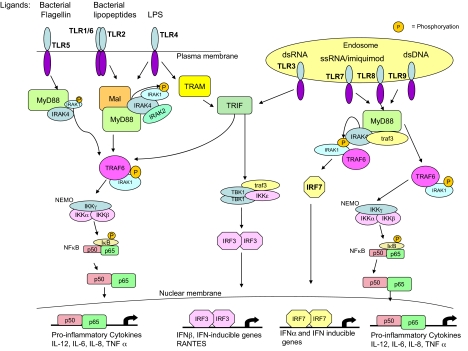
Fig. 1.
TLR signaling pathways. Once activated by their respective ligands, TLRs recruit their specific repertoire of the TIR adapters MyD88, Mal, TRIF, or TRAM, resulting in the recruitment and activation of the IRAKs and TRAF6. This leads to the activation of NF-κB essential modulator (NEMO) and the subsequent phosphorylation and degradation of IκB, the inhibitor of NFκB, rendering NFκB free to translocate from the cytosol to the nucleus and activate κB-dependent genes. IRF7 is also activated downstream of TLRs 7, 8, and 9, leading to its dimerization and translocation into the nucleus and to activation of IFNα and IFN-inducible genes. TLR3 and TLR4 both use TRIF to activate the noncanonical IKKs TBK1 and IKKϵ, resulting in the dimerization and activation of IRF3 and the transcription of IFNβ and IFN-inducible genes.
Studies on TLR-deficient mice have implicated them in multiple pathologic conditions, and the targeting of either the TLRs themselves or the signals they generate is proving to be of great interest. There is sufficient validation around certain TLRs, as we describe in this review, to justify them as therapeutic targets. These validation criteria are standard— expression in disease; activation leading to enhanced disease in models; protection of TLR-deficient mice against disease; and provision of risk factors for disease by single nucleotide polymorphisms in TLRs or their adapters. Finally, a key output from TLRs are inflammatory cytokines such as TNF and IL-6, which have proven to be excellent targets for inflammatory diseases such as rheumatoid arthritis. Targeting TLRs will therefore in all likelihood prevent the induction of many immune and inflammatory proteins. The wide tissue distribution of TLRs, however, may make it difficult to determine whether an agonist or an antagonist will be most effective therapeutically. In this review, we systematically describe and discuss the potential role of each TLR in disease and speculate on the prospect of future targeting of TLRs. The hope is that given the level of validation around them, they should prove to be a very interesting new class of targets for diseases where there is still an unmet medical need.
II. Toll-Like Receptor 4: Agonism and Antagonism
TLR4 was the first TLR identified (Medzhitov et al., 1997) and was characterized as a pattern recognition receptor through the study of the lipopolysaccharide (LPS)-resistant C3H/HeJ and C57BL/10ScCr mice strains. Mapping and sequencing identified the Tlr4 gene as a candidate site for the mutation causing LPS resistance. In C3H/HeJ mice, the Tlr4 gene has a single A-to-C point mutation, resulting in a P712H substitution in the TIR domain of TLR4 (Poltorak et al., 1998; Qureshi et al., 1999) conferring dominant-negative activity on TLR4 in these mice (Vogel et al., 1999). The C57BL/10ScCr strain is homozygous for a null mutation of Tlr4 (Poltorak et al., 1998). The role of TLR4 in LPS signaling was confirmed in TLR4(-/-) mice that were hyporesponsive to LPS (Hoshino et al., 1999). Mutations in the human Tlr4 gene, corresponding to D299G and T399I, were shown to associate with hyporesponsiveness to inhaled LPS (Arbour et al., 2000), and expression of these mutants in vitro shows reduced activation in response to LPS (Rallabhandi et al., 2008). Expression of TLR4 alone does not confer responsiveness of cells to LPS. TLR4 was found to require an additional protein, MD-2, with which it has to be associated to be activated by LPS (Shimazu et al., 1999), and mice lacking MD-2 do not respond to LPS (Nagai et al., 2002). A number of MD-2 polymorphisms have been identified that alter LPS binding and/or activation (Hamann et al., 2004; Gu et al., 2007; Vasl et al., 2008). LPS interaction with MD-2/TLR4 involves at least two other proteins. LPS binds first to lipopolysaccharide binding protein in serum (Schumann et al., 1990) and is then transferred to CD14 (Wright et al., 1990). The major role for CD14 is to enhance the sensitivity of the MD-2/TLR4 signaling complex, dropping the binding affinity for LPS to picomolar concentrations (Gioannini et al., 2004). Mice without CD14 are resistant to endotoxic shock (Haziot et al., 1996).
A. Ligand Recognition at Myeloid Differentiation Factor 2/Toll-Like Receptor 4 Protein Complex: A Basis for Antagonism?
The best characterized ligand for the MD-2/TLR4 complex is lipid A (the biologically active component of LPS). The lipid A domain of LPS consists of a disaccharide to which various substituents, including acyl chains of variable length and number, are attached (Raetz and Whitfield, 2002). Escherichia coli lipid A is usually hexaacylated, whereas a tetra-acylated lipid A, lipid IVa, is also produced by E. coli as an intermediate in the lipid A biosynthetic pathway (Raetz and Whitfield, 2002). Different lipid A structures may be agonists or antagonists at the MD-2/TLR4 (Walsh et al., 2008). Subtle alterations in lipid A structure profoundly alter its biological activity, such that a synthetic compound CRX-527 is an agonist, but decreasing the secondary acyl chain length below 6 or increasing it above 14 results in a loss of agonist activity (Stöver et al., 2004).
Binding of lipid A to MD-2/TLR4 (Raetz et al., 2006) induces structural rearrangements that trigger oligomerisation of TLR4 and initiate signal transduction (Re and Strominger, 2002, 2003; Visintin et al., 2003; Gangloff and Gay, 2004; Viriyakosol et al., 2006). MD-2 binds to lipid A (Viriyakosol et al., 2001) and was therefore thought to be the key player in lipid A recognition, whereas TLR4, unlike other TLRs, was not thought not to participate directly in lipid A binding (Viriyakosol et al., 2001). Lipid A is recognized by MD-2 after transfer from CD14, which does not participate in the signaling complex (Gioannini et al., 2004). The first ligand bound structures for MD-2 (Ohto et al., 2007) and TLR4/MD-2 (Kim et al., 2007) were both complexes bound to antagonists. These studies led to the hypothesis that lipid A induces MD-2 to change shape, which would result in a change in conformation of TLR4 to trigger signaling. Very recently, lipid A in complex with MD-2 was crystallized, however, and these data show that MD-2 does not change shape when bound to an agonist (Park et al., 2009). The structure of the TLR4/MD-2 antagonist-bound complex is shown in Fig. 2.
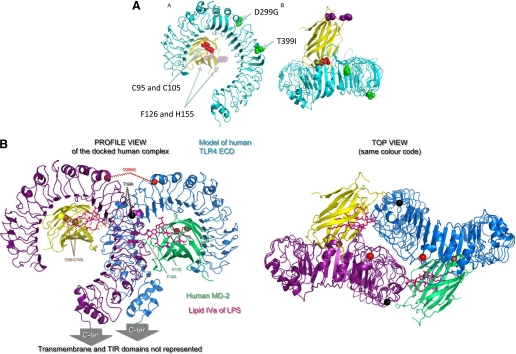
Fig. 2.
The structure of TLR4/MD-2: molecular basis for ligand binding. A, the structure of human TLR4 (turquoise) bound to MD-2 (yellow) is taken from the crystal structure (Kim et al., 2007). The single nucleotide polymorphisms in TLR4 (D299G and T399I) are shown in green, the cysteine residues in MD-2 critical for LPS binding (Cys95 and Cys105) are shown in red, and the residues in MD-2 (Phe126 and His155) critical for receptor dimerization in response to LPS are shown in pink. B, a model to suggest the structural basis of ligand activation of TLR4/MD-2 (lateral and top views). Using the structural data, a model was made to explain how TLR4/MD-2 might dimerize to form an active complex (Walsh et al., 2008). The two TLR4 molecules are represented in purple and turquoise and the two MD-2 molecules in yellow and green. In this model, there are contacts between the two TLR4 proteins, and each MD-2 touches both TLR4 proteins (see the top view). TLR4 SNP D299G is indicated in red and T399I is indicated in black.
The first crystal structure for human MD-2 is of the protein bound to lipid IVa (an antagonist at human MD-2/TLR4). In this structure, the four acyl chains of lipid IVa fills the deep hydrophobic cavity formed by the two β sheets in MD-2. The phosphorylated glucosamine backbone is located at the entrance to the hydrophobic cavity (Ohto et al., 2007). In the MD-2/TLR4 complex, MD-2 is complexed to another antagonist, eritoran. Similar to the MD-2-lipid IVa structure, the four acyl chains of Eritoran occupies approximately 90% of the solvent-accessible volume of the pocket. Two of the acyl chains are fully extended conformation within the binding pocket, but two of the acyl chains are bent in the middle. The di-glucosamine backbone of Eritoran, like the diglucosamine backbone of lipid IVa, is fully exposed to solvent (Kim et al., 2007). What happens to the extra acyl chains in lipid A structures that have more than 4 acyl chains, such as hexaacylated lipid A? Do the extra acyl chains somehow associate with TLR4?
To answer these questions many mutagenesis, structural modeling and crytallisation studies have been performed. There was controversy as to whether TLR4 participates directly in ligand binding and discrimination. TLR4 could play a secondary role in ligand binding, as residues in MD-2 (C95 and C105) important for TLR4 binding (Mullen et al., 2003; Re and Strominger, 2003), are located at the rim of the ligand-binding cavity (Ohto et al., 2007). This was supported by the higher LPS affinity of the MD-2/TLR4 complex (with a Kd of around 3 nM (Akashi et al., 2003)) compared with MD-2 (Kd of 65 nM (Viriyakosol et al., 2001)) on its own. MD-2 binds to TLR4 on a lateral surface of the TLR4 solenoid near to the N terminus (Kim et al., 2007). One approach to understand how active MD-2/TLR4 complexes are formed is to exploit the marked mammalian species differences in the activity of different types of lipid A that behave as agonists or antagonists at the MD-2/TLR4 complex (Akashi et al., 2001; Kawasaki et al., 2001; Muroi and Tanamoto, 2006). Lipid IVa is an agonist in the mouse, a partial agonist in the horse and is an antagonist for human cells (Akashi et al., 2001; Sauter et al., 2007). Using chimeric constructs made from human and horse TLR4 and MD-2, sequences have been identified in both proteins that are required for lipid IVa to signal. A molecular model using this data predicted that interchain contacts occur between MD-2 and TLR4 and explain why two highly conserved residues in MD-2 (F126 and H155) are critical for receptor dimerization in response to LPS (Kobayashi et al., 2006) by contributing to TLR4 cross-linking. The model also predicted the presence of TLR4 receptor-receptor contacts. The assembly of the active TLR4 complexes was predicted to be a stepwise process, with initial MD-2/TLR4 contacts induced by binding of lipid A promoting the subsequent homodimerization of the receptor ectodomains (Walsh et al., 2008). Crystallization of LPS bound to MD-2/TLR4 showed that the predictions of the mutagenesis data and the modeling were remarkably accurate. This fascinating structure shows the main dimerization face of TLR4 being between leucine-rich repeats 15–17. It also shows that five of the LPS acyl chains are fully accommodated in MD-2, the sixth acyl chain is exposed to interact with TLR4, and the LPS phosphate groups interact with the positively charged residues in TLR4 (Park et al., 2009). Several questions remain unanswered, however, including how the TLR4 single-nucleotide polymorphisms (D299G and T399I) in human TLR4, which are not in the N-terminal MD-2 binding site or in the dimerization interface on TLR4, reduce lipid A responsiveness (Arbour et al., 2000; Rallabhandi et al., 2006). The mechanism for this is unclear, but the mutations could either affect the cooperative binding of lipid A or alter the conformational changes that occur during ligand-induced signal transduction.
The interaction of lipid A with the MD-2/TLR4 complex is increasingly well understood, but a number of ligands other than lipid A have been identified as TLR4 agonists. These include endogenous ligands [such as high mobility group box 1 protein (HMGB1), 60-kDa heat shock protein (HSP60), HSP70, type III repeat extra domain of fibronectin (EDA), hyaluronic acid oligosaccharides, heparin sulfate polysaccharide fragments, and fibrinogen], other pathogen-derived ligands (such as Streptococcus pneumoniae pneumolysin, Chlamydia pneumoniae HSP60, mouse mammary tumor virus envelope proteins, and respiratory syncytial virus fusion protein), and plant ligands (paclitaxel) (Gay and Gangloff, 2007). The molecular nature of how these ligands interact with TLR4 and whether MD-2 is required is not well understood. It does seem that EDA (Okamura et al., 2001), C. pneumoniae HSP60, and respiratory syncytial virus fusion protein (Rallabhandi et al., 2006) do require MD-2 for activation of TLR4 although the molecular basis for this is unclear. Some ligands, such as oxidized low-density lipoprotein and β-amyloid, may induce heterotrimeric complexes of CD36/TLR4/TLR6 (Stewart et al., 2008), but whether the formation of these type of complexes is common for TLR4 remains to be clarified. Until crystallographic evidence shows that these protein ligands bind to the receptor to induce a conformational change and activate signaling, it remains controversial as to whether these proteins are true ligands for TLR4.
B. Efficacy at Toll-Like Receptor 4: Recruitment of Adapter Proteins
Efficacy at TLR4 induced by ligand binding involves dimerization or oligomerization of receptor chains (Saitoh et al., 2004). This in turn probably causes protein conformational changes in the receptor, resulting in the association of two receptor TIR domains (Gay et al., 2006). Fluorescence resonance energy transfer microscopy showed that the TLR9 TIR domains undergo a large positional change on ligand binding (Lorenz et al., 2002); therefore, it is reasonable to assume that this may occur with other TLRs on dimerization. The association of the receptor TIR domains would provide a new scaffold that allows the recruitment of specific adapter proteins to form a postreceptor signaling complex. Five adapter proteins function in TLR signaling, and they all have TIR domains (O'Neill and Bowie, 2007). Activated TLR4 recruits two distinct adapter protein pairs, Mal/MyD88 and TRAM/TRIF. These molecules are thought to engage directly with the receptor and to act as “bridging adapters” for the recruitment of MyD88 and TRIF, respectively. Mal is required for rapid activation of the NFκB transcription factor and the production of proinflammatory cytokines such as TNFα. TRAM stimulates sustained NFκB activation and a different signaling pathway, leading to activation of IRF3. IRF3 induces expression of a set of genes distinct from that of NFκB, such as IFNβ and the chemokine RANTES (regulated on activation normal T cell expressed and secreted) (O'Neill and Bowie, 2007).
Mutagenesis and molecular modeling studies suggest that ligand-induced dimerization of the TLR4 extracellular domains leads to concerted protein conformational changes that in turn lead to self-association or rearrangement of the receptor TIR, thereby creating a new molecular surface for the recruitment of signaling adapter proteins (Núñez Miguel et al., 2007). This model predicts that Mal and TRAM bind to the same region in the TLR4 dimer interface, thus explaining why cell-permeant blocking peptides compete out both Mal- and TRAM-directed responses simultaneously (Toshchakov and Vogel, 2007). The model does not, however, resolve the question of whether 1) a single activated receptor dimer can stimulate both the Mal- and TRAM-directed pathways simultaneously or 2) adapter engagement is mutually exclusive (something that would require positive cooperativity). Each activated receptor will have two symmetry-related adapter binding sites; in principle, either hypothesis is feasible.
Both Mal and TRAM are regulated by covalent modification. Mal is phosphorylated by Bruton's tyrosine kinase (Gray et al., 2006). This is required for Mal to signal but subsequently leads to recruitment of suppressor of cytokine signaling 1 (SOCS1) and degradation of Mal (Mansell et al., 2006). Mal also contains a phosphatidylinositol bisphosphate binding domain that localizes it to the plasma membrane (Kagan and Medzhitov, 2006). TRAM is myristoylated (Rowe et al., 2006) in its N terminus, which localizes it to the plasma membrane. It undergoes phosphorylation by protein kinase C-ϵ, which is required for it to signal. It has also been shown that TLR4 traffics to the early endosome (Kagan et al., 2008); it is here that TRIF is recruited to activate the IRF3 pathway in a manner analogous to the nucleic acid-sensing TLRs (see section V), all of which signal from the early endosomes.
C. Diseases Linked with Toll-Like Receptor 4
The range of ligands (both pathogen-related and endogenous) identified as agonists of TLR4 suggest that this receptor is likely to be associated with a number of diseases. Many published studies suggest that TLR4 is linked to a range of diseases, including infectious disease, atherosclerosis, asthma, cardiac disease, liver disease, renal disease, inflammatory bowel disease, obesity, diabetes (types I and II), rheumatoid arthritis, Alzheimer's disease, Parkinson's disease, and multiple sclerosis. Genetic data are emerging to support the association of TLR4 with several of these diseases. Two receptor polymorphisms were originally identified (D299G and T399I) as decreasing responsiveness to inhaled LPS (Arbour et al., 2000). This resulted in a number of studies looking for associations between these polymorphisms and infectious diseases, but much of the data were conflicting (Schröder and Schumann, 2005; Ferwerda et al., 2008). This may be because most of the studies consider either the D299G or the T399I polymorphism but neglect the fact that these polymorphisms also exist in a cosegregated (D299G/T399I) way, which implies that there are four haplotypes: wild type/wild type, D299G/wild type, T399I/wild type, and D299G/T399I (Ferwerda et al., 2008). Recent data suggest that only the D299G haplotype differs in phenotype from wild-type TLR4, LPS-stimulated blood samples from this population of people showing increased, rather than blunted, TNF-α response (Ferwerda et al., 2007).
1. Toll-Like Receptor 4 and Infectious Diseases.
Studies in knockout mice have indicated a role for TLR4 in protection against endotoxemia (Hoshino et al., 1999), but an increased susceptibility of TLR4 mutant mice to systemic Gram-negative infections, such as Salmonella typhimurium (O'Brien et al., 1980; Weiss et al., 2004). This is because activation of TLR4 is required for protective immunity against infections but also mediates the effects of systemic endotoxin/infections. Studies of a number of Gram-negative pathogens in mouse infection models have shown a role for TLR4, including Neisseria meningitides, E. coli, Haemophilus influenzae, Klebsiella pneumoniae, and Brucella abortus (Schnare et al., 2006). Mouse models have also shown that TLR4 is important for infection with other pathogens, including S. pneumoniae and Mycobacteria tuberculosis (Schnare et al., 2006). TLR4 has also been linked to several viral infections, including respiratory syncytial virus (Kurt-Jones et al., 2000), the murine retroviruses mouse mammary tumor virus and murine leukemia virus (Rassa et al., 2002), as well as the picornavirus Coxsackievirus B4 (Triantafilou and Triantafilou, 2004). The role of TLR4 in human infectious disease is emerging. There are now a great number of published studies of polymorphisms in TLR4 and their association with many infectious diseases, including sepsis, Gram-negative infections, other bacterial diseases (including tuberculosis, malaria, and infections with respiratory syncytial virus and Candida spp.) (Ferwerda et al., 2008). The data probably conflict because of the different populations of people studied and the variety of haplotypes involved. The strongest association of TLR4 polymorphisms with an infectious disease is with respiratory syncytial virus infection, where high risk infants heterozygous for D299G and T399I polymorphisms showed an increased susceptibility to infection (Awomoyi et al., 2007). There is also increased risk of severe malaria in Ghanaian children with the TLR-4–D299G and TLR-4–T399I variants (Mockenhaupt et al., 2006) although there is no association between the D299G and tuberculosis in a Gambian population (Newport et al., 2004). An association of the D299G haplotype was found only in the group of patients with septic shock, whereas the D299G/T399I haplotype was found equally in both patients and control subjects, although patients with this geneotype had a higher prevalence of Gram-negative infections (Lorenz et al., 2002).
2. Toll-Like Receptor 4 and Noninfectious Diseases.
TLR4 and TLR4 receptor polymorphisms have been implicated in a number of noninfectious diseases. This is perhaps unsurprising given the range of endogenous ligands identified for TLR4 and the number of diseases (cancer, atherosclerosis, and autoimmune conditions) that are now believed to have an inflammatory etiology. The D299G SNP is implicated in gastric cancer, atherosclerosis, sepsis, and asthma, and a G11481C mutation has been linked to prostate cancer (El-Omar et al., 2008). A number of studies also suggest a possible role for TLR4 in cardiovascular disease (Frantz et al., 2007; Satoh et al., 2008), inflammatory bowel disease (Fukata and Abreu, 2007), Alzheimer's disease (Balistreri et al., 2008), rheumatoid arthritis (van den Berg et al., 2007), renal disease (Anders et al., 2004), obesity, and diabetes types I and II (Kim, 2006); whether the genetic evidence will support the disease tissue and model observations remains to be proven. In mouse models, for example, inhibition of TLR4 is beneficial in mouse models of rheumatoid arthritis (Eder et al., 2004), and patients with the disease carrying the D299G mutation have altered macrophage responses to LPS (Roelofs et al., 2008), but a clear genetic link between TLR4 and rheumatoid arthritis has yet to be found. It is possible that the genetic data will also be useful in predicting the success of chemotherapeutic regimes (e.g., in cancer chemotherapy). The interaction of HMGB1, released from dying tumor cells, with TLR4 on dendritic cells promoted tumor-specific cytotoxic T-cell responses, and patients with breast cancer who have the D299G polymorphism relapsed earlier after chemotherapy (Apetoh et al., 2008). Whether endogenous ligands or the involvement of infectious disease is the underlying cause of the involvement of TLRs in the susceptibility to these diseases remains to be clarified.
A most interesting recent finding in relation to TLR4 and disease concerns allergy caused by airborne allergens. Derp2, the key allergen from the house dust mite, has been shown to be structurally similar to MD-2 and acts to deliver LPS to TLR4 in airways, thereby provoking inflammation. This might be a common mechanism, because several airborne allergens are lipid-binding proteins and might act analogously. This makes TLR4 a very interesting target for allergy in the airways (Trompette et al., 2009).
3. Myeloid Differentiation Factor 2 and Disease.
Studies in the MD-2 knockout mice have been very much more limited compared with the TLR4 knockout mice. Three human polymorphisms have been described: T35A (Hamann et al., 2004), C1625G in the MD-2 promoter (Gu et al., 2007), and G56R (Vasl et al., 2008). The promoter polymorphism may be linked to increased susceptibility to complications such as organ dysfunction and sepsis after major trauma (Gu et al., 2007), whereas the other polymorphisms have yet to show any disease association.
4. Cluster of Differentiation 14 and Disease.
A SNP in the 5′ genomic region of CD14 at position -159 (Martinez, 2007) is associated with infectious diseases, asthma, and allergy (Wiertsema et al., 2006). A number of other diseases have also been linked to this polymorphism, from cardiovascular disease to autoimmunity and from infections to malignancies (Martinez, 2007). It would seem that diseases linked to CD14 will overlap those linked to TLR4, and possibly to TLR2, suggesting that therapeutic intervention with either CD14 or TLR4 should benefit patients who have genetic susceptibilities in either of these genes.
D. Pharmacological Manipulation of Myeloid Differentiation Factor 2/Toll-Like Receptor 4
The association of TLR4 with many diseases emphasizes the importance of MD-2/TLR4 as a therapeutic target. A number of different strategies could be considered to pharmacologically alter TLR4, including receptor agonists, receptor antagonists, and signal transduction inhibitors. Some of these are illustrated in Fig. 3. The two discreet signaling pathways (Mal/MyD88 and TRAM/TRIF) offer targets for selective modulation of TLR4 activity. The precise clinical goal of modifying TLR4 activity remains an interesting question. In patients with sepsis, for example, it may be better to use a partial agonist rather than an antagonist to decrease TLR4 activity, such that some activation of TLR4 remains to stimulate protective immunity. To regain adjuvant activity, a partial agonist or a drug that selectively stimulates TRAM/TRIF signaling would be safer than a full agonist, which might activate a systemic inflammatory response. Species differences in the response to different agonists at TLR4 suggest that care will be needed in developing safe new drugs.
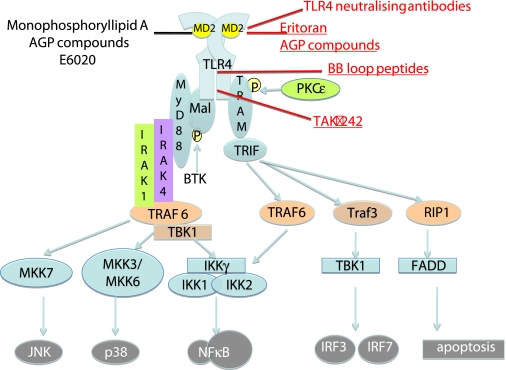
Fig. 3.
Drugs targeting the TLR4/MD-2 signaling pathway. Activation of TLR4 recruits the adapter pairs Mal/MyD88 and TRAM/TRIF. Signaling through Mal/Myd88 recruits IRAK1, IRAK4, Traf6, and TAK1-binding protein (TAB) to activate the NFκB signaling pathway. Signaling through TRAM/TRIF activates NFκB through TRAF6, but also activates signaling through IRF1 and IRF3 through TRAF3. Several drugs now target the TLR4/MD-2 signaling pathway. Eritoran and the AGP compounds bind to the TLR4/MD-2 lipid IA binding site, monoclonal antibodies (for example the neutralizing antibody from NovImmune, Geneva, Switzerland) bind to TLR4. Soluble peptides, such as the BB loop peptides, target the BB loop on the TIR domain, the region of the protein important in receptor dimerization. The small molecular inhibitor TAK-242 targets the signaling domain of the TIR.
TLR4 antagonists are currently undergoing clinical trials for treatment of sepsis but may well be useful for the treatment of a range of other conditions. Antagonists of lipid A have been under development since before the discovery of TLRs as treatments for Gram-negative sepsis and endotoxemia. Early work identified a number of lipid A analogs (lipid IVa and Rhodobacter sphaeroides lipid A) (Golenbock et al., 1991). In 1995, a synthetic form of Rhodobacter capsulatus lipid A was generated that antagonized E. coli lipid A and formed the basis for E5531 (Christ et al., 1995). Modification of E5531 generated the stable analog E5564 (eritoran), which is currently undergoing clinical trials for use in treating Gram-negative endotoxemia and sepsis (Mullarkey et al., 2003). E5564 is also able to antagonize the interaction of the protein ligand EDA with TLR4 (Okamura et al., 2001), suggesting that this receptor antagonist may be useful for conditions other than sepsis and endotoxemia. Other antagonists at TLR4 include curcumin, auranofin (an antirheumatic gold compound), cinnamaldehyde, and acrolein, all of which prevent homodimerization of TLR4 (Youn et al., 2006a,b, 2008; Lee et al., 2008), although how specific these agents are is uncertain. Small molecules that inhibit MyD88 binding to TLR4 are also emerging (Lee et al., 2007). Cell-penetrating peptides fused with the BB loop sequences of TLR2 and TLR4 also inhibit LPS-induced signaling, probably by interfering with either receptor dimerization or adapter recruitment (Toshchakov et al., 2007). Treatment of patients with sepsis with anti-inflammatory therapies has so far not been beneficial to improving clinical disease (Rittirsch et al., 2008); therefore, it will be very interesting to ascertain the clinical efficacy of inhibiting TLR4/MD-2 activity in sepsis.
TLR4 agonists are currently being developed as immunomodulators and adjuvants. The development of safe and efficacious vaccines remains a major goal in global public health. For these reasons, TLR ligands have become a focus in therapeutic studies for their potential use as adjuvants in vaccine formulations with the systematic development of vaccines that coordinately engage the innate and adaptive immune systems by incorporating a TLR ligand into the vaccine construct. The majority of vaccines comprise two components, the antigen of therapeutic interest and an adjuvant, a component that enhances the immune response to the antigen, a process known as immunogenicity. The nature of adjuvants varies greatly; many elicit adverse side effects. Therefore, the only adjuvant approved and licensed for human use remains limited to aluminum hydroxide. However, the simultaneous delivery of a TLR ligand and an antigen of interest would be more in accordance with natural infection than vaccination with a cocktail of adjuvant and antigen. By physically linking the TLR ligand and antigen, each antigen would be delivered to a vesicle with an activated TLR in a host antigen-presenting cell, potentially achieving optimal antigen processing and presentation (Blander and Medzhitov, 2004; Blander, 2007).
Monophosphoryl lipid A (MPL) was generated by detoxifying Salmonella minnesota lipid A (Qureshi et al., 1982) and was shown to be safe and to have good adjuvant activity. The basis of its adjuvant effect is the stimulation of the TRAM/TRIF signal transduction pathway of TLR4 and deactivation of Mal/MyD88 signaling (Mata-Haro et al., 2007), thereby acting as a partial rather than full agonist at the receptor. MPL has recently been licensed for use as a vaccine adjuvant (Casella and Mitchell, 2008). TLR4 agonists were also generated during the chemical synthetic program to make E5531 and E5564; some of these compounds, such as E6020 (Przetak et al., 2003), have good adjuvant activity (Hawkins et al., 2002). Another class of compounds, the aminoalkyl glucosaminide phosphates (AGP), have been developed as immunomodulators that activate TLR4 (Stöver et al., 2004). Members of the AGP family of lipid A mimetics also have good adjuvant activity and can confer protection against Listeria monocytogenes or influenza virus challenge in the absence of coadministration of the microbe itself or microbial antigen (Cluff et al., 2005).
We have recently found a splice variant of TRAM that we have termed TRAM adapter with gold domain (TAG) (E. Palsson-McDermott, S. L. Doyle, A. F. McGettrick, M. Hardy, H. Husebye, K. Banahan, M. Gong, D. Golenbock, T. Espevik, and L. A. J. O'Neill, manuscript submitted). TAG is localized to late endosomes where it acts to inhibit TRAM and specifically block the MyD88-independent signaling pathway. Interference with TAG would therefore boost this pathway but not the MyD88-dependent pathway. This could be useful in the effort to promote adjuvancy without causing inflammation, because the MyD88-dependent pathway is required for adjuvancy by LPS (Hoebe et al., 2003).
The activation of Mal/MyD88 and TRAM/TRIF signaling pathways offers opportunities to selectively inhibit or activate separate arms of the TLR4 signaling pathway. The success of MPL as an adjuvant in selectively activating the TRIF pathway offers some proof of concept for this pharmacological approach (Mata-Haro et al., 2007). The TLR4 signaling pathways lend themselves to screening with small-molecule inhibitors. The first to be described is the novel cyclohexene derivative TAK-242, a small molecular inhibitor of TLR4 but not TLR2 signaling that probably works by directly inhibiting the intracellular signaling domain of TLR4 (Ii et al., 2006; Kawamoto et al., 2008). Selective agonists and antagonists of TLR4 signaling are likely to be potent therapeutic compounds. The compounds currently available have largely been restricted to being used as antagonists in patients with sepsis and as adjuvant, but as our understanding of the role of TLR4 in disease is clarified, it is likely that these compounds will be useful in a range of diseases. The availability of structural data for TLR4 and MD-2 is likely to speed up the process of compound design and production.
III. Toll-Like Receptor 2: Agonism and Antagonism
TLR2 was originally identified as the LPS receptor (Kirschning et al., 1998; Yang et al., 1998), but the TLR2 activation by LPS was subsequently attributed to the bacterial lipoprotein contamination of the LPS preparations (Lee et al., 2002; Hellman et al., 2003). TLR2 recognizes a wide range of ligands, many of which are from Gram-positive bacteria (Takeuchi et al., 1999), and it signals not as a homodimer but as a heterodimer with either TLR1, TLR6 (Ozinsky et al., 2000; Takeuchi et al., 2001), or TLR10 (Hasan et al., 2005). Mice without TLR2 are hyporesponsive to Gram-positive bacterial cell wall components (Takeuchi et al., 1999). A number of SNPs in TLR2 have been reported in the extracellular domain [R753Q (Lorenz et al., 2000), Y715K, and Y715stop (Merx et al., 2007)] and the cytoplasmic domain [P631H (Smirnova et al., 2003)]; although the extracellular domain mutations result in decreased activity of TLR2 to Gram-positive bacterial ligands (Lorenz et al., 2000; Schröder et al., 2003), the genetic evidence linking these SNPs with a susceptibility to Gram-positive infections is unclear (Schröder and Schumann, 2005). Twelve SNPs in TLR1 [of these, S248N, H305L, P315L (Johnson et al., 2007), and I602S (Johnson et al., 2007) have defective signaling] and 14 SNPs in TLR6 (Johnson et al., 2007) have also been identified. It is unclear whether TLR2 heterodimerization leads to the activation of differential signaling, and although this is an attractive hypothesis, there is currently no evidence to support it (Farhat et al., 2008). TLR2 also cooperates with other receptors for pathogen recognition; for example, cooperation between TLR2 and Dectin 1 allows recognition of the yeast particles such as zymosan (Brown et al., 2003; Gantner et al., 2003). In a manner analogous to TLR4, other proteins have been implicated as coreceptors for TLR2, such as CD14 (Jiang et al., 2005) and CD36 (Hoebe et al., 2005).
A. Ligand Recognition at Toll-Like Receptor 2
A wide range of structurally diverse ligands, including those derived from microbial, fungal, and endogenous sources, is believed to be recognized by TLR2 (Zähringer et al., 2008). Many of these ligands are glycolipids, lipopeptides, or glycosylphosphatidylinisotol-anchored structures [e.g., lipoteichoic acid (LTA) from Gram-positive bacteria, lipoarabinomannan from mycobacteria, and glycosylphosphatidylinositol-anchored lipids from Trypanosoma cruzi] that, like LPS, contain a significant hydrophobic component (Zahringer et al., 2008). Well characterized ligands of TLR2 include Mycoplasma fermentan macrophage activating lipopeptide (MALP-2), which interacts with TLR2/TLR6 (Takeuchi et al., 2001), triacylated lipopeptides (such as Pam3CSK4 at TLR2/TLR1), and diacylated lipids/lipopeptides (LTA, Pam2CSK4) (Ozinsky et al., 2000; Takeuchi et al., 2001). Like the recognition of LPS by TLR4, the number of acyl chains linked to a lipoprotein plays an important role in ligand recognition by TLR2. Other properties of lipoproteins, such as the ester bonds present in the acyl chains and the nature of the amino acids, are also discriminated by TLR2 heterodimers (Buwitt-Beckmann et al., 2005a,b), and some lipopeptides may be recognized by TLR2 independently of TLR1 or TLR6 (Buwitt-Beckmann et al., 2006).
The molecular basis for lipoprotein recognition by TLR2 was largely explained by the solving of the crystal structure of the TLR1/TLR2 heterodimer bound to Pam3CSK4 (Jin et al., 2007). In this structure, the extracellular domains of TLR1 and TLR2 form an “M”-shaped heterodimer, with the two N termini extending outward in opposite directions. The lipid chains of Pam3CSK4 bridge the two TLRs, therefore playing a crucial role in the formation of the heterodimer. Two of the three lipid chains of Pam3CSK4 interact with a hydrophobic pocket in TLR2, and the amide-bound lipid chain lies in a hydrophobic channel within TLR1. The ligand-bound complex of TLR1 and TLR2 is stabilized by protein-protein interactions at the interface near the ligand-binding pocket. The TLR1 Pro315Leu polymorphism is located at the TLR1-TLR2 interface, which probably explains why it reduces TLR1/TLR2 signaling (Jin et al., 2007). This structure also explains mutagenesis data identifying the leucine-rich repeat region 9 to 12 of TLR1/TLR6 as being important in recognizing lipopeptides (Omueti et al., 2005; Andersen-Nissen et al., 2007). The nature of ligand binding at TLR2/TLR6 is unknown, but presumably it will be similar to that at TLR1/TLR2. How coreceptors such as CD14 or CD36 fit in with the structural data remains unclear. CD36 contributes to diacylglyceride recognition only at TLR2/TLR6 (Hoebe et al., 2005); it is unknown how CD14 interacts structurally with the MD-2/TLR4 complex, but CD14 is critical for TLR4 signaling. It may be some time before it is clear how coreceptors such as CD14 and CD36 interact with TLR2 signaling complexes.
The data on lipoprotein recognition at TLR2 are clear but do not explain why this receptor recognizes such a wide array of different ligands. Peptidoglycan and LTA both bind to TLR2, but precisely which structures in these ligands interact with the receptor is unclear. LTA, like LPS, is a glycolipid with repeating carbohydrate units from Gram-positive bacteria, and it has been suggested that the d-alanylation in the Gro-P repeating units of this lipid are recognized by TLR2 (Morath et al., 2002), although this is controversial (Zähringer et al., 2008). TLR2 is one of several receptors believed to be important for peptidoglycan recognition, but the structural motif in peptidoglycan recognized by TLR2 is unknown. It is possible that the peptidoglycan recognition attributed to TLR2 is due to contamination of the peptidoglycan preparation with lipoproteins (Zähringer et al., 2008). A range of LPS structures has also been suggested to be TLR2 ligands, such as lipid A from Porphyromonas gingivalis (Hirschfeld et al., 2001), Leptospira interrogans (Werts et al., 2001), and Legionella pneumophila (Girard et al., 2003). This remains a controversial topic. Contaminating lipoproteins in the LPS from P. gingivalis could explain why the LPS from this bacterium can activate TLR2, but results from studies of this subject remain confusing (Hashimoto et al., 2004). Given the structural data, however, it is difficult to understand how LPS structures with more than three acyl chains, such as the LPS from P. gingivalis, could be accommodated within the TLR2 binding site. The molecular mechanisms underlying how ligands such as whole bacteria [e.g., Francicella tularensis (Cole et al., 2007)] and fungal ligands [e.g., zymosan (Gantner et al., 2003)] are recognized by TLR2 or TLR2/dectin 1, respectively, remain unknown.
B. Efficacy at Toll-Like Receptor 2: Recruitment of Adapter Proteins
There is clear evidence to support the TIR domain of TLR2 heterodimerizing with the TIR of TLR1 or TLR6 to induce signaling (Ozinsky et al., 2000). TLR2 activates only MyD88-dependent signaling and requires Mal to recruit MyD88 to the TIR domain (Fitzgerald et al., 2001; Horng et al., 2001, 2002; Yamamoto et al., 2002). This means that TLR2 signaling induces expression of only a subset of genes activated by TLR4 (Hirschfeld et al., 2001). There are limited data on how the TIR domains of TLR2 and TLR1 or TLR6 interact. One study used molecular modeling and mutagenesis analysis of the TLR2/TLR1 heterodimer and showed that 4 residues are important: Arg748, Phe749, Leu752, and Arg753. The model suggested that, of these residues, Arg748 and Phe749 in TLR2 (in the DD loop) were in close contact with Gly676 from TLR1 (in the BB loop), and mutation of Gly676 or Gly676 in TLR1 or Gly676 in TLR2 reduced Pam3CSK4-induced signaling (Gautam et al., 2006); however, further structural work is required to verify these observations. A similar analysis has not been performed for TLR2/TLR6. The regulation of signaling via Mal and MyD88 is likely to be similar to that induced by TLR4.
C. Diseases Linked with Toll-Like Receptor 2
The range of ligands recognized by TLR2 would suggest that this receptor is likely to play an important role in many diseases and therefore be a useful therapeutic target.
1. Toll-Like Receptor 2 and Infectious Disease.
An early phenotype of the TLR2 knockout mice was an increased susceptibility to high-dose but not low-dose infection with Staphylococcus aureus (Takeuchi et al., 2000). Subsequent studies suggest that TLR2 plays a role in infection pathogenesis with other Gram-positive pathogens (S. pneumoniae meningitis, group B streptococcus, Bacillus subtilis, L. monocytogenes), and other bacterial species (Chlamydia trachomatis, spirochetes, Mycobacterium tuberculosis, and Yersinia enterocolitica) (Marra and Brigham, 2001). Viral [herpesvirus (Compton et al., 2003; Kurt-Jones et al., 2004) and paramyxovirus (Bieback et al., 2002)] and fungal diseases have also been linked to TLR2. The first SNP [in the C-terminal region of human TLR2 (R753E)] was identified in 2000 (Lorenz et al., 2000). This polymorphism had decreased activation by TLR2 ligands when transfected into human embryonic kidney 293 cells (Lorenz et al., 2000). Similar to the frequency of the TLR4 polymorphisms D299G and T399I (among white persons), the occurrence of the R753E SNP as homozygote seems to be rare [e.g., 9.4% heterozygous for R753E and no homozygotes in a white population (n = 319)] (Schröder et al., 2003). The R753E SNP is now no longer thought to be associated with the severe diseases caused by S. aureus (Moore et al., 2004), but may be associated with Candida spp. sepsis (Misch et al., 2008). The R753E SNP does seem to be linked to tuberculosis (Ogus et al., 2004) and also to acute rheumatic fever in children (Berdeli et al., 2005) and end stage Lyme disease (Schröder et al., 2005). An R677W SNP, a mutation that also decreases TLR2 activation by TLR2 ligands in vitro, has been shown to be associated with lepromatous leprosy in Asian people, but this SNP is now believed to be a polymerase chain reaction artifact from a TLR2 pseudogene (Malhotra et al., 2005). The TLR2 P631H SNP is less frequent in people with meningitis (Smirnova et al., 2003). The TLR1 I602S SNP not only decreases signaling to TLR2 ligands but also may prevent trafficking of this receptor to the cell surface and is associated with susceptibility to meningeal tuberculosis and leprosy (Hawn et al., 2006; Johnson et al., 2007). In Nepalese patients with leprosy, the 1805G allele of TLR1 is associated with protection against leprosy (Misch et al., 2008). Hypermorphic genetic variation in TLR1, particularly the G allele of TLR1–7202A/G (rs5743551), is associated with increased susceptibility to Gram-positive infection in sepsis (Wurfel et al., 2008).
2. Toll-Like Receptor 2 and Noninfectious Disease.
There has been extensive analysis of murine disease models in the TLR2 knockout mice. Atherosclerosis models have been studied extensively with TLR2, and TLR4 is possibly associated with lesion development in mice (Tobias and Curtiss, 2008); much evidence supports a role for both TLR2 and TLR4 in atherosclerosis and ischemic coronary artery disease in humans (Satoh et al., 2008). Asthma and atopy have been linked to TLR2, and a promoter polymorphism TLR2 (-16,934 A/T) in children of European farmers was associated with a lower risk of developing these diseases (Eder et al., 2004). Polymorphisms in TLR1, TLR2, TLR6, and TLR10 may protect against asthma (Kormann et al., 2008). The TLR2 R753Q polymorphism was also present in a subgroup of people with severe atopic dermatitis (Ahmad-Nejad et al., 2004) and arthritis (Tsui et al., 2008). The genetic evidence linking diabetes types I and II to TLR2 is controversial (Park et al., 2004; Santin et al., 2006), but experimental evidence suggests that TLR2 may be a useful therapeutic target in some forms of this disease (Caricilli et al., 2008). TLR2 may also be important in the pathogenesis of renal disease, particularly in infection and toxic injury (Anders et al., 2004). There is also some evidence to link a TLR2 guanine-thymine microsatellite repeat polymorphism in the second intron to susceptibility to sporadic colorectal cancer (Boraska Jelavić et al., 2006).
TLR2 has also recently been implicated in SLE and also in ischemia/reperfusion injury in kidney (Leemans et al., 2005; Urbonaviciute et al., 2008). Prostate cancer has also been linked to TLR2 through its association with TLR10 (Kormann et al., 2008; Stevens et al., 2008).
D. Pharmacological Manipulation of Toll-Like Receptor 2
Currently, the major use for compounds that activate TLR2 are as adjuvants. The synthetic compounds, such as Pam3CSK4 and MALP-2, could be developed for adjuvant usage. TLR2 should be a useful therapeutic target for the development of antagonists given the range of diseases with which this receptor is associated. A series of novel synthetic phospholipids that are antagonists at TLR2 have been made, but there are few data on the use of these compounds (Spyvee et al., 2005). Inhibition of TLR2-induced signaling through Mal/MyD88 would also result in partial inhibition of TLR4 signal transduction. This might be a useful approach, particularly targeting Mal, given that it binds exclusively to TLR2 and TLR4. A number of diseases, such as sepsis, diabetes, rheumatoid arthritis, and cardiovascular diseases, seem to be linked to both TLR2 and TLR4; therefore, Mal seems to be an attractive therapeutic target for these diseases.
Another approach to blocking TLR2 is with a neutralizing antibody. One such antibody, T2.5, has been shown to prevent sepsis induced by TLR2 ligands (Meng et al., 2004); furthermore, when T2.5 is used in combination with an anti-TLR4/MD-2 antibody, it protects mice against sepsis induced by Salmonella enterica or E. coli when given with antibiotics (Spiller et al., 2008). This latter finding suggests that a combination approach involving anti-TLR4 and anti-TLR2 might be a very useful adjunct to antibiotics in the prevention of sepsis.
IV. Toll-Like Receptor 5: Agonism and Antagonism
TLR5 is the receptor for bacterial flagellin monomers and is the only TLR that recognizes a protein ligand (Hayashi et al., 2001). The region of flagellin that TLR5 recognizes is highly conserved among microbial species and therefore allows TLR5 to detect a wide variety of microbes. TLR5 signals by recruiting the TIR adapter MyD88, leading to the activation of the IKK complex and subsequent activation of the proinflammatory transcription factor NFκB, which in turn results in the increased expression of pro-inflammatory genes. In addition, TLR5 ligation activates a number of antiapoptotic genes, allowing cells to stay alive in response to challenges that would otherwise result in cell death. In this way, TLR5 activation is cytoprotective (Zeng et al., 2006). This characteristic of TLR5 has recently been harnessed to protect cells against ionizing radiation. Ionizing radiation, the primary therapy for cancer patients, is a double-edged sword. Although it destroys tumor cells, it also causes healthy cells to undergo apoptosis. Tumor cells can persist because they can block apoptosis by activating NFκB, and much of the research in the cancer field involves developing methods to inhibit NFκB in tumor cells. Burdelya et al. (2008) have instead attempted to activate NFκB in normal cells to give these radiosensitive tissue cells a higher chance of surviving radiation therapy by suppressing apoptosis. The toxicity of high-dose ionizing radiation is associated with the induction of acute radiation symptoms involving the hematopoietic system and the gastrointestinal (GI) tract. To activate NFκB in these radiosensitive cells without inducing acute inflammatory responses, Burdelya et al. (2008) focused on TLR5 because of its activation in the gut in response to the protective role played by benign commensal microorganisms in the GI tract. An engineered flagellin derivative named CBLB502 was found to have potent NFκB activation and reduced immunogenic characteristics. A single injection of CBLB502 before lethal total body irradiation protected mice and rhesus monkeys from both GI and hematopoietic acute radiation symptoms and resulted in improved survival and yet, importantly, did not decrease tumor radiosensitivity. These results imply that TLR5 agonists may be valuable as adjuvants for cancer radiotherapy (Burdelya et al., 2008).
The activation of TLR5 has also been recently reported to be an efficient adjuvant for an influenza A vaccine. Development of influenza vaccines is challenging because of the genetic instability of the commonly used antigens hemagglutinin and neuraminidase; therefore, these vaccines require annual reformulation. A promising genetically stable influenza antigen is the ectodomain of the matrix2 protein (M2e). However, although the sequence of M2e is stable across all influenza A isolates, dating as far back as a pandemic strain in 1918, studies have shown M2e to be poorly immunogenic. As described previously, immunogenicity of an antigen can be improved upon by the delivery of the antigen in combination with adjuvant. However, in the past, several strategies have been tested in an attempt to produce a universal influenza vaccine incorporating the conserved antigen M2e, but its poor immunogenicity could not be overcome with any efficacy. However, M2e was recently fused with the TLR5 ligand S. typhimurium flagellin (STF2). The resulting fusion protein can activate cells in a TLR5-dependent manner and elicits potent antibody responses in mice. This study demonstrates that a recombinant protein containing a consensus M2e sequence linked to the TLR5 ligand provides an effective approach to developing vaccines against wide-spread epidemic and pandemic influenza (Huleatt et al., 2008). These findings indicate that activating the TLR5 signaling pathway may have broad therapeutic applications, not only in its role as a linker adjuvant candidate for vaccines, but also as a dampener of excessive apoptosis in acute radiation syndromes, a characteristic that may be extended for use in degenerative diseases and ischemia reperfusion injury as well.
However, the over-activation of TLR5 may have a negative effect on certain diseases of the gut. Crohn's disease and ulcerative colitis, two related chronic inflammatory diseases, are known collectively as inflammatory bowel disease (IBD). IBD is thought to be the product of a combination of genetic and environmental factors that result in the abnormal regulation of the immune responses. Experimental studies suggest that IBD is a T-cell driven process that results from defects in the T-cell-mediated regulatory processes that would normally prevent and or terminate inflammatory responses (Himmel et al., 2008). There is also strong evidence to suggest that abnormal responses to commensal bacteria are also central to the development of IBD (Strober et al., 2002). The GI tract is a unique organ, in that although it maintains the ability to mount an immune response to pathogens, it needs to remain tolerant to dietary antigens and commensal bacteria. Many studies have indicated that CD4+ T-regulatory cells expressing forkhead box P3 (FOXP3) or IL-10 have a fundamental role in maintaining gut immune homeostasis (Himmel et al., 2008); however, TLRs are also thought to be necessary for maintaining tolerance (Crellin et al., 2005). In the case of IBD, TLRs can also amplify inappropriate immune responses that ultimately cause chronic inflammation. Accumulating data suggest that TLR stimulation on T cells, presumably by commensal bacteria, has a significant role in the development of IBD (Himmel et al., 2008). Additional evidence for the role of TLRs in IBD comes from studies of flagellin and TLR5. A common polymorphism in TLR5 that produces a dominant-negative receptor is protective against Crohn's disease (but not ulcerative colitis) (Gewirtz et al., 2006). This is in contrast to mice, where TLR5-deficient mice develop spontaneous colitis, suggesting that TLR5-flagellin ligation would normally have a protective role (Vijay-Kumar et al., 2007), this difference may be due to the differential expression of TLR5 in mice and humans. Further studies have indicated that a difference in the concentration of flagellin can have opposite effects on T-cell function, suggesting a model for how flagellin could influence the balance between regulatory and effector T cells. At low concentrations, flagellin can stimulate TLR5 on CD4+ T cells and enhance the expression of FOXP3, allowing for an increased suppressive capacity of regulatory T cells, whereas high concentrations stimulate T-effector function (Crellin et al., 2005). In the case of IBD, the latter would probably prevail with increased effector cell levels, causing the eventual loss of all regulatory T cell function. Because of the ability of the human TLR5 mutation to protect against Crohn's disease, pharmacological targeting of this pathway antagonistically may turn out to be therapeutically beneficial.
V. Toll-Like Receptors That Recognize Nucleic-Acid Ligands
TLRs 3, 7, 8, and 9 are all nucleic acid-recognizing TLRs expressed on endosomal membranes. Nucleic acid recognition has so far been resolved for double-stranded RNA binding to TLR3. Each extracellular domain of TLR3 binds to dsRNA at two sites located at opposite ends of the TLR3 horseshoe. There is also an intermolecular contact between the two TLR3 extracelluar C-terminal domains that coordinates and stabilizes the TLR3 dimer. This structural arrangement can then mediate downstream signaling by dimerizing the cytoplasmic TIR domains. If there is a common mechanism for nucleic acid recognition at TLRs, then recognition of nucleic acids differs from that of the bacterial lipids quite considerably (Liu et al., 2008).
Cytokine profiles produced by activated TLRs are dependent on the type of ligand they recognize, which allows for an immune response fine-tuned for each microbe. In general, however, nucleic acid-based agonists of TLRs induce Th1-type immune responses (Agrawal and Kandimalla, 2007).
A. Toll-Like Receptor 3 Activation in Vaccine Adjuvancy and Antitumor Immunity
TLR3 has been shown to be the receptor for viral dsRNA and the dsRNA mimic poly(I:C) (Alexopoulou et al., 2001). Most viruses synthesize dsRNA at some point during their replicative cycle; therefore, TLR3 is an important detector of viral infection and initiator of the antiviral immune response. Once activated, TLR3 signals through its TIR adapter TRIF to both the IKK complex IKKα/IKKβ/IKKγ and the noncanonical IKK complex of TBK1/IKKϵ, culminating in the activation of proinflammatory and antiviral transcription factors. This signal cascade leads to changes in gene expression in a Th1-type pattern, resulting in the activation and maturation of antigen-presenting cells such as dendritic cells (DCs) and monocytes, allowing for the regulated processing and presentation of antigens, the up-regulation of major histocompatibility complex, and costimulatory molecules and secretion of proinflammatory chemokines and cytokines. These events then mediate the activation of antigen-specific T- and B-cell responses. In these ways, TLR signaling mediates an effective immune response by contributing to the priming and the type of the adaptive immune response. Because DCs are potent inducers of T-cell-mediated immunity and are activated through TLR ligation, they are attractive targets to improve vaccine efficacy.
Subsequently, a potential therapeutic use for stimulating TLR3 with an agonist is that of vaccine adjuvancy. Poly(I:C) shows potential as an adjuvant for DC-targeted vaccines. One group interested in improving the efficacy of T-cell-mediated immunity induced by HIV vaccines has demonstrated that when vaccine antigens were administered along with poly(I:C) protective specific CD4+, T-cell immunity was induced and was long-lasting in a lung infection model (Trumpfheller et al., 2008). It has also recently been reported that mDCs exposed to TLR3/TLR4 ligands up-regulated their expression of 1α-hydroxylase, an enzyme that converts the precursor of vitamin D3 precursor into calcitriol, the active form. Calcitriol-dependent mechanisms allow for the ability of DCs to migrate from skin sites of vaccination to mucosal lymphoid organs. Therefore, vaccines containing TLR3/TLR4 ligands and the specific antigen administered subcutaneously can function as effective mucosal adjuvants because they stimulate the metabolism of vitamin D3 (Enioutina et al., 2008).
Activation of TLR3 is also potentially promising as an anticancer therapy. Th1 immune activation is optimized for fighting intracellular infection such as viruses and involves the activation of natural killer (NK) cells and T cells that can lyse infected cells. This Th1-pattern of cytokine and chemokine secretion is highly desirable for cancer therapy. TLRs can be expressed on cancer cells and are therefore implicated as possible options in cancer-based therapy. However, the expression patterns of TLRs in human cancer tissues are largely unknown; studies to date stimulating cancer tissues with TLR agonists have shown that the effects of stimulation may be either pro- or antiapoptotic in different cases. An example of this is the ligation of TLR9; although it inhibits the progression of growth in prostrate cancer cells, it causes increased growth in breast cancer cells (Huang et al., 2005). A TLR3 agonist, however, may prove useful as an anticancer agent in a number of cases, because functional TLR3 has been shown to be expressed in breast cancer cells and to a very high extent in both primary and metastatic clear-cell renal cell carcinoma, one of the most drug-refractory cancers known. Poly(I:C) induced TLR3-dependent IFNβ production and exerted a growth inhibitory effect against clear-cell renal cell carcinoma cells and breast cancer cells. These studies are examples of reports that cancer cells themselves express functional TLR3 in vivo and that poly(I:C) is acting directly through them and not solely through the stimulation of DCs leading to anticancer effects (Salaun et al., 2006). Furthermore, retrospective analysis of an old clinical trial of a TLR3 ligand, poly-AU, in breast cancer patients has shown that there was an improved survival rate in the subset of patients whose tumor cells themselves expressed TLR3 (Andre et al., 2004). It is noteworthy that the adverse effects of administering TLR3 agonists to cancer patients might be limited; although there is a high level of TLR3 expression in these cancer cells, there is low TLR3 expression elsewhere in the body.
Although TLR3 activation may be desirable in increasing vaccine efficacy and as an anticancer therapy, it can be an unwanted contraindication of a different therapeutic mechanism. RNA interference (RNAi) or RNA silencing uses dsRNA to exploit a natural antiviral intracellular pathway to induce the knockdown or “silencing” of a specific gene. The silencing mechanism is triggered when dsRNA interacts with the endoribonuclease Dicer, which cleaves the aberrant dsRNA into 21-base pair fragments; these are known as small interfering RNA (siRNA), which are incorporated into an RNA-induced silencing complex that unwinds the siRNA and anneals it to the complementary RNA target, subsequently cleaving and degrading it. The use of nucleic acid-induced gene silencing, such as RNAi, as a therapy has been a focus since the discovery of the antiviral machinery for silencing specific target genes in the late 1990s (Fire et al., 1998) and has led to the hope that specific diseases may benefit therapeutically from knockdown of a single gene, especially when initial studies suggested that in contrast to long dsRNA, siRNA did not induce a nonspecific antiviral response (Karpala et al., 2005). In fact, Reich et al. (2003) and Shen et al. (2006) reported that siRNAs targeting VEGFa or the VEGF receptor 1 effectively inhibited ocular choroidal neovascularization (CNV) in mouse models. CNV occurs at a late stage in age-related macular degeneration (which afflicts 30 to 50 million people globally), in which the retina is invaded by choroidal vessels, causing blindness. Because of these studies, initial clinical trials were set up using intraocular injection in patients with CNV. More recently, however, various groups have demonstrated that the antiviral response is activated upon introduction of siRNA implicating TLR3 activation by siRNA (Karikó et al., 2004). In fact, Kleinman et al. (2008) have since claimed that suppression of neovascularization is a generic property of siRNAs independent of sequence, target, and internalization. They showed that numerous synthetic nontargeted 21-nucleotide duplex siRNAs, siRNAs targeting nonmammalian genes, or nonocular genes all suppressed CNV in mice as effectively as VEGFa siRNA. These data indicate that angio-inhibition is a siRNA-class effect because, although nuclease digestion abolished the angioinhibitory effect of siRNA-induced CNV suppression, a chemically modified siRNA unable to interact with the RNA-induced silencing complex could no longer effect CNV inhibition. Kleinman et al. (2008) clearly show that siRNA suppression of CNV, whether using targeted siRNA against VEGFa/VEGF receptor 1 or nontargeting sequences, is via TLR3 activation, because nontargeting siRNA could not suppress CNV in Tlr3(-/-) mice, whereas poly(I:C) and dsRNA were shown to suppress CNV in wild-type mice but not in Tlr3 (-/-) mice. The angioinhibitory effect was similarly shown to require the TIR adapter TRIF and the induction of IL-12 and IFN-γ, both known for their antiangiogenic abilities. It is possible that a difference in the method of siRNA introduction could explain the different results observed between the study by Reich et al. (2003) and that by Kleinman et al. (2008). The siRNAs in the former were injected subretinally, whereas in the latter, the siRNA was introduced by intravitreous injection. It is more probable, however, that the difference is due to the differing lengths of siRNA used by Reich et al. (2003). Kleinman et al. (2008) show that the siRNA needs to be a minimum of 21 nt to engage and activate TLR3; however, although the VEGF-targeting siRNA in Reich's study was 21 nt long, EGFP-targeting siRNA was used as the control in this case, and although the antisense strand of this siRNA was 21 nt long, the sense strand was only 18 nt. This would effectively halve the dose of TLR3-activating siRNA available in the control experiments. The discrepancies between the study by Kleinman et al. (2008) and that of Shen et al. (2006) are more difficult to reconcile because the studies used the same method of introduction and the siRNAs were 21 nt long. The differences again are likely to come down to choice of control siRNA. Shen et al. (2006) show data for one control siRNA, whereas Kleinman et al. (2008) describe 12 control siRNAs. However, why the control siRNA used in by Shen et al. (2006) did not activate TLR3 and inhibit angiogenesis whereas those used by Kleinman et al. (2008) study did remains unclear. The study by Kleinman et al. (2008) has high-reaching implications for the development of siRNAs as a method for treatment of disease in general, in that they may induce unanticipated vascular or undesirable antiviral effects. However, it was necessary for the siRNA molecules to be at least 21 nt in length to induce a TLR3 response; therefore, it might be possible to enhance the therapeutic specificity of siRNAs and abrogate TLR3 activation by reducing the siRNAs to under 21 nt (Kleinman et al., 2008). Nonetheless, therapeutic use of RNAi needs careful consideration because there is potential for wider impact on the system as a result of the associated stimulation of the antiviral signaling pathways.
B. A Potential Case for Toll-Like Receptor 3 Antagonism
TLR3 antagonism may be beneficial in treating West Nile virus (WNV) infection. Infection of macrophages or DCs by WNV in peripheral lymphoid tissue induces TLR3-dependent secretion of TNFα and results in a transient increase in the permeability of the blood-brain barrier (BBB), facilitating the penetration of WNV across the BBB and into the CNS. Although the exact mechanism of entry into the CNS remains unclear, TNFα may alter endothelial cell tight junctions allowing either WNV itself across the BBB or leukocytes carrying the virus to pass between the endothelial cells of the BBB; it is clear, however, that that TLR3 activation is vital to the passage of the virus into the CNS. TLR3-deficient mice have an increased survival rate after WNV infection, lower viral titers in the brain, and decreased BBB leakiness as a result of reduced levels of TNFα (Wang et al., 2004). Therefore, inhibition of TLR3 signaling and the subsequent reduction in TNFα could be a potential means of treating persons infected with WNV.
C. Toll-Like Receptors 7 and 8: Small-Molecule Targets
As mentioned previously, DCs are essential for mediating an effective immune response. There are two types of DCs: myeloid DCs (mDCs) and plasmacytoid DCs (pDCs). pDCs are particularly important in the antiviral response because of their ability to produce large amounts of type I IFNs upon viral infection. This function of human pDCs is due to their selective expression of TLR7 and TLR9. TLR8, however, is preferentially expressed by mDCs and monocytes. Like TLR3, TLRs 7 and 8 are antiviral TLRs. In this case TLRs 7 and 8 recognize single-stranded RNA sequences containing GU-rich or poly-U sequences as their natural ligands, but they are also activated by synthetic small-molecular-weight compounds of the imidazoquinoline family, such as resiquimod and imiquimod (Lee et al., 2003; Diebold et al., 2004; Heil et al., 2004). Upon ligation, TLRs 7 and 8 recruit the TIR adapter MyD88 and initiate a signaling cascade, resulting in the activation of both pro-inflammatory transcription factors such as NFκB and antiviral factors such as the IRF family of transcription factors. They are localized intracellularly to endosomal membranes and act as potent activators of innate immune responses upon viral infection. So, it is not surprising that the therapeutic potential in targeting TLR7 and 8 has focused on developing TLR7/TLR8 agonists as “antiviral agents.” Imidazoquinolines were originally developed as such antiviral agents, and many such small-molecule compounds have been tested for their ability to induce TLR7/TLR8-mediated cytokine induction. Imiquimod is the first approved topically active TLR7 agonist. It is prescribed for treatment of external virus-induced skin lesions, such as the genital and perianal warts resulting from papillomavirus infections (Gupta et al., 2004). Although the exact mechanism of action is unknown and may involve other receptors, cellular responses to imiquimod include the induction of cytokines such as IFNα, IL-12, and TNFα and chemokines such as IL-8, macrophage inflammatory proteins 1α and 1β, and macrophage chemotactic protein 1. Production of such mediators allows for the activation of and attraction of effector cells such as DCs and cytotoxic T cells to the lesion site. NFκB-dependent IFNγ induction and the induction of 2′,5′-oligoadenylate synthetase has also been demonstrated resulting in the stimulation of NK cells, which are integral to antiviral and antitumor activity (Miller, 2002; Navi and Huntley, 2004).
Therapeutic interest in TLR7/TLR8 for cancer treatment came about because of the antitumoral activity of TLR7/TLR8 agonists (Sidky et al., 1992). As such, imiquimod is now also used as a treatment for cancer and has shown itself to be efficacious against primary skin tumors and cutaneous metastases (Schön and Schön, 2008). In fact, imiquimod has been approved for therapeutic use in a number of oncological and virus-associated diseases, including external genital warts, precancerous actinic keratoses, basal cell carcinomas (the most common of skin cancers), and on lesions formed as a result of metastatic melanoma. In addition, it has been shown to be an effective treatment for herpes simplex virus in some cases, offering an alternative therapy for persons who are resistant to conventional treatment (Gupta et al., 2004; Miller et al., 2008). Hepatitis C virus (HCV) has also been under investigation as a potential target disease that would benefit therapeutically from TLR7 agonists. Chronic HCV infection affects 3% of the world's population, and the current therapy is based on a combination of pegylated IFNα and the nucleoside analog ribavirin (Schultheiss and Thimme, 2007). However, less than 50% of the population responds to this treatment, and novel strategies for treating HCV are therefore needed. A number of studies suggest that activation of TLR7 would be beneficial in persons infected with HCV. Indeed, one study has shown that TLR7 is expressed in normal and HCV-infected hepatocytes, and activation of TLR7 alone reduces HCV mRNA and protein levels (Lee et al., 2006). This was associated with stimulation of IRF7 and could not be neutralized by the addition of an IFNα receptor antibody, suggesting that TLR7 ligation can inhibit HCV replication by direct activation of antiviral mechanisms within the hepatocytes and not just via IFNα production. Further evidence of a role for TLR7 comes from a study demonstrating that a variant TLR7, due to a SNP c.1–120T>G, was associated with less inflammation and liver fibrosis in male patients. The increased IL-6 secretion observed in peripheral blood mononuclear cells from these patients is associated with a decrease in fibrosis progression. Because TLR7 is located on the X chromosome, it is not surprising that this effect was only observed in male patients expressing the variant (Schott et al., 2007). These combined studies clearly support a role for TLR7 in natural HCV infection. However, a study was carried out in which patients were administered resiquimod orally, and although low doses were well tolerated, they had little antiviral effect; higher doses seemed to decrease HCV levels after the first dose, but the higher dosing resulted in severe side effects (Pockros et al., 2007). To date, patients have responded best to the injectable TLR7 agonist isatoribine in a proof-of-concept study that resulted in reduced viral load in chronically infected patients. An oral prodrug of isatoribine, ANA975, was developed for the treatment of chronic HCV infection and initial results were promising (Horsmans et al., 2005); however, further trials were suspended when toxicity developed in long-term animal studies (Fletcher et al., 2006). Further studies are therefore necessary to determine whether there will be any advantage of TLR therapy over the current option.
An aqueous imidazoquinoline that could be administered systemically would be potentially important for the treatment of severe diseases such as chronic lymphocyte leukemia and solid-tumor cancers. Recent studies have focused on improving the stability of the single-stranded RNA-based TLR7/TLR8 agonists and enhancing their delivery for systemic use (Agrawal and Kandimalla, 2007). The resulting stabilized immune modulatory RNA compounds have since been shown to induce T cell, monocyte, and NK cell activation and migration and to induce elevated levels of Th1-type cytokine in the plasma of nonhuman primates (Lan et al., 2007). Another group has synthesized a TLR7 agonist that can be coupled to many different chemical entities, potentially allowing for directed systemic use of this TLR7 agonist (Wu et al., 2007). Yet another group has synthesized an aqueous imidazoquinoline, known as 852A, that is potentially promising for treating cancers of the blood (Dudek et al., 2007).
D. Toll-Like Receptor 9
At present, TLR9 is the only TLR for which a systemically administered specific agonist has shown substantial evidence of antitumor activity in human clinical trials. TLR9 has evolved to recognize unmethylated CpG dinucleotides (CpG ODN) that are prevalent in viral and bacterial DNA but not in vertebrate genomes. TLR9 is expressed on B cells and pDCs in humans and is pivotal to the production of type I IFN, which in turn is essential to control viral replication and eradicate infected cells. CpG ODNs are spontaneously taken up by most immune cells; upon uptake, TLR9 translocates to the same compartment, allowing for ligation and initiation of TLR9-dependent signal transduction. TLR9 stimulation activates innate immunity in a predominantly Th1 pattern. In this way, TLR9 activation is very similar to TLR3/TLR7/TLR8 activation and results in increased expression levels of costimulatory molecules such as TNF-related apoptosis-inducing ligand (TRAIL), which can induce tumor cell death, and CC chemokine receptor 7, which when activated causes cell trafficking to the T-cell zone of the lymph nodes. Together, these innate immune effects of TLR9 activation can promote tumor regression directly through the action of TNF-related apoptosis-inducing ligand or indirectly through the activation of NK cell-mediated tumor killing (Krieg, 2007).
The pattern with which CpG ODNs activate the immune system suggests the use of such TLR9 agonists as effective vaccine adjuvants. Indeed CpG ODNs have been shown to be beneficial in animal models as a vaccine adjuvant not only for infectious diseases (Jurk and Vollmer, 2007), but also as a tumor vaccine adjuvant. CpG ODNs seem to be the most promising of all adjuvants currently in preclinical development; they induce a Th1 environment and can synergize with other adjuvants, such as liposomes, which allow for enhanced uptake (Jurk and Vollmer, 2007). In fact, not only are CpG ODNs excellent adjuvants for normal therapeutic vaccination, but they also have the ability to help vaccine-hyporesponsive populations, such as persons positive for HIV, to benefit from vaccination (Cooper et al., 2005). There are also promising results from studies into the adjuvant activity of CpG ODN for tumor vaccination. CD8+ T cells play a major role in effective tumor vaccination, and strong peptide specific T cell responses have been reported when patients have been vaccinated with a combination of GpG ODN and antigen (Speiser et al., 2005; Cornet et al., 2006).
Because TLR9 stimulation by CpG ODN activates the Th1 response, it could be reasoned that the induction of this Th1 response should influence the progress of diseases that present with a dominant Th2 pattern, such as that found in asthma and allergy. Indeed, the use of CpG ODN has been successfully used as an adjuvant in combination with a portion of the ragweed allergen, demonstrating how TLR9 ligation can specifically redirect the allergic Th2 response toward a nonallergic Th1 response (Simons et al., 2004). Although such CpG ODN-allergy combination vaccines have huge therapeutic potential, they can provide only allergen-specific redirection of allergic responses. The main goal remains the development of an inhaled CpG ODN therapy that could treat or prevent allergic airway responses in a broad spectrum of patients with allergies.
E. Inhibiting Toll-Like Receptors 7, 8, and 9
Apart from having potential as a target for adjuvants, TLRs 7, 8, and 9 have been strongly implicated in autoantibody production (Leadbetter et al., 2002; Lau et al., 2005). Immune complexes containing self-DNA and or self-RNA in mouse models of SLE have been shown to activate self-IgG–specific B-cells as a result of costimulation through TLR 7, 8, or 9 and the B-cell receptor. Both DNA immune complexes and RNA immune complexes from patients with SLE result in a Th1 pattern of cytokine production. It is probable that TLRs 7, 8, and 9 are activated by products of damaged tissue and provide the stimulus that gives rise to autoreactive B-cell proliferation, autoantibody production, and the subsequent development of autoimmune disease (Lau et al., 2005). It has long been known that patients with SLE have increased serum levels of IFNα, and recent studies have demonstrated that this increase correlates with disease activity. In fact, TLRs 7, 8, and 9 may play a dual role in SLE, because it has been shown that pDCs are the probable source of this IFNα, and the ligation of circulating RNA- or DNA-containing immune complexes with TLRs 7, 8, and 9 on the pDCs induce this IFNα expression (Vallin et al., 1999).
However, the role of TLR9 in SLE remains incomplete. Since the initial report that TLR9 was required for autoantibody formation in vivo, a number of studies have reported that loss of TLR9 in models of SLE actually aggravates the disease, suggesting a possible regulatory role for constitutive TLR9 expression in autoimmune disease (Christensen et al., 2005; Bauer et al., 2008). A more prominent role has been demonstrated for TLR7 in the pathogenesis of SLE, with recent reports demonstrating that the expression levels of TLR7 alone can influence autoimmune disease (Pisitkun et al., 2006). Pisitkun et al. (2006) tracked SLE susceptibility in mice to a Y-chromosome linked “autoimmune accelerator” locus termed Yaa. Yaa is not due to a mutation in a normal Y chromosome gene; instead, it is due to a duplication of a segment of X chromosomal DNA that has been transposed onto the Y chromosome. This effectively doubles the TLR7 gene dosage and responsiveness of B cells to TLR7 ligands, redirecting the disease from DNA- to RNA-associated pattern of autoantibodies. These results demonstrate that merely doubling the level of TLR7 expression can induce autoimmunity. Furthermore, it was reported that SLE-prone mice deficient in TLR7 showed reduced severity of autoimmune disease and that antagonism of TLR7 prevented autoimmune lung and kidney injury (Christensen et al., 2006; Pawar et al., 2007).
Overall these studies into SLE imply that a TLR9 and/or TLR7 antagonist would be predicted to have dual therapeutic benefits by inhibiting the major source of IFNα from pDCs and by inhibiting the costimulation and therefore activation of RNA/DNA immune complex-specific B cells, with the consequential inhibition of autoantibody production. In fact, chloroquine and its related compounds have been used since the 1950s to treat SLE, and their mechanism of action is now known to be inhibition of TLRs 7, 8, and 9, probably by blocking their ability to signal by increasing the pH of the endo-/lysosomes where they reside (Kalia and Dutz, 2007). Several types of inhibitory or suppressive ODNs have been described recently, some of which have been shown to block IFNα and reduce symptoms in SLE murine models, therefore representing a promising therapeutic agent for treatment of SLE (Dong et al., 2005; Barrat et al., 2007).
A point to take account of when considering TLR7/TLR8/TLR9 agonists as therapeutic agents is the potential for the induction of autoimmune disease. Because TLR7/TLR8/TLR9 agonists induce the secretion of IFNα and activate a Th1-weighted immune response, a hallmark feature of autoimmune diseases, there is the possibility that their use may result in the induction of autoimmunity. However, CpG ODN has been given to millions of people in the form of Bacillus Calmette-Guérin, and there have been no reported cases of autoimmunity to date (Krieg and Vollmer, 2007). If this trend proves to be extrapolated to the activation of the other endosomal TLRs (i.e., 3, 7, and 8), then the therapeutic potential for TLR agonists is practically unlimited and may allow for the targeting of a broad range of disease, including cancers, asthma, allergies, and infections.
VII. Future Considerations in Therapeutic Development
Table 1 provides a summary of the major diseases in which TLRs have been implicated. There are clearly many options for the targeting of TLRs, because the key function of TLRs is to induce cytokines, which are well validated in these diseases, and indeed successfully being targeted in the clinic. Why would TLRs represent a better set of targets than cytokines or other downstream processes in inflammation? They occur early in these pathways and so inhibiting them might be more potent. They may be, in fact, at the initiation of pathologic conditions, because it is possible that the conditions listed are triggered by infections, with the ensuing inflammation leading to the production of endogenous ligands for TLRs, which further propagate the inflammation. With a genetic background in which TLRs are overactive (or their inhibitors are underactive), this might pivot into chronicity, and so targeting TLRs might therefore lead to remission from this chronic inflammation.
TABLE 1.
TLR targets in different diseases
|
TLR |
Tissue |
Therapeutic Potential/Use |
Action |
|---|---|---|---|
| TLR2 | Heart/vasculature | Atherosclerosis | Antagonistic |
| Lung | Asthma | Antagonistic | |
| Kidney | Ischemia/reperfusion | Antagonistic | |
| Pancreas | Diabetes | Antagonistic | |
| TLR3 | Systemic | Vaccine adjuvant | Agonistic |
| Breast | Anti-cancer | Agonistic | |
| Renal system | Anti-cancer | Agonistic | |
| Eye | CNV inhibition in AMD | Agonistic | |
| Brain | Anti-viral (WNV) | Antagonistic | |
| TLR4 | Heart/vasculature | Atherosclerosis | Antagonistic |
| Lung | Asthma | Antagonistic | |
| Kidney | Ischemia/reperfusion | Antagonistic | |
| Pancreas | Diabetes | Antagonistic | |
| Joints | Rheumatoid arthritis | Antagonistic | |
| Brain | Alzheimer's disease, Parkinson's disease | Antagonistic | |
| Systemic | Sepsis | Antagonistic | |
| Vaccine adjuvant | Agonistic | ||
| TLR5 | Hematopoietic system and GI tract | Radio-protective | Agonistic |
| Systemic | Vaccine-adjuvant (influenza virus) | Agonistic | |
| GI tract | Treatment of Crohn's disease | Antagonistic | |
| TLR7/8 | Skin | Treatment of viral induced lesions (caused by papilloma virus and Herpes simplex virus) | Agonistic |
| Primary tumors | Agonistic | ||
| Cutaneous metastases | Agonistic | ||
| Anti-viral (HCV) | Agonistic | ||
| Blood | Chronic lymphocyte leukemia | Agonistic | |
| Systemic | SLE | Antagonistic | |
| TLR9 | Prostate | Anticancer | Agonistic |
| Systemic | Vaccine-adjuvant | Agonistic | |
| Systemic | Tumor-vaccine-adjuvant | Agonistic | |
| Lung/systemic |
Asthma/allergy |
Agonistic |
In terms of how to target them, different approaches are being taken. Neutralizing antibodies to TLRs might be possible, but only for those on the cell surface, such as TLR2, TLR4, and TLR5. Small-molecule antagonists (e.g., eritoran against TLR4 or ODN-based inhibitors of TLR7) might be a better prospect, although these are not traditional “drug-like” molecules, and so it is hard to predict off-target effects and efficacy. Because there are kinases on the signaling pathways, these might also be amenable to inhibition. One concern here, however, is that such inhibitors might block multiple TLRs and therefore give rise to unwanted immunosuppression. Monotherapies against a specific TLR might not have this problem; based on knockout mouse studies, there seem to be less redundancy in TLRs in relation to inflammation, which may not be the case with infection, where multiple TLRs can engage with a given pathogen.
Adjuvancy may continue to yield new agents. Imiquimod is already approved for its antiviral effects, whereas MPL is approved as a vaccine adjuvant. We can expect further adjuvants to emerge that might improve vaccine efficacy or have antitumor effects. In terms of antagonism, we have data beyond phase II for only one TLR inhibitor—eritoran. As described in section II.D, its effects were significant but somewhat marginal. We therefore eagerly await trials with other TLR inhibitors, possibly in combination in the case of sepsis. In which indication might we see the first agent? SLE is of considerable interest here, because TLR7 has been implicated in disease pathogenesis and an antagonist might prove very useful.
We have come a long way from the discovery of the first Toll in the fruit fly. The intense interest around TLRs, shared by immunologists, biomedical researchers, and pharmacologists, should surely yield badly needed therapies for major pathologic conditions.
Acknowledgments
This work was supported by the Science Foundation Ireland; the Irish Health Research Board; the Wellcome Trust [Grant WT081744MA]; the Biotechnology and Biological Sciences Research Council Research Councils UK [Grant BB\C005503\1]; and the Horserace Betting Levy Board. The authors would like to thank Monique Gangloff and Tom Monie for the structural models in Fig. 2, A and B, respectively.
Footnotes
This article is available online at http://pharmrev.aspetjournals.org .
doi:10.1124/pr.109.001073.
Abbreviations: 852A, N-(4-(4-amino-2-ethyl-1H-imidazo(4,5c)-quinolin-1-yl)butyl)methanesulfonamide; BBB, blood-brain barrier; CD14, cluster of differentiation 14; CNS, central nervous system; CNV, choroidal neovascularization; DC, dendritic cell; dsRNA, double-stranded RNA; EDA, type III repeat extra domain of fibronectin; FOXP3, forkhead box P3; GI, gastrointestinal; HCV, hepatitis C virus; HSP, heat-shock protein; IBD, inflammatory bowel disease; IFN, interferon; IKK, IκB kinase complex; IL-1, interleukin; IRAK, IL-1 receptor-associated kinase; IRF3, interferon regulatory factor 3; LPS, lipopolysaccharide; LTA, lipoteichoic acid; M2e, ectodomain of the matrix2 protein; Mal, MyD88 adapter-like; MAP, mitogen-activated protein; MD-2, myeloid differentiation factor 2; mDC, myeloid dendritic cell; MPL, monophosphoryl lipid A; NFκB, nuclear factor-κB; NK, natural killer; nt, nucleotides; ODN, dinucleotides; pDC, plasmacytoid dendritic cell; RNAi, RNA interference; siRNA, small interfering RNA; SLE, systemic lupus erythematosus; SNP, single nucleotide polymorphism; TAG, TRAM adapter with gold domain; TAK-242, ethyl 6-(N-(2-chloro-4-fluorophenyl)sulfamoyl)cyclohex-1-ene-1-carboxylate; TIR, Toll-IL-1 receptor; TLR, Toll-like receptor; TNF-α, tumor necrosis factor-α; TRAM, TRIF-related adapter protein; TRIF, Toll/IL-1R domain-containing adapter inducing IFN-β; VEGF, vascular endothelial growth factor; WNV, West Nile virus.
References
- Agrawal S and Kandimalla ER (2007) Synthetic agonists of Toll-like receptors 7, 8 and 9. Biochem Soc Trans 35:1461-1467. [DOI] [PubMed] [Google Scholar]
- Ahmad-Nejad P, Mrabet-Dahbi S, Breuer K, Klotz M, Werfel T, Herz U, Heeg K, Neumaier M, and Renz H (2004) The toll-like receptor 2 R753Q polymorphism defines a subgroup of patients with atopic dermatitis having severe phenotype. J Allergy Clin Immunol 113:565-567. [DOI] [PubMed] [Google Scholar]
- Akashi S, Nagai Y, Ogata H, Oikawa M, Fukase K, Kusumoto S, Kawasaki K, Nishijima M, Hayashi S, Kimoto M, et al. (2001) Human MD-2 confers on mouse Toll-like receptor 4 species-specific lipopolysaccharide recognition. Int Immunol 13:1595-1599. [DOI] [PubMed] [Google Scholar]
- Akashi S, Saitoh S, Wakabayashi Y, Kikuchi T, Takamura N, Nagai Y, Kusumoto Y, Fukase K, Kusumoto S, Adachi Y, et al. (2003) Lipopolysaccharide interaction with cell surface Toll-like receptor 4-MD-2: higher affinity than that with MD-2 or CD14. J Exp Med 198:1035-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulou L, Holt AC, Medzhitov R, and Flavell RA (2001) Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413:732-738. [DOI] [PubMed] [Google Scholar]
- Anders HJ, Banas B, and Schlöndorff D (2004) Signaling danger: toll-like receptors and their potential roles in kidney disease. J Am Soc Nephrol 15:854-867. [DOI] [PubMed] [Google Scholar]
- Andersen-Nissen E, Smith KD, Bonneau R, Strong RK, and Aderem A (2007) A conserved surface on Toll-like receptor 5 recognizes bacterial flagellin. J Exp Med 204:393-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre F, Slimane K, Bachelot T, Dunant A, Namer M, Barrelier A, Kabbaj O, Spano JP, Marsiglia H, Rouzier R, et al. (2004) Breast cancer with synchronous metastases: trends in survival during a 14-year period. J Clin Oncol 22:3302-3308. [DOI] [PubMed] [Google Scholar]
- Apetoh L, Tesniere A, Ghiringhelli F, Kroemer G, and Zitvogel L (2008) Molecular interactions between dying tumor cells and the innate immune system determine the efficacy of conventional anticancer therapies. Cancer Res 68:4026-4030. [DOI] [PubMed] [Google Scholar]
- Arbour NC, Lorenz E, Schutte BC, Zabner J, Kline JN, Jones M, Frees K, Watt JL, and Schwartz DA (2000) TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet 25:187-191. [DOI] [PubMed] [Google Scholar]
- Awomoyi AA, Rallabhandi P, Pollin TI, Lorenz E, Sztein MB, Boukhvalova MS, Hemming VG, Blanco JC, and Vogel SN (2007) Association of TLR4 polymorphisms with symptomatic respiratory syncytial virus infection in high-risk infants and young children. J Immunol 179:3171-3177. [DOI] [PubMed] [Google Scholar]
- Balistreri CR, Grimaldi MP, Chiappelli M, Licastro F, Castiglia L, Listì F, Vasto S, Lio D, Caruso C, and Candore G (2008) Association between the polymorphisms of TLR4 and CD14 genes and Alzheimer's disease. Curr Pharm Des 14:2672-2677. [DOI] [PubMed] [Google Scholar]
- Barrat FJ, Meeker T, Chan JH, Guiducci C, and Coffman RL (2007) Treatment of lupus-prone mice with a dual inhibitor of TLR7 and TLR9 leads to reduction of autoantibody production and amelioration of disease symptoms. Eur J Immunol 37:3582-3586. [DOI] [PubMed] [Google Scholar]
- Bauer S, Pigisch S, Hangel D, Kaufmann A, and Hamm S (2008) Recognition of nucleic acid and nucleic acid analogs by Toll-like receptors 7, 8 and 9. Immunobiology 213:315-328. [DOI] [PubMed] [Google Scholar]
- Berdeli A, Celik HA, Ozyürek R, Dogrusoz B, and Aydin HH (2005) TLR-2 gene Arg753Gln polymorphism is strongly associated with acute rheumatic fever in children. J Mol Med 83:535-541. [DOI] [PubMed] [Google Scholar]
- Bieback K, Lien E, Klagge IM, Avota E, Schneider-Schaulies J, Duprex WP, Wagner H, Kirschning CJ, Ter Meulen V, and Schneider-Schaulies S (2002) Hemagglutinin protein of wild-type measles virus activates toll-like receptor 2 signaling. J Virol 76:8729-8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blander JM (2007) Coupling Toll-like receptor signaling with phagocytosis: potentiation of antigen presentation. Trends Immunol 28:19-25. [DOI] [PubMed] [Google Scholar]
- Blander JM and Medzhitov R (2004) Regulation of phagosome maturation by signals from toll-like receptors. Science 304:1014-1018. [DOI] [PubMed] [Google Scholar]
- Boraska Jelavić T, Barisić M, Drmic Hofman I, Boraska V, Vrdoljak E, PeruzovićM, Hozo I, Puljiz Z, and Terzić J (2006) Microsatelite GT polymorphism in the toll-like receptor 2 is associated with colorectal cancer. Clin Genet 70:156-160. [DOI] [PubMed] [Google Scholar]
- Brown GD, Herre J, Williams DL, Willment JA, Marshall AS, and Gordon S (2003) Dectin-1 mediates the biological effects of beta-glucans. J Exp Med 197:1119-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdelya LG, Krivokrysenko VI, Tallant TC, Strom E, Gleiberman AS, Gupta D, Kurnasov OV, Fort FL, Osterman AL, Didonato JA, et al. (2008) An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science 320:226-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buwitt-Beckmann U, Heine H, Wiesmüller KH, Jung G, Brock R, Akira S, and Ulmer AJ (2005a) Toll-like receptor 6-independent signaling by diacylated lipopeptides. Eur J Immunol 35:282-289. [DOI] [PubMed] [Google Scholar]
- Buwitt-Beckmann U, Heine H, Wiesmüller KH, Jung G, Brock R, Akira S, and Ulmer AJ (2006) TLR1- and TLR6-independent recognition of bacterial lipopeptides. J Biol Chem 281:9049-9057. [DOI] [PubMed] [Google Scholar]
- Buwitt-Beckmann U, Heine H, Wiesmüller KH, Jung G, Brock R, and Ulmer AJ (2005b) Lipopeptide structure determines TLR2 dependent cell activation level. FEBS J 272:6354-6364. [DOI] [PubMed] [Google Scholar]
- Caricilli AM, Nascimento PH, Pauli JR, Tsukumo DM, Velloso LA, Carvalheira JB, and Saad MJ (2008) Inhibition of toll-like receptor 2 expression improves insulin sensitivity and signaling in muscle and white adipose tissue of mice fed a high-fat diet. J Endocrinol 199:399-406. [DOI] [PubMed] [Google Scholar]
- Casella CR and Mitchell TC (2008) Putting endotoxin to work for us: monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell Mol Life Sci 65:3231-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ WJ, Asano O, Robidoux AL, Perez M, Wang Y, Dubuc GR, Gavin WE, Hawkins LD, McGuinness PD, and Mullarkey MA (1995) E5531, a pure endotoxin antagonist of high potency. Science 268:80-83. [DOI] [PubMed] [Google Scholar]
- Christensen SR, Kashgarian M, Alexopoulou L, Flavell RA, Akira S, and Shlomchik MJ (2005) Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J Exp Med 202:321-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, and Shlomchik MJ (2006) Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity 25:417-428. [DOI] [PubMed] [Google Scholar]
- Cluff CW, Baldridge JR, Stöver AG, Evans JT, Johnson DA, Lacy MJ, Clawson VG, Yorgensen VM, Johnson CL, Livesay MT, et al. (2005) Synthetic toll-like receptor 4 agonists stimulate innate resistance to infectious challenge. Infect Immun 73:3044-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole LE, Shirey KA, Barry E, Santiago A, Rallabhandi P, Elkins KL, Puche AC, Michalek SM, and Vogel SN (2007) Toll-like receptor 2-mediated signaling requirements for Francisella tularensis live vaccine strain infection of murine macrophages. Infect Immun 75:4127-4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton T, Kurt-Jones EA, Boehme KW, Belko J, Latz E, Golenbock DT, and Finberg RW (2003) Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J Virol 77:4588-4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper CL, Davis HL, Angel JB, Morris ML, Elfer SM, Seguin I, Krieg AM, and Cameron DW (2005) CPG 7909 adjuvant improves hepatitis B virus vaccine seroprotection in antiretroviral-treated HIV-infected adults. AIDS 19:1473-1479. [DOI] [PubMed] [Google Scholar]
- Cornet S, Menez-Jamet J, Lemonnier F, Kosmatopoulos K, and Miconnet I (2006) CpG oligodeoxynucleotides activate dendritic cells in vivo and induce a functional and protective vaccine immunity against a TERT derived modified cryptic MHC class I-restricted epitope. Vaccine 24:1880-1888. [DOI] [PubMed] [Google Scholar]
- Crellin NK, Garcia RV, Hadisfar O, Allan SE, Steiner TS, and Levings MK (2005) Human CD4+ T cells express TLR5 and its ligand flagellin enhances the suppressive capacity and expression of FOXP3 in CD4+CD25+ T regulatory cells. J Immunol 175:8051-8059. [DOI] [PubMed] [Google Scholar]
- Diebold SS, Kaisho T, Hemmi H, Akira S, and Reis e Sousa C (2004) Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303:1529-1531. [DOI] [PubMed] [Google Scholar]
- Dong L, Ito S, Ishii KJ, and Klinman DM (2005) Suppressive oligodeoxynucleotides delay the onset of glomerulonephritis and prolong survival in lupus-prone NZB x NZW mice. Arthritis Rheum 52:651-658. [DOI] [PubMed] [Google Scholar]
- Dudek AZ, Yunis C, Harrison LI, Kumar S, Hawkinson R, Cooley S, Vasilakos JP, Gorski KS, and Miller JS (2007) First in human phase I trial of 852A, a novel systemic toll-like receptor 7 agonist, to activate innate immune responses in patients with advanced cancer. Clin Cancer Res 13:7119-7125. [DOI] [PubMed] [Google Scholar]
- Eder W, Klimecki W, Yu L, von Mutius E, Riedler J, Braun-Fahrländer C, Nowak D, and Martinez FD (2004) Toll-like receptor 2 as a major gene for asthma in children of European farmers. J Allergy Clin Immunol 113:482-488. [DOI] [PubMed] [Google Scholar]
- El-Omar EM, Ng MT, and Hold GL (2008) Polymorphisms in Toll-like receptor genes and risk of cancer. Oncogene 27:244-252. [DOI] [PubMed] [Google Scholar]
- Enioutina EY, Bareyan D, and Daynes RA (2008) TLR ligands that stimulate the metabolism of vitamin D3 in activated murine dendritic cells can function as effective mucosal adjuvants to subcutaneously administered vaccines. Vaccine 26:601-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhat K, Riekenberg S, Heine H, Debarry J, Lang R, Mages J, Buwitt-Beckmann U, Röschmann K, Jung G, Wiesmüller KH, et al. (2008) Heterodimerization of TLR2 with TLR1 or TLR6 expands the ligand spectrum but does not lead to differential signaling. J Leukoc Biol 83:692-701. [DOI] [PubMed] [Google Scholar]
- Ferwerda B, McCall MB, Alonso S, Giamarellos-Bourboulis EJ, Mouktaroudi M, Izagirre N, Syafruddin D, Kibiki G, Cristea T, Hijmans A, et al. (2007) TLR4 polymorphisms, infectious diseases, and evolutionary pressure during migration of modern humans. Proc Natl Acad Sci U S A 104:16645-16650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferwerda B, McCall MB, Verheijen K, Kullberg BJ, van der Ven AJ, Van der Meer JW, and Netea MG (2008) Functional consequences of toll-like receptor 4 polymorphisms. Mol Med 14:346-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, and Mello CC (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391:806-811. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KA, Palsson-McDermott EM, Bowie AG, Jefferies CA, Mansell AS, Brady G, Brint E, Dunne A, Gray P, Harte MT, et al. (2001) Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature 413:78-83. [DOI] [PubMed] [Google Scholar]
- Fletcher S, Steffy K, and Averett D (2006) Masked oral prodrugs of toll-like receptor 7 agonists: a new approach for the treatment of infectious disease. Curr Opin Investig Drugs 7:702-708. [PubMed] [Google Scholar]
- Frantz S, Ertl G, and Bauersachs J (2007) Mechanisms of disease: Toll-like receptors in cardiovascular disease. Nat Clin Pract Cardiovasc Med 4:444-454. [DOI] [PubMed] [Google Scholar]
- Fukata M and Abreu MT (2007) TLR4 signalling in the intestine in health and disease. Biochem Soc Trans 35:1473-1478. [DOI] [PubMed] [Google Scholar]
- Gangloff M and Gay NJ (2004) MD-2: the Toll `gatekeeper' in endotoxin signalling. Trends Biochem Sci 29:294-300. [DOI] [PubMed] [Google Scholar]
- Gantner BN, Simmons RM, Canavera SJ, Akira S, and Underhill DM (2003) Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med 197:1107-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam JK, Ashish, Comeau LD, Krueger JK, and Smith MF Jr (2006) Structural and functional evidence for the role of the TLR2 DD loop in TLR1/TLR2 heterodimerization and signaling. J Biol Chem 281:30132-30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay NJ and Gangloff M (2007) Structure and function of Toll receptors and their ligands. Annu Rev Biochem 76:141-165. [DOI] [PubMed] [Google Scholar]
- Gay NJ, Gangloff M, and Weber AN (2006) Toll-like receptors as molecular switches. Nat Rev Immunol 6:693-698. [DOI] [PubMed] [Google Scholar]
- Gewirtz AT, Vijay-Kumar M, Brant SR, Duerr RH, Nicolae DL, and Cho JH (2006) Dominant-negative TLR5 polymorphism reduces adaptive immune response to flagellin and negatively associates with Crohn's disease. Am J Physiol Gastrointest Liver Physiol 290:G1157-G1163. [DOI] [PubMed] [Google Scholar]
- Gioannini TL, Teghanemt A, Zhang D, Coussens NP, Dockstader W, Ramaswamy S, and Weiss JP (2004) Isolation of an endotoxin-MD-2 complex that produces Toll-like receptor 4-dependent cell activation at picomolar concentrations. Proc Natl Acad Sci U S A 101:4186-4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard R, Pedron T, Uematsu S, Balloy V, Chignard M, Akira S, and Chaby R (2003) Lipopolysaccharides from Legionella and Rhizobium stimulate mouse bone marrow granulocytes via Toll-like receptor 2. J Cell Sci 116:293-302. [DOI] [PubMed] [Google Scholar]
- Golenbock DT, Hampton RY, Qureshi N, Takayama K, and Raetz CR (1991) Lipid A-like molecules that antagonize the effects of endotoxins on human monocytes. J Biol Chem 266:19490-19498. [PubMed] [Google Scholar]
- Gray P, Dunne A, Brikos C, Jefferies CA, Doyle SL, and O'Neill LA (2006) MyD88 adapter-like (Mal) is phosphorylated by Bruton's tyrosine kinase during TLR2 and TLR4 signal transduction. J Biol Chem 281:10489-10495. [DOI] [PubMed] [Google Scholar]
- Gu W, Shan YA, Zhou J, Jiang DP, Zhang L, Du DY, Wang ZG, and Jiang JX (2007) Functional significance of gene polymorphisms in the promoter of myeloid differentiation-2. Ann Surg 246:151-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta AK, Cherman AM, and Tyring SK (2004) Viral and nonviral uses of imiquimod: a review. J Cutan Med Surg 8:338-352. [DOI] [PubMed] [Google Scholar]
- Hamann L, Kumpf O, Müller M, Visintin A, Eckert J, Schlag PM, and Schumann RR (2004) A coding mutation within the first exon of the human MD-2 gene results in decreased lipopolysaccharide-induced signaling. Genes Immun 5:283-288. [DOI] [PubMed] [Google Scholar]
- Hasan U, Chaffois C, Gaillard C, Saulnier V, Merck E, Tancredi S, Guiet C, Brière F, Vlach J, Lebecque S, et al. (2005) Human TLR10 is a functional receptor, expressed by B cells and plasmacytoid dendritic cells, which activates gene transcription through MyD88. J Immunol 174:2942-2950. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Asai Y, and Ogawa T (2004) Separation and structural analysis of lipoprotein in a lipopolysaccharide preparation from Porphyromonas gingivalis. Int Immunol 16:1431-1437. [DOI] [PubMed] [Google Scholar]
- Hawkins LD, Ishizaka ST, McGuinness P, Zhang H, Gavin W, DeCosta B, Meng Z, Yang H, Mullarkey M, Young DW, et al. (2002) A novel class of endotoxin receptor agonists with simplified structure, toll-like receptor 4-dependent immunostimulatory action, and adjuvant activity. J Pharmacol Exp Ther 300:655-661. [DOI] [PubMed] [Google Scholar]
- Hawn TR, Dunstan SJ, Thwaites GE, Simmons CP, Thuong NT, Lan NT, Quy HT, Chau TT, Hieu NT, Rodrigues S, et al. (2006) A polymorphism in Toll-interleukin 1 receptor domain containing adaptor protein is associated with susceptibility to meningeal tuberculosis. J Infect Dis 194:1127-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, and Aderem A (2001) The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410:1099-1103. [DOI] [PubMed] [Google Scholar]
- Haziot A, Ferrero E, Köntgen F, Hijiya N, Yamamoto S, Silver J, Stewart CL, and Goyert SM (1996) Resistance to endotoxin shock and reduced dissemination of gram-negative bacteria in CD14-deficient mice. Immunity 4:407-414. [DOI] [PubMed] [Google Scholar]
- Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, and Bauer S (2004) Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 303:1526-1529. [DOI] [PubMed] [Google Scholar]
- Hellman J, Tehan MM, and Warren HS (2003) Murein lipoprotein, peptidoglycan-associated lipoprotein, and outer membrane protein A are present in purified rough and smooth lipopolysaccharides. J Infect Dis 188:286-289. [DOI] [PubMed] [Google Scholar]
- Himmel ME, Hardenberg G, Piccirillo CA, Steiner TS, and Levings MK (2008) The role of T-regulatory cells and Toll-like receptors in the pathogenesis of human inflammatory bowel disease. Immunology 125:145-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschfeld M, Weis JJ, Toshchakov V, Salkowski CA, Cody MJ, Ward DC, Qureshi N, Michalek SM, and Vogel SN (2001) Signaling by toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect Immun 69:1477-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebe K, Georgel P, Rutschmann S, Du X, Mudd S, Crozat K, Sovath S, Shamel L, Hartung T, Zähringer U, et al. (2005) CD36 is a sensor of diacylglycerides. Nature 433:523-527. [DOI] [PubMed] [Google Scholar]
- Hoebe K, Janssen EM, Kim SO, Alexopoulou L, Flavell RA, Han J, and Beutler B (2003) Upregulation of costimulatory molecules induced by lipopolysaccharide and double-stranded RNA occurs by Trif-dependent and Trif-independent pathways. Nat Immunol 4:1223-1229. [DOI] [PubMed] [Google Scholar]
- Horng T, Barton GM, Flavell RA, and Medzhitov R (2002) The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature 420:329-333. [DOI] [PubMed] [Google Scholar]
- Horng T, Barton GM, and Medzhitov R (2001) TIRAP: an adapter molecule in the Toll signaling pathway. Nat Immunol 2:835-841. [DOI] [PubMed] [Google Scholar]
- Horsmans Y, Berg T, Desager JP, Mueller T, Schott E, Fletcher SP, Steffy KR, Bauman LA, Kerr BM, and Averett DR (2005) Isatoribine, an agonist of TLR7, reduces plasma virus concentration in chronic hepatitis C infection. Hepatology 42:724-731. [DOI] [PubMed] [Google Scholar]
- Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, and Akira S (1999) Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol 162:3749-3752. [PubMed] [Google Scholar]
- Huang B, Zhao J, Li H, He KL, Chen Y, Chen SH, Mayer L, Unkeless JC, and Xiong H (2005) Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res 65:5009-5014. [DOI] [PubMed] [Google Scholar]
- Huleatt JW, Nakaar V, Desai P, Huang Y, Hewitt D, Jacobs A, Tang J, McDonald W, Song L, Evans RK, et al. (2008) Potent immunogenicity and efficacy of a universal influenza vaccine candidate comprising a recombinant fusion protein linking influenza M2e to the TLR5 ligand flagellin. Vaccine 26:201-214. [DOI] [PubMed] [Google Scholar]
- Ii M, Matsunaga N, Hazeki K, Nakamura K, Takashima K, Seya T, Hazeki O, Kitazaki T, and Iizawa Y (2006) A novel cyclohexene derivative, ethyl (6R)-6-[N-(2-Chloro-4-fluorophenyl)sulfamoyl]cyclohex-1-ene-1-carboxylate (TAK-242), selectively inhibits toll-like receptor 4-mediated cytokine production through suppression of intracellular signaling. Mol Pharmacol 69:1288-1295. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Georgel P, Du X, Shamel L, Sovath S, Mudd S, Huber M, Kalis C, Keck S, Galanos C, et al. (2005) CD14 is required for MyD88-independent LPS signaling. Nat Immunol 6:565-570. [DOI] [PubMed] [Google Scholar]
- Jin MS, Kim SE, Heo JY, Lee ME, Kim HM, Paik SG, Lee H, and Lee JO (2007) Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a triacylated lipopeptide. Cell 130:1071-1082. [DOI] [PubMed] [Google Scholar]
- Johnson CM, Lyle EA, Omueti KO, Stepensky VA, Yegin O, Alpsoy E, Hamann L, Schumann RR, and Tapping RI (2007) Cutting edge: A common polymorphism impairs cell surface trafficking and functional responses of TLR1 but protects against leprosy. J Immunol 178:7520-7524. [DOI] [PubMed] [Google Scholar]
- Jurk M and Vollmer J (2007) Therapeutic applications of synthetic CpG oligodeoxynucleotides as TLR9 agonists for immune modulation. BioDrugs 21:387-401. [DOI] [PubMed] [Google Scholar]
- Kagan JC and Medzhitov R (2006) Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell 125:943-955. [DOI] [PubMed] [Google Scholar]
- Kagan JC, Su T, Horng T, Chow A, Akira S, and Medzhitov R (2008) TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol 9:361-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia S and Dutz JP (2007) New concepts in antimalarial use and mode of action in dermatology. Dermatol Ther 20:160-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karikó K, Bhuyan P, Capodici J, and Weissman D (2004) Small interfering RNAs mediate sequence-independent gene suppression and induce immune activation by signaling through toll-like receptor 3. J Immunol 172:6545-6549. [DOI] [PubMed] [Google Scholar]
- Karpala AJ, Doran TJ, and Bean AG (2005) Immune responses to dsRNA: implications for gene silencing technologies. Immunol Cell Biol 83:211-216. [DOI] [PubMed] [Google Scholar]
- Kawamoto T, Ii M, Kitazaki T, Iizawa Y, and Kimura H (2008) TAK-242 selectively suppresses Toll-like receptor 4-signaling mediated by the intracellular domain. Eur J Pharmacol 584:40-48. [DOI] [PubMed] [Google Scholar]
- Kawasaki K, Akashi S, Shimazu R, Yoshida T, Miyake K, and Nishijima M (2001) Involvement of TLR4/MD-2 complex in species-specific lipopolysaccharide-mimetic signal transduction by Taxol. J Endotoxin Res 7:232-236. [PubMed] [Google Scholar]
- Kim HM, Park BS, Kim JI, Kim SE, Lee J, Oh SC, Enkhbayar P, Matsushima N, Lee H, Yoo OJ, et al. (2007) Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell 130:906-917. [DOI] [PubMed] [Google Scholar]
- Kim JK (2006) Fat uses a TOLL-road to connect inflammation and diabetes. Cell metabolism 4:417-419. [DOI] [PubMed] [Google Scholar]
- Kirschning CJ, Wesche H, Merrill Ayres T, and Rothe M (1998) Human toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J Exp Med 188:2091-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman ME, Yamada K, Takeda A, Chandrasekaran V, Nozaki M, Baffi JZ, Albuquerque RJ, Yamasaki S, Itaya M, Pan Y, et al. (2008) Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature 452:591-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Saitoh S, Tanimura N, Takahashi K, Kawasaki K, Nishijima M, Fujimoto Y, Fukase K, Akashi-Takamura S, and Miyake K (2006) Regulatory roles for MD-2 and TLR4 in ligand-induced receptor clustering. J Immunol 176:6211-6218. [DOI] [PubMed] [Google Scholar]
- Kormann MS, Depner M, Hartl D, Klopp N, Illig T, Adamski J, Vogelberg C, Weiland SK, von Mutius E, and Kabesch M (2008) Toll-like receptor heterodimer variants protect from childhood asthma. J Allergy Clin Immunol 122:86-92, 92.e1–8 [DOI] [PubMed] [Google Scholar]
- Krieg AM (2007) Development of TLR9 agonists for cancer therapy. J Clin Invest 117:1184-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg AM and Vollmer J (2007) Toll-like receptors 7, 8, and 9: linking innate immunity to autoimmunity. Immunol Rev 220:251-269. [DOI] [PubMed] [Google Scholar]
- Kurt-Jones EA, Chan M, Zhou S, Wang J, Reed G, Bronson R, Arnold MM, Knipe DM, and Finberg RW (2004) Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc Natl Acad Sci U S A 101:1315-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, Walsh EE, Freeman MW, Golenbock DT, Anderson LJ, et al. (2000) Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol 1:398-401. [DOI] [PubMed] [Google Scholar]
- Lan T, Kandimalla ER, Yu D, Bhagat L, Li Y, Wang D, Zhu F, Tang JX, Putta MR, Cong Y, et al. (2007) Stabilized immune modulatory RNA compounds as agonists of Toll-like receptors 7 and 8. Proc Natl Acad Sci U S A 104:13750-13755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau CM, Broughton C, Tabor AS, Akira S, Flavell RA, Mamula MJ, Christensen SR, Shlomchik MJ, Viglianti GA, Rifkin IR, et al. (2005) RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med 202:1171-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, and Marshak-Rothstein A (2002) Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 416:603-607. [DOI] [PubMed] [Google Scholar]
- Lee HK, Brown SJ, Rosen H, and Tobias PS (2007) Application of beta-lactamase enzyme complementation to the high-throughput screening of toll-like receptor signaling inhibitors. Mol Pharmacol 72:868-875. [DOI] [PubMed] [Google Scholar]
- Lee HK, Lee J, and Tobias PS (2002) Two lipoproteins extracted from Escherichia coli K-12 LCD25 lipopolysaccharide are the major components responsible for Toll-like receptor 2-mediated signaling. J Immunol 168:4012-4017. [DOI] [PubMed] [Google Scholar]
- Lee J, Chuang TH, Redecke V, She L, Pitha PM, Carson DA, Raz E, and Cottam HB (2003) Molecular basis for the immunostimulatory activity of guanine nucleoside analogs: activation of Toll-like receptor 7. Proc Natl Acad Sci U S A 100:6646-6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Wu CC, Lee KJ, Chuang TH, Katakura K, Liu YT, Chan M, Tawatao R, Chung M, Shen C, et al. (2006) Activation of anti-hepatitis C virus responses via Toll-like receptor 7. Proc Natl Acad Sci U S A 103:1828-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Lee JY, Lee MY, Hwang DH, and Youn HS (2008) Acrolein with an alpha, beta-unsaturated carbonyl group inhibits LPS-induced homodimerization of toll-like receptor 4. Mol Cells 25:253-257. [PubMed] [Google Scholar]
- Leemans JC, Stokman G, Claessen N, Rouschop KM, Teske GJ, Kirschning CJ, Akira S, van der Poll T, Weening JJ, and Florquin S (2005) Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J Clin Invest 115:2894-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Botos I, Wang Y, Leonard JN, Shiloach J, Segal DM, and Davies DR (2008) Structural basis of toll-like receptor 3 signaling with double-stranded RNA. Science 320:379-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz E, Mira JP, Cornish KL, Arbour NC, and Schwartz DA (2000) A novel polymorphism in the toll-like receptor 2 gene and its potential association with staphylococcal infection. Infect Immun 68:6398-6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz E, Mira JP, Frees KL, and Schwartz DA (2002) Relevance of mutations in the TLR4 receptor in patients with gram-negative septic shock. Arch Intern Med 162:1028-1032. [DOI] [PubMed] [Google Scholar]
- Malhotra D, Relhan V, Reddy BS, and Bamezai R (2005) TLR2 Arg677Trp polymorphism in leprosy: revisited. Hum Genet 116:413-415. [DOI] [PubMed] [Google Scholar]
- Mansell A, Smith R, Doyle SL, Gray P, Fenner JE, Crack PJ, Nicholson SE, Hilton DJ, O'Neill LA, and Hertzog PJ (2006) Suppressor of cytokine signaling 1 negatively regulates Toll-like receptor signaling by mediating Mal degradation. Nat Immunol 7:148-155. [DOI] [PubMed] [Google Scholar]
- Marra A and Brigham D (2001) Streptococcus pneumoniae causes experimental meningitis following intranasal and otitis media infections via a nonhematogenous route. Infect Immun 69:7318-7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FD (2007) CD14, endotoxin, and asthma risk: actions and interactions. Proc Am Thorac Soc 4:221-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, and Mitchell TC (2007) The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science 316:1628-1632. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Preston-Hurlburt P, and Janeway CA Jr (1997) A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388:394-397. [DOI] [PubMed] [Google Scholar]
- Meng G, Rutz M, Schiemann M, Metzger J, Grabiec A, Schwandner R, Luppa PB, Ebel F, Busch DH, Bauer S, et al. (2004) Antagonistic antibody prevents toll-like receptor 2-driven lethal shock-like syndromes. J Clin Invest 113:1473-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merx S, Neumaier M, Wagner H, Kirschning CJ, and Ahmad-Nejad P (2007) Characterization and investigation of single nucleotide polymorphisms and a novel TLR2 mutation in the human TLR2 gene. Hum Mol Genet 16:1225-1232. [DOI] [PubMed] [Google Scholar]
- Miller R (2002) Imiquimod stimulates innate and cell mediated immunity which controls virus infections and tumors. Int J Dermatol 41 (Suppl 1):3-6. [DOI] [PubMed] [Google Scholar]
- Miller RL, Meng TC, and Tomai MA (2008) The antiviral activity of Toll-like receptor 7 and 7/8 agonists. Drug News Perspect 21:69-87. [DOI] [PubMed] [Google Scholar]
- Misch EA, Macdonald M, Ranjit C, Sapkota BR, Wells RD, Siddiqui MR, Kaplan G, and Hawn TR (2008) Human TLR1 Deficiency Is Associated with Impaired Mycobacterial Signaling and Protection from Leprosy Reversal Reaction. PLoS Negl Trop Dis 2:e231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockenhaupt FP, Cramer JP, Hamann L, Stegemann MS, Eckert J, Oh NR, Otchwemah RN, Dietz E, Ehrhardt S, Schröder NW, et al. (2006) Toll-like receptor (TLR) polymorphisms in African children: common TLR-4 variants predispose to severe malaria. J Commun Dis 38:230-245. [PubMed] [Google Scholar]
- Moore CE, Segal S, Berendt AR, Hill AV, and Day NP (2004) Lack of association between Toll-like receptor 2 polymorphisms and susceptibility to severe disease caused by Staphylococcus aureus. Clin Diagn Lab Immunol 11:1194-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morath S, Stadelmaier A, Geyer A, Schmidt RR, and Hartung T (2002) Synthetic lipoteichoic acid from Staphylococcus aureus is a potent stimulus of cytokine release. J Exp Med 195:1635-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullarkey M, Rose JR, Bristol J, Kawata T, Kimura A, Kobayashi S, Przetak M, Chow J, Gusovsky F, Christ WJ, et al. (2003) Inhibition of endotoxin response by e5564, a novel Toll-like receptor 4-directed endotoxin antagonist. J Pharmacol Exp Ther 304:1093-1102. [DOI] [PubMed] [Google Scholar]
- Mullen GE, Kennedy MN, Visintin A, Mazzoni A, Leifer CA, Davies DR, and Segal DM (2003) The role of disulfide bonds in the assembly and function of MD-2. Proc Natl Acad Sci U S A 100:3919-3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muroi M and Tanamoto K (2006) Structural regions of MD-2 that determine the agonist-antagonist activity of lipid IVa. J Biol Chem 281:5484-5491. [DOI] [PubMed] [Google Scholar]
- Nagai Y, Akashi S, Nagafuku M, Ogata M, Iwakura Y, Akira S, Kitamura T, Kosugi A, Kimoto M, and Miyake K (2002) Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol 3:667-672. [DOI] [PubMed] [Google Scholar]
- Navi D and Huntley (2004) Imiquimod 5 percent cream and the treatment of cutaneous malignancy. Dermatol Online J 10:4. [PubMed] [Google Scholar]
- Newport MJ, Allen A, Awomoyi AA, Dunstan SJ, McKinney E, Marchant A, and Sirugo G (2004) The toll-like receptor 4 Asp299Gly variant: no influence on LPS responsiveness or susceptibility to pulmonary tuberculosis in The Gambia. Tuberculosis 84:347-352. [DOI] [PubMed] [Google Scholar]
- Núñez Miguel R, Wong J, Westoll JF, Brooks HJ, O'Neill LA, Gay NJ, Bryant CE, and Monie TP (2007) A dimer of the Toll-like receptor 4 cytoplasmic domain provides a specific scaffold for the recruitment of signalling adaptor proteins. PLoS ONE 2:e788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien AD, Rosenstreich DL, Scher I, Campbell GH, MacDermott RP, and Formal SB (1980) Genetic control of susceptibility to Salmonella typhimurium in mice: role of the LPS gene. J Immunol 124:20-24. [PubMed] [Google Scholar]
- Ogus AC, Yoldas B, Ozdemir T, Uguz A, Olcen S, Keser I, Coskun M, Cilli A, and Yegin O (2004) The Arg753GLn polymorphism of the human toll-like receptor 2 gene in tuberculosis disease. Eur Respir J 23:219-223. [DOI] [PubMed] [Google Scholar]
- Ohto U, Fukase K, Miyake K, and Satow Y (2007) Crystal structures of human MD-2 and its complex with antiendotoxic lipid IVa. Science 316:1632-1634. [DOI] [PubMed] [Google Scholar]
- Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, Chow JC, and Strauss JF 3rd (2001) The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem 276:10229-10233. [DOI] [PubMed] [Google Scholar]
- Omueti KO, Beyer JM, Johnson CM, Lyle EA, and Tapping RI (2005) Domain exchange between human toll-like receptors 1 and 6 reveals a region required for lipopeptide discrimination. J Biol Chem 280:36616-36625. [DOI] [PubMed] [Google Scholar]
- O'Neill LA and Bowie AG (2007) The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol 7:353-364. [DOI] [PubMed] [Google Scholar]
- Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, Schroeder L, and Aderem A (2000) The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci U S A 97:13766-13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BS, Song DH, Kim HM, Choi BS, Lee H, and Lee JO (2009) The structural basis of lipopolysaccharide recognition by the TLR4–MD-2 complex. Nature 458:1191-1195. [DOI] [PubMed] [Google Scholar]
- Park Y, Park S, Yoo E, Kim D, and Shin H (2004) Association of the polymorphism for Toll-like receptor 2 with type 1 diabetes susceptibility. Ann N Y Acad Sci 1037:170-174. [DOI] [PubMed] [Google Scholar]
- Pawar RD, Ramanjaneyulu A, Kulkarni OP, Lech M, Segerer S, and Anders HJ (2007) Inhibition of Toll-like receptor-7 (TLR-7) or TLR-7 plus TLR-9 attenuates glomerulonephritis and lung injury in experimental lupus. J Am Soc Nephrol 18:1721-1731. [DOI] [PubMed] [Google Scholar]
- Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, and Bolland S (2006) Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science 312:1669-1672. [DOI] [PubMed] [Google Scholar]
- Pockros PJ, Guyader D, Patton H, Tong MJ, Wright T, McHutchison JG, and Meng TC (2007) Oral resiquimod in chronic HCV infection: safety and efficacy in 2 placebo-controlled, double-blind phase IIa studies. J Hepatol 47:174-182. [DOI] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, et al. (1998) Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- Przetak M, Chow J, Cheng H, Rose J, Hawkins LD, and Ishizaka ST (2003) Novel synthetic LPS receptor agonists boost systemic and mucosal antibody responses in mice. Vaccine 21:961-970. [DOI] [PubMed] [Google Scholar]
- Qureshi N, Takayama K, and Ribi E (1982) Purification and structural determination of nontoxic lipid A obtained from the lipopolysaccharide of Salmonella typhimurium J Biol Chem 257:11808-11815. [PubMed] [Google Scholar]
- Qureshi ST, Larivière L, Leveque G, Clermont S, Moore KJ, Gros P, and Malo D (1999) Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4). J Exp Med 189:615-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz CR, Garrett TA, Reynolds CM, Shaw WA, Moore JD, Smith DC Jr, Ribeiro AA, Murphy RC, Ulevitch RJ, Fearns C, et al. (2006) Kdo2-Lipid A of Escherichia coli, a defined endotoxin that activates macrophages via TLR-4. J Lipid Res 47:1097-1111. [DOI] [PubMed] [Google Scholar]
- Raetz CR and Whitfield C (2002) Lipopolysaccharide endotoxins. Annu Rev Biochem 71:635-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rallabhandi P, Awomoyi A, Thomas KE, Phalipon A, Fujimoto Y, Fukase K, Kusumoto S, Qureshi N, Sztein MB, and Vogel SN (2008) Differential activation of human TLR4 by Escherichia coli and Shigella flexneri 2a lipopolysaccharide: combined effects of lipid A acylation state and TLR4 polymorphisms on signaling. J Immunol 180:1139-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rallabhandi P, Bell J, Boukhvalova MS, Medvedev A, Lorenz E, Arditi M, Hemming VG, Blanco JC, Segal DM, and Vogel SN (2006) Analysis of TLR4 polymorphic variants: new insights into TLR4/MD-2/CD14 stoichiometry, structure, and signaling. J Immunol 177:322-332. [DOI] [PubMed] [Google Scholar]
- Rassa JC, Meyers JL, Zhang Y, Kudaravalli R, and Ross SR (2002) Murine retroviruses activate B cells via interaction with toll-like receptor 4. Proc Natl Acad Sci USA 99:2281-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re F and Strominger JL (2002) Monomeric recombinant MD-2 binds toll-like receptor 4 tightly and confers lipopolysaccharide responsiveness. J Biol Chem 277:23427-23432. [DOI] [PubMed] [Google Scholar]
- Re F and Strominger JL (2003) Separate functional domains of human MD-2 mediate Toll-like receptor 4-binding and lipopolysaccharide responsiveness. J Immunol 171:5272-5276. [DOI] [PubMed] [Google Scholar]
- Reich SJ, Fosnot J, Kuroki A, Tang W, Yang X, Maguire AM, Bennett J, and Tolentino MJ (2003) Small interfering RNA (siRNA) targeting VEGF effectively inhibits ocular neovascularization in a mouse model. Mol Vis 9:210-216. [PubMed] [Google Scholar]
- Rittirsch D, Flierl MA, and Ward PA (2008) Harmful molecular mechanisms in sepsis. Nat Rev Immunol 10:776-787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofs MF, Wenink MH, Toonen EJ, Coenen MJ, Joosten LA, van den Berg WB, van Riel PL, and Radstake TR (2008) The functional variant (Asp299gly) of toll-like receptor 4 (TLR4) influences TLR4-mediated cytokine production in rheumatoid arthritis. J Rheumatol 35:558-561. [PubMed] [Google Scholar]
- Rowe DC, McGettrick AF, Latz E, Monks BG, Gay NJ, Yamamoto M, Akira S, O'Neill LA, Fitzgerald KA, and Golenbock DT (2006) The myristoylation of TRIF-related adaptor molecule is essential for Toll-like receptor 4 signal transduction. Proc Natl Acad Sci U S A 103:6299-6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh S, Akashi S, Yamada T, Tanimura N, Kobayashi M, Konno K, Matsumoto F, Fukase K, Kusumoto S, Nagai Y, et al. (2004) Lipid A antagonist, lipid IVa, is distinct from lipid A in interaction with Toll-like receptor 4 (TLR4)-MD-2 and ligand-induced TLR4 oligomerization. Int Immunol 16:961-969. [DOI] [PubMed] [Google Scholar]
- Salaun B, Coste I, Rissoan MC, Lebecque SJ, and Renno T (2006) TLR3 can directly trigger apoptosis in human cancer cells. J Immunol 176:4894-4901. [DOI] [PubMed] [Google Scholar]
- Santin I, Bilbao JR, de Nanclares GP, Calvo B, and Castaño L (2006) No association of TLR2 and TLR4 polymorphisms with type I diabetes mellitus in the Basque population. Ann N Y Acad Sci 1079:268-272. [DOI] [PubMed] [Google Scholar]
- Satoh M, Ishikawa Y, Minami Y, Takahashi Y, and Nakamura M (2008) Role of Toll like receptor signaling pathway in ischemic coronary artery disease. Front Biosci 13:6708-6715. [DOI] [PubMed] [Google Scholar]
- Sauter KS, Brcic M, Franchini M, and Jungi TW (2007) Stable transduction of bovine TLR4 and bovine MD-2 into LPS-nonresponsive cells and soluble CD14 promote the ability to respond to LPS. Vet Immunol Immunopathol 118:92-104. [DOI] [PubMed] [Google Scholar]
- Schnare M, Rollinghoff M, and Qureshi S (2006) Toll-like receptors: sentinels of host defence against bacterial infection. Int Arch Allergy Immunol 139:75-85. [DOI] [PubMed] [Google Scholar]
- Schön MP and Schön M (2008) TLR7 and TLR8 as targets in cancer therapy. Oncogene 27:190-199. [DOI] [PubMed] [Google Scholar]
- Schott E, Witt H, Neumann K, Taube S, Oh DY, Schreier E, Vierich S, Puhl G, Bergk A, Halangk J, et al. (2007) A Toll-like receptor 7 single nucleotide polymorphism protects from advanced inflammation and fibrosis in male patients with chronic HCV-infection. J Hepatol 47:203-211. [DOI] [PubMed] [Google Scholar]
- Schröder NW and Schumann RR (2005) Single nucleotide polymorphisms of Toll-like receptors and susceptibility to infectious disease. Lancet Infect Dis 5:156-164. [DOI] [PubMed] [Google Scholar]
- Schröder NW, Diterich I, Zinke A, Eckert J, Draing C, von Baehr V, Hassler D, Priem S, Hahn K, Michelsen KS, et al. (2005) Heterozygous Arg753Gln polymorphism of human TLR-2 impairs immune activation by Borrelia burgdorferi and protects from late stage Lyme disease. J Immunol 175:2534-2540. [DOI] [PubMed] [Google Scholar]
- Schröder NW, Hermann C, Hamann L, Göbel UB, Hartung T, and Schumann RR (2003) High frequency of polymorphism Arg753Gln of the Toll-like receptor-2 gene detected by a novel allele-specific PCR. J Mol Med 81:368-372. [DOI] [PubMed] [Google Scholar]
- Schultheiss M and Thimme R (2007) Toll like receptor 7 and hepatitis C virus infection. J Hepatol 47:165-167. [DOI] [PubMed] [Google Scholar]
- Schumann RR, Leong SR, Flaggs GW, Gray PW, Wright SD, Mathison JC, Tobias PS, and Ulevitch RJ (1990) Structure and function of lipopolysaccharide binding protein. Science 249:1429-1431. [DOI] [PubMed] [Google Scholar]
- Shen J, Samul R, Silva RL, Akiyama H, Liu H, Saishin Y, Hackett SF, Zinnen S, Kossen K, Fosnaugh K, et al. (2006) Suppression of ocular neovascularization with siRNA targeting VEGF receptor 1. Gene Ther 13:225-234. [DOI] [PubMed] [Google Scholar]
- Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, and Kimoto M (1999) MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med 189:1777-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidky YA, Borden EC, Weeks CE, Reiter MJ, Hatcher JF, and Bryan GT (1992) Inhibition of murine tumor growth by an interferon-inducing imidazoquinolinamine. Cancer Res 52:3528-3533. [PubMed] [Google Scholar]
- Simons FE, Shikishima Y, Van Nest G, Eiden JJ, and HayGlass KT (2004) Selective immune redirection in humans with ragweed allergy by injecting Amb a 1 linked to immunostimulatory DNA. J Allergy Clin Immunol 113:1144-1151. [DOI] [PubMed] [Google Scholar]
- Smirnova I, Mann N, Dols A, Derkx HH, Hibberd ML, Levin M, and Beutler B (2003) Assay of locus-specific genetic load implicates rare Toll-like receptor 4 mutations in meningococcal susceptibility. Proc Natl Acad Sci U S A 100:6075-6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speiser DE, Liénard D, Rufer N, Rubio-Godoy V, Rimoldi D, Lejeune F, Krieg AM, Cerottini JC, and Romero P (2005) Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J Clin Invest 115:739-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller S, Elson G, Ferstl R, Dreher S, Mueller T, Freudenberg M, Daubeuf B, Wagner H, and Kirschning CJ (2008) TLR4-induced IFN-gamma production increases TLR2 sensitivity and drives Gram-negative sepsis in mice. J Exp Med 205:1747-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyvee MR, Zhang H, Hawkins LD, and Chow JC (2005) Toll-like receptor 2 antagonists. Part 1: preliminary SAR investigation of novel synthetic phospholipids. Bioorg Med Chem Lett 15:5494-5498. [DOI] [PubMed] [Google Scholar]
- Stevens VL, Hsing AW, Talbot JT, Zheng SL, Sun J, Chen J, Thun MJ, Xu J, Calle EE, and Rodriguez C (2008) Genetic variation in the toll-like receptor gene cluster (TLR10-TLR1-TLR6) and prostate cancer risk. Int J Cancer 123:2644-2650. [DOI] [PubMed] [Google Scholar]
- Stewart C, Stuart L, Halle A, Deng J, Wilkinson K, Golenbock DT and Moore K (2008) Endogenous danger signals trigger sterile inflammation via a heterotrimeric complex of CD36, TLR4 and TLR6; 2008 Sep 24–27; Lisbon, Portugal. University of Massachusetts, Worcester, MA.
- Stöver AG, Da Silva Correia J, Evans JT, Cluff CW, Elliott MW, Jeffery EW, Johnson DA, Lacy MJ, Baldridge JR, Probst P, et al. (2004) Structure-activity relationship of synthetic toll-like receptor 4 agonists. J Biol Chem 279:4440-4449. [DOI] [PubMed] [Google Scholar]
- Strober W, Fuss IJ, and Blumberg RS (2002) The immunology of mucosal models of inflammation. Annu Rev Immunol 20:495-549. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Hoshino K, and Akira S (2000) Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol 165:5392-5396. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, and Akira S (1999) Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Kawai T, Mühlradt PF, Morr M, Radolf JD, Zychlinsky A, Takeda K, and Akira S (2001) Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int Immunol 13:933-940. [DOI] [PubMed] [Google Scholar]
- Tobias PS and Curtiss LK (2008) TLR2 in murine atherosclerosis. Semin Immunopathol 30:23-27. [DOI] [PubMed] [Google Scholar]
- Toshchakov VY and Vogel SN (2007) Cell-penetrating TIR BB loop decoy peptides a novel class of TLR signaling inhibitors and a tool to study topology of TIR-TIR interactions. Expert Opin Biol Ther 7:1035-1050. [DOI] [PubMed] [Google Scholar]
- Toshchakov VY, Fenton MJ, and Vogel SN (2007) Cutting Edge: Differential inhibition of TLR signaling pathways by cell-permeable peptides representing BB loops of TLRs. J Immunol 178:2655-2660. [DOI] [PubMed] [Google Scholar]
- Triantafilou K and Triantafilou M (2004) Coxsackievirus B4-induced cytokine production in pancreatic cells is mediated through toll-like receptor 4. J Virol 78:11313-11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trompette A, Divanovic S, Visintin A, Blanchard C, Hegde RS, Madan R, Thorne PS, Wills-Karp M, Gioannini TL, Weiss JP, et al. (2009) Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature 457:585-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumpfheller C, Caskey M, Nchinda G, Longhi MP, Mizenina O, Huang Y, Schlesinger SJ, Colonna M, and Steinman RM (2008) The microbial mimic poly IC induces durable and protective CD4+ T cell immunity together with a dendritic cell targeted vaccine. Proc Natl Acad Sci U S A 105:2574-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui FW, Xi N, Rohekar S, Riarh R, Bilotta R, Tsui HW, and Inman RD (2008) Toll-like receptor 2 variants are associated with acute reactive arthritis. Arthritis Rheum 58:3436-3438. [DOI] [PubMed] [Google Scholar]
- Urbonaviciute V, Fürnrohr BG, Meister S, Munoz L, Heyder P, De Marchis F, Bianchi ME, Kirschning C, Wagner H, Manfredi AA, et al. (2008) Induction of inflammatory and immune responses by HMGB1-nucleosome complexes: implications for the pathogenesis of SLE. J Exp Med 205:3007-3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallin H, Perers A, Alm GV, and Rönnblom L (1999) Anti-double-stranded DNA antibodies and immunostimulatory plasmid DNA in combination mimic the endogenous IFN-alpha inducer in systemic lupus erythematosus. J Immunol 163:6306-6313. [PubMed] [Google Scholar]
- van den Berg WB, van Lent PL, Joosten LA, Abdollahi-Roodsaz S, and Koenders MI (2007) Amplifying elements of arthritis and joint destruction. Ann Rheum Dis 66 (Suppl 3):iii45-iii48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasl J, Prohinar P, Gioannini TL, Weiss JP, and Jerala R (2008) Functional activity of MD-2 polymorphic variant is significantly different in soluble and TLR4-bound forms: decreased endotoxin binding by G56R MD-2 and its rescue by TLR4 ectodomain. J Immunol 180:6107-6115. [DOI] [PubMed] [Google Scholar]
- Vijay-Kumar M, Sanders CJ, Taylor RT, Kumar A, Aitken JD, Sitaraman SV, Neish AS, Uematsu S, Akira S, Williams IR, et al. (2007) Deletion of TLR5 results in spontaneous colitis in mice. J Clin Invest 117:3909-3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viriyakosol S, Tobias PS, and Kirkland TN (2006) Mutational analysis of membrane and soluble forms of human MD-2. J Biol Chem 281:11955-11964. [DOI] [PubMed] [Google Scholar]
- Viriyakosol S, Tobias PS, Kitchens RL, and Kirkland TN (2001) MD-2 binds to bacterial lipopolysaccharide. J Biol Chem 276:38044-38051. [DOI] [PubMed] [Google Scholar]
- Visintin A, Latz E, Monks BG, Espevik T, and Golenbock DT (2003) Lysines 128 and 132 enable lipopolysaccharide binding to MD-2, leading to Toll-like receptor-4 aggregation and signal transduction. J Biol Chem 278:48313-48320. [DOI] [PubMed] [Google Scholar]
- Vogel SN, Johnson D, Perera PY, Medvedev A, Larivière L, Qureshi ST, and Malo D (1999) Cutting edge: functional characterization of the effect of the C3H/HeJ defect in mice that lack an Lpsn gene: in vivo evidence for a dominant negative mutation. J Immunol 162:5666-5670. [PubMed] [Google Scholar]
- Walsh C, Gangloff M, Monie T, Smyth T, Wei B, McKinley TJ, Maskell D, Gay N, and Bryant C (2008) Elucidation of the MD-2/TLR4 interface required for signaling by lipid IVa. J Immunol 181:1245-1254. [DOI] [PubMed] [Google Scholar]
- Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, and Flavell RA (2004) Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med 10:1366-1373. [DOI] [PubMed] [Google Scholar]
- Weiss DS, Raupach B, Takeda K, Akira S, and Zychlinsky A (2004) Toll-like receptors are temporally involved in host defense. J Immunol 172:4463-4469. [DOI] [PubMed] [Google Scholar]
- Werts C, Tapping RI, Mathison JC, Chuang TH, Kravchenko V, Saint Girons I, Haake DA, Godowski PJ, Hayashi F, Ozinsky A, et al. (2001) Leptospiral lipopolysaccharide activates cells through a TLR2-dependent mechanism. Nat Immunol 2:346-352. [DOI] [PubMed] [Google Scholar]
- Wiertsema SP, Khoo SK, Baynam G, Veenhoven RH, Laing IA, Zielhuis GA, Rijkers GT, Goldblatt J, Lesouëf PN, and Sanders EA (2006) Association of CD14 promoter polymorphism with otitis media and pneumococcal vaccine responses. Clin Vaccine Immunol 13:892-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, and Mathison JC (1990) CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249:1431-1433. [DOI] [PubMed] [Google Scholar]
- Wu CC, Hayashi T, Takabayashi K, Sabet M, Smee DF, Guiney DD, Cottam HB, and Carson DA (2007) Immunotherapeutic activity of a conjugate of a Toll-like receptor 7 ligand. Proc Natl Acad Sci U S A 104:3990-3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurfel MM, Gordon AC, Holden TD, Radella F, Strout J, Kajikawa O, Ruzinski JT, Rona G, Black RA, Stratton S, et al. (2008) Toll-like receptor 1 polymorphisms affect innate immune responses and outcomes in sepsis. Am J Respir Crit Care Med 178:710-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Sanjo H, Uematsu S, Kaisho T, Hoshino K, Takeuchi O, Kobayashi M, Fujita T, et al. (2002) Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature 420:324-329. [DOI] [PubMed] [Google Scholar]
- Yang RB, Mark MR, Gray A, Huang A, Xie MH, Zhang M, Goddard A, Wood WI, Gurney AL, and Godowski PJ (1998) Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature 395:284-288. [DOI] [PubMed] [Google Scholar]
- Youn HS, Lee JK, Choi YJ, Saitoh SI, Miyake K, Hwang DH, and Lee JY (2008) Cinnamaldehyde suppresses toll-like receptor 4 activation mediated through the inhibition of receptor oligomerization. Biochem Pharmacol 75:494-502. [DOI] [PubMed] [Google Scholar]
- Youn HS, Lee JY, Saitoh SI, Miyake K, and Hwang DH (2006a) Auranofin, as an anti-rheumatic gold compound, suppresses LPS-induced homodimerization of TLR4. Biochem Biophys Res Commun 350:866-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn HS, Saitoh SI, Miyake K, and Hwang DH (2006b) Inhibition of homodimerization of Toll-like receptor 4 by curcumin. Biochem Pharmacol 72:62-69. [DOI] [PubMed] [Google Scholar]
- Zähringer U, Lindner B, Inamura S, Heine H, and Alexander C (2008) TLR2 - promiscuous or specific? A critical re-evaluation of a receptor expressing apparent broad specificity. Immunobiology 213:205-224. [DOI] [PubMed] [Google Scholar]
- Zeng H, Wu H, Sloane V, Jones R, Yu Y, Lin P, Gewirtz AT, and Neish AS (2006) Flagellin/TLR5 responses in epithelia reveal intertwined activation of inflammatory and apoptotic pathways. Am J Physiol Gastrointest Liver Physiol 290:G96-G108. [DOI] [PMC free article] [PubMed] [Google Scholar]