Abstract
Growing evidence shows that C1q modulates the growth and function of cells committed to the monocyte-derived dendritic cell (DC) lineage. Because C1q regulates both innate and acquired immune responses, we postulated that C1q modulates the transition from monocytes to DCs, i.e. the interface between innate and acquired immunity. Human peripheral blood monocytes cultured with soluble C1q and DC growth factors (GM-CSF + IL-4) failed to down-regulate monocyte-associated (CD14, CD16) and up-regulate DC-associated (CD83, CD86) markers. Impaired DC differentiation was not due to apoptosis; further analysis revealed the development of CD14hiCD11chiCD16+/− cells that have previously been associated with both innate and acquired immunity. Monocyte–DC precursors expressed gC1qR, the receptor for globular heads of C1q, from the outset, while cC1qR, the receptor for the collagen tails of C1q, was expressed at low levels. Notably, the binding pattern of monoclonal antibodies specific to the globular heads of C1q indicated that C1q is bound to monocytes via globular heads, presumably through gC1qR. Moreover, gC1qR levels decreased, while cC1qR levels were dramatically amplified as monocytes differentiated into immature DC. Thus, specific C1q/C1q receptor (R) interactions may control the transition from the monocyte state (innate immunity) toward the professional antigen-presenting cell state (adaptive immunity).
Keywords: dendritic cells, complement, C1q, C1q receptors, innate immunity
Introduction
Dendritic cells (DCs) are a complex lineage of antigen-presenting cells (APCs) that orchestrate a variety of immune responses.1–7 In their immature form, DCs are very efficient at capturing and processing antigen, while fully mature DCs are equipped to display these antigens on their surface via major histocompatibility (MHC) molecules and thus deliver signals to T cells.4,8 The differentiation of DCs from human peripheral blood (PB) monocytes is marked by the rapid loss of CD14, and the simultaneous up-regulation of CD11c, HLA-DR (MHC class II), as well as co-stimulatory and maturation associated molecules, such as CD86, CD80, and CD83.3–6
Dendritic cells display remarkable heterogeneity in phenotype and function, even within a specific lineage, such as the monocyte-derived DC subtype.3,6 Distinct subsets of DC precursor populations have been described to arise from human PB monocytes treated with GM-CSF + IL-4, including the major subset of CD14−CD11c+ cells, and minor subsets, such as CD14−CD11c− and CD16+/−CD14+CD11c+.7,9 Phenotypic diversity among DC subsets is thought to reflect a functional dissociation, imparting them with different immunoregulatory capabilities.9 Despite the great range of origin, phenotype and function, all DCs have a potent capacity to control B- and T-cell, as well as natural killer cell activity, thus serving as a fundamental link between innate and adaptive immunity.1,2,10
Because of the immense biological significance of DC function, their activity is highly regulated.11 Dendritic cells express an array of complement receptors on their surface,12–14 and also secrete many components of the complement cascade, including C1q.12,15 Interestingly, although the majority of complement components are secreted by the liver, C1q is primarily produced by immature DCs (iDCs) and macrophages.14–16 While C1q is traditionally known as the recognition unit of the classical complement pathway,17 it also plays a role in the antibody-dependent adaptive and antibody-independent innate arm of immunity. For example, C1q induces an antiproliferative signal in T cells, thereby modulating peripheral immunity.18 Furthermore, C1q deficiency is often associated with autoimmune diseases, such as systemic lupus erythematosus (SLE) and rheumatoid arthritis, suggesting functions for C1q in the maintenance of immune tolerance. Interestingly, some pathogens also mimic C1q activity and/or produce C1q-like molecules to alter host immune function. For example, hepatitis C virus (HCV) core protein binds to gC1qR on T cells, resulting in reduced T cell responses19–21 and DCs isolated from patients infected with HCV lack the capacity to stimulate T cells in a mixed leukocyte reaction (MLR).22 These biological responses are mediated by the interaction of C1q with cell surface structures and receptors.
There are at least two C1q receptors (R), which individually or together mediate a plethora of immunological functions. The first is cC1qR, which binds to the collagen region of C1q,23 as well as to some members of the collectin family of proteins (mannan binding lectin [MBL], lung surfactant proteins A and D [SP-A, SP-D])24 and is identical to the secreted chaperone protein calreticulin (CR). The second receptor is gC1qR (p33), a ubiquitously expressed, functionally diverse molecule involved in inflammation, infection and cancer.23,25,26 In addition to binding to the globular heads of C1q, gC1qR also binds to several plasma proteins, such as high molecular mass kininogen, multimeric vitronectin, as well as a wide range of pathogen-associated surface proteins.23 Although both surface gC1qR and cC1qR are capable of transducing ligand-mediated intracellular signals,23,25 they lack a consensus motif for a transmembrane domain. The apparent lack of direct signaling conduit is, however, circumvented by the formation of signaling/docking complexes with transmembrane proteins, such as the β1-integrin/gC1qR complex on endothelial cells,27 or the cC1qR/CD91 partnership that forms upon C1q ligation.28
Recently, C1q has emerged as an important regulator of DC activity. Exogenously supplied C1q has been shown to modulate the maturation of fully committed DCs after 5–7 days in culture and alter cytokine production and allogeneic T cell stimulatory ability of the cells.29–31 However, the correlation between the effects of C1q and the role of its receptors at earlier stages of DC growth is yet to be determined. Many DC-secreted proteins control DC differentiation/maturation via ‘loop-back’ mechanisms,3,32,33 and iDCs (but not mature DCs) are a major source of C1q production.12,15 Because C1q regulates both innate and acquired immune responses, the present studies were undertaken to test the hypothesis that C1q modulates immune activity by regulating the transition from monocytes to DCs (the interface between innate/ acquired immunity) via a C1q/C1qR coupled system. In support of this premise we show that C1q impairs GM-CSF + IL4–induced DC differentiation events in vitro, as evidenced by sustained expression of monocyte markers CD14 and CD16, reduced expression of DC maturation markers (CD86, CD83). The effect of C1q was most potent when added at the onset of culture period and was associated with temporal variations in the type of C1q receptors expressed on the cell surface. Based on our findings, we suggest the novel concept that, within the myelodendritic lineage, distinct C1q/ C1qR interactions promote responses associated with either innate or adaptive immunity.
Materials and Methods
Chemicals and reagents
The following reagents and chemicals were purchased or obtained from the sources indicated: Lymphoprep (Axis-Shield, Oslo, Norway); 100× penicillin/streptomycin, RPMI 1640 (Gibco-Invitrogen, Grand Island, NY, USA); heat inactivated fetal bovine serum (FBS; Hyclone, Logan, UT, USA); human serum albumin (HSA; Immuno-US, Rochester, MI, USA); human recombinant (r) granulocyte-macrophage colony-stimulating factor (GM-CSF), human rIL-4, human recombinant macrophage colony-stimulating factor (M-CSF; Peprotech, Rocky Hill, NJ, USA); annexin V-FITC (Becton-Dickinson, Mountain View, CA, USA); C1q (CompTech, Tyler, TX, USA); human IgG, fluorescein-conjugated dextran (Sigma-Aldrich, St Louis, MO, USA); Detoxi-Gel Endotoxin Removing Gel, p-nitrophenyl phosphate (pNPP; Pierce, Rockford, IL, USA); and Immu-Mount (Thermo Fisher, Waltham, MA, USA). Antibodies used were against: CD14 and CD83 (Biolegend, San Diego, CA, USA); CD16, HLA-DR, CD86, CD11c (BD), cC1qR (Serotec, Raleigh, NC, USA); monoclonal (mAb) and polyclonal antibody (pAb) against C1q (Quidel, Santa Clara, CA, USA); FITC conjugated goat anti-mouse IgG F(ab')2 or sheep anti-rabbit IgG F(ab')2 (Invitrogen, Carlsbad, CA, USA); and alkaline phosphatase (AP)–conjugated rabbit anti-goat IgG (Pierce).
Monoclonal and polyclonal antibodies to gC1qR
The production, characterization and purification of various monoclonal as well as polyclonal antibodies to the gC1qR protein as well as to various peptides derived from the molecule have been previously described.34
Generation of monocyte-derived DCs
Mononuclear cells were isolated from heparinized whole blood of normal, apparently healthy, human donors using Lymphoprep density gradient centrifugation. The cells were then cultured at a concentration of 106 cells/ml in RPMI 1640 containing 10% heat inactivated FBS, 100 U/ml penicillin/streptomycin, supplemented with 50 U/ml rGM-CSF and 50 ng/ml rIL-4 in Teflon vials to generate monocyte-DCs. As previously reported,35 this suspension culture system coupled with flow cytometric analysis, allows accurate assessment of the effects of the various stimuli on developing DC, monocyte, and lymphoid subpopulations. After purification of mononuclear cells using Lymphoprep, monocyte/DCs comprised 15–20% of the culture system. In addition to monocyte/DCs, lymphocytes were present, while all other contaminating cell constituents (platelets, red blood cells, polymorphonuclear cells) were absent from the culture, as assessed by scatter profiles and Wright stain analysis. Monocyte/DC subset gates were calculated on the basis of forward and side light scatter profiles (Fig. 1D) and expression patterns of myeloid cell/DC associated markers (HLA-DR, CD86, CD83, CD14, CD16, and CD11c). Lymphocytes were characterized by distinct light scatter patterns and lack of myeloid/DC markers. Highly purified C1q at a concentration of 25 μg/ml or as indicated in the experimental procedure was added to the culture starting from day 0. The cells were harvested every day for flow cytometric analysis. All cell culture conditions and reagents were low in endotoxin and viability of cells was usually ≥95%. Because of the sensitivity of DCs to endotoxin and contaminating PAMPs, highly purified and endotoxin-poor reagents and proteins were purchased when possible. In addition, special efforts were made to ensure that proteins such as C1q and antibodies used in the cell culture were low in endotoxin by passage over Detoxi-Gel columns using pyrogen-free buffers. The endotoxin removal efficiency in one passage is ≥99% (Pierce). Finally, each experiment performed using the suspension cell system was repeated with purified adherent monocytes (n = 2–3) yielding similar results (data not included).
Fig. 1.
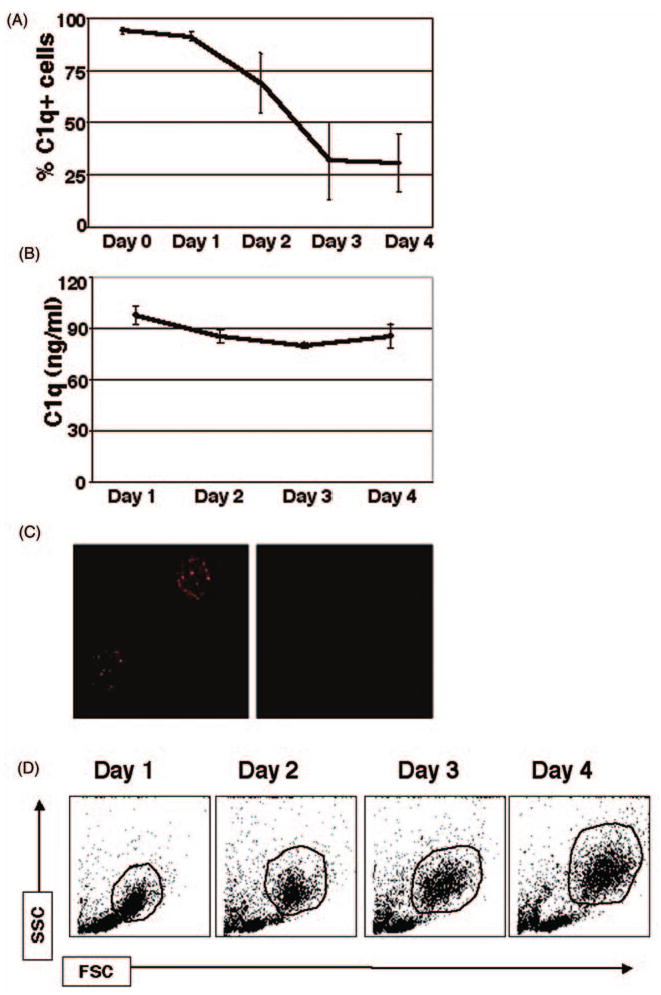
Kinetics of extracellular C1q expression during the monocyte-to-DC transition (surface [A,C], secreted [B]). Mononuclear cells (MNCs) isolated from PB and cultured in the presence of GM-CSF + IL-4 were analyzed for surface expression and secretion of C1q. (A) Cells were analyzed by flow cytometry for surface bound C1q on days 0–4. Cell surface expression of C1q was highest on days 0–2, and it was greatly reduced by day 4. Isotype-matched Ab was used as a negative control; cells were gated on the HLA-DR+ population. (n = 4) (B) Cell supernatants were assessed for secreted C1q by sandwich ELISA. C1q secretion remained at a steady level of 90 ± 3 ng/ml throughout days 1–4. (n = 4). (C) Detection of surface C1q on fresh PB monocytes by immunofluorescent microscopy. MNCs were isolated and immediately analyzed for surface bound C1q. Isotype-matched Ab was used as a negative control (n = 3). (D) Light scatter profile analysis of peripheral blood mononuclear cells on days 1–4 in culture in the presence of DC growth factors. One representative experiment is shown. The gated populations indicated (HLA-DR+) were used for subsequent analysis (n = 20). FSC, forward scatter; SSC, side scatter.
Detection of C1q in DC culture supernatants
Culture supernatants were collected on days 1–4, and the presence of C1q was tested by enzyme-linked immunosorbent assay (ELISA). Microtiter plates (MaxiSorb, Nunc, Denmark) were coated with C1q-specific mAb or the appropriate isotype-matched control at a concentration of 5 μg/ml in coating buffer (100 mM Na2CO3/NaHCO3, pH 9.6) for 2 h at 37°C. Non-specific binding sites were blocked using 3% heat-inactivated (56°C, 60 min) BSA in PBS (1 h, 37°C). In our experience, we have found that even the highest grade BSA can contain trace amounts of C1q. Therefore, we routinely use heat-inactivated and micro-filtered BSA. Because bovine complement is unusually resistant to heat inactivation, we use a 90-min incubation at 56°C to ensure destruction of C1q activity. Highly purified serum C1q was used at concentrations ranging from 1–5000 ng/ml as positive control and to establish a standard curve. Medium alone was used as a negative control. Next, 100 μl of each sample were added (1 h, 37°C), followed by a C1q-specific polyclonal Ab (1 h, 37°C). For detection of the reaction, alkaline phosphatase–conjugated rabbit anti-goat IgG was used (1 h, 37°C). All these steps were performed in ELISA buffer (PBS, 1% BSA, 0.05% Tween 20) and each step was followed by 3 washes with PBS/0.05% Tween 20. Enzyme activity was assessed by the addition of the substrate pNPP. The optical density (OD) at 415 nm was measured using a kinetic microplate reader (μQuant; Bio-Tek Instruments, Winooski, VT, USA) at various time points, and the values of the negative control were deducted from the experimental values. The sensitivity of the ELISA was 5 ng/ml.
Flow cytometry assisted analysis of cell surface markers
Cells were removed from the culture daily and washed twice in PBA staining buffer (PBS containing 1% BSA and 0.01% NaN3). The cells were incubated with 1 mg/ml human IgG in 100 μl of 1 × 106 cells PBA (30 min, 4°C), followed by addition of primary Abs conjugated to FITC, PE or APC fluorochromes or the appropriate isotype-matched controls (30 min, 4°C). For unconjugated primary Abs, the cells were first incubated with the primary Abs or their isotype-matched controls, then washed twice in PBA buffer and further incubated with FITC conjugated goat anti-mouse IgG F(ab')2 or sheep anti-rabbit IgG F(ab')2 (30 min, 4°C). The cells were then washed twice in cold PBA, fixed in 1% formalin and assessed by flow cytometric analysis using FACSCalibur (Becton-Dickinson, Mountain View, CA, USA). For each analysis, 10,000 events were collected and the data obtained was analyzed using CellQuest Pro software (BD).
Immunofluorescent microscopy
Indirect cell surface staining of freshly obtained mononuclear cells was performed as described above for flow cytometric analysis. Cells fixed for 5 min in 1% formalin were washed twice with cold PBA, concentrated, and applied to microscope slides. Subsequently, the slides were allowed to air-dry in the dark for 5 min, and cover slips were mounted onto the slides using Immu-mount mounting solution. The slides were viewed on a Zeiss Axiovert 200 M digital deconvolution microscope, followed by image capture at ×63 (oil) magnification and analysis with Axiovision v.4.5 software. The results represent the analysis of 100 cells in three independent experiments. Monocytes were identified by co-staining for monocyte specific markers in addition to anti-C1q.
Multiparametric analysis of apoptosis
Cells were removed from the culture daily, washed twice in cation-free low endotoxin PBS, resuspended in RPMI 1640 containing 1% human serum albumin (1% HSA/RPMI), and incubated with fluorochrome labeled antibodies or isotype controls (20 min, 4°C). After labeling, cells were washed twice in 1% HSA/RPMI, resuspended in 100 μl 1% HSA/RPMI, followed by 400 μl of 1 × binding buffer (1 mM HEPES, pH 7.4; 140 mM NaCl; 2.5 mM CaCl2), and incubated with Annexin V-FITC (20 min, 4°C). The cells were finally fixed in 1% PBS buffered formalin and acquired by flow cytometric analysis as described above. Apoptotic events were also assessed by morphological analysis of Wright stained cells using light microscopy.
Dextran uptake
Mononuclear cells were isolated as previously indicated; monocytes were further separated by plastic adherence (2 h, 37°C) and cultured in DC growth factors ± C1q (25 μg/ml) for 3 days. On day 3, the cells were washed twice in PBS and resuspended in RPMI + 10% heat-inactivated FBS (1 × 106 cells/ml). The cells were then incubated with FITC-dextran (1 mg/ml; 30 min, 37°C or 4°C). The incubation was stopped by washing the cells three times with ice-cold PBA (PBS containing 1% BSA and 0.01% NaN3). After fixing the cells with 1% formaldehyde, phagocytic uptake was analyzed using a FACSCalibur device (Becton Dickinson).
Statistical analysis
Student's t-tests were performed using statistical software (Excel; Microsoft, Redmond, WA, USA). A value of P = 0.05 was considered to be a significant difference. (n values represent separate experiments performed using different donors)
Results
Extracellular expression of C1q during the monocyte-DC transition
To investigate the possible role of C1q in regulating DC activity, we first analyzed its surface expression and secretion during the monocyte-to-DC transition. Peripheral blood (PB) monocytes from healthy individuals were used as precursors for monocyte-derived DCs. Surprisingly, C1q was present on the surface of monocytes from the outset, as confirmed by flow cytometric (Fig. 1A) analyses. What is more surprising is the finding that even freshly isolated monocytes that were not exposed to GM-CSF/IL-4 had C1q on their surface. Surface expression remained elevated until day 1, but was followed by a rapid decline on days 2–3. Importantly, this decline coincided with firm commitment to the DC lineage on day 3, as assessed by the up-regulation of DC maturation markers (CD86, HLA-DR, CD11c; Fig. 4) and the loss of CD14 – a monocyte-specific marker (Fig. 2). Since C1q expression on the surface of fresh PB monocytes has not been previously shown, we further confirmed our results using immunofluorescent microscopy. In subsequent experiments, we used two different polyclonal antibodies to the whole C1q molecule to observe surface C1q expression after isolation of mononuclear cells from whole blood. Analysis of surface C1q on monocytes using these Abs revealed a punctate pattern evenly distributed over the cellular membrane (Fig. 1C). Similar results were obtained for both antibodies, whereas the isotype control showed no staining (Fig. 1C).
Fig. 4.
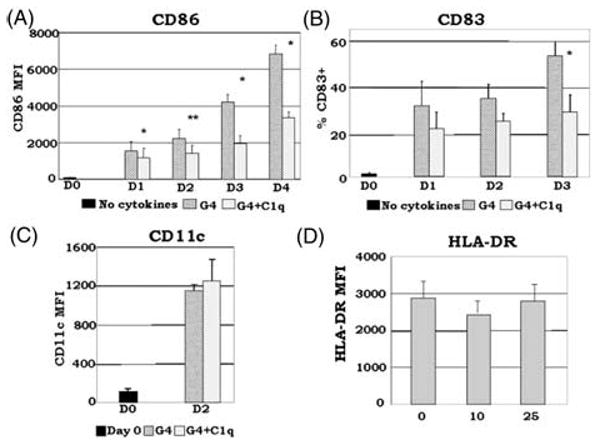
C1q alters GM-CSF + IL-4 induced DC differentiation. Monocyte-DCs were isolated and cultured in the presence of GM-CSF + IL-4 (G4) and with or without 25 μg/ml C1q (A–C). For the dose-response experiments, several concentrations of C1q were added as indicated (D). (A) C1q significantly decreased CD86 expression in monocyte-DCs compared to G4. *P <0.05, **P <0.01 (n = 4). (B) Monocyte-DCs cultured in the presence of C1q showed a decrease in the percentage of CD83+ cells in comparison with cells cultured in G4 alone. While CD83 expression was detected on the surface of these cells, their MFI remained low throughout the days with or without the addition of C1q (data not shown). *P <0.05 (n = 4). (C) CD11c expression was increased by day 2 with the addition of C1q compared to day 0. There was no significant difference in CD11c expression levels on cells cultured with or without C1q (n = 6). (D) Dose-response analysis revealed that there was little or no difference in the distribution of HLA-DR between C1q treated versus untreated cells on day 2 (n = 3). Cells were gated on HLA-DR+ cells for all experiments.
Fig. 2.
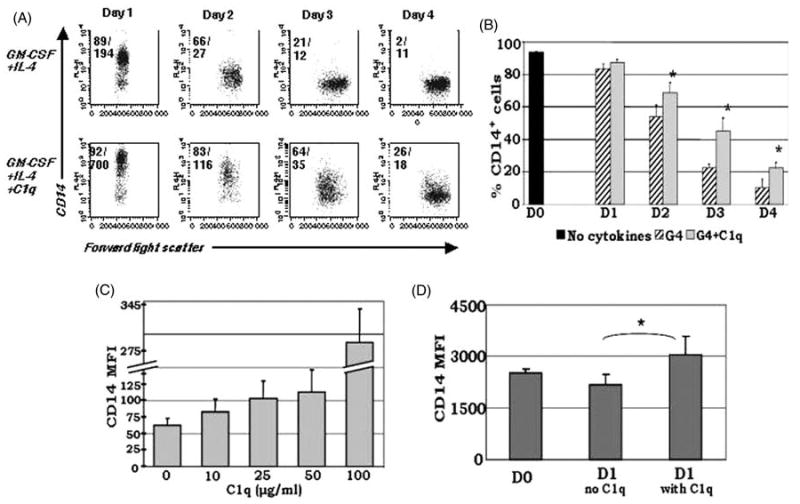
C1q sustains CD14 expression on monocyte-derived DCs in culture. MNCs isolated from PB by density gradient centrifugation were cultured in the presence of GM-CSF + IL-4 ± 25 μg/ml C1q (A,B), or without the addition of cytokines ± 25 μg/ml C1q (D). For the dose-response analysis, several concentrations of C1q were added as indicated (C). Cells were analyzed on days 0–4 for the expression of CD14. (A) Dendritic cells cultured in the presence of C1q retained CD14 on their surface and maintained elevated CD14 expression until day 4. The numbers in the upper corners of the plots represent percentage of positive cells/MFI. One representative experiment illustrated by dot plot analysis is shown. (B) Temporal analysis performed by flow cytometry revealed significantly higher levels of CD14 expression in C1q treated cultures (G4 + C1q) compared to G4 alone until day 4. *P < 0.05, **P < 0.01 (n = 4) (C) Dose-response analysis confirmed that the MFI of CD14+ cells correlates positively with increasing doses of C1q on day 2 (n = 3). (D) Baseline CD14 expression (day 0) on monocytes increased after 24 h of C1q treatment without the addition of DC growth factors (n = 3). Cells were gated on the HLA-DR+ population for all experiments.
Despite decreasing cell surface expression between days 1–4, C1q levels remained relatively high in cell-free supernatants during this period (90 ± 3 ng/ml; Fig. 1B). The latter may be due to altered expression patterns favoring secreted versus membrane anchored C1q and/or masking of epitopes occurring with changing cell surface protein profile.
Exogenous C1q sustains the expression of the monocyte marker CD14 on monocyte-DCs in culture
In order to gain insight into the role of extracellular C1q during the monocyte-to-DC transition, monocytes were cultured in the presence of exogenously supplied C1q in addition to GM-CSF + IL4. The C1q utilized in these studies was depleted of endotoxin and free of antigenic ‘cargo’. In support of altered DC differentiation, under these conditions, monocyte-derived DCs retained CD14 on their surface, and this level of enhanced surface expression was maintained until day 4 in culture (Fig. 2A,B). Mean fluorescence intensity (MFI) analysis revealed a 72% loss of CD14 molecules by day 1 with GM-CSF + IL-4 alone, whereas C1q treatment resulted in only a 26% loss of CD14 molecules (Fig. 2A). The pattern of increased CD14 MFI between the two culture conditions was sustained until day 4. In further support of altered differentiation, the percentage of CD14+ cells was significantly higher with the addition of C1q on days 2–4 (Fig. 2B). Preliminary analysis revealed no differences in CD14 between the two culture conditions beyond day 4. Dose-response analyses, which included C1q within physiological range (10–50 μg/ml), revealed that the effects of C1q on CD14 expression occurred in a dose-dependent fashion (Fig. 2C).
To elucidate whether increased CD14 expression in the presence of C1q was in response to the effects of C1q alone or in combination with the supplied DC growth factors, we cultured monocytes in media without cytokines, with and without the addition of C1q. Intriguingly, baseline CD14 expression (day 0) increased in response to C1q, and there was a significant difference of CD14 levels between cells cultured with and without C1q on day 1 (Fig. 2D), indicating that CD14 expression is not only maintained, but increased with C1q treatment. Together, these data suggest that C1q is capable of altering monocyte-to-DC differentiation and its effects are most potent during the earliest stages of monocyte-DC growth.
In order to eliminate the possibility that increased CD14 expression in the presence of C1q was due to selective cell death of CD14− cells, we monitored apoptosis of CD14+/− cells by Annexin V staining on days 1–4. In Figure 3A, we show that the inclusion of C1q does not increase apoptotic events on day 3. Similar results were obtained for days 1, 2 and 4 (data not shown). Furthermore, flow cytometric analysis of C1q-treated monocyte-DCs revealed light scatter patterns consistent with viable cells and Annexin V/propidium iodide staining confirmed the lack of apoptosis and secondary necrosis (data not shown). Wright stain analysis also showed that C1q treated monocyte-DCs did not undergo increased cell death in culture (Fig. 3B).
Fig. 3.

C1q induced changes in DC differentiation are not due to selective DC death. Mononuclear cells were isolated from PB and cultured with or without 25 μg/ml C1q. Cells were collected on days 1–4 and analyzed for the co-expression of CD14 and annexin V. (A) Annexin V analysis revealed that monocyte-DCs cultured in the presence of C1q did not have higher percentage apoptosis of CD14− or CD14+ cells than those cultured in G4 alone on day 3. A typical experiment is shown illustrated by dot plots gated on DR+ cells (n = 3). (B) Microscopic observation of monocyte-DCs cultured in the presence of C1q (right panel) did not show increased number of apoptotic cells by Wright stain analysis. C1q-treated cells (right panel) were smaller in size and displayed monocyte-like morphology, lacking the typical extending membrane processes (arrow) characteristic of iDCs developing in the presence of GM-CSF + IL-4 alone (left panel).
Through morphological analysis (Wright stain) of cells cultured with DC growth factors (GM-CSF + IL-4), we identified cells exhibiting typical features of iDCs, including extended membrane processes (Fig. 3B, left panel). With the addition of C1q, cells lacked DC-like membrane processes, were smaller in size, and exhibited monocyte-like features (Fig. 3B, right panel). The monocyte-like morphology is consistent with increases in monocyte-associated CD14 on these cells. Together, these data confirm that C1q induced altered DC growth was not due to selective survival of CD14+ cells and indicate that C1q influences differentiation events during the monocyte-to-DC transition (days 1–3).
C1q alters the expression of monocyte-derived DC maturation markers
We analyzed the expression of several DC maturation-dependent markers by flow cytometry in the presence of C1q. C1q treatment significantly diminished CD86 expression, a required co-stimulatory molecule for T cell activation (Fig. 4A). For CD83, cells were dimly positive and there was no statistically significant difference between MFI values in the presence or absence of C1q (data not shown). Nonetheless, the percentage of CD83+ cells was significantly decreased by day 3 with C1q treatment (Fig. 4B). Levels of CD11c increased over time with the addition of C1q compared to day 0. Furthermore, there was no significant difference in CD11c levels on cells with or without C1q treatment (Fig. 4C). With HLA-DR, 100% of the cells were positive under both conditions, and no statistically significant differences occurred in MFI values on day 2 (Fig. 4D). Thus, the addition of exogenous C1q at the onset of monocyte to DC transition alters DC differentiation/maturation, even in the presence of growth factors that strongly support the DC lineage.
C1q promotes the development of CD14hiCD11chiCD16+/− iDC subsets
As monocytes transition into DCs under the influence of GM-CSF + IL-4, certain monocyte-associated molecules, including CD14 and CD16, are down-regulated on the cell surface. CD11c is present on PB monocytes from the outset; with GM-CSF + IL-4, rapid up-regulation of CD11c molecules marks progression toward the DC lineage. Because we noted that C1q sustained CD14 expression (Fig. 2A–C), and others have demonstrated the unusual distribution of CD14/CD16/CD11c when GM-CSF + IL-4 triggered DC growth is altered by various physiological stimuli,36–40 we investigated the pattern of CD14/CD16/CD11c expression with or without C1q treatment. Consistent with monocyte-derived DC differentiation events, with both treatments, CD11c levels increased over time and there were no significant differences between the culture conditions (Figs 4C and 5B). With C1q treatment, CD16, like CD14, was retained on the cell surface (total CD16+ cells = 42% vs 24%, respectively, on day 1; Fig. 5A). Furthermore, multiparametric analysis revealed increased co-expression of these molecules in the presence of C1q (Fig. 5A). By day 3, CD16 levels decreased (data not shown), while CD14 levels were still elevated (Fig. 2A,B). Because DC maturation markers were also present on C1q treated cells (Fig. 4), these data indicate that the cells acquire features of both monocytes and DCs. Collectively, phenotypic analysis reveals the development of CD14hiCD11chiCD16+/−HLA-DRhiCD86dim cells in the presence of C1q versus CD14−CD11chiCD16−HLA-DRhiCD86hi cells, which develop without C1q addition. Taken together, the data support that C1q plays a fundamental role in regulating DC differentiation by favoring the development of specific precursors, such as the CD14hiCD11chiCD16+/− subsets, instead of fully committed iDCs.
Fig. 5.
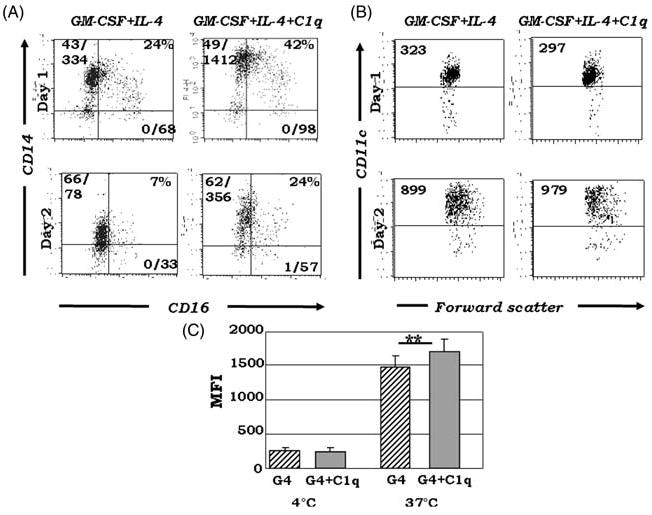
C1q promotes the development of distinct iDC subsets and increases phagocytic capacity of iDCs. Immature DCs were generated from PB cultured in the presence of GM-CSF + IL-4 ± 25 μg/ml C1q. (A) Multiparametric analysis reveals increased expansion of CD14hiCD16+/− cells on days 1 and 2 in the presence of C1q. These cells represent distinct subsets of iDCs. While CD14 expression was sustained until day 4, CD16 levels decreased by day 3 (data not shown). The numbers in the upper left and lower right quadrants of the plots represent percentage positive/MFI values of CD14 and CD16, respectively; the numbers in the upper right corners indicate percentage positive cells co-expressing CD14 and CD16 (n = 4). (B) There was little or no difference in MFI values with or without C1q on days 1 and 2, and 100% of the cells were CD11chi with C1q treatment. The numbers in the dot plots represent MFI values of CD11c. One typical experiment is shown; cells were gated on HLA-DR+ cells (n = 4). (C) C1q treatment significantly increased phagocytic uptake of fluorescein-labeled dextran on day 3. Monocytes were cultured for 3 days in the presence of GM-CSF + IL-4 (G4) ± 25 μg/ml C1q. On day 3, C1q-treated cells showed significantly increased endocytic capacity than cells cultured with DC growth factors alone (n = 7). *P <0.05 represents significant difference as compared to control.
Since iDCs are characterized by high endocytic capacity, we next sought to investigate the effects of C1q on receptor-mediated antigen uptake using fluorescently labeled dextran. Cells cultured both with and without C1q exhibited low basal dextran uptake at 4°C, and dextran uptake levels significantly increased when the cells were incubated at 37°C. Furthermore C1q treated cells exhibited significantly higher dextran uptake as compared to non-treated cells (Fig. 5C), suggesting that the presence of C1q affects the regulation of the endocytic and antigen presenting pathways in these cells.
Regulation of DC maturation by C1q may depend on the varied expression of C1q receptors
In Figure 1, we established the declining cell surface distribution and sustained release of C1q as monocytes transition into DCs. Exogenously supplied C1q altered differentiation events during this time frame (Figs 4 and 5). Based on our results, we postulated that extracellular C1q (including that derived from DC) might interact with DC-associated C1qRs to regulate DC differentiation. Therefore, in order to gain insight into which C1qR might be associated with the proposed role of C1q on DC growth during the monocyte-to-DC transition we analyzed the expression of two C1qRs: gC1qR and cC1qR on monocyte-DCs cultured in the presence of GM-CSF + IL-4. On day 0, nearly all monocytes expressed gC1qR (Fig 6B), while cC1qR was more variable within the population (Fig. 6A). Even though there was a modest reduction in gC1qR+ cells by day 4 (Fig. 6B), the percentage of cC1qR+ cells increased compared to day 0 (Fig. 6A). Furthermore, MFI analysis revealed that the amount of cC1qR was dramatically amplified after day 2 (Fig. 6C), whereas the amount gC1qR remained at relatively steady levels (Fig. 6D). Thus, at the precise period (∼day 3) corresponding to firm commitment to the DC lineage, there is an inverse correlation between gC1qR and cC1qR expression on the cell surface as detected by flow cytometry.
Fig. 6.

Varied expression of C1q receptors and specific binding orientation of surface bound C1q on monocyte-DC precursors may regulate DC differentiation events. Mononuclear cells cultured in the presence of GM-CSF + IL-4 were analyzed for the expression of cC1qR (A,C) and gC1qR (B,D) expression, and C1q binding orientation (E). (A) The percentage of cC1qR expression was variable on monocytes, but by day 2 nearly all monocyte-DCs had the receptor on their surface (n = 4). (B) On day 0, gC1qR was present on almost all the cells, and its expression was only slightly reduced by day 4 (n = 4). (C) Mean fluorescence analysis revealed that cC1qR expression was dramatically amplified by days 3 and 4 (n = 4). (D) Mean fluorescence intensity of gC1qR remained at relatively steady levels throughout the days (n = 4). (E) C1q is bound to the monocyte and DC surface via its globular head regions, while on M-CSF treated monocyte-macrophages its orientation is reversed. Binding orientation of C1q was determined using monoclonal antibodies specific to the globular head regions of C1q as well as polyclonal antibodies to the whole protein, and assessed by flow cytometry (n = 3). Experiments were gated on HLA-DR+ cells. *P<0.05, **P<0.01.
We next investigated whether the distinct pattern of C1qR expression was associated with the binding orientation of C1q on the surface of monocytes and iDCs. To this end, we utilized monoclonal antibodies specific to the globular head regions of C1q as well as polyclonal antibodies to the whole protein, and monitored C1q binding by flow cytometry. In agreement with our previous results (Fig. 1), pAb binding confirmed that fresh monocytes express C1q (>75%; Fig. 6E). Furthermore, compared to baseline C1q levels (day 0), M-CSF treated monocyte-macrophages sustained surface C1q expression, while in the presence of DC growth factors (GM-CSF + IL-4) C1q levels showed a 50% decrease by day 3 (Fig. 6E). In contrast, surface C1q was detected on monocytes and iDCs at much lower levels (2.5–10%) using mAbs specific to the globular heads, suggesting that C1q might be bound to these cells via its globular heads (Fig. 6E). In contrast, M-CSF treated monocyte-macrophages showed strong binding to the mAb, confirming that C1q is displayed on these cells with the globular regions exposed, as previously described.16 Taken together, these data suggest that the regulatory effects of C1q on DC differentiation and function depend on specific C1q/C1qR interactions. Additionally, cytokines that support innate immune functions (e.g. M-CSF) may also sustain C1q expression on monocytes.
Discussion
C1q belongs to the expanding TNF/C1q family of proteins that recognize injurious stimuli such as foreign antigens, cell debris and apoptotic cells, and regulate the innate immune system by mediating their removal by phagocytosis.41,42 Similar to the role of other innate immune system molecules, C1q can also induce inflammatory responses that are associated with adaptive immunity.43–45
The data presented in these studies show that monocyte-DC precursors express high levels of surface C1q during the monocyte-to-DC transition (days 1–2) until firm commitment to DC growth is achieved (day 3), while secreted C1q levels remain steady on days 1–4. Previous studies have shown that macrophages and maturing dendritic cells, but not monocytes, synthesize and express C1q.12,16 However, our results clearly show that fresh monocytes carry C1q on their surface even at day 0, when they have not been exposed to cytokines such as GM-CSF and/or IL-4. Indeed, what remains to be determined is whether monocytes, which are extraordinarily sensitive to in vitro manipulations, react to these stimuli by translocation of intracellular pool of C1q to the surface, or the C1q on the surface is normally carried by the circulating monocyte and used as a molecular sensor of ‘danger’. Even if the surface C1q is captured from the circulating pool of free C1q, it would still be interesting to know if this C1q would function as an ‘early warning’ molecular sensor. Our studies show that surface expression of C1q noted on day 0 could be sustained beyond day 3 when the precursors were cultured with a mono-macrophage specific growth factor (M-CSF), rather than with DC growth factors (GM-CSF + IL-4; Fig. 6E). This is consistent with previously published results showing that C1q is present on the surface of macrophages.16 The distinct pattern of extracellular C1q expression during the monocyte to DC transition (days 3–4; Fig. 1) is in agreement with previous observations (including our own unpublished data) demonstrating that C1q is detected on the cell surface and/or in cell-free supernatants of iDCs between days 5–7.46
It is well documented that, at different stages of development, DCs are specialized in either Ag capture/processing (iDC) or Ag presentation (mature DC). Our studies provide new insight into how a C1q/C1qR system could be envisioned to influence these activities during the critical innate/acquired immunity interface (day 0–3). The release of C1q at this point may act as an autocrine sensor of danger and would reflect the earliest ability of C1q to retrieve extracellular ‘danger’ Ag for re-entry into DC for Ag processing. Persistent release of C1q after the DC lineage is firmly established (days 5–7) correlates with the enhanced ability of iDCs to capture and process antigens in an inflammatory and/or infectious setting. Finally, the lack of C1q and C1qRs on fully mature DCs (as previously observed by us and others15) is consistent with their advancement into cells specialized in Ag presentation, rather than in Ag capture/processing.
In contrast to C1q released from iDCs, C1q on the monocyte surface would not function to retrieve Ag in the extracellular space. Instead, we propose that C1q on the surface of monocyte-DC precursors reflects an as yet unidentified built-in regulatory mechanism that sustains innate immune functions. Like the suppressive effects of C1q on T cells which act through gC1qR/C1q globular head interactions,20 we hypothesize that the regulatory effects of C1q on monocyte/DC precursors may occur via engagement of globular regions of C1q. Due to the swift nature of the monocyte-to-DC transition, regulatory effects of a C1q/C1qR system would occur within a narrow time frame and would be influenced by the micro-environment (steady state/infection/inflammation), as demonstrated for C1qR.12
Our data support the notion that altered distribution of C1q and C1qRs during the monocyte-to-DC transition leads to distinct C1q/C1qR interactions. The inverse relationship between cC1qR (up-regulated) and gC1qR (down-regulated) on the cell surface (Fig. 6) may also reflect preferential signaling through the receptors at distinct stages of DC growth. As outlined in an existing pro-inflammatory induction model, the globular heads of extracellular C1q bind to pathogen-associated molecular patterns (PAMPs) or self-derived immunogenic material, and thus the collagen tails remain available for stimulating cC1qR on the surface of phagocytic cells. cC1qR may then potentially initiate cell signaling via either CD9141,47 or other potential cell surface signaling partners. Such antigen-bound C1q is likely to be aggregated, which is optimal for induction of the pro-inflammatory response through cC1qR. A normal response to danger may involve up-regulation of cC1qR levels on iDC to ensure uptake of noxious agents utilizing the Ag-retrieving functions of C1q. In the context of inflammatory stimuli, DC maturation would ensue, allowing adaptive immune responses toward the initiating agent. During normal physiology, steady-state levels of C1q/C1qR on monocytes would resume once pathogen/danger has been cleared.
Although alternate explanations such as steric hindrance or inaccessibility of the antibody due to the molecular canopy of the cell surface have not been ruled out, our results using C1q domain-specific antibodies seem to show that the C1q on the monocyte surface is probably bound via the globular heads, presumably to gC1qR. This may, therefore, represent a steady-state immunoregulatory interaction. The question to be asked is: what is the significance of these observations and what functional difference would it make if C1q is bound by its head or tail? In autoimmune diseases such as SLE, where C1q deficiency is considered to predispose individuals to develop SLE,48,49 a disruption of C1q/gC1qR interactions including those on the monocyte-DC precursor might represent the loss of an important regulatory function. While we recognize the important role of C1q in the clearance of apoptotic cells, multiple alternate mechanisms are available for apoptotic uptake into iDCs. These include phosphatidylserine recognition, scavenger receptors, and β2-integrins.42 In light of these alternative mechanisms, the loss of antigen retrieving functions of C1q may not be a primary pathogenic event. Instead, a lack of C1q might result in dysregulated progression toward the DC lineage and inappropriate acquired (auto)immunity resulting in autoimmune diseases such as SLE.
The data presented here suggest that soluble C1q that is free of antigenic or apoptotic ‘cargo’ may serve as an autocrine signal that regulates differentiation of monocytes into DCs, as evidenced by retention of CD14 and reduced expression of DC maturation markers and co-stimulatory molecules (CD86, CD83). A possible explanation for the increased CD14 levels on C1q-treated monocyte–DCs is up-regulated gene expression or translation. Alternatively, since CD14 is a GPI-anchored molecule that is routinely shed into the extracellular milieu, the kinetics of shedding may be delayed or reduced. C1q did not operate by inducing selective apoptosis of CD14− DCs as is the case with IL-10,32 suggesting that C1q alters DC differentiation via a separate pathway than IL-10. The effect of C1q was rapid (within 1–2 days) and was most efficient during the earliest stages of DC growth. C1q induced the development of CD14hiCD11chiCD16+/− cells (Fig. 5). Intriguingly, a similar population of CD14hiCD16+ iDCs has recently been described to exhibit characteristics of both monocytes and DCs, possibly enabling these cells to activate innate, as well as adaptive, immune responses.9,50,51 Aside from elevated IL-12 and TNF-α production and high expression of T-cell stimulatory molecules, these cells displayed high phagocytic activity and retained CD14 on their surface, indicating signaling potential via monocyte- and macrophage-associated receptors.9,50,52 Interestingly, while there are physiological conditions in which this subset is expanded to >20%,52,53 the role of this subset in immunopathogenic events is unclear. We are currently investigating the biological function of these cells.
Evidence of the effects of C1q on monocyte-DC development so far has been conflicting. Some studies report that C1q promotes DC maturation when added to iDCs,30,54 while others describe decreased DC function in an MLR with C1q treatment.29 The differences in these observations may reflect distinct culture conditions and temporal analysis. For instance, soluble C1q, such as that employed in this study, may be expected to have very different effects on monocyte-DC development than immobilized C1q. Such fixed C1q might resemble C1q bound to immune complexes, various antigen or apoptotic debris, resulting in induction of DC maturation (possibly via cC1qR). Furthermore, our preliminary data indicate that the effects of C1q on monocyte-DCs are most pronounced when it is added to the cells from the outset (data not shown).
Conclusions
Based on the data presented here, we propose that the differential expression of receptors targeting functionally and structurally distinct regions of the C1q molecule during the monocyte–DC transition may determine the nature and specificity of the cells' response to C1q. Moreover, the distinct binding orientation of C1q on the cell surface of monocytes and iDCs further suggests that specific C1q/C1qR interactions may regulate cells as they transition from the monocyte state (innate immunity) toward the professional APC state (adaptive immunity).
Acknowledgments
This work was supported, in part, by grants from the National Institutes of Health (R01 AI 060866 to BG; and R03 AR05396, to FSS). It represents part of KKH's work, performed in partial fulfilment of the PhD degree in Genetics. The results were presented, in part, at the FASEB 2008 meeting, 5–9 April 2008, San Diego, CA. FSS and BG contributed equally in the supervision of this work. The authors thank Sylvia Samaniego for reading the manuscript.
Abbreviations
- DCs
dendritic cells
- iDC
immature DC
- C1qR
receptor for C1q
- gC1qR
receptor for the globular heads of C1q
- cC1qR
receptor for the collagen tail of C1q
- CR
calreticulin
- SLE
systemic lupus erythematosus
- PB
peripheral blood
- PAMPs
pathogen-associated molecular patterns
- MNC
mononuclear cell
- MHC
major histocompatibility complex
- G4
IL-4/GM-CSF
- HLA-DR
human leukocyte antigen class II, subregion DR
References
- 1.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 4.Reid SD, Penna G, Adorini L. The control of T cell responses by dendritic cell subsets. Curr Opin Immunol. 2000;12:114–121. doi: 10.1016/s0952-7915(99)00059-x. [DOI] [PubMed] [Google Scholar]
- 5.Santiago-Schwarz F, Anand P, Liu S, Carsons SE. Dendritic cells (DCs) in rheumatoid arthritis (RA): progenitor cells and soluble factors contained in RA synovial fluid yield a subset of myeloid DCs that preferentially activate Th1 inflammatory-type responses. J Immunol. 2001;167:1758–1768. doi: 10.4049/jimmunol.167.3.1758. [DOI] [PubMed] [Google Scholar]
- 6.Shortman K, Caux C. Dendritic cell development: multiple pathways to nature's adjuvants. Stem Cells. 1997;15:409–419. doi: 10.1002/stem.150409. [DOI] [PubMed] [Google Scholar]
- 7.MacDonald KP, Munster DJ, Clark GJ, Dzionek A, Schmitz J, Hart DN. Characterization of human blood dendritic cell subsets. Blood. 2002;100:4512–4520. doi: 10.1182/blood-2001-11-0097. [DOI] [PubMed] [Google Scholar]
- 8.Steinman RM. Dendritic cells: understanding immunogenicity. Eur J Immunol. 2007;37(Suppl 1):S53–S60. doi: 10.1002/eji.200737400. [DOI] [PubMed] [Google Scholar]
- 9.Grage-Griebenow E, Flad HD, Ernst M. Heterogeneity of human peripheral blood monocyte subsets. J Leukoc Biol. 2001;69:11–20. [PubMed] [Google Scholar]
- 10.Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM, Munz C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med. 2002;195:343–351. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santiago-Schwarz F. Dendritic cells: friend or foe in autoimmunity? Rheum Dis Clin North Am. 2004;30:115–134. doi: 10.1016/S0889-857X(03)00108-X. [DOI] [PubMed] [Google Scholar]
- 12.Vegh Z, Goyarts EC, Rozengarten K, Mazumder A, Ghebrehiwet B. Maturation-dependent expression of C1q-binding proteins on the cell surface of human monocyte-derived dendritic cells. Int Immunopharmacol. 2003;3:345–357. doi: 10.1016/S1567-5769(02)00234-5. [DOI] [PubMed] [Google Scholar]
- 13.Bajtay Z, Csomor E, Sandor N, Erdei A. Expression and role of Fc- and complement-receptors on human dendritic cells. Immunol Lett. 2006;104:46–52. doi: 10.1016/j.imlet.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 14.Reis ES, Barbuto JA, Isaac L. Complement components, regulators and receptors are produced by human monocyte-derived dendritic cells. Immunobiology. 2007;212:151–157. doi: 10.1016/j.imbio.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Castellano G, Woltman AM, Nauta AJ, et al. Maturation of dendritic cells abrogates C1q production in vivo and in vitro. Blood. 2004;103:3813–3820. doi: 10.1182/blood-2003-09-3046. [DOI] [PubMed] [Google Scholar]
- 16.Kaul M, Loos M. Expression of membrane C1q in human monocyte-derived macrophages is developmentally regulated and enhanced by interferon-gamma. FEBS Lett. 2001;500:91–98. doi: 10.1016/s0014-5793(01)02592-3. [DOI] [PubMed] [Google Scholar]
- 17.Tacnet P, Cheong EC, Goeltz P, et al. Trimeric reassembly of the globular domain of human C1q. Biochim Biophys Acta. 2008;1784:518–529. doi: 10.1016/j.bbapap.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen A, Gaddipati S, Hong Y, Volkman DJ, Peerschke EI, Ghebrehiwet B. Human T cells express specific binding sites for C1q. Role in T cell activation and proliferation. J Immunol. 1994;153:1430–1440. [PubMed] [Google Scholar]
- 19.Kittlesen DJ, Chianese-Bullock KA, Yao ZQ, Braciale TJ, Hahn YS. Interaction between complement receptor gC1qR and hepatitis C virus core protein inhibits T-lymphocyte proliferation. J Clin Invest. 2000;106:1239–1249. doi: 10.1172/JCI10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao ZQ, Nguyen DT, Hiotellis AI, Hahn YS. Hepatitis C virus core protein inhibits human T lymphocyte responses by a complement-dependent regulatory pathway. J Immunol. 2001;167:5264–5272. doi: 10.4049/jimmunol.167.9.5264. [DOI] [PubMed] [Google Scholar]
- 21.Yao ZQ, Ray S, Eisen-Vandervelde A, Waggoner S, Hahn YS. Hepatitis C virus: immunosuppression by complement regulatory pathway. Viral Immunol. 2001;14:277–295. doi: 10.1089/08828240152716547. [DOI] [PubMed] [Google Scholar]
- 22.Kanto T, Hayashi N, Takehara T, et al. Impaired allostimulatory capacity of peripheral blood dendritic cells recovered from hepatitis C virus-infected individuals. J Immunol. 1999;162:5584–5591. [PubMed] [Google Scholar]
- 23.Ghebrehiwet B, Peerschke EI. cC1q-R (calreticulin) and gC1q-R/p33: ubiquitously expressed multi-ligand binding cellular proteins involved in inflammation and infection. Mol Immunol. 2004;41:173–183. doi: 10.1016/j.molimm.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 24.Sim RB, Moestrup SK, Stuart GR, et al. Interaction of C1q and the collectins with the potential receptors calreticulin (cC1qR/collectin receptor) and megalin. Immunobiology. 1998;199:208–224. doi: 10.1016/s0171-2985(98)80028-4. [DOI] [PubMed] [Google Scholar]
- 25.Ghebrehiwet B, Lim BL, Kumar R, Feng X, Peerschke EI. gC1q-R/p33, a member of a new class of multifunctional and multicompartmental cellular proteins, is involved in inflammation and infection. Immunol Rev. 2001;180:65–77. doi: 10.1034/j.1600-065x.2001.1800106.x. [DOI] [PubMed] [Google Scholar]
- 26.Peerschke EI, Ghebrehiwet B. The contribution of gC1qR/p33 in infection and inflammation. Immunobiology. 2007;212:333–342. doi: 10.1016/j.imbio.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng X, Tonnesen MG, Peerschke EI, Ghebrehiwet B. Cooperation of C1q receptors and integrins in C1q-mediated endothelial cell adhesion and spreading. J Immunol. 2002;168:2441–2448. doi: 10.4049/jimmunol.168.5.2441. [DOI] [PubMed] [Google Scholar]
- 28.Bohlson SS, Fraser DA, Tenner AJ. Complement proteins C1q and MBL are pattern recognition molecules that signal immediate and long-term protective immune functions. Mol Immunol. 2007;44:33–43. doi: 10.1016/j.molimm.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 29.Castellano G, Woltman MA, Schlagwein N, et al. Immune modulation of human dendritic cells by complement. Eur J Immunol. 2007;37:2803–2811. doi: 10.1002/eji.200636845. [DOI] [PubMed] [Google Scholar]
- 30.Csomor E, Bajtay Z, Sandor N, et al. Complement protein C1q induces maturation of human dendritic cells. Mol Immunol. 2007;44:3389–3397. doi: 10.1016/j.molimm.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 31.Baruah P, Dumitriu IE, Malik TH, et al. C1q enhances IFN-gamma production by antigen specific T cells via the CD40 co-stimulatory pathway on dendritic cells. Blood. 2009;113:3485–3493. doi: 10.1182/blood-2008-06-164392. [DOI] [PubMed] [Google Scholar]
- 32.Chang WL, Baumgarth N, Eberhardt MK, et al. Exposure of myeloid dendritic cells to exogenous or endogenous IL-10 during maturation determines their longevity. J Immunol. 2007;178:7794–7804. doi: 10.4049/jimmunol.178.12.7794. [DOI] [PubMed] [Google Scholar]
- 33.Santiago-Schwarz F. Positive and negative regulation of the myeloid dendritic cell lineage. J Leukoc Biol. 1999;66:209–216. doi: 10.1002/jlb.66.2.209. [DOI] [PubMed] [Google Scholar]
- 34.Ghebrehiwet B, Lu PD, Zhang W, et al. Identification of functional domains on gC1Q-R, a cell surface protein that binds to the globular ‘heads’ of C1Q, using monoclonal antibodies and synthetic peptides. Hybridoma. 1996;15:333–342. doi: 10.1089/hyb.1996.15.333. [DOI] [PubMed] [Google Scholar]
- 35.Santiago-Schwarz F, Tucci J, Carsons SE. Endogenously produced interleukin 6 is an accessory cytokine for dendritic cell hematopoiesis. Stem Cells. 1996;14:225–231. doi: 10.1002/stem.140225. [DOI] [PubMed] [Google Scholar]
- 36.Almeida J, Bueno C, Alguero MC, et al. Comparative analysis of the morphological, cytochemical, immunophenotypical, and functional characteristics of normal human peripheral blood lineage(−)/CD16(+)/HLA-DR(+)/CD14(−/lo) cells, CD14(+) monocytes, and CD16(−) dendritic cells. Clin Immunol. 2001;100:325–338. doi: 10.1006/clim.2001.5072. [DOI] [PubMed] [Google Scholar]
- 37.Ancuta P, Weiss L, Haeffner-Cavaillon N. CD14+CD16++ cells derived in vitro from peripheral blood monocytes exhibit phenotypic and functional dendritic cell-like characteristics. Eur J Immunol. 2000;30:1872–1883. doi: 10.1002/1521-4141(200007)30:7<1872::AID-IMMU1872>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 38.Avila-Moreno F, Lopez-Gonzalez JS, Galindo-Rodriguez G, Prado-Garcia H, Bajana S, Sanchez-Torres C. Lung squamous cell carcinoma and adenocarcinoma cell lines use different mediators to induce comparable phenotypic and functional changes in human monocyte-derived dendritic cells. Cancer Immunol Immunother. 2006;55:598–611. doi: 10.1007/s00262-005-0060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rivas-Carvalho A, Meraz-Rios MA, Santos-Argumedo L, Bajana S, Soldevila G, Moreno-Garcia ME, Sanchez-Torres C. CD16+ human monocyte-derived dendritic cells matured with different and unrelated stimuli promote similar allogeneic Th2 responses: regulation by pro- and anti-inflammatory cytokines. Int Immunol. 2004;16:1251–1263. doi: 10.1093/intimm/dxh127. [DOI] [PubMed] [Google Scholar]
- 40.Schlitt A, Heine GH, Blankenberg S, et al. CD14+CD16+ monocytes in coronary artery disease and their relationship to serum TNF-alpha levels. Thromb Haemost. 2004;92:419–424. doi: 10.1160/TH04-02-0095. [DOI] [PubMed] [Google Scholar]
- 41.Paidassi H, Tacnet-Delorme P, Garlatti V, et al. C1q binds phosphatidylserine and likely acts as a multiligand-bridging molecule in apoptotic cell recognition. J Immunol. 2008;180:2329–2338. doi: 10.4049/jimmunol.180.4.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maniati E, Potter P, Rogers NJ, Morley BJ. Control of apoptosis in autoimmunity. J Pathol. 2008;214:190–198. doi: 10.1002/path.2270. [DOI] [PubMed] [Google Scholar]
- 43.Kishore U, Gaboriaud C, Waters P, et al. C1q and tumor necrosis factor superfamily: modularity and versatility. Trends Immunol. 2004;25:551–561. doi: 10.1016/j.it.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 44.Wallach D, Varfolomeev EE, Malinin NL, Goltsev YV, Kovalenko AV, Boldin MP. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu Rev Immunol. 1999;17:331–367. doi: 10.1146/annurev.immunol.17.1.331. [DOI] [PubMed] [Google Scholar]
- 45.Fraser DA, Bohlson SS, Jasinskiene N, et al. C1q and MBL, components of the innate immune system, influence monocyte cytokine expression. J Leukoc Biol. 2006;80:107–116. doi: 10.1189/jlb.1105683. [DOI] [PubMed] [Google Scholar]
- 46.Castellano G, Woltman AM, Schlagwein N, et al. Immune modulation of human dendritic cells by complement. Eur J Immunol. 2007;37:2803–2811. doi: 10.1002/eji.200636845. [DOI] [PubMed] [Google Scholar]
- 47.Trouw LA, Blom AM, Gasque P. Role of complement and complement regulators in the removal of apoptotic cells. Mol Immunol. 2008;45:1199–1207. doi: 10.1016/j.molimm.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 48.Walport MJ, Davies KA, Botto M. C1q and systemic lupus erythematosus. Immunobiology. 1998;199:265–285. doi: 10.1016/S0171-2985(98)80032-6. [DOI] [PubMed] [Google Scholar]
- 49.Ghebrehiwet B, Peerschke EI. Role of C1q and C1q receptors in the pathogenesis of systemic lupus erythematosus. Curr Dir Autoimmun. 2004;7:87–97. doi: 10.1159/000075688. [DOI] [PubMed] [Google Scholar]
- 50.Grage-Griebenow E, Zawatzky R, Kahlert H, Brade L, Flad H, Ernst M. Identification of a novel dendritic cell-like subset of CD64(+)/CD16(+) blood monocytes. Eur J Immunol. 2001;31:48–56. doi: 10.1002/1521-4141(200101)31:1<48::aid-immu48>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 51.Passlick B, Flieger D, Ziegler-Heitbrock HW. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood. 1989;74:2527–2534. [PubMed] [Google Scholar]
- 52.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol. 2007;81:584–592. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 53.Scherberich JE, Nockher WA. CD14++ monocytes, CD14+/CD16+ subset and soluble CD14 as biological markers of inflammatory systemic diseases and monitoring immunosuppressive therapy. Clin Chem Lab Med. 1999;37:209–213. doi: 10.1515/CCLM.1999.039. [DOI] [PubMed] [Google Scholar]
- 54.Gardai SJ, Xiao YQ, Dickinson M, et al. By binding SIRPalpha or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell. 2003;115:13–23. doi: 10.1016/s0092-8674(03)00758-x. [DOI] [PubMed] [Google Scholar]


