Abstract
Alkylating agents, topoisomerase II inhibitors, ionizing radiation, and other hematotoxins induce DNA damage in hematopoietic stem cells that results in lesions such as balanced and unbalanced chromosome rearrangements, −5/del(5q) and/or −7/del(7q), as well as other submicroscopic genetic lesions. Together with epigenetic alterations, these result in dysplasia, clonal expansion, and ultimately myeloid leukemia. Combinations of lesions are required to induce overt leukemia. Altering a small subset of signaling pathways leads to disruption of normal self-renewal, proliferation, differentiation, and apoptotic mechanisms that control the development of hematopoietic stem cells and their differentiation into mature effector cells. Recent studies have shown that cytogenetically normal (CN-) AML is quite heterogeneous at the molecular level. Patients with CN-AML harboring mutations in NPM1, FLT3, CEBPA, WT1 or expressing high levels of BAALC, ERG, or MN1 have distinctly different clinical outcomes. NPM1 mutations are independently associated with higher remission rates and longer disease-free and overall survival in AML. Copy number alterations (CNA) are deletions or amplifications of single genes. CNAs have been found at the breakpoints of known chromosomal translocations. Fewer CNAs have been detected in AML than in pediatric ALL. MicroRNAs (miRs) are non-coding small RNA molecules containing about 22 nucleotides that are typically encoded within introns. They hybridize to complementary mRNA targets and modulate protein expression by inhibiting translation and/or inducing degradation of target messenger RNAs. This new class of genes has recently been shown to play a pivotal role in malignant transformation. miRs are down-regulated in many tumors and thus appear to function as tumor suppressor genes. Distinctive genome-wide miR expression profiles have been associated with different subsets of AML. A miR signature that is associated with clinical outcome in patients with high-risk molecular features of AML (those who have FLT3-ITD or wild-type NPM1) has been reported. This subgroup constitutes approximately 65% of patients with CN-AML and one-third of all patients with AML < 60 years old. Down-regulation of the miR-181 family contributes to an aggressive leukemia phenotype through mechanisms associated with the activation of pathways of innate immunity mediated by toll-like receptors and interleukin-1β.
INTRODUCTION
Current concepts seeking to explain the etiology of acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) begin with hematotoxins that induce DNA damage in hematopoietic stem cells. Alkylating agents, topoisomerase II inhibitors, ionizing radiation, and environmental factors induce DNA damage that results in lesions such as balanced and unbalanced chromosomal rearrangements, −5/del(5q) and/or −7/del(7q), as well as other submicroscopic genetic lesions. Together with epigenetic alterations, these result in dysplasia, clonal expansion, and ultimately myeloid leukemia. It is hypothesized that environmental factors such as benzene, smoking, and radiation exposure act through similar pathways to generate leukemia. It appears that combinations of lesions are required to induce overt leukemia. Altering a small but variable subset of signaling pathways leads to disruption of normal self renewal, proliferation, differentiation, and apoptotic mechanisms that control the development of hematopoietic stem cells and their differentiation into mature effector cells. The heterogeneous clinical and pathological features of AML result from these multiple different pathways and combinations of genetic lesions. Will a better understanding of the clinical phenotypes and biological features of AML provide insight into the etiology of AML?
A variety of genomic aberrations leading to malignancy have been identified in leukemia as well as other cancers. These include a change in the DNA sequence through single gene mutations or chromosomal translocations as well as copy number alterations (CNA). CNAs are deletions or amplifications of single genes. Recent studies have shown that cytogenetically normal (CN-) AML is quite heterogeneous at the molecular level.(1) Patients with CN-AML harboring mutations in NPM1, FLT3, CEBPα, or WT1 genes, or expressing high levels of BAALC, ERG, or MN1 have distinctly different outcomes.(2-9) NPM1 mutations, for example, are independently associated with higher remission rates and longer disease-free and overall survival in AML across the entire adult age spectrum.(2,10-14) Additional genomic alterations leading to malignancy include changes in the organization of the genome through epigenetic methylation or alterations in chromatin organization by histone acetylation. Epigenetic changes likely play an important role in AML, working in concert with genetic alterations to alter a wider range of biological processes to induce overt leukemia.
It has been known for several decades that specific chromosomal abnormalities are recurrent in human AML and are in some cases closely associated with characteristic morphology, response to specific therapeutic agents, and outcome.(15,16) Balanced translocations and other structural rearrangements appear to arise in early myeloid progenitor cells. The t(8;21), for example, identifies a subset of AML characterized by increased myeloblasts with partial maturation in the granulocytic lineage (subtype M2). Auer rods are usually observed together with characteristic cytologic features. Acute myelomonocytic leukemia with abnormal eosinophils (subtype M4Eo) is characterized by an inv(16) or t(16;16). This malignant process involves both monocyte and granulocyte lineages including eosinophils. Some monoblastic leukemias are characterized by balanced translocations involving chromosome band 11q23 and the MLL gene. The genes involved at the chromosome breakpoints in most of these translocations or inversions have been identified.(16) In about 20% of newly diagnosed AML cases, no detectable cytogenetic abnormality is found. These cytogenetically normal cases have been the focus of intense investigation looking for submicroscopic genetic lesions.
Because of its rapid proliferative rate, AML typically presents earlier in its natural history than more slowing growing epithelial tumors. The large number of mutations frequently observed within cancer cells may reflect inherent genomic instability or multiple mutational events over a prolonged tumor lifespan. In contrast, the development of AML may require fewer genetic alterations than other cancers, and a limited number of biological processes may need to be altered in hematopoietic stem cells or committed myeloid progenitors to convert them from a normal cell into a myeloid leukemia cell.
COPY NUMBER ALTERATIONS
Studies of genetic variation in humans have revealed an abundance of submicroscopic copy number variation of DNA segments ranging from kilobases (kb) to megabases (Mb) in size.(17) These consist of deletions, insertions, duplications, and complex multi-site variants and are collectively termed copy number variations (CNVs) or copy number polymorphisms. They are found in humans and in all other mammals that have been examined. A CNV can be simple, such as a tandem duplication, or it may involve complex gains or losses of homologous sequences at multiple sites in the genome. CNVs influence normal gene expression and phenotypic variation by disrupting genes and altering gene dosage. They can also cause disease.
Acquired copy number alterations (CNAs) have been found at the break points of known chromosomal translocations. High resolution genome-wide copy number analysis has identified genes that are the target of cryptic chromosomal translocations not visible by light microscopy. Simultaneous analysis of both tumor and corresponding germline DNA has revealed large numbers of CNAs in pediatric ALL.(18) Both precursor-B and precursor-T cell ALL have recurring CNAs that target genes regulating lymphocyte development, tumor suppression, cell cycle regulation, apoptosis, signaling, and responsiveness to drug therapy.
In work done at Washington University in St. Louis by Walter and colleagues, 86 adult patients with de novo AML were studied with paired bone marrow leukemia samples and normal skin DNA samples.(19) The paired samples enabled acquired CNAs to be distinguished from inherited copy number variations. All together, 201 acquired CNAs were identified in the 86 AML genomes using single nucleotide polymorphism (SNP) arrays. These occurred in 38 of the 86 AML genomes. There was a mean of 2.34 CNAs per AML genome (range, 0–30; median, 0). Deletions were more common than amplifications (1.23 to 1). The 201 CNAs were distributed across all morphological subtypes of AML although FAB-M6 and M7 subtypes contain significantly more alterations per genome compared with all other subtypes (mean of 21 vs 1.4; p=0.02).
Of the 201 alterations, 125 (62%) corresponded to changes also detected by cytogenetics, and 76 (38%) were detected by SNP array only. Thirty-two of these were less than 1 Mb in size. Of the 201 alterations, 198 (99%) contained known genes, and 154 loci (77%) contained at least one gene that had previously been associated with cancer and/or AML or MDS. There were 12 chromosomal regions (8 deletions and 4 amplifications) that were significantly altered in multiple AML genomes. Most of these regions contained at least one gene previously implicated in cancer and/or AML or MDS. The total number of alterations per patient was not predictive of event-free or overall survival independent of cytogenetic classification. Known cancer and leukemia-related genes were significantly enriched in the CNA loci, and their mRNA expression levels were often altered, suggesting that these genes may contribute to AML pathogenesis. Fifty-one recurrent alterations less than 5 Mb in size in the 86 genomes and 3 recurrent alteration regions less than 210 Kb in size in an independent set of 38 AML genomes were identified. Copy-neutral loss of heterozygosity occurred infrequently in AML (only 7 of the 86 genomes) but was more common in CN-AML.
CYTOGENETICALLY NORMAL AML
Gene expression arrays measuring messenger RNA transcripts clearly indicate that AML is a heterogeneous disorder at the molecular level. However, within cytogenetic or molecular subsets, there is considerable homogeneity in biological and clinical features. A variety of genetic and epigenetic events are probably responsible for the heterogeneity.
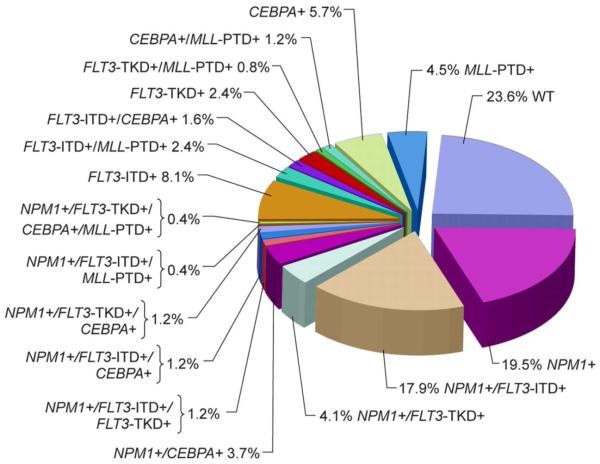
This is well illustrated by evaluating specific mutations detected in CN-AML, a subtype that accounts for about 20% of all de novo AML. Several large cohorts of newly diagnosed patients with CN-AML have been studied by a number of researchers.(1,3,7-9,20) The frequency of detection of mutations in NPM1, FLT3, CEBPα and MLL are shown in Figure 1 from a large series reported by the Cancer and Leukemia Group B (CALGB).(1) Only 24% of patients in this cohort had no detectable mutation in any of these genes (i.e. wild-type alleles). Schlenk and colleagues in Germany have reported a similar frequency of these gene mutations.(3) Among 438 newly diagnosed AML patients, hypothetical class II mutations that are thought to impair differentiation were seen in 54% with an NPM1 mutation, 14% with a CEBPα mutation, and 7% with MLL partial tandem duplication. Class I mutations believed to confer a proliferative advantage were seen in 32% of patients with a FLT3 internal tandem duplication (FLT3-ITD), 12% with a FLT3 tyrosine kinase domain mutation (FLT3-TKD), and 14% with an NRAS mutation. Of interest, FLT3 mutations sometimes occur in combination with NPM1 mutations in the same patient, but CEBPα mutations were rarely observed in combination with FLT3 mutations.(3,6,7) These genotypes are predictive of relapse-free survival and overall survival with high statistical significance. The most favorable outcomes have been observed in patients with a NPM1 mutation as a sole abnormality or a mutant CEBPα, either alone or with other abnormalities.
Figure 1. Mutations of NPM1, FLT3, CEBPα, and MLL in cytogenetically normal AML patients studied by the Cancer and Leukemia Group B (1).
A “molecular high-risk” subset of CN-AML has been defined to be those patients either with a FLT3 mutation or with wild-type NPM1 alleles (NPM1-wt) and no other abnormality.(7,21,22) However, in work published by Marcucci and colleagues in the CALGB, the combined presence of a CEBPα mutation in this molecular high-risk subset is associated with a favorable outcome to treatment.(7) In multivariable analysis, the hazard rate for CEBPα mutated AML patients for event-free survival was 0.3 (p < 0.001). Patients with a CEBPα mutation were observed to have a unique gene signature among the molecular high-risk CN-AML patients. The overall survival of 29 CEBPα mutation patients was 60% at 5 years compared to only 25% for 86 patients with wild-type CEBPα within the molecular high-risk subset. These investigators also reported a unique micro-RNA expression signature associated with CEBPα mutational status in these patients.
MICRO-RNAS
Micro-RNAs (miRs) constitute a newly discovered pathway that regulates gene expression and tissue development and acts as a regulator of malignant and normal hematopoiesis. The Nobel Prize was awarded to Fire and Mello in 2006 for their identification of RNA interference, the mechanism of suppression of gene expression by RNA molecules. miRs are short, noncoding, single-stranded RNAs of about 22 nucleotides that regulate an estimated 30% of human genes. They are a naturally occurring form of RNA interference. They were first discovered in C. elegans in about 1993. They are highly conserved in mammals. Some are expressed ubiquitously and some are tissue restricted. More than 500 have been identified so far, and each miR may target several hundred genes due to imperfect base pairing and binding to different regions. miRs are encoded throughout the genome, often in introns.
miRs guide the RNA-induced silencing complex (RISC) onto the 3′ untranslated regions of target messenger RNAs by complimentary base pairing of at least 6–8 nucleotides. This acts to destabilize the mRNA or block its translation. The net effect is to reduce protein synthesis from the target mRNAs. More extensive base pairing favors mRNA cleavage with near complete loss of gene expression via mRNA loss. This provides one explanation for the lack of correlation between mRNA expression and protein levels. This micro-regulation is critical for normal tissue function and development, and misregulation may contribute to cancer. Some miRs are controlled by epigenetic alterations such as DNA methylation and histone modification. Activation of tumor suppressor miRs by chromatin modifying drugs may lead to downregulation of target oncogenes and could be a novel strategy for the treatment of cancer.(23)
Micro-RNA expression profiles have been shown to classify human cancers by Lu and colleagues and by others.(24,25) They found distinct micro-RNA profiles for different tumor types. Micro-RNA expression patterns reflect the developmental origin of the tumor. The micro-RNA profile can be better than the mRNA profile for classification. Specific micro-RNA signatures correlate with clinical features such as ALL vs AML, or normal blood cells vs malignant cells.(25) They provide mechanistic insights by identifying key oncogenic pathways. Many major questions exist for micro-RNA biology. First, what is their role in normal tissue differentiation and hematopoiesis? Secondly, what is their role in cancer, especially leukemias and lymphomas? Thirdly, what are the environmental influences on micro-RNAs? What regulates the production of specific micro-RNAs? What are the critical targets for each micro-RNA? Specific micro-RNA signatures may be prognostic and may elucidate new treatment strategies.
This new class of genes has recently been shown to play a pivotal role in malignant transformation.(26) miRs may act as oncogenes. However, miRs are down-regulated in many tumors and thus appear to function as tumor suppressor genes (Table 1). The BCL2 oncogene is targeted by miR-15a and miR-16. Genome-wide miR expression profiles have been associated with different subsets of AML. A miR signature that is associated with clinical outcome in patients with molecular high-risk features of AML (those who have FLT3-ITD or wild-type NPM1 or both) has been reported.(21) This high-risk subgroup constitutes approximately 65% of patients with CN-AML and one-third of all patients with AML less than 60 years old.
Table 1. Aberrantly expressed miRNAs in AML (26).
| miRNA | Location | Function | Targets |
|---|---|---|---|
| miR-155 | 21q21.3 | Oncogene |
AGTR1, FGF7,
ZNF537, ZIC3, IKBKE |
| miR-181a | 1q31.3 (miR-181a-1) | TSG |
BCL2, TLR4, CARD8,
CASP1 |
| 9q33.3 (miR-181a-2) | TSG |
IL1B, SKC11A1,
MSR1M, CD64 |
|
| miR-181b | 1q31.3 (miR-181b-1) | TSG |
TCL1, TLR4, CARD8,
CASP1 |
| 9q33.3 (miR-181b-2) | TSG |
IL1B, SLC11A1,
MSR1, CD64 |
|
| Let-7b | 22q13.31 | Oncogene/TSG | RAS |
| miR-223 | Xq12 | Oncogene | N/A |
| miR-204 | 9q21.11 | TSG | HOXA10, MEISI |
| miR-34b | 11q23.1 | TSG | CREB |
TSG denotes tumor suppressor gene.
Modified from Fanini et al (26).
Recently, Schwind and colleagues from the CALGB reported on miR-181a expression in 187 de novo CN-AML patients < 60 years old.(22) They were enrolled prospectively and treated on CALGB protocols 9621 and 19808 with standard daunorubicin, cytarabine, and etoposide therapy. One hundred twenty-two patients had molecular high-risk AML with a FLT3 mutation or NPM1 wild-type genotype. Sixty-five patients had molecular low-risk AML, defined as having an NPM1 mutation but no mutation in FLT3. They found that higher miR-181a expression was significantly associated with a higher percentage of blasts in the blood, M1/M2 phenotype, no extramedullary disease, higher hemoglobin levels at diagnosis, CEPBα mutation, NPM1 wild-type alleles, and low expression levels of ERG and BAALC. Higher miR-181a expression was associated with a favorable outcome, including achievement of complete remission (CR), and prolonged disease-free and overall survival (Table 2). Among the 122 patients with molecular high-risk AML, the survival for 68 patients with high miR-181a expression was 50% at five years compared to 20% for the 54 patients with low miR-181a expression. In multivariable analysis, higher miR-181a expression used as a continuous variable independently predicted outcome with higher CR rates and longer disease-free and overall survival. These data suggest a role of miR-181a in determining the aggressiveness of the disease phenotype. There was no significant association between miR-181a expression and outcome in the molecular low risk CN-AML patients.
Table 2. Association of clinical outcomes with microRNA-181a expression in 187 patients with CN-AML <60 years old (22).
| Outcome | Higher expression | Lower expression | P |
|---|---|---|---|
| Compete remission | 84% | 72% | 0.07 |
| 5-year Disease-free survival | 43% | 18% | 0.05 |
| 5-year Survival | 48% | 19% | 0.01 |
For descriptive purposes, the percentages are reported for patients dichotomized at the median, but miR-181a expression was analyzed statistically as a continuous variable.
Is miR-181a a tumor suppressor? Can small molecule therapeutics increase miR-181a expression in AML blasts? It is known that increased miR-181a expression leads to decreased expression of Toll-like Receptor 4 (TLR4) and Interleukin-1β (IL1β), genes associated with the innate immune system.(21) This results in decreased NFκB expression and decreased miR-155 expression, which in turn leads to decreased proliferation and decreased survival of neutrophils and monocytes. Conversely, decreased miR-181a expression leads to increased miR-155 expression. Higher expression of miR-155 is strongly and independently associated with FLT3-ITD mutations.(27) Agents that increase miR-181a expression induce apoptosis of AML blasts.
NPM1 GENE MUTATION, EXPRESSION ARRAYS, AND CLINICAL OUTCOME
The nucleophosmin gene (NPM1) is located on chromosome 5q35.1 and on rare occasion is rearranged by translocations involving this chromosome band. It encodes a nucleocytoplasmic shuttling protein.(2,10) The wild-type protein remains in the nucleus. The mutated protein is found in the cytoplasm, due in part to loss of a nucleolar localization signal and the introduction of a novel nuclear export signal by a recurring mutation usually involving exon 12. It has been hypothesized that NPM1 is a haplo-insufficient tumor suppressor since NPM1+/- mice develop an MDS-like syndrome.
AML with a mutated NPM1 gene is provisional entity in the 2008 WHO classification of leukemia.(11-14, 16) This is the most common recurring genetic lesion in AML. Its prevalence increases with age: 2–8% in children, and 27–35% in adults. About 50% – 60% of adult CN-AML have NPM1 mutations. These cases are usually de novo AML, and there is a female predominance. Although mutated NPM1 was reported in 10 of 140 t-MN cases, these cases lacked the more typical features of therapy-related disease.(28) NPM1 mutated AML most often has myelomonocytic features; 80–90% of monocytic AML have NPM1 mutations. CD34 is generally not expressed. Importantly, about 40% of AML with NPM1 mutations also have FLT3- ITD mutations, and thus fall into the molecular high-risk category.
Investigators in the CALGB recently reported on the significance of NPM1 mutation in 148 adults aged ≥60 years with de novo CN-AML.(11) All of these patients were enrolled prospectively and treated on CALGB protocols 9720 and 10201, using standard daunorubicin and cytarabine chemotherapy regimens.(29,30) Fifty-six percent of patients had NPM1 mutations. Their clinical outcomes are shown in Table 3. In multivariable analyses, NPM1 mutations remained independent predictors for higher CR rates (P<0.001), longer DFS (P=0.004), and longer OS (P<0.001), after adjustment for other prognostic clinical and molecular variables. Unique NPM1 mutation-associated gene-expression and microRNA-expression signatures were identified, and these were age-independent. Genetic changes were characterized by up-regulation of HOX genes and their embedded microRNAs, and downregulation of the prognostically adverse MN1, BAALC, and ERG genes.
Table 3. Association of clinical outcomes with NPM1 mutation status in 148 older adults with de novo CN-AML (11).
| Outcomes | NPM1 mutated | NPM1 wild-type | P |
|---|---|---|---|
| Complete remission | 84% | 48% | <0.001 |
| 3-year Disease-free survival | 23% | 10% | 0.047 |
| 3-year Survival | 35% | 8% | <0.001 |
AML is a heterogeneous syndrome made up of genetically homogeneous diseases. Studying the phenotype and genotype of overt leukemia may provide clues as to the initiating events for malignant transformation. Environmental factors impacting on hematopoietic stem cells lead to chromosomal rearrangements, gene copy number alterations, single gene mutations, and dysregulation of microRNAs. Each of these events, acting alone or in combination, may contribute to the initiation a myeloid neoplasm. Some of these pathways may be susceptible to therapeutic interventions to prevent AML or to treat AML.
ACKNOWLEDGEMENTS
I thank Dr. Michelle M. Le Beau at the University of Chicago for helpful discussions and my many colleagues in the CALGB for their collaborative translational research on AML.
Supported in part by NCI grants CA14599, CA 31946, and CA40046.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Mrózek K, Marcucci G, Paschka P, et al. Clinical relevance of mutations and gene-expression changes in adult acute myeloid leukemia with normal cytogenetics: Are we ready for a prognostically prioritized molecular classification? Blood. 2007;109:431–448. doi: 10.1182/blood-2006-06-001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falini B, Mecucci C, Tiacci E, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med. 2005;352:254–266. doi: 10.1056/NEJMoa041974. [DOI] [PubMed] [Google Scholar]
- 3.Schlenk RF, Döhner K, Krauter J, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1909–1918. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 4.Whitman SP, Ruppert AS, Radmacher MD, et al. FLT3 D835/I836 mutations are associated with poor disease-free survival and a distinct gene-expression signature among younger adults with de novo cytogenetically normal acute myeloid leukemia lacking FLT3 internal tandem duplications. Blood. 2008;111:1552–1559. doi: 10.1182/blood-2007-08-107946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: Association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99:4326–4335. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- 6.Gale RE, Green C, Allen C, et al. The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood. 2008;111:2776–2784. doi: 10.1182/blood-2007-08-109090. [DOI] [PubMed] [Google Scholar]
- 7.Marcucci G, Maharry K, Radmacher MD, et al. Prognostic significance of, and gene and microRNA expression signatures associated with, CEBPA mutations in cytogenetically normal acute myeloid leukemia with high-risk molecular features: A Cancer and Leukemia Group B study. J Clin Oncol. 2008;26:5078–5087. doi: 10.1200/JCO.2008.17.5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paschka P, Marcucci G, Ruppert AS, et al. Wilms’ tumor 1 gene mutations independently predict poor outcome in adults with cytogenetically normal acute myeloid leukemia: A Cancer and Leukemia Group B study. J Clin Oncol. 2008;26:4595–4602. doi: 10.1200/JCO.2007.15.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baldus CD, Tanner SM, Ruppert AS, et al. BAALC expression predicts clinical outcome of de novo acute myeloid leukemia patients with normal cytogenetics: A Cancer and Leukemia Group B study. Blood. 2003;102:1613–1618. doi: 10.1182/blood-2003-02-0359. [DOI] [PubMed] [Google Scholar]
- 10.Falini B, Bolli N, Liso A, et al. Altered nucleophosmin transport in acute myeloid leukaemia with mutated NPM1: molecular basis and clinical implications. Leukemia. 2009 doi: 10.1038/leu.2009.124. 10.1038/leu.2009.124. [DOI] [PubMed] [Google Scholar]
- 11.Becker H, Marcucci G, Maharry K, et al. Favorable prognostic impact of NPM1 mutations in older patients with cytogenetically normal de novo AML. J Clin Oncol. 2009 doi: 10.1200/JCO.2009.25.1496. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Döhner K, Schlenk RF, Habdank M, et al. Mutant nucleophosmin (NPM1) predicts favorable prognosis in younger adults with acute myeloid leukemia and normal cytogenetics: Interaction with other gene mutations. Blood. 2005;106:3740–3746. doi: 10.1182/blood-2005-05-2164. [DOI] [PubMed] [Google Scholar]
- 13.Schnittger S, Schoch C, Kern W, et al. Nucleophosmin gene mutations are predictors of favorable prognosis in acute myelogenous leukemia with a normal karyotype. Blood. 2005;106:3733–3739. doi: 10.1182/blood-2005-06-2248. [DOI] [PubMed] [Google Scholar]
- 14.Thiede C, Koch S, Creutzig E, et al. Prevalence and prognostic impact of NPM1 mutations in 1485 adult patients with acute myeloid leukemia. Blood. 2006;107:4011–4020. doi: 10.1182/blood-2005-08-3167. [DOI] [PubMed] [Google Scholar]
- 15.Byrd JC, Mrózek K, Dodge RK, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: Results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100:4325–4336. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 16.Arber DA, Vardiman JW, Brunning RD, et al. Acute myeloid leukaemia with recurrent genetic abnormalities. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC; Lyon, France: 2008. pp. 110–123. [Google Scholar]
- 17.Redon R, et al. Global variation in copy number in the human genome. Nature. 2006;444:444–454. doi: 10.1038/nature05329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mullighan CG, Downing JR. Genome-wide profiling of genetic alterations in acute lymphoblastic leukemia: recent insights and future directions. Leukemia. 2009;23:1209. doi: 10.1038/leu.2009.18. [DOI] [PubMed] [Google Scholar]
- 19.Walter JM, Payton JE, Ries RE, et al. Acquired copy number alterations in adult acute myeloid leukemia genomes. Proc Natl Acad Sci USA. 2009;106:12950–12955. doi: 10.1073/pnas.0903091106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radmacher MD, Marcucci G, Ruppert AS, et al. Independent confirmation of a prognostic gene-expression signature in adult acute myeloid leukemia with a normal karyotype: A Cancer and Leukemia Group B study. Blood. 2006;108:1677–1683. doi: 10.1182/blood-2006-02-005538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marcucci G, Radmacher RD, Maharry K, et al. MicroRNA expression in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1919–1928. doi: 10.1056/NEJMoa074256. [DOI] [PubMed] [Google Scholar]
- 22.Schwind S, Marcucci G, Maharry K, Radmacher MD, et al. CALGB MicroRNA 181a expression as a prognosticator in cytogenetically normal acute myeloid leukemia. J Clin Oncol. 2009;27:15s. for the. abstr 7001. [Google Scholar]
- 23.Saito Y, Jones RA. Epigenetic activation of tumor suppressor microRNAs in human cancer cells. Cell Cycle. 2006;5:2220–2222. doi: 10.4161/cc.5.19.3340. [DOI] [PubMed] [Google Scholar]
- 24.Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 25.Li Z, Lu J, Sun M, et al. Distinct microRNA expression profiles in acute myeloid leukemia with common translocations. Proc Natl Acad Sci USA. 2008;105:15535–15540. doi: 10.1073/pnas.0808266105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fanini F, Vannini I, Faabbri M. MicroRNAs: tiny players with a big role in the pathogenesis of leukemias and lymphomas. Hematology Reviews. 2009;1:40–45. [Google Scholar]
- 27.Garzon R, Garofalo M, Martelli MP, et al. Distinctive microRNA signature of acute myeloid leukemia bearing cytoplasmic mutated nucleophosmin. Proc Natl Acad Sci USA. 2008;105:3945–3950. doi: 10.1073/pnas.0800135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andersen MT, Andersen MK, Christiansen DH, Pedersen-Bjergaard J. NPM1 mutations in therapy-related acute myeloid leukemia with uncharacteristic features. Leukemia. 2008;22:951–955. doi: 10.1038/leu.2008.17. [DOI] [PubMed] [Google Scholar]
- 29.Baer MR, George SL, Dodge RK, et al. Phase 3 study of the multidrug resistance modulator PSC-833 in previously untreated patients 60 years of age and older with acute myeloid leukemia: Cancer and Leukemia Group B study 9720. Blood. 2002;100:1224–1232. [PubMed] [Google Scholar]
- 30.Marcucci G, Moser B, Blum W, et al. A phase III randomized trial of intensive induction and consolidation chemotherapy ± oblimersen, a pro-apoptotic Bcl-2 antisense oligonucleotide in untreated acute myeloid leukemia patients >60 years old. J Clin Oncol. 2007;25:360s. abstr 7012. [Google Scholar]