Abstract
The interplay between genes and environment is complex, particularly when it comes to cancer. Studies on breast cancer cells have shown that environmental influences dominate over genotype in their effect on phenotype, and can cause cancerous cells to revert to a non-malignant phenotype, while remaining genotypically malignant. Using breast tissue in three-dimensional cell culture has proved a better model than traditional two-dimensional cell culture in that different cell types can be seen to behave differently to the same pro–apoptotic signal, with normal cells surviving.
Keywords: breast cancer, cell branching, cell culture model, β1 integrin, mammary gland acinus, matrix metalloproteinase
Introduction
This short review describes how the physiological context determines how oncogenes function and how cancer develops, using examples taken mainly from studies in my laboratory on the function of normal and malignant mammary gland. I started my postdoctoral training studying RSV (Rous sarcoma virus), which causes tumours in chickens. Rous discovered this virus in 1911 and won a share in the Nobel Prize for Physiology or Medicine for this work more than 50 years later, in 1966.
I am essentially a bacteriologist, with a background in chemistry and bacterial genetics, and initially I had difficulty understanding how a single viral gene could cause cancer. When I had my own laboratory we started a series of experiments in which David Dolberg, a fellow in my laboratory at that time, injected RSV into chicken embryos and found that the embryos did not develop tumours. However, when the infected embryos were dissociated and the tissues were put in a Petri dish, oncogenic transformation occurred almost immediately [1,2]. This indicated clearly that the expression of oncogenes or inhibition of tumour-suppressor genes was not sufficient to cause tumours and that the context, or the microenvironment, determines what an oncogene can do.
The interplay between genes and the microenvironment within an organism is complex. Every cell in an organism contains the same genetic material, yet the specificity of each tissue and organ is usually maintained throughout the organism’s life. This ‘programming’ is lost in cancer, when cells lose specificity, de-differentiate and proliferate to form a tumour, and it is also lost to some extent even in normal aging. Discovering the mechanism of programming in normal tissue and how it is lost in cancer and aging may lead to useful developments in therapy for cancer and degenerative diseases.
The mammary gland as a model system
The human mammary gland can be thought of as ‘an organism within an organism’. It is one of the few organs that develops almost completely after an individual is born. Before puberty, the breasts are similarly undeveloped in both boys and girls. The mammary anlages, or the cells that serve as a foundation for the milk-producing cells, are the same in both sexes, but, when a girl reaches puberty, these develop further, growing out into the fat pads within the breasts. When the woman becomes pregnant, acini, which look like tiny bunches of grapes, form on the ends of these structures. It is these that develop further and produce the milk during the lactation period; when lactation ceases the gland involutes, decreases in size and reorganizes, so they are not much different from how they were before pregnancy. Mammary gland tissues are therefore constantly changing during a woman’s reproductive years, and this makes the gland a very useful modulatable system for studying the problem of tissue specificity.
In vivo, the mammary epithelial cells (the cells that produce the milk) are complex well-differentiated cells containing organized nuclei and fat droplets and are surrounded by myoepithelial cells and a basement membrane. However, when these cells are placed in culture, even with lactogenic hormones in the medium, they de-differentiate, change their shape and lose the ability to make milk within 3 days [3]. This led us to argue that the cell function is being affected by something in vivo that does not exist in cell culture. We argued further that this was most likely to be the basement membrane or the ECM (extracellular matrix).
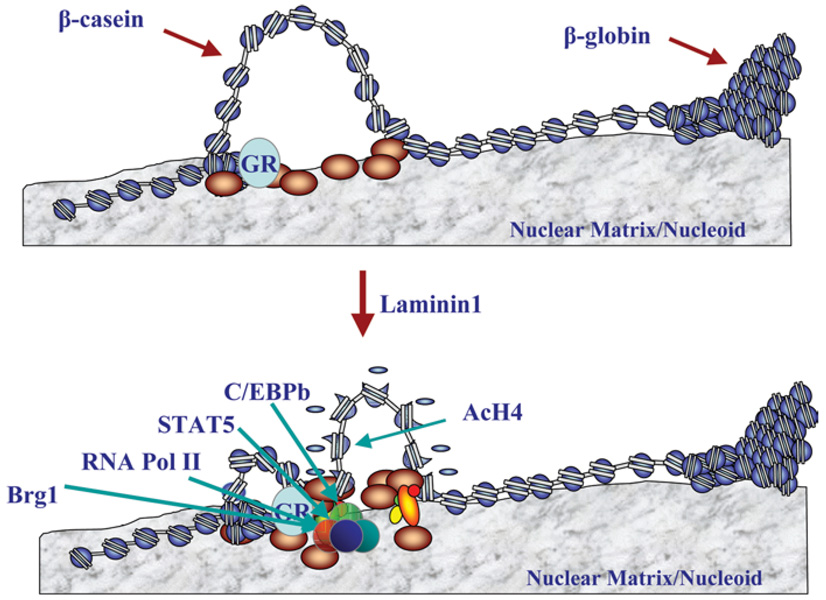
We therefore developed a cell culture model consisting of mouse epithelial cells on a laminin-rich gel as a model for the mammary acini (Figure 1). The cells developed into approximately spherical structures about the same size as normal acini in vivo. After 4 days, the cells in the centre of the spheres underwent apoptosis, creating hollow lumina into which milk was secreted [4]. Knowing the importance of the basement membrane in initiating milk production, however, raised the further question of how signals are passed from there into epithelial cells and into their nuclei in order to switch on expression of milk proteins, such as β-casein. We discovered an ECM-response element in the promoter of the β-casein gene and showed, using mutagenesis, that particular transcription factors are necessary for β-casein expression and also that the ECM changed the structure of the chromatin [5]. We found further that the presence of both ECM and hormones was necessary for this response element to initiate β-casein transcription. In summary, we now believe that there are both biochemical and physical connections between ECM and chromatin, and that the transcription factors and other components of the complex only reorganize to initiate expression of the genes leading to milk production if both lactogenic hormones and laminins, the major non-collagenous components of basement membranes, are present (Figure 2).
Figure 1. Formation of model mouse acini in cell culture on a laminin-rich ECM.
Similarities can be seen between acini in vivo and in three-dimensional cell culture. Reproduced from [4] with permission.
Figure 2. Schematic diagram showing the effect of laminin on the expression of genes involved in milk production in mammary epithelial cells.
Figure based on unpublished work by R. Xu and M.J. Bissell. AcH4, acetylated histone H4; C/EBP, CCAAT/enhancer-binding protein; GR, glucocorticoid receptor; RNA Pol II, RNA polymerase II; STAT5, signal transducer and activator of transcription 5.
Modelling breast cancer development
Our models for normal breast tissue development, and the insights that these have generated, can also be used to study breast cancer. Our model of the mammary acinus as an ‘experimental organism’ described above was originally developed in the mouse. However, the model functions in a very similar manner if human breast tissue is used, i.e. it is possible to generate the three-dimensional acinar structures from normal human breast epithelium if a laminin-rich gel is present. If human breast cancer cells are used instead, the cells simply grow and proliferate and form masses without ordered structures. This finding led to a very simple assay which can differentiate normal from malignant breast cells after only 6–7 days’ growth (depending on the type of assay used), and to a progression model for transforming human breast cells into malignant ones [6].
Our understanding of the importance of the tissue structure and the cellular microenvironment in the development of both normal and cancerous cells led us to propose and set out to prove a hypothesis. This states, first, that tumour cells with abnormal genomes should be capable of becoming phenotypically normal if their normal microenvironment can be restored, and, secondly (and conversely), that the destruction of tissue structure itself could be an oncogenic event, even in the absence of initial genetic mutation. Both types of events would require the transfer of signals into the cells from the external environment.
In order to test the first part of this hypothesis, Valerie Weaver, a talented postdoctoral fellow in my group, studied normal and malignant breast cells using flow cytometry, and found that, although both cell types expressed EGFR (epidermal growth factor receptor), β1 integrin and ECM receptors, the levels were different, with the malignant cells overexpressing β1 integrin, a number of other integrins and EGFR [7]. Reduction of the level of β1 integrin in the cancer cells to that of the normal cells using an inhibitory antibody showed that tumour cells reverted completely to a normal phenotype [8] (Figure 3). Since then, we have illustrated this effect in a mouse model of human mammary tumours, showing tumour regression within a few weeks with little toxicity when the mice were treated with antibodies against β1 integrin [9]. However, the genotype of the reverted cells was still malignant, and the malignant phenotype was restored when they were dissociated on a Petri dish and replated in three-dimensions without antibody present.
Figure 3. Effect of inhibitory antibodies to β1 integrin on malignant breast cells.
Inhibition of β1 integrin causes the cells to revert to a normal phenotype although their genotype remains unchanged. Reproduced from The Journal of Cell Biology, 1997, 137:231–245. Copyright 1997 The Rockefeller University Press.
These repeated transitions of the same cells between normal and malignant phenotypes, in response to signals from their environment, emphasizes the dominance of phenotype over genotype. This leads to a view of cancer that is actually quite hopeful, in that it should be possible to keep cancer development in check, even in individuals with oncogenic mutations in genes such as BRCA-1 and BRCA-2, by altering the environment of the affected cells.
Therefore growth and malignant behaviour of cells appear to be regulated at the level of the overall tissue organization. The essential three-dimensionality of this behaviour is underlined when cells in the model systems are compared with the same cells in tissue culture plastic. In the three-dimensional acini models, adding anti-(β1 integrin) antibody to malignant cells reduces their β1 integrin and EGFR levels, restoring a normal phenotype. However, if the same antibody is added to a monolayer of the same cells, they stop growing, but the expression levels of these key proteins are not reduced. Another example of the same phenomenon, illustrating how cell signalling is tissue-specific and entirely different in appropriate three-dimensional gels from those on two dimensions, comes from experiments we did with adenovirus infection in collaboration with Michael Korn of UCSF [10]. The CAR (coxsackievirus and adenovirus receptor) is expressed at the same level in both normal and malignant cells in two-dimensional cultures, whereas to three-dimensional acini, the level is dramatically down-regulated in normal cells, but remains high in tumour cells. Not only anti-(β1 integrin) antibody, but also a number of other signalling inhibitors, can revert the malignant phenotype of the T4-2 cells and down-regulate related receptors, a number of signalling proteins and reduce tumour growth, but only in laminin-rich ECM, delineating once again the differences between two- and three-dimensions [11].
Turning to the second part of the hypothesis, we have investigated whether destruction of tissue structure itself could be carcinogenic in the absence of genetic changes. The normal cycle of the mammary gland can give insights into this, since the involution of the acini at the end of lactation involves a loss of tissue structure and function. We discovered initially that the loss of milk production during involution could be correlated with down-regulation of MMP (matrix metalloproteinase) inhibitors, up-regulation of MMP-3, and loss of the basement membrane. We went on to show that, when metalloproteinase expression was induced in a normal pregnant mouse, its mammary glands involuted as if lactation was over, and that involution could be prevented if we inserted an Elvak pellet that contained an MMP inhibitor. Thus mice not only lost functional differentiation before involution set in, but also developed breast tumours as they aged [12].
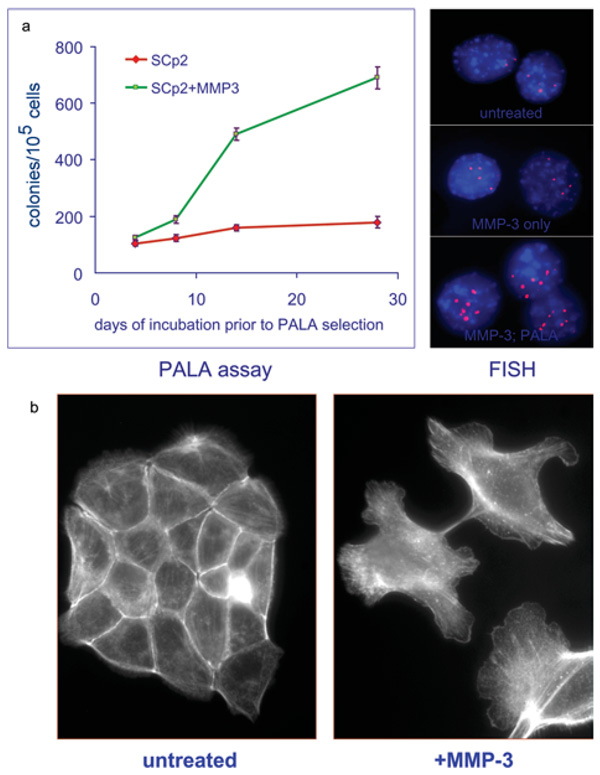
Derek Radisky, a former member of my group who is now a professor in the Mayo Clinic in Florida, showed by measuring the rate of amplification at the CAD locus using a PALA (partition affinity ligand assay) that expression of MMP-3 increased genomic instability in mouse mammary epithelial cells [13] (Figure 4a). Metalloprotease expression also caused alterations in the actin cytoskeleton, leading to cell shape changes (Figure 4b) and differences in Rac splicing to Rac-1b. Increased Rac-1b production leads to increased production of mitochondrial reactive oxygen species and thence to DNA damage and the observed genomic instability. We now believe that these effects arise from the cleavage of E-cadherin by the metalloproteinase.
Figure 4. Effects of increased MMP-3 expression on mouse mammary epithelial cells.
(a) Increased genomic instability, shown as an increase in the rate of amplification at the CAD locus. FISH, fluorescence in situ hybridization; PALA, partition affinity ligand assay. (b) Cell shape changes caused by MMP-3-induced alterations in the actin cytoskeleton. Reproduced with permission from [13]. © 2005 Nature Publishing Group; http://www.nature.com
Three-dimensional cell culture models
We have proved that we can make malignant cells phenotypically “normal”, and normal ones malignant, just by changing the structure of their environments. This highlights the fact that cancer is an organ-specific polymorphism-dependent disease. Personalized combination therapies for individual cases are likely to prove more successful in curing it than generic treatments could be, but we do not have enough patients or resources for the number of clinical tests that would be required. Alternative models of human disease that are more complex and reproducible than conventional tissue culture models may be able to partly fill this gap. Three-dimensional models such as those described here can be useful, although more complete and complex models, including whole-organ models, are still needed.
The following three papers give proof of the principle that three-dimensional cell culture models are more accurate than conventional monolayer tissue cultures. Valerie Weaver in my laboratory applied six different pro-apoptotic agents with different known mechanisms to normal and malignant cells in two- and three-dimensional tissue cultures. She found that, in the monolayers, all the normal and malignant cells went into apoptosis, whereas normal cells in three-dimensional cultures could withstand the pro-apoptotic stimuli. She showed further that normal cells that had been ‘disorganized’ went into apoptosis, whereas malignant ones that had been ‘reverted’ by adding β1 integrin inhibitors were apoptosis-resistant [14]. This proof that response to chemotherapeutic agents is dependent on cell architecture and tissue polarity led Jacks and Weinberg to write in a recent review that “all of a sudden, studying cancer cells in two dimensions appears to be quaint, if not archaic” [15].
The second example showing proof of principle is the efficacy of using inhibitory antibodies against β1 integrin in mice with induced human breast cancer cell tumours [9]. We developed a variation of our previous assays where the cancer cell lines were growing on top of the gels with a Matrigel drip in the medium, and showed that the three-dimensional morphology of the cells was correlated with their phenotype (for example, if they were metastatic or produced differentiated tumours), as well as with their gene expression profiles. It was possible to assign each cell type precisely to a phenotypic category based only on cell morphology and the results of a β1 integrin inhibition assay, without reference to the gene expression data.
My final example is some very recent work by Marcia Fournier, Paraic Kenny and co-workers in my laboratory [16], again taking normal acinus development as a model. We hypothesized that genes that were significantly over- or under-expressed in acinus morphogenesis could be likely to also be used in breast cancer prognosis. Acinus development involves a gain in tissue organization and growth control (which is opposite to the transition that occurs in early tumorigenesis), so it seemed likely that the expression of genes that were up-regulated in acinus development would be predictive of good outcome and vice versa. We correlated gene expression changes in carcinogenesis with those in acinus development and described a 19-gene signature that was well correlated with a good outcome in breast cancer.
Regulation of invasion and metastasis: lessons from mammary branching
More recently, a new technique was developed by Celeste Nelson, a bioengineering fellow in my laboratory, to investigate how cells know where to branch and why they do not breach the basement membrane in the normal gland. These studies, similar to the ones above, could be used to learn something about how it is that the tumour cells do not understand this and do invade the basement membrane and eventually metastasize. We wanted to develop an assay for cell branching that would specify a priori and uniformly the three-dimensional locations of cells and allow quantification and analysis of the statistically significant data. She used a technique called micropatterning where mammary epithelial tubules are immobilized between two layers of collagen, and quantified their positions by stacking 50 images on top of each other. She found, surprisingly, that branching occurred only at the corners of the tubules; altering the geometry of the tubules altered the position of the branch sites, a square branched from the corners and a circular tubule gave rise to branching all around. The theoretical biologists Dan Fletcher and his fellow Martijn van Duijn modelled this for us and suggested that this pattern could arise if branching was being affected by different concentrations of an inhibitor. We found that branching positions were, in fact, consistent with local minima in the concentration of the inhibitor TGF-β (transforming growth factor β) [17].
In summary, whereas we understand much of how genes influence cancer development, we still have a lot to learn about the tissue and organ context in which cancer arises. Organ specificity is plastic, dynamic and context-dependent; homoeostasis is all about balance in signalling and maintenance of tissue structure. Thus half of the secret of the cell lies outside the cell.
Acknowledgments
The studies from my laboratory were supported by grants from the OBER (Office of Biological and Environmental Research) of the U.S. DOE (Department of Energy), the U.S. NCI (National Cancer Institute) and the U.S. DOD (Department of Defense) BCRP (Breast Cancer Research Program). I am a recipient of an Innovator Award from the U.S. DOD BCRP and a Distinguished Fellowship from the OBER of the DOE. This lecture was dedicated to my daughter, Yalda T. Uhls, on the occasion of her birthday.
Abbreviations used
- ECM
extracellular matrix
- EGFR
epidermal growth factor receptor
- MMP
matrix metalloproteinase
- RSV
Rous sarcoma virus.
Biography

Mina J. Bissell
References
- 1.Dolberg DS, Bissell MJ. Nature. 1984;309:552–556. doi: 10.1038/309552a0. [DOI] [PubMed] [Google Scholar]
- 2.Stoker AW, Hatier C, Bissell MJ. J. Cell Biol. 1990;111:217–228. doi: 10.1083/jcb.111.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emerman JT, Pitelka DR. In Vitro. 1977;13:316–328. doi: 10.1007/BF02616178. [DOI] [PubMed] [Google Scholar]
- 4.Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Development. 1989;105:223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myers CA, Schmidhauser C, Mellentin-Michelotti J, Fragoso G, Roskelley CD, Casperson G, Mossi R, Pujuguet P, Hager G, Bissell MJ. Mol. Cell. Biol. 1998;18:2184–2195. doi: 10.1128/mcb.18.4.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ. Proc. Natl. Acad. Sci. U.S.A. 1992;89:9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang F, Weaver VM, Petersen OW, Larabell CA, Dedhar S, Briand P, Lupu R, Bissell MJ. Proc. Natl. Acad. Sci. U.S.A. 1997;95:14821–14826. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. J. Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park CC, Zhang H, Pallavicini M, Gray JW, Baehner F, Park CJ, Bissell MJ. Cancer Res. 2006;66:1526–1535. doi: 10.1158/0008-5472.CAN-05-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anders M, Hansen R, Ding RX, Rauen KA, Bissell MJ, Korn WM. Proc. Natl. Acad. Sci. U.S.A. 2003;100:1943–1948. doi: 10.1073/pnas.0337599100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bissell MJ, Rizki A, Mian IS. Curr. Opin. Cell Biol. 2003;15:753–762. doi: 10.1016/j.ceb.2003.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, Pinkel D, Bissell MJ, Werb Z. Cell. 1999;98:137–146. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, et al. Nature. 2005;436:123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weaver VM, Lelievre S, Lakins JN, Chrenek MA, Jones JC, Giancotti F, Werb Z, Bissell MJ. Cancer Cell. 2002;2:205–216. doi: 10.1016/s1535-6108(02)00125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacks T, Weinberg RA. Cell. 2002;111:923–925. doi: 10.1016/s0092-8674(02)01229-1. [DOI] [PubMed] [Google Scholar]
- 16.Fournier MV, Martin KJ, Kenny PA, Xhaja K, Bosch I, Yaswen P, Bissell MJ. Cancer Res. 2006;66:7095–7102. doi: 10.1158/0008-5472.CAN-06-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson CM, VanDuijn MM, Inman JL, Fletcher DA, Bissell MJ. Science. 2006;314:298–300. doi: 10.1126/science.1131000. [DOI] [PMC free article] [PubMed] [Google Scholar]